Abstract
Involuntary movements and parkinsonism have been interesting and important topics in neurology since the last century. The development of anatomical and physiological studies of the neural circuitry of motor systems has encouraged the study of movement disorders by means of pathophysiology and brain imaging.
Multichannel electromyography from affected muscles has generated objective and analytical data on chorea, ballism, athetosis, and dystonia. Studies using floor reaction forces revealed the pathophysiology of freezing of gait in parkinsonism. Akinesia and bradykinesia are attributable to dysfunctions in the basal ganglia, frontal lobe, and parieto-occipital visual association cortex.
Reciprocal innervation is an essential mechanism of smooth voluntary movement. Spinal reflexes on reciprocal innervation has been investigated in awake humans, and the pathophysiology of spasticity and Parkinson’s disease were revealed as a result. Clinical applications for the treatment and evaluation of status have been developed.
For future studies, detailed neural mechanisms underlying the development of motor disorders in basal ganglia diseases and recovery by interventions including surgery and neurorehabilitation are important.
Keywords: Basal ganglia, involuntary movements, posture, EMG, behavioral study, H-reflex
Introduction
The basal ganglia are located at the base of the forebrain (cerebrum) and have attracted attention in medicine for various disturbances that appear with dysfunctions caused by diseases or trauma.
Various involuntary movements, including chorea, ballism, athetosis, dystonia, and difficulty in the execution of voluntary or natural movement in parkinsonism have been described clinically, and their correlation with pathological changes in the basal ganglia were established in the first half of the 20th century.
The neural mechanisms of such curious symptoms have been studied via neurophysiology, neuropathology, single neuron recording, and various neuroimaging techniques, including computed tomography, magnetic resonance imaging, single-photon emission computed tomography, and positron emission tomography.
Despite the fact that the actual neural processes underlying various motor disturbances have not yet been clarified, research on the basal ganglia in movement, posture, and cognitive functions based on neuronal activities has progressed in recent decades. In this review, the functions and dysfunctions of the basal ganglia in humans and in non-human primates will be described from a standpoint of neurology.
The basal ganglia — history of disease symptomatology and research in functions
In early anatomical definitions of the central nervous system, the basal ganglia included the thalamus, caudate nucleus, putamen, and globus pallidus because these are located in the base of the forebrain. Soon, the thalamus was removed from the definition, but the subthalamic nucleus in the diencephalon and the substantia nigra in the midbrain were included, because these two nuclei have close associations with the globus pallidus. The caudate nucleus and the putamen together are called the striatum using their macroscopic appearance, and the putamen and the globus pallidus together are called the lenticular or lentiform nucleus.
The basal ganglia have been considered as a motor center since the end of the eighteenth century. The classically established symptoms are involuntary movements including tremor, chorea, ballism, athetosis and dystonia; muscular rigidity; hypotonia; disturbances in standing equilibrium; gait; speech; and akinesia (lack of natural movement in daily living).1)
In the first half of the 20th century, the correlation between motor symptoms and the pathology of the basal ganglia was made,2) and the motor pathway through the basal ganglia was designated as the “extrapyramidal system”.3)
Jakob3) described the symptoms of basal ganglia disorders in detail, and correlated involuntary movement with underlying muscle tone, thus “hypotonisch-hyperkinetische Syndrom” representing chorea and “hypokinetisch-hypertonische Syndrom” for parkinsonism were divided.
The pathogenesis and pathophysiology of various involuntary movements remained unclear, because only naked eye observation or cinema-recording was available for the diagnosis of different patterns of involuntary movements until the middle of the second half of the 20th century.
Animal experiments on “extrapyramidal” disorders succeeded in the production of forward bending posture with akinesia by destruction of the bilateral globus pallidus.1,4,5) Instead, the production of involuntary movements met with serious difficulty, and only “choreoid hyperkinesia” as a model of ballism was succeeded by partial destruction of the contralateral subthalamic nucleus,6) and athetosis by partial destruction of the anterior and posterior putamen in infant monkeys.5)
Limitations in the analysis of motor disturbances in patients and difficulty in the production of animal models for basal ganglia disorders invited David Marsden, in the “Robert Wartenberg Lecture” at the annual meeting of the American Academy of Neurology in 19807) with the title “The Mysterious Motor Function of the Basal Ganglia”, to describe that “the basal ganglia were still retained in darkness as the characteristic of basement” by quoting the words by Kinnier Wilson (the discoverer of Wilson’s disease) in the Croonian Lecture in 1925.8)
Thereafter, progress has been gradual. In the 1960s, an anatomical substratum for neural networks involving the basal ganglia was developed by the invention with the Nauta method. Nauta established a staining method to trace fine projecting fibers from their target nucleus to the origin nuclei using a silver impregnation method. Then, a method with the retrograde transport of horseradish peroxidase was developed.
The striatum receives afferent projections from three main sources, the cerebral cortex, thalamus, and midbrain.
Kemp and Powell (1970)9) clarified projections from the cerebral cortex to the striatum in monkey. It was found that the whole of the cerebral cortex sends fibers to both the caudate nucleus and putamen, and the projection is arranged essentially on a topographic basis, with the heaviest projection from the somatic sensory and motor regions. From the thalamus, centro-median (CM) and parafascicular (PF) nuclei project to the striatum. A third input to the striatum is from the substantia nigra, which is an important substratum for Parkinson’s disease (PD).
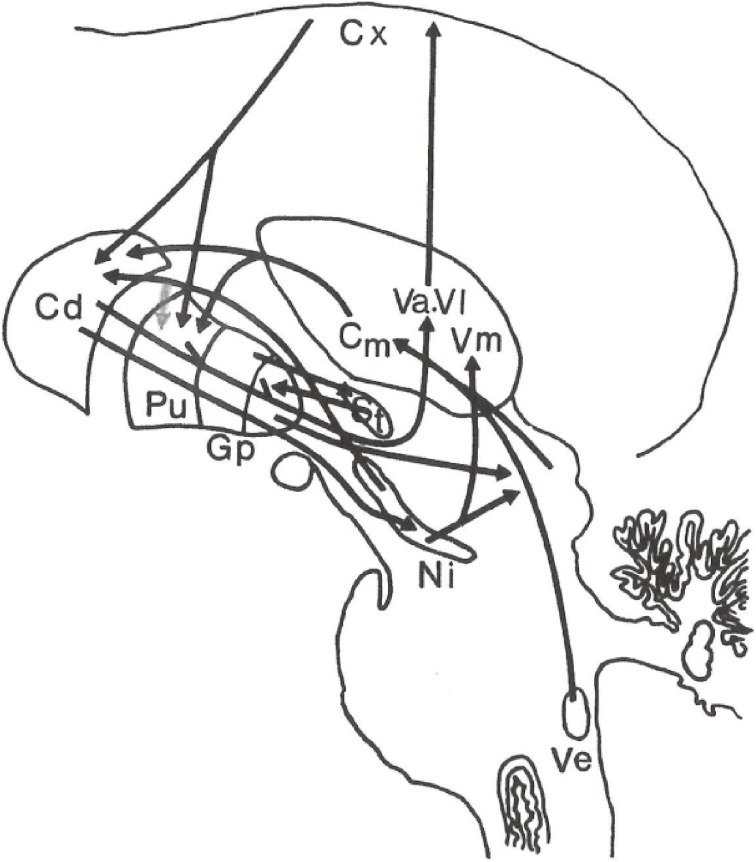
Furthermore, there is a strong projection to the CM nucleus from the medial and lateral vestibular nuclei and from the superior colliculus, which are considered to be related to postural reflex and equilibrium (Fig. 1).
Figure 1.
Principal fiber connections of the basal ganglia. Cx: Cerebral cortex, Cd: Caudate nucleus, Pu: Putamen, Gp: Globus pallidus, St: Subthalamic nucleus, Va, Vl, Vm, Cm: Nucleus ventralis anterior, ventralis lateralis, ventralis medialis, and centromedianus of the thalamus, Ni: Substantia nigra, Ve: Vestibular nucleus.
In the 1970s, physiological studies on involuntary movements started with application of multichannel surface electromyography.10,11) Qualification and quantitation of involuntary movements and their correlation with the detailed neuropathology of the basal ganglia has progressed.
In physiology, Evarts developed a recording method for single neuron activity in the brain in awake, behaving monkeys.12) With his coworkers, the study focused on the basal ganglia, cerebellum, and eye movement system. DeLong and coworkers tackled the basal ganglia, the most difficult target, and accumulated a tremendous amounts of data. They proposed cortico-basal ganglia relations in 1983.13) The new idea in their proposal was 1) introduction of a “loop” in the cortico-basal ganglia-thalamo-cortical circuitry, 2) introduction of a “motor loop” and “complex loop”, thus attributing cognitive function to the basal ganglia. Later, DeLong and collaborators proposed 3) parallel pathways, direct and indirect, in connections within the basal ganglia, and 4) the significance of the subthalamic nucleus.14)
Albin and coworkers also proposed direct and indirect pathways from the striatum to the internal globus pallidus15) independently from DeLong’s group.
DeLong proposed a model of hypokinetic disorders (parkinsonism) by hyperactivity of excitatory subthalamo-pallidal pathway, then an increase in pallido-thalamic inhibitory pathway suppressing thalamo-cortical projections. Hyperkinetic disorders (chorea, ballism) were explained by hyperactivity of the inhibitory putamino-pallidal pathway, resulting in a decrease in the pallido-thalamic inhibitory activity and hyperactivity of the thalamo-cortical pathways.16)
Based on this model, DeLong and coworkers succeeded in the recovery of a MPTP-induced experimental monkey model of parkinsonism by destruction of the subthalamic nucleus,17) which led to deep brain stimulation (DBS) of the subthalamic nucleus for the treatment of drug-resistant parkinsonian patients. As development of a new therapy based on research data and a proposed model, this has been evaluated as a great contribution by the group led by DeLong.
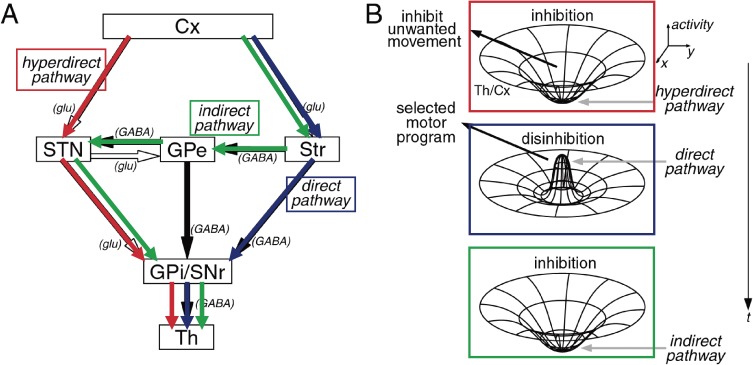
The cortico-basal ganglia-thalamo-cortical loop, especially the “motor loop” proposed by DeLong and coworkers, has been considered a model of basal ganglia function for voluntary movement. Based on this model, Nambu and coworkers proposed the importance of the cortico-subthalamo-pallidal “hyperdirect pathway” for the execution of voluntary movement (Fig. 2).18) Because patterns of elementary movement are considered restored in the cerebral cortex,5) the basal ganglia cause tonic inhibition on thalamo-cortical movement-triggering system at rest. In voluntary movement, the cortico-subthalamo-pallidal “hyperdirect pathway” inhibits all voluntary motor path activities, then disinhibition takes place using a direct pathway, and let the designated motor pattern release and the voluntary movement cease by inhibition of the thalamo-cortical pathway by the action of the indirect pathway. Thus, the inhibition-disinhibition-inhibition system is considered the function of cortico-basal ganglia-thalamo-cortical motor pathway.
Figure 2.
Dynamic model of the basal ganglia function. The direct pathway releases only the selected motor program. Hyperdirect and indirect pathways inhibit other competing programs and help the direct pathway to release only the selected motor program at the appropriate timing. See text for further explanation. (Courtesy of Professor A. Nambu).
Focusing on the prefrontal-basal ganglionic loop and various cognitive disorders in PD, cognitive dysfunction in PD has been a topic in neuropsychology and neuroimaging.
From classical neurology, the basal ganglia and the cerebellum are the two major motor centers controlling voluntary movements. Lesions in the basal ganglia or the cerebellum show distinctly different motor symptoms and signs either by neurodegenerative diseases or local lesions by trauma, neoplasm, or vascular disorders. However, in various movement disorders with affects of the basal ganglia, an increase in metabolic activity in the cerebellum has been reported recently. Furthermore, the existence of direct connections between the basal ganglia and the cerebellum has been revealed in primates.19)
Each center may compensate for dysfunction in the other. Actual processes in acquisition of functions in each motor center in ontogenetic motor learning and compensation for dysfunction of the other center, due to lesions with diseases or trauma, are still unknown.
With the development of various methodologies, the functions of the basal ganglia are being illuminated in detail, but the structure and function from a molecular basis to observable phenomena have not yet been clarified.
Involuntary movements in basal ganglia lesions
1. Short history.
From a historical perspective, chorea as a peculiar involuntary movement has attracted attention in neurology, and Jackson (1868)20) considered the instability of the striatal activities as a cause. Vogts (1920)2) and Jakob (1923)3) both introduced the idea of “release phenomenon” for involuntary movement in basal ganglia lesions. Following these, Hunt (1933)21) proposed that the globus pallidus is the final site of output of basal ganglia function, and differences in lesions of constituents of the putamen and globus pallidus may lead to hyperkinetic-hypotonic involuntary movements or to hypokinetic-hypertonic parkinsonism. This concept was accepted in neurology until the latter half of the 20th century.
Beyond this stage, Denny-Brown introduced an understanding of the peculiar involuntary movements based on the physiology of the Sherrington’s school in Oxford. Denny-Brown (1962)4) proposed ① movement is a change of posture, ② abnormal postures are an exaggeration of various reflexes, and ③ involuntary movement is a conflict of opposing postural reflexes.
2. Nature of involuntary movements revealed with surface electromyograms.
Involuntary movements in basal ganglia diseases include ① tremor, ② chorea, ③ ballism, ④ athetosis, and ⑤ dystonia.
Tremor appears in PD as an initial sign of the illness in 60–70% of patients.22,23) It soon disappears using levodopa medication. Brain circuitry for rhythmic activity involving the primary motor cortex neurons seems to manifest as lesions in the nigrostriatal pathway in PD.
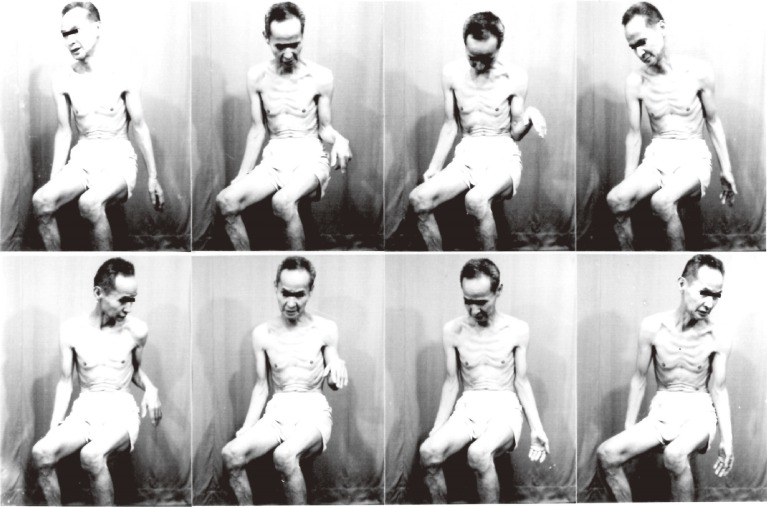
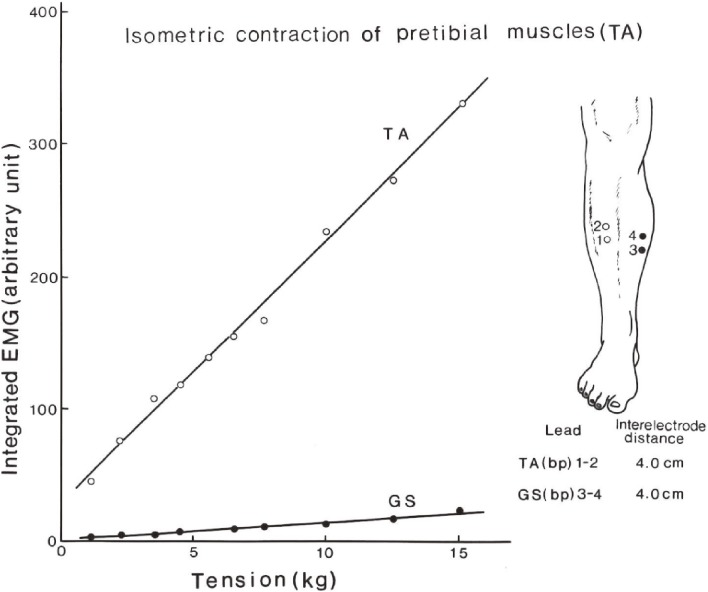
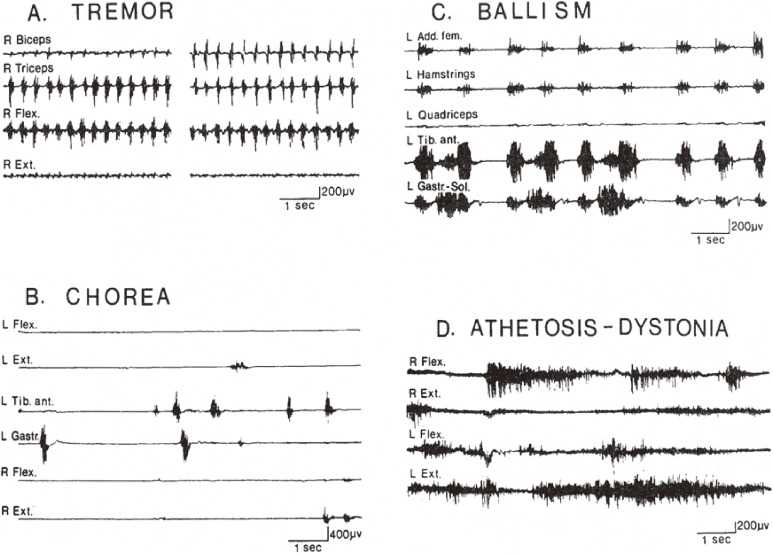
Movie recordings, video or serial photographs give images of peculiar involuntary movements (Fig. 3). Beyond naked-eye observation, electromyograms (EMGs) of the muscles involved give more objective and analytical information on involuntary movements with disorders of the central nervous system. Surface EMG with electrodes placed bipolarly on the skin covering a muscle record action potentials with contraction, and the amount of these potentials has a close correlation with the force produced (Fig. 4).24) With this method, the potential spread from other muscles including antagonists is minimal and can be ignored (Fig. 4).
Figure 3.
Serial photographs of a choreatic movement. From left to right, top to bottom, each frame was taken at 2 seconds intervals.
Figure 4.
Relationship between the amount of surface EMG and tension produced. EMG recorded bipolarly using surface electrodes placed 3–5 cm apart on the skin covering the muscle. A linear relationship exists between the amount of integrated EMG and tension produced (TA). Potential spread to antagonistic muscle can be ignored (GS). TA: pretibial muscles, GS: gastrocnemius-soleus muscles. (From Yanagisawa, 198424)).
With “multichannel surface EMG” for the study of involuntary movements, we usually record EMG from 8 to 12 muscles simultaneously. Recording of agonist and antagonist as a pair is necessary. Examples of EMG patterns in involuntary movements in the basal ganglia disorders are shown in Fig. 5.11) EMG in each type of movement showed unique patterns in terms of the duration of contraction, time course of repeated activities, and reciprocity in activity of antagonistic muscles. In chorea and ballism, in addition to brief involuntary contraction at rest, sudden brief interruption of contraction occurs randomly in tonic isometric voluntary contraction.24) Underlying muscle tone is hypotonic in chorea and ballism.
Figure 5.
Different EMG patterns in various involuntary movements. For explanation, see the text. (From Yanagisawa and Hashimoto, 199211)).
In contrast, athetosis and dystonia show continuous involuntary contraction of fluctuating levels (Fig. 5). Both flexors and extensors contract simultaneously, which means disturbance in reciprocal innervation, and underlying muscle tone is normal to hyperactive.
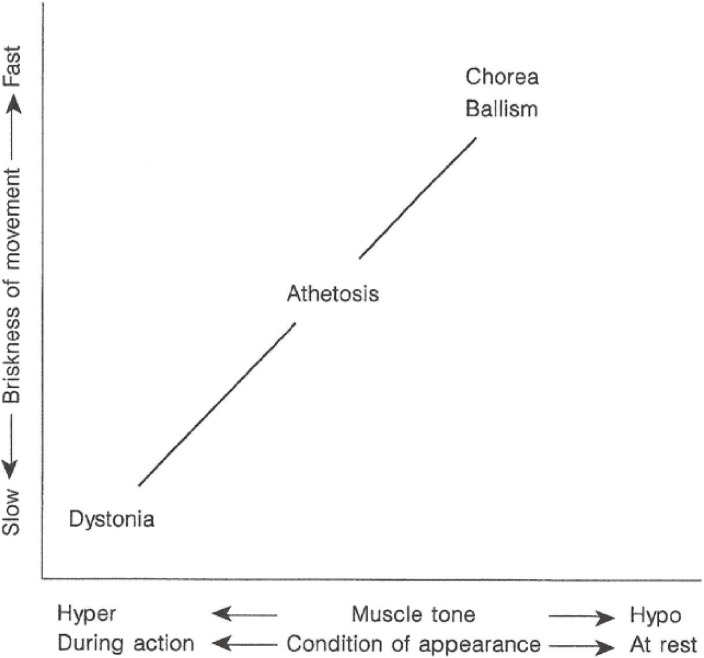
Conditions of appearance, whether the involuntary movement appears at rest or during action, and the underlying muscle tone vary among different types of involuntary movement, whether they are brief or tonic in contraction (Fig. 6).25)
Figure 6.
Relationships between briskness of involuntary movements, underlying muscle tone, and conditions for appearance (from Yanagisawa, 199625)).
In addition to involuntary movements, the responses to passive stretching of limb muscles give valuable information. Tonic stretch reflex (rigidity) or paradoxical contraction in relaxed, shortened muscles (Westphal’s paradoxical contraction) or induction of involuntary contraction can be observed.10,24)
The utility of surface EMGs and the characteristics of EMG patterns in various basal ganglia disorders are shown in Table 1.11)
Table 1.
Correspondences between clinical observation and EMG in basal ganglia disorders
| Clinical observations | EMG |
|---|---|
| Affected parts of the body | Simultaneous recording from many muscles |
| Pattern of movement | Pattern of EMG |
| Briskness | Duration of discharges |
| Irregularity | Irregularity of discharges |
| Amount of movement | Amount of action potentials |
| Underlying muscle tone | Tonic discharges at rest |
| Response to passive stretch | |
| Provoking or inhibiting factors | Recording under different physical or mental conditions |
(From Yanagisawa and Hashimoto, 199211))
Recently, there have been reports that ballism appears in vascular infarctions in the striatum26) instead of the subthalamic nucleus, which is the classical site for hemiballism. Thus, differentiation of ballism and chorea matters in terms of clinical neurology. The characteristic features of ballism are relatively regular movements at around 1 Hz with torsion of the proximal part of limbs. Instead, chorea is characterized by irregularity in the intervals in successive involuntary contractions. Similar characteristics between ballism and chorea are briefness of a single muscular contraction (Fig. 5B, C), underlying muscular hypotonia, and sudden, brief involuntary interruption of tonic voluntary contraction in the affected muscles.24)
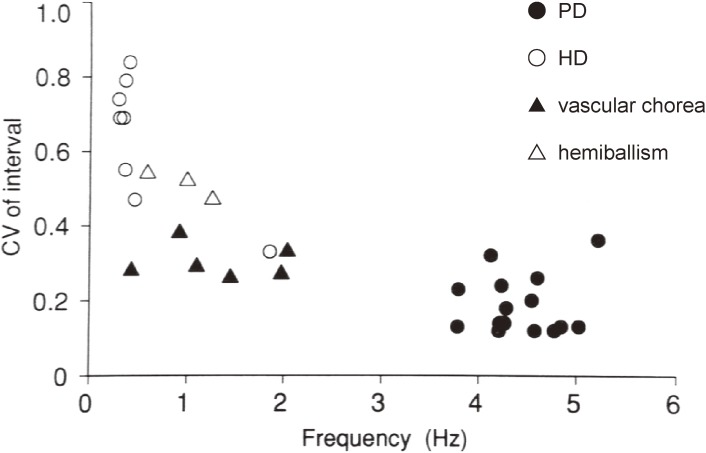
We focused our studies on the regularity of time intervals in successive involuntary contractions among classical hemiballism, Huntington’s chorea, vascular chorea (which included ballistic movements caused by lesions in the striatum), and the resting tremor in PD as a reference.27)
With multi-channel surface EMG, action potentials in the concerned muscles were rectified and integrated. From peak intervals in successive contractions in the most regularly discharged muscles, the coefficient of variation of intervals were calculated. The results are summarized in Fig. 7. The regularity of contractions in vascular chorea was significantly greater than that in Huntington’s chorea, and some patients with vascular chorea showed a regular rhythm, the degree of which approximated that of parkinsonian tremor. Regularity of muscular contractions in hemiballism by lesions in the subthalamic nucleus was greater than that in Huntington’s chorea, but lower than that in vascular chorea (Fig. 7). The high regularity of muscle contraction intervals in vascular chorea and hemiballism may arise from neural circuits that are activated abnormally, as well as those producing tremor of central origin as in PD or cerebellar diseases.
Figure 7.
Regularity of involuntary movements among tremor, ballism, and chorea. PD: Parkinson’s disease. HD: Huntington’s disease. In this figure, vascular chorea includes ballistic movement caused by lesions in the striatum. (From Hashimoto and Yanagisawa, 199427)).
3. Varieties in clinical expression in a disease affecting the basal ganglia.
Huntington’s disease is a hereditary disease with autosomal dominant (Mendelian) inheritance. The abnormal gene was identified in 1993. Common clinical manifestations are typical chorea with muscular hypotonia and dementia in the late stage of the illness. The main pathological change in typical cases (common or classical form) is the degeneration of efferent nerve cells (medium-sized spiny neuron) in the striatum. However, it has been recognized that patients with young onset at 10–20 years of age usually show parkinsonism with muscular rigidity and akinesia, instead of chorea. This type was called “rigid form” or “Westphal’s variant”. Classically, it was claimed that additional pathological changes were responsible for the development of the rigid form. Degeneration of large nerve cells in the striatum (Spielmeyer, 192628)), or nerve cells in the globus pallidus (Jakob, 19233); Denny-Brown, 19624)) were claimed in addition to the efferent striatal neurons.
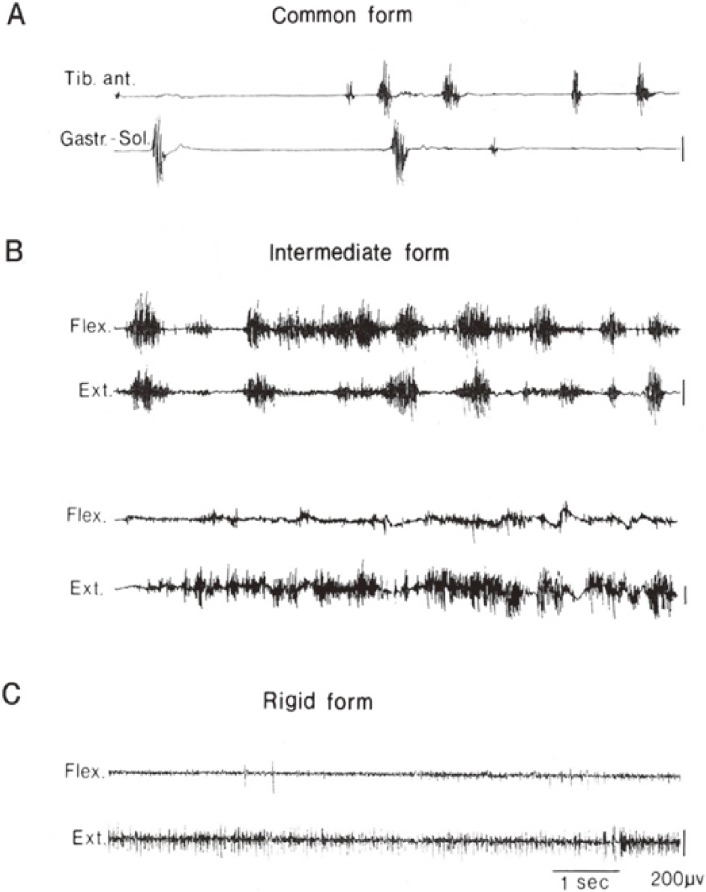
It was shown, however, that the motor manifestations are variable in addition to chorea and parkinsonism. With analysis of involuntary movement and underlying muscle tone using multichannel surface EMG in Huntington’s disease, it was found that there are cases with mixtures of tonic and phasic involuntary contractions in both flexors and extensors simultaneously (nonreciprocally) with normal to hyperactive muscle tone. This pattern is the same as athetosis or dystonia and we called this “the intermediate form” (Fig. 8).29)
Figure 8.
Various spectra of motor disorders in Huntington’s disease. Chorea (A), athetosis or dystonia (B), or parkinsonism (C) appear in different patients with this genetically determined single disease. See text for further explanation. (From Yanagisawa, 199229)).
Variability in clinical forms from chorea to parkinsonism in a gene-determined disease invites discussion on different clinical forms with pathophysiological correlations in the basal ganglia. There are two possibilities to explain the variations in clinical manifestation in Huntington’s disease. First, this is a triplet-repeat disease, and as the numbers of CAG repeats in the variant gene increases, an earlier onset of symptoms and wider range of neural degeneration occur in the striatum and in the globus pallidus. Another possibility is an effect of aging on the dopamine system in the basal ganglia. Activity of the nigrostriatal dopamine neuron has a marked age variation, especially in the first two decades.30) Clinical pictures in hereditary progressive dystonia with marked diurnal fluctuation (Segawa’s disease), which is dopa-responsive dystonia change with age from onset in childhood to the third or fourth decade in each patient.31) As another example, antipsychotic drugs for schizophrenia or for generalized tic have an antagonistic action on dopamine D2 receptors, and their side effects are dystonia in childhood and chorea (dyskinesia) in adulthood.
In Huntington’s disease, differences in clinical types of motor symptoms are well explained by different degrees of effect on each of parallel pathways from the striatum to the globus pallidus. In the rigid form, widespread lesions involve both direct and indirect pathways.32) As a result of the effect on the strio-pallidal inhibitory pathway, the pallido-thalamic inhibitory pathway is activated more than normal and akinesia appears. On the other hand, the direct pathway from the striatum to the internal globus pallidus is maintained in the classical (common) form, which suppress the pallido-thalamic inhibitory pathway, then activation of the thalamo-cortical pathway and motor cortex takes place. As a result, hyperkinesia occurs (Fig. 3). The intermediate form consisting of a mixture of chorea and athetosis (upper pair of EMG traces in Fig. 8B) to dystonia (lower pair of EMG traces in Fig. 8B) is explained well by the different degree of involvement of the strio-pallidal direct pathway.
The basal ganglia and posture
1. Animal experiments on the globus pallidus.
Widespread destruction of the bilateral globus pallidus (both of internal and external segment) in monkeys resulted in severe forward bending and a so-called “flexed posture” (Richter, 194533)), “somersault posture” (Carpenter et al., 19506)), or “pallidal posture” (Denny-Brown, 19624)). Its mechanism was presumed to involve impairment of the vestibular and visual postural righting by interruption of the projection from the thalamus to globus pallidus.5)
Forward bending posture by acute destruction of the globus pallidus was not fixed, but natural posture was resumed by lying on the floor or by visual stimuli.5) On the other hand, long-term inhalation of CS2 gas resulted in selective neuronal death in both the globus pallidus and the substantia nigra in Richter’s experiment,33) which resulted in flexed posture with muscular rigidity. This model could not change posture by vestibular or visual stimuli due to muscular rigidity by destruction of the substantia nigra.
2. Globus pallidus and postures in humans.
Postencephalitic parkinsonism was widespread around the world as a sequelae to the pandemic of von Economo’s encephalitis in the years around the 1920s. In severe cases, a complete forward bending posture was observed, as seen in monkeys with bilateral pallidal destruction.1) In such severe cases, nerve cells in both the globus pallidus and the substantia nigra disappeared almost completely.
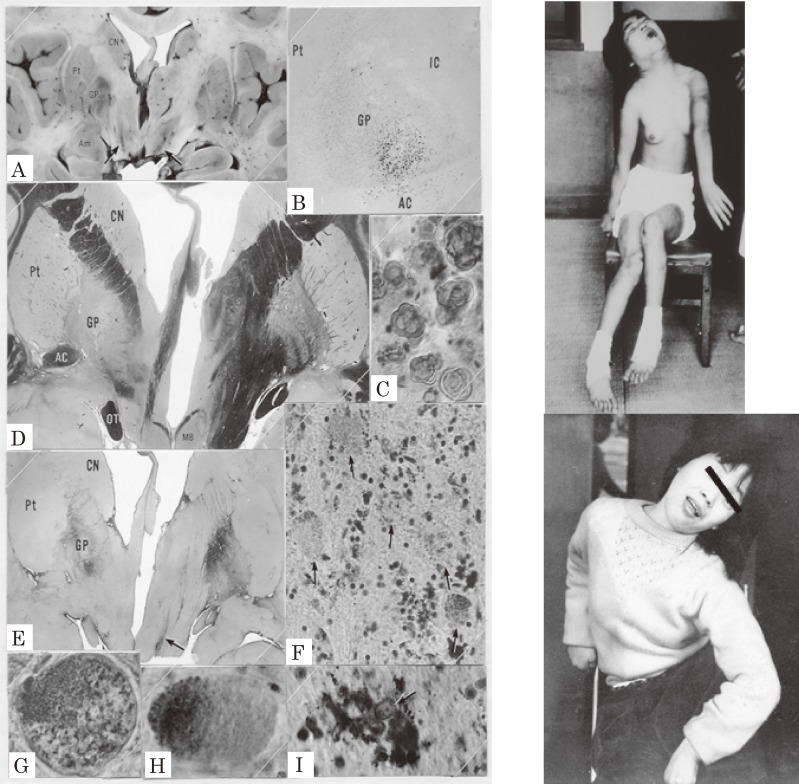
Hallervorden–Spatz (H-S) disease also affects neurons and neuroaxons in the GP and the reticular portion of the substantia nigra selectively. We had a chance to examine sister cases with H-S disease clinically and pathologically first in Japan.34) The clinical features started in childhood and showed dystonic movement and dystonic posture in both cases (Fig. 9). Dystonic movement was a slow twisting involuntary movement affecting the proximal limbs and the trunk. Dystonic posture included a bizarre twisting posture of the neck and the body (Fig. 9) contrasting with the forward bending posture in parkinsonism (cf. Fig. 12). EMG studies on both patients revealed nonreciprocal diffuse activation of the trunk and extremity muscles with grouping contractions (tremor) with the same pattern as the idiopathic torsion dystonia.10) A literature study on H-S disease showed that patients with dystonia showed slighter involvement of the substantia nigra compared with the lesions in the globus pallidus. In contrast, cases with dominant muscular rigidity had more severe involvement of the substantia nigra.34) Similar to PD, rigidity can be attributed to lesions in the substantia nigra.
Figure 9.
Pathology of the globus pallidus and dystonic postures of siblings with Hallervorden–Spatz disease. Pigmentation (A, B, I), demyelination (D), and gliosis (E) in the globus pallidus were marked and localized. Neurospheroids (F, G, H) were observed in the globus pallidus and substantia nigra. CN: caudate nucleus, Pt: putamen, GP: globus pallidus, IC: internal capsule, AC: anterior commissure. Dystonic postures were observed both in the elder (top) and younger (bottom) sisters. (From Yanagisawa et al., 196634)).
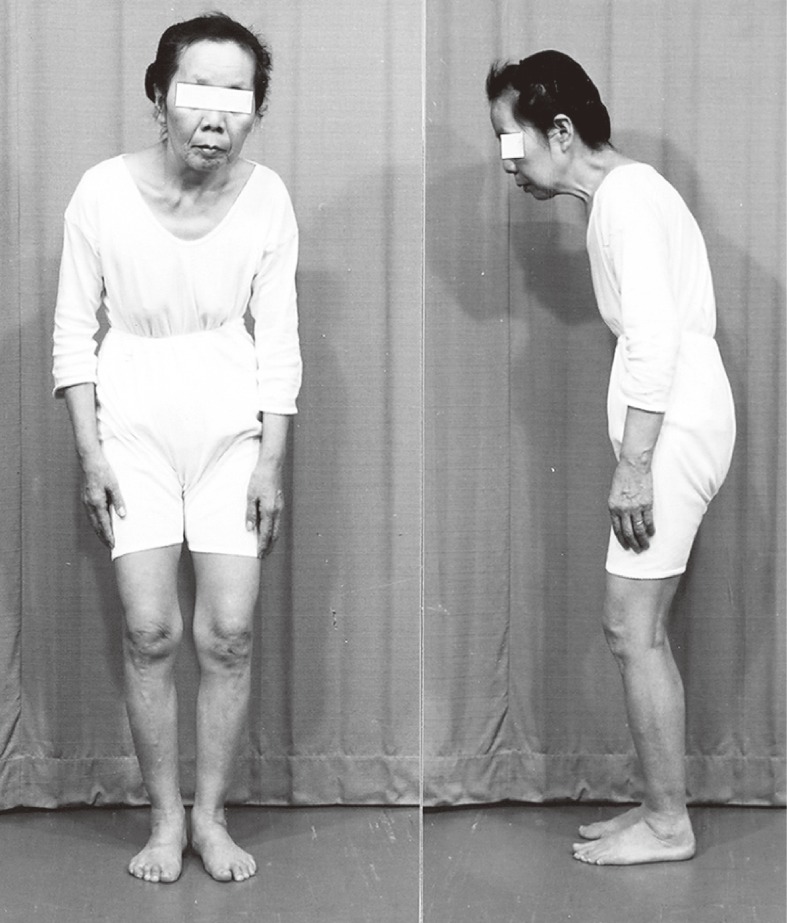
Figure 12.
Typical posture of Parkinson’s disease. Forward bending of the upper body with slight flexion at the knee is a stable, balanced posture.
As to the involvement of the globus pallidus, we experienced a patient aged 10 years old who suffered from complete destruction of the bilateral globus pallidus with the ectopic pinealoma, and showed characteristic dystonia similar to the hereditary torsion dystonia. The patient showed a typical dystonic posture with painful muscular contractions or muscular hypotonia in the same muscles on occasion.35) Changes of muscle tone from hypertonia to hypotonia in a muscle on different occasions was the origin of the term “dystonia”. Oppenheim (1911) coined “dystonia musculorum deformans” for this condition in his original work on hereditary dystonia.36)
Replacement of the whole tissue in the globus pallidus, including nerve cells and subserving structures, by malignant neoplasm was a form of natural experiment of globus pallidus destruction. It is important to note that typical dystonic posture and dystonic movement with changes of muscle tone from hypertonia to hypotonia appeared in childhood by destruction of bilateral globus pallidus alone.
3. Postural abnormalities in torsion dystonia and responsible lesions.
The disease concept of idiopathic torsion dystonia (ITD) was proposed by Oppenheim (1911)36) for a familial disease in mid-Europe, which is now established as the DYT-1 dystonia due to special gene transformations. DYT-1 and other genetic types of ITD exist worldwide, including in Japan.
ITD is characterized by postural abnormalities including the whole body. However, in the first half of the 20th century, the concept of dystonia widened and confusion on the clinical and pathophysiological basis of dystonia occurred. This confusion has been settled by the establishment of the gene abnormalities responsible and progress on pathophysiological research on the clinical symptoms.37)
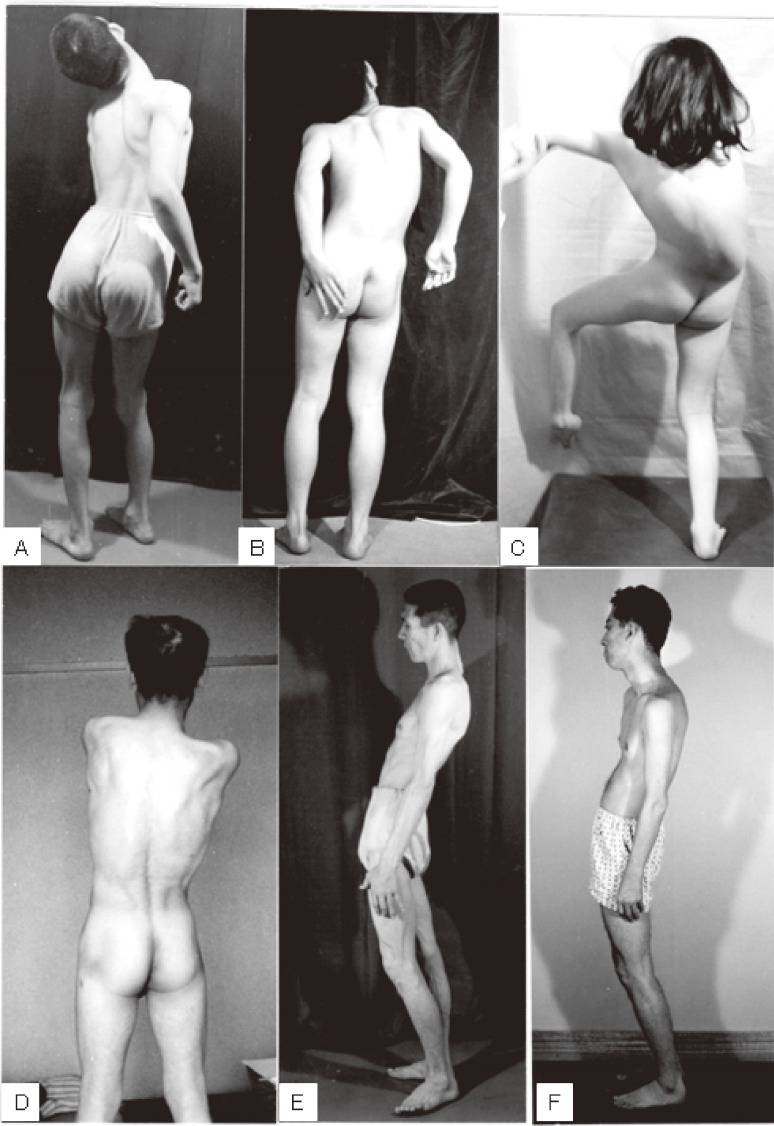
The standing posture of ITD is unique, with torsion of the neck and trunk, as shown in Fig. 10. The word “dystonia musculorum deformans” as proposed by Oppenheim36) is suitable, and imbalance between the developed volume of acting muscles and skeletal deformity results in permanent postural imbalance (Fig. 10C, D). Otherwise, in both children and adults, typical dystonic postures disappear when lying on the floor. Postural abnormality in ITD is a reversible posture attributable to abnormalities in postural reflex in space.5)
Figure 10.
Standing posture in idiopathic torsion dystonia (ITD). Torsion of the neck and trunk is more marked in children (A, B, C) than in adults (D, E, F). Dystonic posture disappears by lying on the floor.
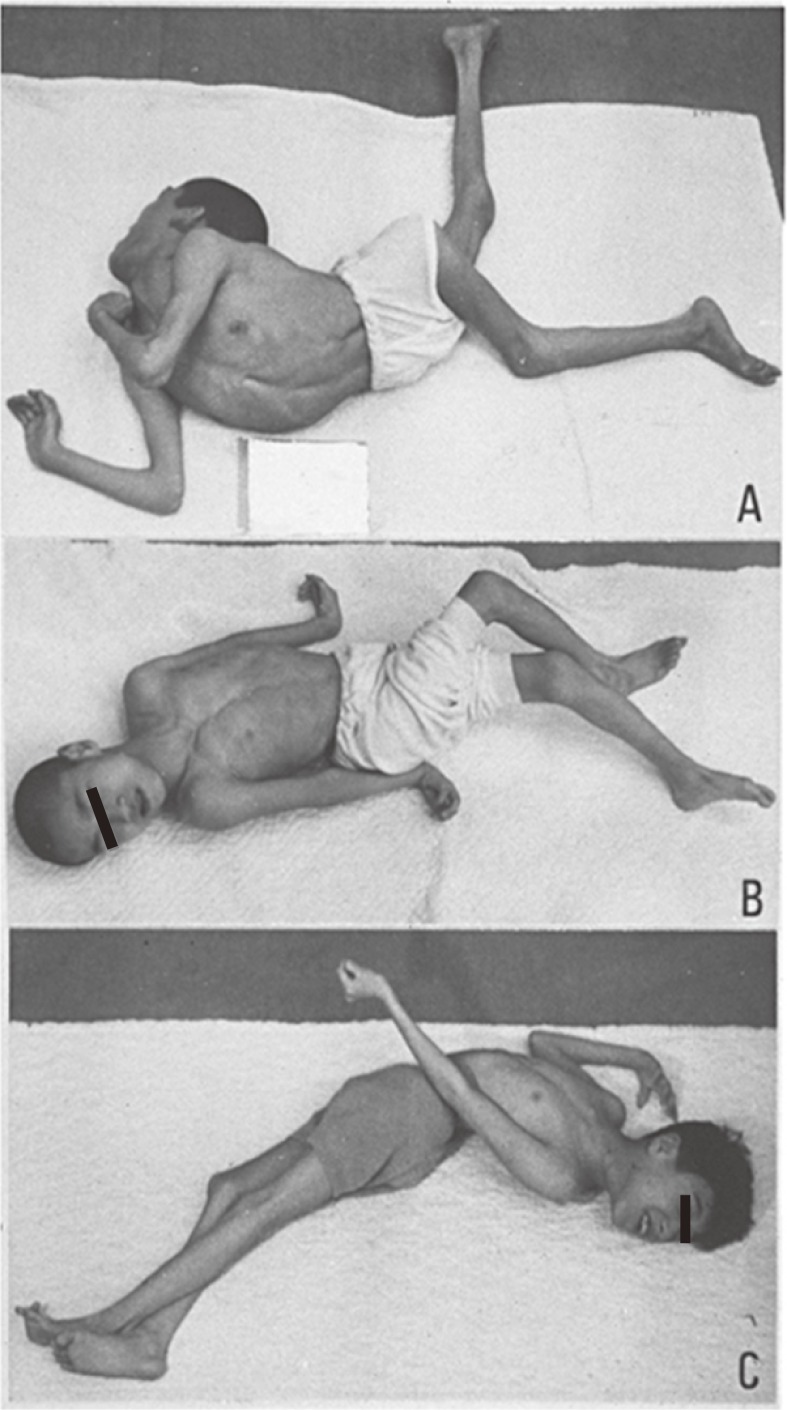
In contrast, dystonic posture in cerebral palsy caused by widespread lesions in the striatum (nucleus caudatus and putamen) is a reflexive, fixed abnormal posture associated with muscular rigidity (Fig. 11). Patients show opisthotonus (A), decerebrate rigidity (B), and tonic neck reflexes (C) as abnormal postures. The three patients shown in Fig. 11 suffered from severe perinatal asphyxia and the fixed posture that could not be changed either by contact stimuli or gravity. Medication including levodopa was ineffective, and stereotaxic operation on the globus pallidus or thalamus produced slight reductions in muscular rigidity, without apparent improvement in voluntary movement.
Figure 11.
Symptomatic dystonia caused by organic lesions in the striatum. Fixed abnormal postures with muscular rigidity are typical of athetotic or dystonic types of cerebral palsy.
Treatment of dystonia has progressed recently, particularly for ITD. Deep brain stimulation (DBS) either on the globus pallidus or the subthalamic nucleus resulted in improvement or abolishment of dystonia, both postural abnormalities and action dystonia (dystonia in voluntary movement). Of note is a fact that therapeutic improvement takes several weeks or months, which indicated the existence of plasticity in the neural networks underlying dystonia.
An enigma in ITD is that no histopathological changes can be detected in nerve cells or interconnecting fibers in the basal ganglia.38) On the other hand, recently developed functional brain imaging techniques have revealed metabolic brain networks associated with various movement disorders. Increases in metabolic activities exist in the posterior putamen, globus pallidus, cerebellum, and supplementary motor cortex in ITD, including DYT-1.39) Furthermore, in hereditary cases with the abnormal gene, the same brain imaging findings were observed before the appearance of clinical signs. This identification of abnormal activity in the brain networks underlining concealed dystonia is an important issue for further research.
4. Abnormal postures in Parkinson’s disease.
In PD, forward bending of the upper body with slight flexion at the knee is a typical posture on standing (Fig. 12). In this natural forward-bending posture, the center of foot pressure (COP) representing the point of gravity against the floor is the same as normal subjects with an upright posture.40) With the effort to stand in an upright posture, PD patients step backwards and fall down. This unique natural posture and instability to keep an upright posture can be easily corrected with levodopa treatment, at least in the early stage of the illness.41)
Disturbance in postural reflex may appear at an early stage of the illness in PD. A slight push on the chest backward leads patients to easily fall backwards without preventative postural reflexes. Forward bending posture with slight knee flexion is a stable balanced posture in humans. Sliding posture on skis or posture in the gait on a slippery floor or on ground such as an icy road resembles this parkinsonian posture to prevent falling backward.
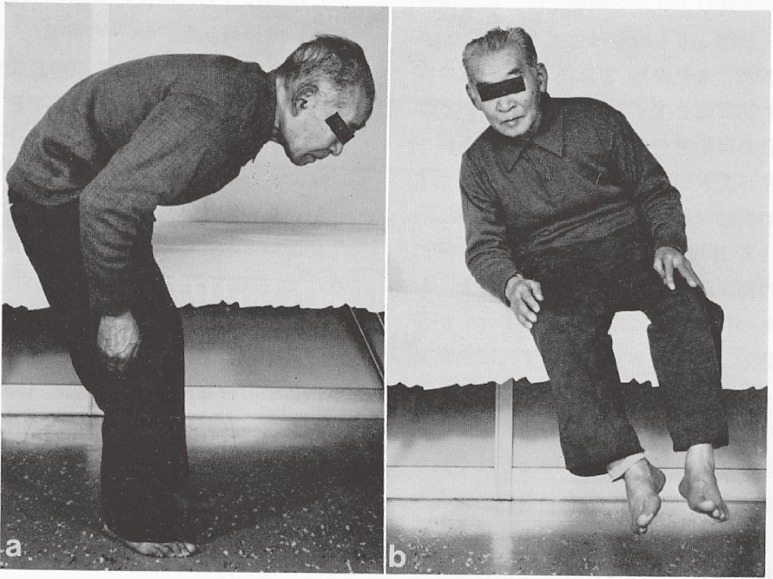
In PD patients with extreme forward bending or tilting posture on sitting (Fig. 13), awareness of self-posture is lacking and rehabilitation for recovery of natural posture is difficult in cases if the patient is unaware of their abnormal posture.
Figure 13.
Extreme forward bending or tilting posture in Parkinson’s disease. Patients are used to and unaware of this abnormality in posture.
In contrast, cerebrovascular parkinsonism (VP) shows an upright standing posture different from PD. VP patients keep an upright posture with both feet wide apart on the floor. However, similar postural disturbances such as in PD appear in response to external stimuli such as a pull or push backwards. The site responsible for lesions in VP comprise diffuse infarction in the cerebral white matter or infarctions in the striatum, particularly the bilateral putamen.
The mechanism of differences in postural disturbances between PD and VP remains unclear.
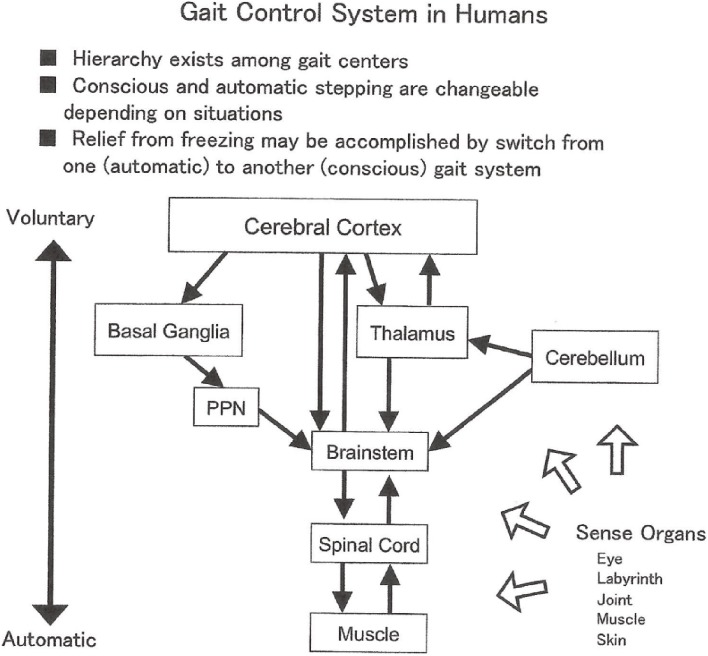
Acquisition of bipedal standing and locomotion with phylogenetic development has resulted in the development of centers for posture and gait from the spinal cord to the cerebrum, especially the frontal lobe, which primarily subserve psychic function, skillful manual motion, and language (Fig. 14). Hierarchical relationships may exist for motor centers in humans for posture and gait.
Figure 14.
Gait control system in humans.
There are supposed vulnerabilities for the human body from the acquisition of bipedal standing and locomotion in comparison with other quadruped mammals. It can easily be assumed by looking at anatomical substrata. In humans, fully developed skeletal musculature supports standing posture and locomotion with sophisticated innervation with developed central and peripheral nervous systems. In bipedal standing and gait, the cervical spine support the heavily developed head. The lumbar spine, hip, and knee joints support the whole body constantly. These structure suffer degeneration and deformation by daily living labor and aging. In addition to specific neural degeneration of locomotor centers in the central nervous system, as observed in parkinsonism, aging effects body structures and the functions of the nervous and musculoskeletal systems, thereby contributing to postural abnormalities and imbalance in diseases affecting the basal ganglia in aged patients.
Locomotor centers and gait disorders
Gait disorders in parkinsonism (hypokinetic-hypertonic syndrome) are short-stepped gait (marche à petit pas), retro- or ante-pulsion, which is forced stepping to falling down backwards or forwards in natural gait or to external mechanical stimuli, and freezing of gait (FOG).
From historical findings, gait analysis with floor reaction forces, EMG, and brain imaging, gait disorders involving basal ganglia lesions are summarized as follows.
1. Historical review.
The original description of the FOG is attributed to Gerstmann and Schilder (1926),42) who described a unique gait disorder in cases with frontal lobe tumors. They considered it as an “apraxia of gait”. This apraxia of gait was characterized by (a) rooting of the feet in the effort of walking, (b) marked disequilibrium, including falling backward, and (c) maintenance of voluntary motion of the extremities. They attributed its causative lesions to the medial-basal part of the frontal lobe. Later, Denny-Brown called this rooting of the feet in gait effort the “slipping clutch syndrome” and evaluated the foot grasp reflex as an important factor in the condition.43) “Foot grasp” is a reflex where the toes flex against the floor from pressure on sole and the surface of the toes. The methodology of the study of gait is listed in Table 2.
Table 2.
Methodology in study of gait
| Record of locomotion |
| Movie, video |
| Position sensor |
| Goniometer |
| Analysis of Gait |
| EMG of leg & trunk muscles |
| Floor reaction forces |
| Foot trace |
| Treadmill |
| Ambulatory gait record with pressure sensitive insoles |
| Center of foot pressure on standing |
| Posturography |
| Brain activity imaging at rest or during walking |
| PET |
| SPECT |
Gait is unique for humans, and analytical studies have been performed to identify the nature of phenomena in gait disorders in basal ganglia lesions from a physiological point of view.
2. Analysis of gait with floor reaction forces.
Small-stepped gait is common in PD and VP despite having different features. In normal subjects, cadence — the frequency cycle of stepping by the right and left feet in natural gait — is 0.85–1.0 Hz. In natural walking, cadence will be slower in old subjects but it did not exceed 1.1 Hz.44)
In PD, the cadence of natural gait was 0.9 ± 0.3 Hz,45) in the same range as in normal subjects. In short-stepped gait and FOG, PD patients showed unique features in gait analysis with floor reaction forces.45,46)
For the study of parkinsonian gait, a floor 4 m in length and 80 cm in width consisting of four force plates was installed in a walkway. Force reactions exerted on the floor by the feet during locomotion were measured in three dimensions (vertical, fore-aft, lateral) simultaneously and independently. The position of the center of body pressure on the floor (COP: gravity), cadence, the distance between the right and left foot in the lateral direction (X), the patterns of foot pressure in the vertical (Z) and fore-aft (Y) axes, the Z/Y vector of foot pressure, and on and off periods of the feet from the floor were measured for forward locomotion over 10 sec or a 4-m walk from the starting point. EMG of paravertebral, gluteal, thigh and leg extensors and flexors on both sides were recorded simultaneously.
Examples of floor reaction studies in normal, shuffling, and frozen gaits are shown in Fig. 15.46) In natural walking by normal subjects (Fig. 15A), 8–10 steps gained 4-meter’s progression (upper record). The step phase of a foot was characterized by two peaks in vertical pressure (Fz, middle record) corresponding to the increase in pressure on stepping in and kicking off from the floor (Z/Y, lower record). As observed in the middle record, during the two peaks of vertical pressure of a step by a foot, the pressure of the other foot was zero, showing the complete shift of the center of pressure produced by the body weight onto the other foot. The vector of floor reaction forces in the Z/Y direction shows the direction and amount of forces exerted on the floor during walking. The lower record shows a regular step-in and kick-off power of each foot in forward locomotion. Vectors below 90 degrees (horizontal line) show the force of step-in and vectors exceeding 90 degrees show the force of kick-back on the floor.
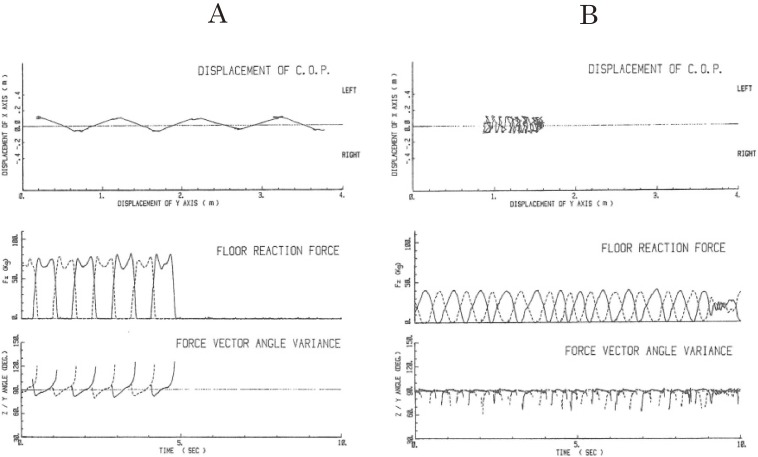
Figure 15.
Gait studies with floor reaction forces. A: sample records of floor reaction force studies in normal walking. Upper records: displacement of the center of pressure in a 4 m walk. Middle records: vertical component of floor reaction forces. The solid line shows the trace of pressure for the right foot and the dotted line for the left. Two peaks of vertical pressure at the beginning and the end of the step phase correspond to the step-in and kick-off forces. Duration of the complete shift of body weight to the foot on stepping is shown by zero pressure on the other foot. Lower records: z/y force vectors showing periodic step-in (below 90°) and kick-off (above 90°) forces. B: traces of locomotion and floor reaction forces in shuffling and frozen gait in a patient with Parkinson’s disease. Upper records: displacement of the center of pressure shows a shuffling gait with little forward movement in most of the record and cessation of locomotion at around the end as shown by dark tracings. Middle records: in a shuffling gait, both vertical pressure on stepping and zero phase in swing phases showed narrow single peaks at frequencies higher than normal. In freezing with no progression, which is shown in the last part of the record, the range of foot pressure became smaller and complete shifts of body weight on one foot were no longer observed. Lower records: z/y force vector showing frequent step-in forces but kick-off power was hardly observed even during shuffling gait. (From Yanagisawa, Ueno and Takami, 199146)).
In contrast, cases with shuffling gait showed changes in patterns (Fig. 15B): 1) The two peaks of vertical pressure in one step were replaced by a narrow single peak; 2) the duration of the zero pressure phase was much shorter (both in the middle record); 3) despite regular alternating shift in vertical pressure on stepping with both feet, forward locomotion was very little and irregular (upper record); 4) Z/Y force vector showed frequent step-in forces but little kick-off power (lower record), which corresponded with little forward locomotion (upper record).
Thus, forward locomotion was less than 1 m with more than 10 steps in the shuffling gait in PD. In FOG with no progression, which is shown at the last part of Fig. 15B, the range of foot pressure change became smaller, and a complete shift of body weight onto one foot was no longer observed (middle and lower record).
VP patients walked with a wider step width between the left and right foot at a faster rhythm around 1.5 Hz. Kick-off vector was small as in PD. Because both PD and VP show postural reflex disturbances, small-stepped gait and little kick-off power on walking may represent acquired gait pattern for defensive posture and prevention of falling.
On FOG, alternating shift in COP on the right and left foot appears at a faster rhythm than normal. A complete shift of COP on one foot becomes impossible as the frequency of cadence increases beyond a certain range. Thus, rooting or inability to lift one foot resulted in FOG.
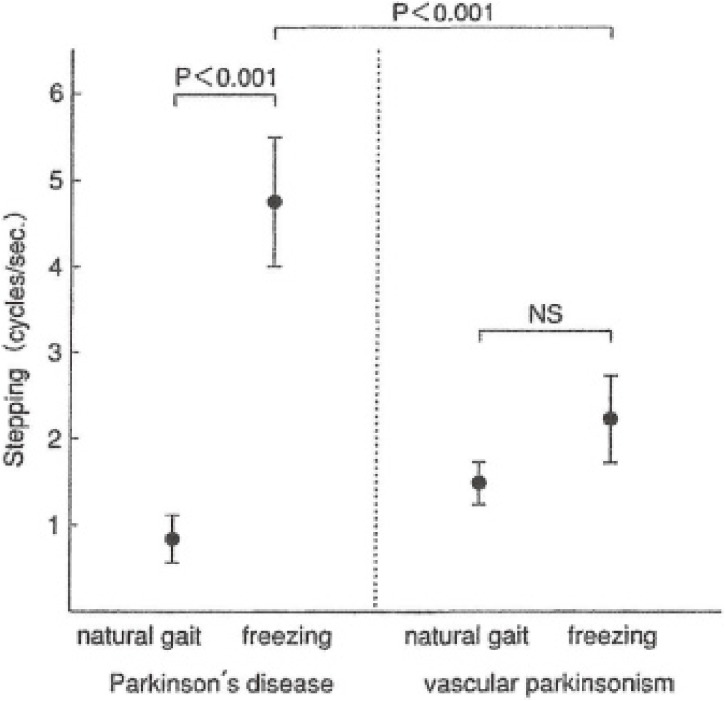
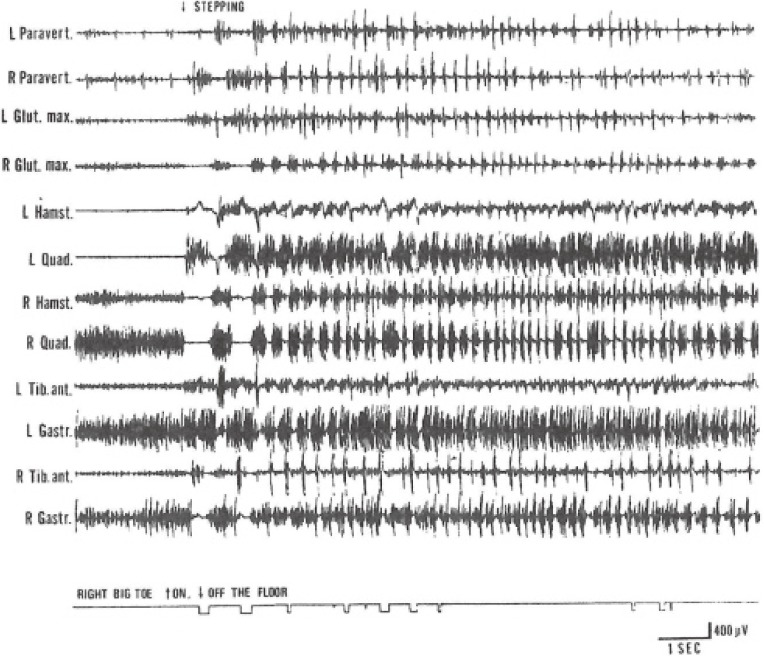
The rhythm of shift of COP in freezing was different in PD and VP. In PD, the rhythm of shift of COP jumped to 4.6 ± 0.8 Hz on freezing, which coincided with the rhythm of resting tremor in PD (Fig. 16).47) Naturally, the rhythm of shift of COP was due to the rhythm of muscular contractions in the thigh and leg muscles (Fig. 17). In PD, freezing of upper extremity motion was first analyzed by Narabayashi and Imai (1976)48) and was attributed to rhythmic contraction of the concerned muscles at 4–5 Hz, the same as in resting tremor.
Figure 16.
Rhythm of steps in Parkinson’s disease (PD) and cerebrovascular parkinsonism (VP). In natural, small-stepped gait, the rhythm of PD is normal and slightly faster in VP. In freezing of gait, the rhythm of one walking cycle in PD was significantly greater than VP, and it coincides with the rhythm of tremor and freezing of repetitive motions in the upper extremities. (Modified from Ueno, Yanagisawa and Takami, 199347)).
Figure 17.
EMG of freezing of gait. From the top downward, EMG from, left (L) and right (R) paravertebral, gluteus maximus, hamstrings, quadricepts, pretibial, and triceps surae muscles. The lowest record shows a period of touching (on) or lifting up (off) from the floor of the plantar surface of the right big toe. On stepping, grouping of muscular contractions at rates of 4–6 Hz developed after initial normal steps, corresponding to trembling of the legs and freezing of steps. (From Yanagisawa, Ueno and Takami, 199146)).
On the other hand, in VP, step rhythm gradually increases in walking, and freezing occurs when the rhythm (cadence) exceeds 1.7 Hz (Fig. 16).47)
FOG in PD may be released by various external or internal rhythmic stimuli. Examples are step-over marks or bars on the floor, auditory stimuli at an adequate rhythm with a metronome or even by voice by patient’s own. Conscious stepping with such rhythmical stimuli release patients from FOG. Because it is an impressively curious phenomenon for ordinary people, even doctors, it was called as “kinésie paradoxale” in French neurology. The famous doctor-novelist Oliver Sacks described release from FOG in parkinsonism patients in a book “Awakening” (1973),49) which described remarkable changes in symptoms in postencephalitic parkinsonism by the introduction of levodopa therapy.
Release from FOG by external rhythmic stimuli is explained by shift in acting locomotion center from a center for automatic stepping to one for voluntary or conscious stepping (Fig. 14).
Disorders in voluntary movement — Akinesia and Bradykinesia
1. History.
Slowness and poverty of natural and purposive movement is a characteristic motor disorder in PD, the most common disease affecting the basal ganglia.
In a famous Croonian Lecture (1925)8) on lesions in the striatum, Wilson listed ① weakness in motion (micrographia), ② delay in initiation, execution, and termination of movement, and ③ poverty of movement, as characteristics of voluntary movement in the striatal diseases and called “akinesis”. Martin (1967)1) described “akinesia” as a negative symptom from various causes in diseases of the basal ganglia. Walton (1977), in a textbook on neurology, called this difficulty in initiation of movement “akinesia” and slowness in the execution of movement as “bradykinesia”.
At present, from understanding of the pathophysiology and adequacy in experimental studies, “akinesia” describes a poverty of natural and purposive movement, and “bradykinesia” involves clumsiness and difficulty in purposive movement because of a delay in the initiation and execution of movement.
Akinesia in PD is characterized by a poverty of natural movement, such as facial expression or natural gestures in conversation. These symptoms do not fit with experimental findings and its mechanisms remain unclear. On the other hand, “bradykinesia” is easily studied; a delay in initiation of simple purposive movement by “reaction time”, and slowness in the execution of movement by prolongation of “movement time”. Furthermore, the inclusion of various paradigms in experiments made presumptions on the neural processes concerned. From the 1970s, studies have progressed in various fields, including physiology, psychology, and brain imaging.
2. Mechanisms of bradykinesia.
At present, the factors involved in bradykinesia revealed in experiments on PD patients are variable, as listed in Table 3. Bradykinesia is observed in natural movement and motor responses to external stimuli both in ordinary life and in experimental conditions.
Table 3.
Factors of bradykinesia
| Delay in reaction time | Prolongation of movement time |
|---|---|
| • Inattention | • Muscular rigidity |
| • Disturbance in shifting aptitude | • Easy fatigability |
| • Executive dysfunction | • Muscular weakness |
| • Delay in visual information processing | • Disturbance in output control |
| • Disturbance in delayed response ※disturbance in maintenance of response criteria | • Difficulty in switching of movement patterns |
| • Abnormality in disinhibition | • Difficulty in termination of movement |
| • Decrease in initial motor output | • Freezing: disturbance in rhythm in movement |
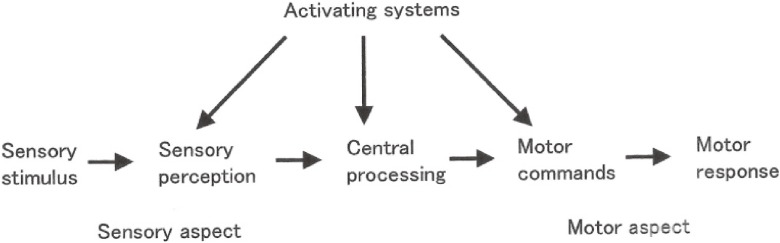
Neural processes in motor responses to external stimuli can be schematized, as shown in Fig. 18. The neural processes comprise perception of sensory stimuli, cognition of sensation and interpretation, selection of movement as the central process, motor commands, and motor responses.
Figure 18.
Scheme of neural processing in motor responses to external stimuli. (From Yanagisawa, 200635)).
(1). Decrease in output amount in ballistic movement.
In a simple rapid movement in PD patients, the first output strength does not reach a target, termed “hypometria”, in contrast with “hypermetria” in the cerebellar ataxia. In normal subjects, the first output reaches a target precisely, which is explained well by “pulse step model” (Schmidt et al., 1988).50) PD patients produce an insufficient amount of the first output and a staircase increase in accumulation of power to reach the target by a few to several steps with the severity of illness, thus the movement time is prolonged.51)
For rapid eye movements, short saccades accumulate to reach the target in PD.52) Thus, tasks requiring a combination of eye and hand movements require further prolongation of both reaction and movement times.53)
(2). Switch from one to another movement.
In PD, in addition to disturbances in eye and hand coordination, switching from one to another pattern of movement requires a longer time than normal in a series of sequential motions using different muscles.54) In such prolongation of switching time from one to another movement, both initiation and termination of each movement are delayed in PD.55)
(3). Rhythm disturbance.
In PD, freezing phenomena appear in the upper extremities in a similar pattern to the gait, as described in the previous chapter. In repetitive motions, such as tooth brushing, freezing may occur with rhythmic contractions of the muscles concerned with frequency at 4–5 Hz, the same frequency as the resting tremor in PD.
Freund stressed the importance of rhythm in voluntary movement in humans.56) Freund claimed that in voluntary movements in normal, a rhythm of 0–8 Hz is selected appropriately depending on the conditions of movement. In PD or cerebellar ataxia, fast movements are disturbed by convergence into tremor rhythm at 4–5 Hz, and slow movements requiring feedback systems goes into a faster rhythm by hastening phenomenon. Voluntary movements in PD are disturbed with a narrowing of rhythm in movement.
Rhythm has an important significance in motion in daily living, such as gait, writing, cooking, and other activities. Abnormalities, such as tremor or freezing, are interesting regarding how and why these appear in actual movements with disturbances in motor centers such as the basal ganglia or cerebellum. Because closed circuits in the nervous system may easily produce oscillation of neuron discharges, lesions in cerebro-cerebellum-cerebral circuits may produce rhythmic activation of motor neurons in the primary motor cortex.
(4). Disturbance in sensory information processing and in cognitive function.
In PD, simple reaction time, which comprises a simple motion by the fingers to visual or auditory go-signal, is normal in the early stages. It becomes prolonged as the disease progress, but improves to normal by gaining attention through a warning signal or a cue.51) This type of improvement in action by gaining attention is considered common to ordinary experiences, in which bradykinesia improves greatly in emergency situations such as a fire or accident.
With improvement by gaining attention, there are common features between PD and frontal lobe lesions. These comprise ① attention deficit, ② masked face, ③ bradykinesia, ④ difficulty in initiation and termination of movement, ⑤ difficulty in simultaneous execution of two different motions by two limbs, and ⑥ FOG. These symptoms and signs in frontal lobe lesions were pointed out before research on PD had progressed.57,58) These findings were understandable, because the frontal lobe is the brain for action and has close relations with the basal ganglia.14)
In addition to frontal lobe functions, PD patients show disturbances in visual cognition,59) visual information processing, and visuomotor responces.51,53) As a base for these disorders, a decrease in blood flow in the occipitoparietal visual association area was detected in PD patients without dementia.60)
For consideration of functional disorders in the cerebral cortex as factors contributing to bradykinesia in PD, there are ample data showing disorders in both the frontal lobe and visual association area. However, in comparison with disturbance in visual processing, as revealed by a reduction in local blood flow, it is not yet established whether there is a primary disturbance in the frontal lobe or it is disturbance through basal ganglia dysfunction.
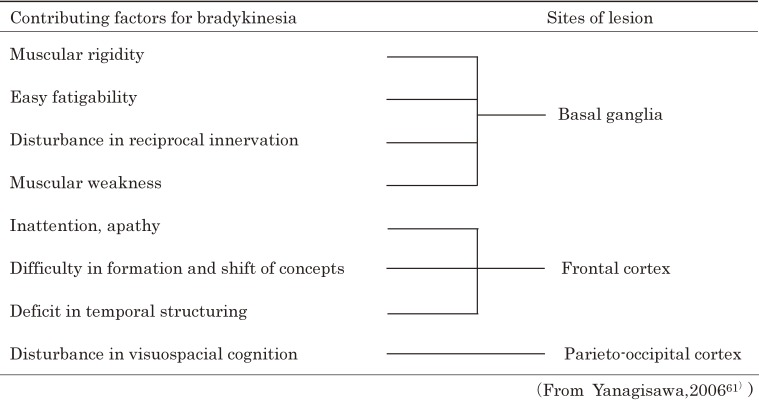
In summary, contributing factors and responsible sites in the brain for bradykinesia, as studied on PD, are listed in Table 4.61)
Table 4.
Contributing factors and brain sites responsible for bradykinesia in Parkinson’s disease
H reflex as a tool for investigation of central nervous disorders in awake humans — reciprocal organization of spinal reflex activities —
1. Reciprocal innervation is essential for smooth movement of limbs.
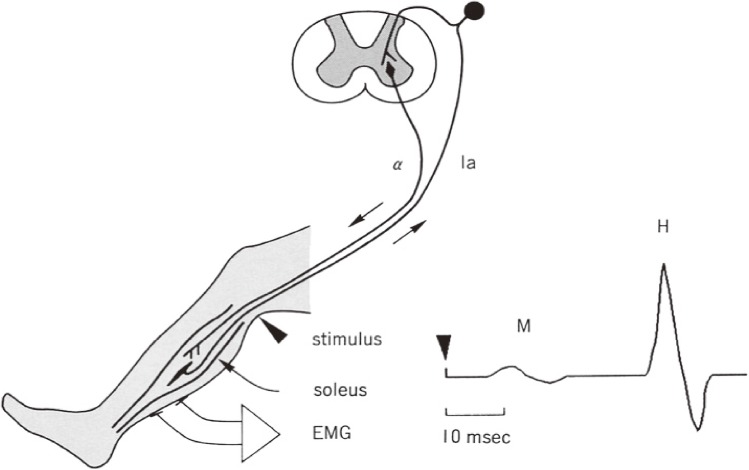
H reflex is homologous to the monosynaptic reflex in the spinal cord,62) which enables us to investigate the motoneuron activity in the spinal cord in humans in an awake state. The method of measurement of H reflex is shown on Fig. 19.
Figure 19.
Method for H reflex study. H reflex in humans has been studied mostly for the triceps surae muscles (soleus and gastrocnemius) in a sitting position on a reclining chair supported at the ankle. Electrical pulse stimuli are given to the tibial nerve at the popliteal fossa. The tibial nerve is a mixed nerve containing Ia afferent fibers from and alpha efferent fibers to the triceps surae. The threshold for excitation of Ia fibers is lower than alpha motor fibers with stimulation with square wave electric pulses of 1–3 milliseconds duration. Thus, with gradual increases in stimulus intensity, the appearance and gradual increase of the H reflex (monosynaptic reflex from Ia afferent fibers) is followed by the appearance of an M wave, which is caused by direct activation of alpha motor fibers. Studies on reciprocal reflex connections and influences from descending motor tracts are made with the stimulus condition for the maximal amplitude of H reflex at rest.
It is a useful tool to investigate the pathophysiological mechanism of motor disorders in disturbances of central motor control systems.
Reciprocal innervation is an essential mechanism for movement of the limbs. Flexors and extensors exist around a joint in the limbs, and contraction of either of them with relaxation of others (antagonists) enables smooth movement around the joint. Simultaneous contraction of both flexors and extensors fix the joint firmly. Rene Descartes (1596–1650) already pointed out that “the only reason for every movement of limbs is shortening of a muscle by contraction associated with elongation of its antagonistic muscle. The only reason for contraction of a muscle against its antagonists is because larger amount of ‘pneuma’ comes from the brain to the muscle”.63) Reciprocal innervation of skeletal muscles is essential as a function of the cerebral motor cortex as established by Sherrington in 1906.64)
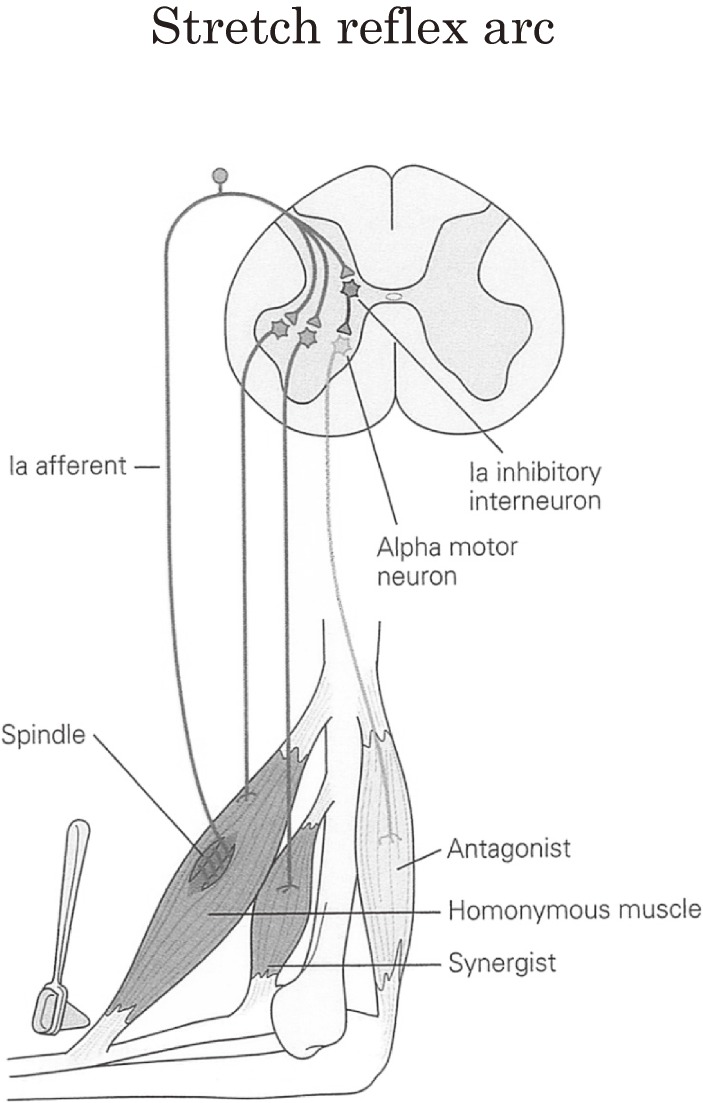
In mammalian spinal reflexes, large-diameter afferent fibers from muscle spindles (Ia) makes monosynaptic facilitation to the homonymous and synergist muscles, which is the main constituent of stretch reflex, and disynaptic inhibition to the antagonistic muscles (Fig. 20). These reciprocal reflex connections were well established by Lloyd and Laporte in the feline spinal cord.65,66) The interneurons, which mediate this reciprocal inhibition, Ia inhibition, are referred to as ‘Ia inhibitory interneurons’. These Ia inhibitory interneurons are under the influence of various descending pathways, including the pyramidal tract.67,68)
Figure 20.
Stretch reflex and reciprocal innervation. Stretching of skeletal muscles around a joint produces contraction of the stretched muscle via excitation of Ia afferent fibers and relaxation of its antagonists by reciprocal Ia inhibition via Ia inhibitory interneurons. For further explanations see text.
In spite of a large number of studies on monosynaptic or stretch reflex activity, little had been discovered about the reciprocal inhibitory pathway in the human spinal cord.
2. H reflex for study of reciprocal innervation in human motor disorders of central origin.
The first study on spinal reflex mechanisms of reciprocal innervation using H reflex in awake humans was published in 1971 on healthy subjects and the bilateral athetosis of cerebral palsy.69) Cerebral palsy with athetosis or dystonia shows marked disability in voluntary movement, with disturbances in reciprocal innervation on limb muscles.10) However, marked reciprocal Ia inhibition from the common peroneal nerve to the triceps surae motoneurons was observed in adult cerebral palsy (Fig. 21, right), whereas reciprocal Ia inhibition on the triceps surae muscles was not observed in normal subjects at rest (Fig. 21, left).69) As reciprocal innervation in limb muscles in ordinary voluntary movement is greatly disturbed in cerebral palsy, the existence of marked reciprocal Ia inhibition was considered as a sign of compensation or plasticity for voluntary motor control acquired in daily living in adult cerebral palsy.69)
Figure 21.
Reciprocal reflex connections in humans. For the study of reciprocal reflex connection in humans, the H reflex is used as an indicator of activity level of motoneurons. Reciprocal reflex effects are measured by giving stimuli to the nerve innervating their antagonists (conditioning stimuli) at various time intervals preceding a test H reflex and at various intensities measured by threshold of the M wave (XMT). Effects of reciprocal reflex connections at rest are shown for a normal subject on the left and an adult cerebral palsy subject on the right. Left figure. A normal subject. The time course of effects of the antagonist muscle nerve stimulation on the H reflex in the calf muscle is shown. The amplitudes of the test H reflexes are expressed as a percentage of the control amplitude, which is indicated on the ordinate with its standard deviation at the right side of graphs A and B. The abscissa shows the time interval between conditioning and test stimuli on a logarithmic scale. A: conditioning stimulus intensity was 1.05 XMT. B: 1.34 XMT. C: diagram of the time course of ankle joint movement by a strong conditioning volley on the same time scales as that of A and B. Upward deflection indicates ankle dorsiflexion. Right figure. A patient with bilateral athetosis. A: similar illustration to the left figure. The scale of the abscissa is different. Strong inhibition is observed within 5 msec of the conditioning-test stimuli intervals. The intensity of conditioning stimulation was 1.3 XMT. B: photographic records of the control (c) and effects at earlier intervals less than 7 msec (1–4). C: potential spread to the calf muscle (soleus and gastrocnemius) from the evoked EMG in pretibial muscles by conditioning stimulus alone. (From Mizuno, Tanaka and Yanagisawa, 197169)).
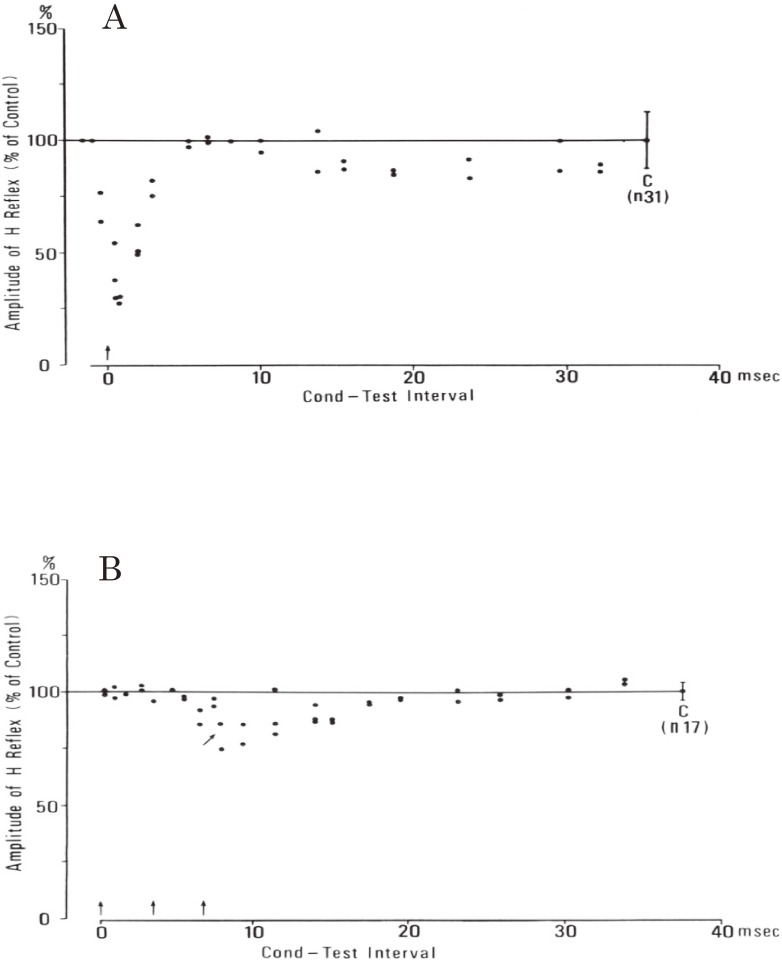
In the next stage, we studied reciprocal Ia inhibition on spastic hemiplegia caused by vascular lesions involving the internal capsule.70) Cerebrovascular lesions involving the internal capsule are commonly characterized by Wernicke–Mann’s posture. In this condition, the physiological extensors of the lower limb (e.g. quadriceps femoris and triceps surae) develop marked spasticity with relative preservation of muscle power, in contrast to marked loss of strength in the physiological flexors (hamstrings and pre-tibial muscles). This reciprocal pattern of motor disorder, extensor spasticity, and flexor weakness is a direct expression of the abnormal balance of spinal reflexes affected by chronic lesions of the higher centers, which involve amongst others the descending pathways, the corticospinal or pyramidal tract. Therefore, analysis of spinal reflex activity was highly beneficial in understanding the pathophysiology of this condition.
In the chronic stage of capsular hemiplegia, H reflex and even the tendon reflex are elicited in pretibial muscles in addition to hyperactive tendon reflex with ankle clonus in the triceps surae muscles. H reflex, analogous to monosynaptic reflex, is inhibited by stimulation of low threshold group Ia fibers from antagonistic muscles, both in pretibial muscles (physiological flexors) and triceps surae muscles (physiological extensors) (Fig. 22). The amount of reciprocal Ia inhibition was markedly different between flexors and extensors. Pre-tibial H reflex showed early and marked depression by very weak single stimulation of the tibial nerve, which was attributable to Ia reciprocal inhibition (Fig. 22A). In contrast, the effect of stimulation of the peroneal nerve on the H reflex of the triceps surae was absent or very weak. In a small number of cases, high frequency repetitive stimulation of the peroneal nerve produced a weak depression of the H reflex of the triceps surae at short latency (Fig. 22B).70)
Figure 22.
Reciprocal Ia inhibition in spastic hemiplegia. In both A and B, the ordinate shows the amplitude of the test H reflex expressed as a percentage of the control amplitude (at C with standard deviation). The abscissa shows the time interval between the first conditioning and the test stimuli. Conditioning stimuli are indicated by arrows. A: from extensor (tibial) nerve to flexor (pretibial muscle) H reflex. Single conditioning stimulus with a strength of 0.75 XMT produced marked inhibition with short latency, which is attributable to Ia inhibition. B: from flexor (peroneal) nerve to extensor (triceps surae) H reflex. The inhibitory effect was very weak compared with A. Three peroneal stimuli at 1.56 XMT, 300 Hz produced slight inhibition from 8.0 msec (arrow). (From Yanagisawa, Tanaka and Ito, 197670)).
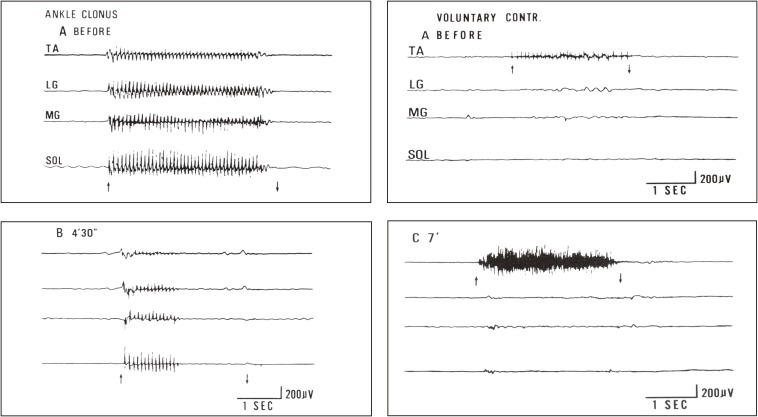
With the purpose of balancing, we made temporal alcohol blocks to the afferent large fibers (involving Ia fibers) in the extensor nerves. The results showed a decrease in ankle clonus because of excessive hyperreflexia in extensors (triceps surae) (Fig. 23, left) and an increase in voluntary muscle power in flexors (pretibial muscles) (Fig. 23, right).70) It was concluded that Ia reciprocal inhibition reduced the muscle power in the pretibial flexors in the natural course of capsular hemiplegia and blocking of Ia afferents from the extensors increased voluntary power of pretibial flexors. This physiological data explained well why conventional orthopedic surgery, such as tendon elongation or tenotomy of ankle extensors, improved ankle joint fixation or stiff foot without a flaccid ankle joint and helped in the rehabilitation of capsular hemiplegia.
Figure 23.
Effects of nerve block to spastic muscles in capsular hemiplegia. Changes in ankle clonus (left figures) and voluntary muscle power in pretibial muscles (right figures) before (A) and after (B, C) nerve block to motor points of the medial and lateral gastrocnemius muscles are shown. Simultaneous recording from four muscles using wire electrodes. From top downwards; TA: tibialis anterior. LG: lateral gastrocnemius. MG: medial gastrocnemius. SOL: soleus muscles. Left figures: EMG of leg muscles during stretching to evoke maximal ankle clonus (between arrows). Records before (A) and 4.5 min after (B) injection of 1 ml of 50% ethyl alcohol into the gastrocnemius motor points. Right figures: EMG of leg muscles at maximal voluntary contraction of the pretibial muscle (TA) before (A) and 7 minutes after (C) alcohol block of the lateral and medial gastrocnemius muscles. After extensor (gastrocnemius) block, a marked increase in EMG and actual strength occurred in the flexor pretibial muscle (TA), whereas the blocked gastrocnemius muscle showed no reduction in strength but resulted in smoother contraction. (From Yanagisawa, Tanaka and Ito, 197670)).
3. Elaboration of physiological studies in humans using H reflex.
The H reflex is composed of a mass electrical muscle response resulting from the firing of the motoneuron group innervating a muscle or synergistic muscle group (Fig. 19). Activity of the motoneuron pool recorded as H reflex may have contamination from activation of different sources of afferent roots or muscles. Shindo and coworkers recorded motor unit firing compared with H reflex and elaborated a method for the evaluation of central effects on the spinal motoneurons.71) Computer control of complicated data with single motor unit discharges gave a new method for evaluating the activity level of motoneurons, which were designated as “firing probability index” or “critical firing threshold” method.72)
Using these renewed methods, Shindo, Morita, and coworkers accumulated data on reciprocal Ia inhibition, presynaptic inhibition, Ib inhibition, or Ia facilitation in PD,73,74) spasticity,75,76) normal aging,77) and normal gait.78)
What is the role of the basal ganglia
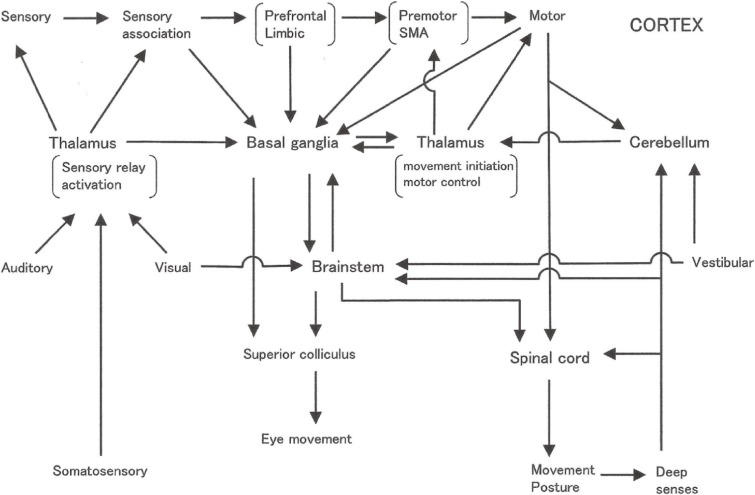
The basal ganglia have been considered as the center of voluntary movement. In humans, the final output of voluntary movement is the motor cortex (area 4 of Brodmann). Circuitry involving motor-related cortices, including the premotor area, supplementary motor area–the basal ganglia–thalamus–motor, and related cortices is considered the site of selection, decision and execution of voluntary movement.14)
As the precise mechanisms at the neuron level have not yet been clarified for voluntary movement, involuntary movement, bradykinesia, and akinesia, we have to consider the neural processes from phylogenetic and ontogenetic stand points. Considering the effects of motor training in physical development and in athletics, and effects of rehabilitation with lesions in the brain area normally involved in the execution of movement, flexibility and plasticity of the central nervous system should be taken into consideration when we consider the role of the nerve centers before the final common path (area 4).
1. Selection of information and decision of pattern of movement.
The basal ganglia receive somatotopical projections from the whole cerebral cortex,9) especially strong projections from the frontal lobe, the limbic system, and the sensory association area, especially for vision. Furthermore, the basal ganglia receive projections from the central part of the thalamus (centromedian [CM] and parafascicular [PF] nuclei).79) After information processing within the basal ganglia, the final output projects through the globus pallidus to the motor-related cortices, frontal lobe, limbic cortex, and brainstem mainly to the pedunclo-pontine nucleus (PPN).
Present understanding of the function of the basal ganglia in humans involves the selection and execution of movement and posture. The principal activity of the globus pallidus to the thalamo-cortical system is tonic inhibition and phasic disinhibition.18,80) Usually, various motor patterns with the potential to be executed are prepared but are under tonic inhibition, and the release of one motor pattern by disinhibition leads to execution. One merit of disinhibition is in its rapidity.
At present, the patterns of voluntary movement are restored in the cerebral cortex5) and restored patterns of movement are acquired mainly through activities of the basal ganglia and the cerebellum by learning in ontogenetic development, exercise, and experiences. Then, the basal ganglia and cerebellum go into action in the selection and execution of a movement. This type of function of the basal ganglia may resemble the function of the hippocampus in the restoration and reproduction of memory.
2. Phylogenetical development of the brain.
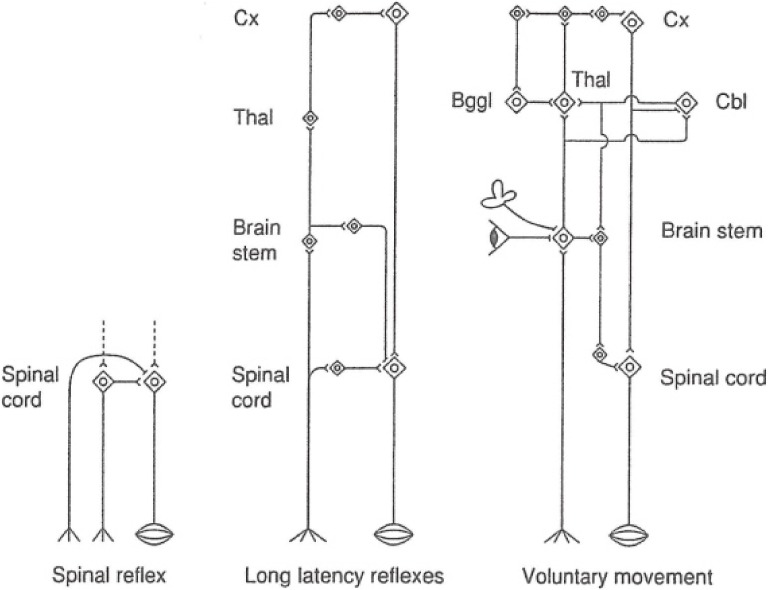
The neural circuitry of motor systems in phylogenetic development is presented in Fig. 24. The principal, most-elementary reflex is a local reflex, such as the spinal reflex in mammals. The second stage is a long latency reflex. Pathways for a long latency reflex encompass the spinal cord–brain stem–spinal cord, and include respiration and whole body movement as their functions. They are still unconscious and animal-like reflexes. The third stage is a system for voluntary movement, and this includes the basal ganglia and the cerebellum, and there are motor centers that function only in the presence of the cerebral cortex.
Figure 24.
Phylogenetic development of motor nervous systems.
Neural circuitry for voluntary movement may implicate the primary origin in the frontal lobe or the limbic cortex, then the basal ganglia and cerebellum, to the thalamus and back to the motor-related cortices and the primary motor cortex (area 4 of Brodmann). Voluntary movement may vary–simple or complex, short- or long-lasting, accustomed or new. Contribution of the basal ganglia to any of the above patterns will be analyzed in normal subjects or in PD in the future.
3. Motor patterns and the basal ganglia.
(1). Simple, quick movement does not need the basal ganglia.
Simple responses such as pressing a button or flexion or extension of a finger in response to light or sound as a go-signal is normal in PD patients in the early to middle stages of illness. In the advanced stage, even such a simple response has time delays, but a warning signal or a cue ahead the go-signal improves the reaction time in PD to normal.51) The effect of a cue is to provoke attention or provide interest for the examinee, and this is considered as activation of the frontal lobe. The simple reaction time is 250–400 ms in this paradigm, and the pre-movement cortical potential (contingent negative variation [CNV]) which is attributed to activity of the basal ganglia takes a longer time than the reaction time.
Simple reflex-like purposive movement, such as catching a ball suddenly thrown to a subject is a well-known surprise for a PD patient with severe bradykinesia. Because principal motor function of the basal ganglia is the selection of a suitable pattern of movement on competitive basis, and motor circuitry for simple reflex-like movement would not include the basal ganglia.
(2). Silence is an active neural process.
Keeping the body at rest in an awake state is a state maintained by active processes in the nervous system. Either in conscious or unconscious conditions, the resting state is a preparatory state for quick, purposeful motion. In an awake state, skeletal muscles, especially trunk muscles, are in active state through postural reflexes.
In a standing position, stable immobile posture is maintained with reflexes by deep senses and vestibular senses. Disturbances in postural reflex and/or vestibular dysfunction may cause asymmetric or dystonic postures. With the loss of deep senses or vibratory sense, slow, irregular, involuntary movement of the arms and/or fingers, called pseudoathetosis, may occur. Sensory ataxia, which is ataxia caused by the loss of deep senses, results in swaying of the standing body when visual information is shut down, known as a Romberg sign.
Chorea, or ballism, involves involuntary movement appearing at rest, and is considered as a purposeless movement by release phenomenon from inhibitory processes caused by disorders of the basal ganglia.
(3). Voluntary movement may appear through variable neural circuitry. Significance of compensation and plasticity.
In PD, purposive movement become difficult because of bradykinesia. However, movement itself is time-consuming but possible. The patient’s ability of daily living becomes narrower rather than from a loss of motivation.
In the experiment by Hore, Brooks and coworkers,81) a monkey trained to exhibit slow and quick movements could accomplish trained movements after blocking of globus pallidus activity by cooling of the tissue. However, additional interruption of visual information resulted in inability to execute trained movement in globus pallidus-cooled monkeys. If the globus pallidus was intact, the trained movements could be accomplished well even with the interruption of visual information. This experiment verified that a trained movement can be accomplished without the final output of the basal ganglia (globus pallidus) as a result of visual information processing.
Motor responses to external stimuli are fundamental in human behavior, and the variable neural circuitries are shown in Fig. 25. As in chronic basal ganglia diseases such as PD, gradual progression of disease processes may allow intact nervous systems to reestablish the neural circuitries for motion by compensation and plasticity with training in activities of daily living. In this respect, cortico–ponto–cerebello–thalamo–cortical circuits and/or cerebello–thalamo–basal ganglia circuits may play important roles.
Figure 25.
Sensori-motor integration circuitry. (Modified from Yanagisawa, 200635)).
(4). Motor patterns are restored in the cerebral cortex. Simple or complex? Feedback or feedforward?
A series of motions, such as picking up a candy from a box on a table in front of eyes and bring it to mouth and eating might be typical successive motion seen in ordinary life and even in monkey experiments. Rizzolatti and coworkers82) found a neuron named “grasping with the hand and the mouth” in area 6 (premotor cortex), which responded to all somatic sensations, visual stimuli, and is related to motor execution.
This kind of serial movements, however, may be interrupted at any moment by an unexpected noise. Furthermore, the behavior of hungry child or polite young lady may be different in speed and in the amount of muscular contractions concerned with the same purposive movement. In daily life activities, in response to visual, auditory, somatic, or deep senses, or vestibular sense, the selection of a series of voluntary movements will occur in succession with judgment for the selection of each elementary movement and finally accomplish a purposeful action.
Considering a series of elementary movements for a purposive movement, it is easily assumed that patterns of motion restored in the cortex are not concrete movement patterns. However, the image of actual purposeful motion will be restored in the cortex.
Other than phylogenetic and ontogenetic development, a series of complex movements may be restored in the cerebral cortex through particular training. Complex gymnastics, figure skating, free style skiing such as moguls are a series of feedforward movements acquired by repeated training. They would not allow feedback correction in the middle of serial different patterns of motions. Except for these unusual examples, ordinary motions in the daily living are usually accomplished with feedback correction in succession with a series of elementary motions.
Considering the experiment by Hore et al.,81) who showed the achievement of trained purposive movements by visual information with inactivation of the globus pallidus, the final output of the basal ganglia; difficulty in switching movement patterns from one to another in PD patients;53,54) observations by Rizzolatti et al.82) on the area 6 neurons on trained complex purposive movements, the function of the basal ganglia is considered to include a realization of elementary motor patterns successively with sensory feedback to produce a series of motions for a purposeful action stored in the cerebral cortex.
Summary
1. For voluntary movement.
① The basal ganglia and the cerebellum play important roles in the learning of patterns of purposive movements.
② Purposive movements in daily living are composed of successions of elementary motions, each of which is a response to external information through visual, auditory, or somatic senses.
③ Switching from one to another movement pattern causes delays in the basal ganglia disorders.
④ The function of the basal ganglia is in the selection of one pattern and the suppression of others from many motor patterns acquired by learning and stored in the cerebral cortex, thus fulfilling an appropriate movement. Importance of disinhibition.
⑤ In an awake state, stillness does not mean a cessation of action in motor nervous systems but is an active state with continuous suppression of motor preparedness.
⑥ Rhythm disturbance is a factor for the disturbance of voluntary movement in basal ganglia diseases (freezing).
⑦ Phasic involuntary movement such as chorea or ballism is considered to result from disinhibition of motor output caused by the disturbance of inhibitory mechanisms in the basal ganglia.
2. On posture and gait.
① Unique abnormal postures appear as a result of lesions either in the globus pallidus or striatum.
② In normal conditions, various postural reflexes are suppressed by functions of the basal ganglia. Disinhibition of these suppressions results in various abnormal postures.
③ Deep brain stimulation of the internal globus pallidus improves dystonic postures remarkably. Among dystonias resulting from basal ganglia disorders, idiopathic torsion dystonia caused by postural reflex disorders improves greatly, whereas symptomatic dystonia with marked muscular rigidity can be modified little by deep brain stimulation.
④ Forward bending posture and disequilibrium in Parkinson’s disease are improved greatly by levodopa at early stages of illness.
⑤ Small-stepped gait and freezing of gait are caused by disturbances in postural reflexes, disequilibrium, and rhythm disturbance.
3. Cognitive dysfunction and higher nervous functions.
① Various higher nervous functions are disturbed in Parkinson’s disease. Frontal lobe dysfunctions are considered as disorders in the frontal lobe-basal ganglia system. For visuocognitive dysfunctions in Parkinson’s disease, inactivity in the occipitoparietal visual association area exists.
② Disturbance in procedural memory can be related to motor dysfunctions covered by the frontal lobe-basal ganglia system.
4. Future problems.
Improving understanding of:
① Neural mechanisms for different types of involuntary movements by the lesions in the basal ganglia.
② Neural mechanisms of dystonia especially action dystonia and dystonic movement, and the components of basal ganglia disorders and the contribution of disturbances in sensory information processing.
③ Neural structures and mechanisms for activity of daily living, particularly systems not involving the basal ganglia, because these may be important in the rehabilitation of Parkinson’s disease.
④ The role of the limbic system-ventral striatum systems as a route for the expression of emotional responses, and the relationship of psychic symptoms by deep brain stimulation of the subthalamic nucleus with the limbic-striatum system.
⑤ The functions of projections from the basal ganglia to the brain stem, particularly projections to the pedunculo-pontine nucleus.
⑥ Interactions between projections from the substantia nigra pars reticulata to the oculomotor system and to the globus pallidus-thalamocortical system.
Profile
Nobuo Yanagisawa was born in Tokyo in 1935 and graduated from Tokyo University School of Medicine in 1960. Then, he graduated from the postdoctoral course of neurophysiology and neuropathology at the Brain Research Institute of Tokyo University School of Medicine in 1965. He engaged in clinical neurology and worked at Harvard Medical School with Derek Denny-Brown from 1969 to 1971.
In the first 30 years of his career, he worked in clinic and research in neurology and made contributions to the clarification of the physiological mechanisms and development of treatments for motor disturbances in basal ganglia disorders and cerebrovascular hemiplegia. He became a professor of medicine at Shinshu University School of Medicine in 1980, and served as the Director General of the Hospital and the Dean of the School of Medicine at Shinshu University from 1993 to 1996. Then, he moved to Chubu National Hospital in Nagoya as the Director General for the establishment of the National Institute of Longevity Sciences and Gerontology, then he moved to Kanto Rosai Hospital for the promotion of Laborer Health and Safety. At present, he is the President of All Japan Labour Welfare Foundation. In academic positions, he has served as the President of the Japanese Society of Neurology from 1996 to 2002, and an International Delegate of the Japanese Society of Clinical Neurophysiology from 1991 to 2001. Internationally, he became a founding member of the International Society of Motor Disturbances (ISMD) in 1985, an International Executive Committee member of the Movement Disorder Society (MDS) from 1997 for 4 years. Based on these, he established the MDS Japan in 2001 and served as the first President for 5 years. He is a Membre Honoraire of Académie Royale de Médecine de Belgique and a Professor Emeritus of Hebei Medical University, China. In addition, he made a unique contribution to the treatment of poisoning by the chemical nerve agent “Sarin”, when the Aum Shinrikyo cult attacked citizens in Matsumoto (1994) and Tokyo (1995), and he received a testimonial from US Government for his medical and social contributions on these occasions.
References
- 1).Martin, P. (1967) The Basal Ganglia and Posture. Pitman Medical, London. [Google Scholar]
- 2).Vogt C., Vogt O. (1920) Zur Lehre der Erkrankungen des striaren Systems. J. Psychol. Neurol. (Lpz.) 25, 627–846. [Google Scholar]
- 3).Jakob, A. (1923) Die Extrapyramidalen Erkrankungen. Julius Springer, Berlin. [Google Scholar]
- 4).Denny-Brown, D. (1962) The Basal Ganglia and Their Relation to Disorders of Movement. Oxford University Press, London, pp. 1–144. [Google Scholar]
- 5).Denny-Brown, D. and Yanagisawa, N. (1976) The role of the basal ganglia in the initiation of movement. In The Basal Ganglia (ed. Yahr, M.D.). Raven Press, New York, pp. 115–149. [PubMed] [Google Scholar]
- 6).Carpenter M.B., Whittier J.R., Mettler F.A. (1950) Analysis of choreoid hyperkinesia in the rhesus monkey. Surgical and pharmacological analysis of hyperkinesia resulting from lesions of the subthalamic nucleus of Luys. J. Comp. Neurol. 92, 293–331. [DOI] [PubMed] [Google Scholar]
- 7).Marsden C.D. (1982) The mysterious motor function of the basal ganglia: The Robert Wartenberg Lecture. Neurology 32, 514–539. [DOI] [PubMed] [Google Scholar]
- 8).Wilson S.A.K. (1925) Disorders of motility and of muscle tone, with special reference to the corpus striatum. Lecture I. Lancet 2, 1–10. [Google Scholar]
- 9).Kemp J.M., Powell T.P.S. (1970) The corticostriate projection in the monkey. Brain 93, 525–546. [DOI] [PubMed] [Google Scholar]
- 10).Yanagisawa N., Goto A. (1971) Dystonia musculorum deformans. Analysis with electromyography. J. Neurol. Sci. 13, 39–65. [DOI] [PubMed] [Google Scholar]
- 11).Yanagisawa, N. and Hashimoto, T. (1992) Quantitation of involuntary movement with electromyography. In Parkinson’s Disease and the Problems of the Clinical Trials (ed. Clifford Rose, F.). Smith-Gordon, London, pp. 131–142. [Google Scholar]
- 12).Evarts E. (1966) Pyramidal tract activity associated with a conditioned hand movement in the monkey. J. Neurophysiol. 29, 1011–1027. [DOI] [PubMed] [Google Scholar]
- 13).DeLong M.R., Georgopoulos A.P., Crutcher M.D. (1983) Cortico-basal ganglia relations and coding of motor performance. Exp. Brain Res. 49 (Suppl. 7), 30–40. [Google Scholar]
- 14).Alexander G.E., Crutcher M.D. (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. [DOI] [PubMed] [Google Scholar]
- 15).Albin R.L., Young A.B., Penney J.B. (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. [DOI] [PubMed] [Google Scholar]
- 16).DeLong M.R. (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. [DOI] [PubMed] [Google Scholar]
- 17).Bergman H., Wichmann T., DeLong M.R. (1990) Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438. [DOI] [PubMed] [Google Scholar]
- 18).Nambu A., Tokuno H., Takada M. (2002) Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci. Res. 43, 111–117. [DOI] [PubMed] [Google Scholar]
- 19).Bostan A.C., Dum R.P., Strick P.L. (2010) The basal ganglia communicate with the cerebellum. Proc. Natl. Acad. Sci. U.S.A. 107, 8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Jackson J.H. (1868) Observations on the physiology and pathology of hemichorea. Edinb. Med. J. 14, 294–303. [PMC free article] [PubMed] [Google Scholar]
- 21).Hunt R. (1933) Primary paralysis agitans (primary atrophy of efferent striatal and pallidal systems). Arch. Neurol. Psychiatry 30, 1332–1349. [Google Scholar]
- 22).Hoehn M.M., Yahr M.D. (1967) Parkinsonism: onset, progression, and mortality. Neurology 17, 427–442. [DOI] [PubMed] [Google Scholar]
- 23).Yanagisawa N. (1988) Educational lecture: Long-term therapy of Parkinson’s disease. J. Jap. Soc. Int. Med. 77, 1378–1382. [DOI] [PubMed] [Google Scholar]
- 24).Yanagisawa, N. (1984) EMG characteristics of involuntary movements. In Dyskinesias (eds. Bruyn, G.W., Buruma, O.J.S. and Roos, R.A.C.). Sandoz, B.V., Uden, pp. 142–159. [Google Scholar]
- 25).Yanagisawa N. (1996) Historical review of research on functions of basal ganglia. Eur. Neurol. 36 (suppl. 1), 2–8. [DOI] [PubMed] [Google Scholar]
- 26).Yahikozawa H., Hanyu N., Yamamoto K., Hashimoto T., Shimozono K., Nakagawa S., et al. (1994) Hemiballism with striatal hyperintensity on T1-weighed MRI in diabetic patients: a unique syndrome. J. Neurol. Sci. 124, 208–214. [DOI] [PubMed] [Google Scholar]
- 27).Hashimoto T., Yanagisawa N. (1994) A comparison of the regularity of involuntary muscle contractions in vascular chorea with that in Huntington’s chorea, hemiballism and parkinsonian tremor. J. Neurol. Sci. 125, 87–94. [DOI] [PubMed] [Google Scholar]
- 28).Spielmeyer W. (1926) Die anatomische Krankheitsforschung am Beispiel einer Huntingtonischen Chorea mit Wilsonischem Symptomenbild. Z. ges. Neurol. Psychiatr. 101, 710–728. [Google Scholar]
- 29).Yanagisawa N. (1992) The spectrum of motor disorders in Huntington’s disease. Clin. Neurol. Neurosurg. 94 (suppl.), 182–184. [DOI] [PubMed] [Google Scholar]
- 30).McGeer, E.G. and McGeer, P.L. (1973) Some brain characteristics of brain tyrosine hydroxylase. In New Concepts in Neurotransmitter Regulation (ed. Mandel, J.). Plenum, New York, London, pp. 53–68. [Google Scholar]
- 31).Segawa, M. and Nomura, Y. (1993) Hereditary progressive dystonia with marked diurnal fluctuation. Pathophysiological importance of the age of onset. In Advances in Neurology Vol. 60, Parkinson’s Disease from Basic Research to Treatment (eds. Narabayashi, H., Nagatsu, T., Yanagisawa, N. and Mizuno, M.). Raven Press, New York, pp. 568–576. [PubMed] [Google Scholar]
- 32).Albin R.L., Reiner A., Anderson K.D., Penney J.B., Young A.B. (1990) Striatal and nigral neuron subpopulations in rigid Huntington’s disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann. Neurol. 27, 357–365. [DOI] [PubMed] [Google Scholar]
- 33).Richter R. (1945) Degeneration of the basal ganglia in monkeys from chronic carbondisulfide poisoning. J. Neuropathol. Exp. Neurol. 4, 324–353. [DOI] [PubMed] [Google Scholar]
- 34).Yanagisawa, N., Shiraki, H., Minakawa, M. and Narabayashi, H. (1966) Clinico-pathological and histochemical studies of Hallervorden-Spatz disease with torsion dystonia with special reference to diagnostic criteria of the disease from the clinic-pathological viewpoint. In Progress in Brain Research 21B, Correlative Neurosciences (eds. Tokizane, T. and Schade J.P.). Elsevier, Amsterdam, pp. 373–425. [DOI] [PubMed] [Google Scholar]
- 35).Yanagisawa, N. (2006) Functions and dysfunctions of the basal ganglia. In Parkinson’s Disease: Clinical Issues (ed. Yamamoto, M.). Chuugai-Igakusha, Tokyo, pp. 2–27. [Google Scholar]
- 36).Oppenheim H. (1911) Uber eine eigenartige Krampfkrankheit des kindlichen und jugentlichen Alters (Dysbasia lordotica progressiva, dystonia musculorum deformans). Neurol. Cbl. 30, 1090–1107. [Google Scholar]
- 37).Fahn, S., Marsden, C.D. and Calne, D.B. (1987) Classification and investigation of dystonia. In Movement Disorders II (eds. Marsden, C.D. and Fahn, S.). Butterworths, London, pp. 332–358. [Google Scholar]
- 38).Zeman W., Dyken P. (1967) Dystonia musculorum deformans. Clinical, genetic and pathoanatomical studies. Psychiatr. Neurol. Neurochir. 70, 77–121. [PubMed] [Google Scholar]
- 39).Eidelberg D. (2003) Brain networks and clinical penetrance: lessons from hyperkinetic movement disorders. Curr. Opin. Neurol. 16, 471–474. [DOI] [PubMed] [Google Scholar]
- 40).Yanagisawa, N., Hayashi, R. and Mitoma, H. (2001) Pathophysiology of frozen gait in parkinsonism. In Advances in Neurology Vol. 87, Gait Disorders (eds. Ruzicka, E., Hallett, M. and Jankovic J.). Lippincott, Williams & Wilkins, Philadelphia, pp. 199–207. [PubMed] [Google Scholar]
- 41).Yanagisawa, N., Fujimoto, S. and Ueno, E. (1985) Neurophysiological aspects of Parkinson’s disease. In Current Problems in Treatment of Parkinson’s Disease (eds. Kuroiwa, Y. and Toyokura, Y.). DMW, Tokyo, pp. 13–22. [Google Scholar]
- 42).Gerstmann J., Schilder P. (1926) Uber eine besondere Gangstorung bei Stirnhirnerkrankung. Wien. Med. Wochenschr. 76, 97–102. [Google Scholar]
- 43).Denny-Brown D. (1958) The nature of apraxia. J. Nerv. Ment. Dis. 126, 9–33. [DOI] [PubMed] [Google Scholar]
- 44).Takami M., Fukui K. (1987) Force-plate study of normal walking. Rehabil. Med. 24, 93–101. [Google Scholar]
- 45).Ueno E. (1989) Clinical and physiological study of apraxia of gait and frozen gait. Clin. Neurol. 29, 275–283. [PubMed] [Google Scholar]
- 46).Yanagisawa, N., Ueno, E. and Takami, M. (1991) Frozen gait of Parkinson’s disease and vascular parkinsonism — A study with floor reaction forces and EMG. In Neurobiological Basis of Human Locomotion (eds. Shimamura, M., Grillner, S. and Edgarton, V.R.). Japan Scientific Societies Press, Tokyo, pp. 291–304. [Google Scholar]
- 47).Ueno, E., Yanagisawa, N. and Takami, M. (1993) Gait disorders in parkinsonism. In Advances in Neurology Vol. 60, Parkinson’s Disease from Basic Research to Treatment (eds. Narabayashi, H., Nagatu, T., Yanagisawa, N. and Mizuno, Y.). Raven Press, New York, pp. 414–418. [PubMed] [Google Scholar]
- 48).Narabayashi, H., Imai, H., Yokochi, M., Hirayama, K. and Nakamura, R. (1976) Cases of pure akinesia without rigidity and tremor and with no effects by L-dopa therapy. In Advances in Parkinsonism (eds. Birkmayer, W. and Hornykiewicz, O.). Roche, Basle, pp. 335–342. [Google Scholar]
- 49).Sacks, O. (1973) Awakenings, Wilie, U.K. [Google Scholar]
- 50).Schmidt R.A., Sherwood D.E., Walter C.B. (1988) Rapid movements with reversals in direction: 1. The control of movement time. Exp. Brain Res. 69, 344–354. [DOI] [PubMed] [Google Scholar]
- 51).Yanagisawa N., Fujimoto S., Tamaru F. (1989) Bradykinesia in Parkinson’s disease: Disorders of onset and execution of fast movement. Eur. Neurol. 29 (suppl. 1), 19–28. [DOI] [PubMed] [Google Scholar]
- 52).White O.B., Saint-Cyr J.A., Tomlinson D., Sharpe J.A. (1983) Ocular motor deficits in Parkinson’s disease: II Control of the saccadic and smooth pursuit systems. Brain 106, 571–587. [DOI] [PubMed] [Google Scholar]
- 53).Warabi T., Yanagisawa N., Shindo R. (1988) Changes in strategy of aiming tasks in Parkinson’s disease. Brain 111, 497–505. [DOI] [PubMed] [Google Scholar]
- 54).Benecke R., Rothwell J.C., Dick J.P.R., Day B.L., Marsden C.D. (1987) Disturbance of sequential movements in patients with Parkinson’s disease. Brain 110, 361–379. [DOI] [PubMed] [Google Scholar]
- 55).Warabi, T., Fukushima, K. and Olley, P.M. (2012) New insights into the mechanism of slowing of voluntary movements in Parkinson’s disease. In Parkinson’s Disease: Diagnosis, Treatment and Prognosis (eds. Yoshida, C. and Ito, A.). Nova Science, New York, pp. 67–97. [Google Scholar]
- 56).Freund H.-J. (1989) Motor dysfunctions in Parkinson’s disease and premotor lesions. Eur. Neurol. 29 (suppl. 1), 33–37. [DOI] [PubMed] [Google Scholar]
- 57).Luria, A.R. (1969) Frontal lobe syndromes. In Handbook of Clinical Neurology (eds. Vinken, R.J. and Bruyn, G.W.). Vol. 2, North Holland, Amsterdam, pp. 725–757. [Google Scholar]
- 58).Damasio, A. (1979) The frontal lobes. In Clinical Neuropsychology (eds. Heilman, E.M. and Valenstein, E.). Oxford University Press, New York, pp. 360–412. [Google Scholar]
- 59).Bodis-Wollner, I. and Tagliati, M. (1993) The visual system in Parkinson’s disease. In Advances in Neurology Vol. 60, Parkinson’s Disease from Basic Research to Treatment (eds. Narabayashi, H., Nagatsu, T., Yanagisawa, N. and Mizuno, Y.). pp. 390–394. [PubMed] [Google Scholar]
- 60).Abe Y., Kachi T., Kato T., Arahata Y., Yamada T., Washimi Y., et al. (2003) Occipital hypoperfusion in Parkinson’s disease without dementia: correlation to impaired cortical visual processing. J. Neurol. Neurosurg. Psychiatry 74, 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Yanagisawa N. (2006) Natural history of Parkinson’s disease: From dopamine to multiple system involvement. Parkinsonism Relat. Disord. 12 (suppl. 1), 40–46. [Google Scholar]
- 62).Magladery J.W., Porter W.E., Park A.M., Teasdall R.D. (1951) Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neuron reflex and identification of certain action potentials from spinal roots and cord. Bull. Johns Hopkins Hosp. 88, 499–519. [PubMed] [Google Scholar]
- 63).Rene Descartes (1649) “Passion of the Soul”.
- 64).Sherrington, C.S. (1906) The Integrative Action of the Nervous System, Yale University Press, New Haven. [Google Scholar]
- 65).Lloyd D.P.C. (1946) Integrative pattern of excitation and inhibition in two-neuron reflex arcs. J. Neurophysiol. 9, 439–444. [DOI] [PubMed] [Google Scholar]
- 66).Laporte Y., Lloyd D.P.C. (1952) Nature and significance of the reflex connection established by large afferent fibers of muscle origin. Am. J. Physiol. 169, 609–621. [DOI] [PubMed] [Google Scholar]
- 67).Lundberg A., Voorhoeve P.E. (1962) Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol. Scand. 56, 201–219. [DOI] [PubMed] [Google Scholar]
- 68).Hultborn H. (1972) Convergence on interneurons in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiol. Scand. Suppl. 375, 1–42. [DOI] [PubMed] [Google Scholar]
- 69).Mizuno Y., Tanaka R., Yanagisawa N. (1971) Reciprocal group I inhibition on triceps surae motoneurons in man. J. Neurophysiol. 34, 1010–1017. [DOI] [PubMed] [Google Scholar]
- 70).Yanagisawa N., Tanaka R., Ito Z. (1976) Reciprocal Ia inhibition in spastic hemiplegia of man. Brain 99, 555–574. [DOI] [PubMed] [Google Scholar]
- 71).Shindo M., Yanagawa S., Morita H., Yanagisawa N. (1995) Increase in reciprocal Ia inhibition during antagonist contraction in the human leg: a study of motor units and the H reflex. J. Physiol. 489, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Shindo M., Yanagawa S., Morita H., Hashimoto T. (1994) Conditioning effect in single human motoneurones: a new method using the unitary H reflex. J. Physiol. 481, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Morita H., Shindo M., Ikeda S., Yanagisawa N. (2000) Decrease in presynaptic inhibition on heteronymous monosynaptic Ia terminals in patients with Parkinson’s disease. Mov. Disord. 15, 830–834. [DOI] [PubMed] [Google Scholar]
- 74).Morita H., Shindo M., Morita S., Hashimoto T., Tada T., Ikeda S. (2002) Abnormal conditioning effect of transcranial magnetic stimulation on soleus H-reflex during voluntary movement in Parkinson’s disease. Clin. Neurophysiol. 113, 1316–1324. [DOI] [PubMed] [Google Scholar]
- 75).Morita H., Crone C., Christenhuis D., Petersen N.T., Nielsen J.B. (2001) Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 124, 826–837. [DOI] [PubMed] [Google Scholar]
- 76).Morita M., Shindo M., Momoi H., Yanagawa S., Ikeda S., Yanagisawa N. (2006) Lack of modulation of Ib inhibition during antagonist contraction in spasticity. Neurology 67, 52–56. [DOI] [PubMed] [Google Scholar]
- 77).Morita M., Shindo M., Yanagawa S., Yoshida T., Momoi H., Yanagisawa N. (1995) Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp. Brain Res. 104, 167–170. [DOI] [PubMed] [Google Scholar]
- 78).Petersen N., Morita H., Nielsen J. (1999) Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J. Physiol. 520, 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Powell T.P.S., Cowan W.H. (1956) A study of thalamo-striate neurons in the monkey. Brain 79, 364–390. [DOI] [PubMed] [Google Scholar]
- 80).Chevalier G., Deniau J.M. (1990) Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 13, 277–280. [DOI] [PubMed] [Google Scholar]
- 81).Hore J., Meyer-Lohman J., Brooks V.B. (1977) Basal ganglia cooling disables learned arm movements of monkeys in the absence of visual guidance. Science 195, 584–586. [DOI] [PubMed] [Google Scholar]
- 82).Rizzolatti G., Camarda R., Fogassi L., Gentilucci M., Luppino G., Matelli M. (1988) Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp. Brain Res. 71, 491–507. [DOI] [PubMed] [Google Scholar]