Abstract
Damage-associated molecular patterns (DAMPs) are endogenous danger molecules that are released from damaged or dying cells and activate the innate immune system by interacting with pattern recognition receptors (PRRs). Although DAMPs contribute to the host's defense, they promote pathological inflammatory responses. Recent studies have suggested that various DAMPs, such as high-mobility group box 1 (HMGB1), S100 proteins, and heat shock proteins (HSPs), are increased and considered to have a pathogenic role in inflammatory diseases. Here, we review current research on the role of DAMPs in inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus, osteoarthritis, atherosclerosis, Alzheimer's disease, Parkinson's disease, and cancer. We also discuss the possibility of DAMPs as biomarkers and therapeutic targets for these diseases.
Keywords: Damage-associated molecular patterns, Inflammation, Pattern recognition receptors, Inflammatory diseases
INTRODUCTION
The innate immune system is the first line of host defense that induces immediate, non-specific immune responses against pathogens (1). Inflammation is part of the innate immune system and is initiated when the innate immune system recognizes invading pathogens or molecules from tissue injury through pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and inflammasomes of the innate immune system (2,3). Although inflammation is a protective response to eliminate harmful stimuli, initiate tissue repair, and restore health, it can also contribute to the development of various diseases, such as autoimmune diseases, cardiovascular diseases, and neurodegenerative diseases, if it is not properly regulated or resolved (4,5).
Damage-associated molecular patterns (DAMPs) are molecules released upon cellular stress or tissue injury and are regarded as endogenous danger signals, because they induce potent inflammatory responses by activating the innate immune system during non-infectious inflammation (6,7). Recently, emerging evidence has indicated that DAMPs play a key role in the pathogenesis of human diseases by inducing inflammation (8). This review describes the role of DAMPs in inflammatory diseases and the possibility of using DAMPs as biomarkers and therapeutic targets for these inflammatory diseases.
ORIGIN AND LIST OF DAMPs
Since the danger model was introduced by Polly Matzinger (9), several DAMPs have been identified, and the number of DAMPs is still increasing (7,10). DAMPs are released from the extracellular or intracellular space following tissue injury or cell death (10). These DAMPs are recognized by macrophages, and inflammatory responses are triggered by different pathways, including TLRs and inflammasomes (10,11). DAMPs can originate from different sources and include extracellular proteins, such as biglycan and tenascin C, and intracellular proteins, such as high-mobility group box 1 (HMGB1), histones, S100 proteins, heat-shock proteins (HSPs), and plasma proteins, like fibrinogen, Gc-globulin, and serum amyloid A (SAA) (10,12,13,14,15). A list of well-characterized DAMPs, along with their origin and receptors, is shown in Table 1.
Table 1. List of DAMPs and their receptors.
| Origin | Major DAMPs | Receptors | |
|---|---|---|---|
| Extracellular matrix | Biglycan | TLR2, TLR4, NLRP3 | |
| Decorin | TLR2, TLR4 | ||
| Versican | TLR2, TLR6, CD14 | ||
| LMW hyaluronan | TLR2, TLR4, NLRP3 | ||
| Heparan sulfate | TLR4 | ||
| Fibronectin (EDA domain) | TLR4 | ||
| Fibrinogen | TLR4 | ||
| Tenascin C | TLR4 | ||
| Intracellular compartments | Cytosol | Uric acid | NLRP3, P2X7 |
| S100 proteins | TLR2, TLR4, RAGE | ||
| Heat shock proteins | TLR2, TLR4, CD91 | ||
| ATP | P2X7, P2Y2 | ||
| F-actin | DNGR-1 | ||
| Cyclophilin A | CD147 | ||
| Aβ | TLR2, NLRP1, NLRP3, CD36, RAGE | ||
| Nuclear | Histones | TLR2, TLR4 | |
| HMGB1 | TLR2, TLR4, RAGE | ||
| HMGN1 | TLR4 | ||
| IL-1α | IL-1R | ||
| IL-33 | ST2 | ||
| SAP130 | Mincle | ||
| DNA | TLR9, AIM2 | ||
| RNA | TLR3, TLR7, TLR8, RIG-I, MDA5 | ||
| Mitochondria | mtDNA | TLR9 | |
| TFAM | RAGE | ||
| Formyl peptide | FPR1 | ||
| mROS | NLRP3 | ||
| ER | Calreticulin | CD91 | |
| Granule | Defensins | TLR4 | |
| Cathelicidin (LL37) | P2X7, FPR2 | ||
| EDN | TLR2 | ||
| Granulysin | TLR4 | ||
| Plasma membrane | Syndecans | TLR4 | |
| Glypicans | TLR4 | ||
ER, endoplasmic reticulum; EDN, eosinophil-derived neurotoxin.
HMGB1, a member of the HMG protein family, which is located in the cell nucleus, has a critical function in gene expression, but when released to the extracellular space, HMGB1 is known to induce inflammation by activating the NF-κB pathway by binding to TLR2, TLR4, TLR9, and the receptor for advanced glycation end products (RAGE) (16). S100 proteins are calcium-binding proteins, and their main function is the management of calcium storage and shuffling (10,17). Although S100 proteins have various functions, which include cell proliferation, differentiation, migration, and energy metabolism under healthy conditions (17), they also act as DAMPs by interacting with TLR2, TLR4, and RAGE after they are released from phagocytes (18). Likewise, HSPs normally function as chaperones and assist with biosynthetic pathways (10), but extracellular HSPs, which are cellular necrosis products, can induce inflammation through the activation of TLR2, TLR4, and CD91 (10,19). Adenosine triphosphate (ATP) and uric acid, which are purine metabolites, also activate NLR family, pyrin domain containing (NLRP) 3 inflammasomes to induce IL-1β and IL-18 (20,21). Finally, some plasma proteins, including SAA, fibrinogen, Gc-globulin, α1-microglobulin, and α2-macroglobulin, are extravasated to the sites of inflammation from the vasculature and function as DAMPs by stimulating macrophages to produce inflammatory cytokines through TLR2 or TLR4 (12,13,14,15).
PRRs
PRRs are important components of the innate immune system. Several families of PRRs have been identified in the diverse compartments of the cell (Table 2). They recognize microbes or tissue damage by specific molecular structures called pathogen-associated molecular patterns (PAMPs) or DAMPs (10,22). The main functions of PRRs are to stimulate phagocytosis and mediate inflammation by sensing various pathogens and molecules from damaged cells (2,23). As a result, PRRs activate inflammatory signaling pathways to induce innate immunity (23).
Table 2. PRRs and their DAMP ligands.
| Family | Major members | DAMP ligands |
|---|---|---|
| TLRs | TLR1–9 | HMGB1, HSPs, S100 proteins, histones, DNA, RNA, mtDNA, syndecans, glypicans, biglycan, decorin, versican, LMW hyaluronan, heparan sulfate, fibrinogen, tenascin C |
| NLRs | NOD1, NOD2, NLRP family | Uric acid, Aβ, mROS, histones, biglycan, LMW hyaluronan |
| RLRs | RIG-I, MDA5, LGP2 | RNA |
| CLRs | DEC-205, MMR, Dectin-1, Dectin-2, Mincle, DC-SIGN, DNGR-1 | SAP130, F-actin |
| CDSs | AIM2-like receptor | DNA |
| Scavenger receptors | CD36, CD44, CD68, CD91, CXCL16, RAGE | HMGB1, HSPs, S100 proteins, calreticulin, versican |
| FPRs | FPR1, FPR2, FPR3 | Formyl peptide, cathelicidin (LL37) |
NLR, NOD-like receptor; CLR, C-type lectin receptor; CDS, cytosolic DNA sensor; FPR, formyl peptide receptor; LMW, low molecular weight.
TLRs are type I transmembrane glycoproteins located at the cell surface (TLR1, 2, 4, 5, 6, and 10) or in intracellular membranes (TLR3, 7, 8, and 9) and recognize various PAMPs or DAMPs (24). TLRs induce the production of proinflammatory cytokines and type I interferons (IFNs) through the myeloid differentiation factor 88 (MyD88)-dependent signaling pathway or the toll/interferon response factor (TRIF)-dependent signaling pathway (24). NOD-like receptors (NLRs) are cytoplasmic PRRs that include NODs, NLRPs, and the IPAF subfamily (25,26). NOD1 and NOD2 initiate proinflammatory signaling by activating NF-κB (25), and NLRP3 stimulation by DAMPs, such as extracellular ATP, hyaluronan, and uric acid, can activate caspase-1 and induce the release of IL-1β and IL-18 through the formation of an inflammasome (26). RIG-like receptors (RLRs), including RIG-I, MDA5, and LGP2, detect viral RNA and self RNA in the cytoplasm (27). RLRs induce the production of IFNs by interacting with IPS-1; furthermore, RLR signaling cross-talks with the TLR or the inflammasome signaling pathway (27). C-type lectin receptors (CLRs), expressed by dendritic cells (DCs), promote NF-κB activation by modulating TLR signaling or directly through the spleen tyrosine kinase (SYK) and RAF1 pathways (28). Scavenger receptors consist of a large family of proteins and recognize various patterns. RAGE, one of the scavenger receptors, interacts with PAMPs or DAMPs, such as advanced glycation end products (AGEs), HMGB1, and S100 proteins, thereby mediating inflammation, oxidative stress, and apoptosis (29).
DAMPs IN AUTOIMMUNE DISEASES
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease (30). Swelling, pain, and stiffness of joints are the main symptoms of RA that result from inflammation of the synovial membrane of joints (31). Although the pathology of RA is not well understood, it is clear that DAMPs are associated with RA (30). S100A8/9/11/12 proteins were upregulated in the synovial tissue, synovial fluid, or serum of RA patients (32,33). In addition, the expression of HMGB1 was increased in the serum and synovial fluid of RA patients (34,35). On the other hand, when RA patients were treated with methotrexate (MTX), a common medication for RA, HMGB1 and cartilage degradation enzymes, matrix metalloproteinase (MMP)-2 and MMP-13, were decreased compared to the levels in the RA patients without MTX treatment (36). Furthermore, neutralization of HMGB1 can protect cartilage from degradation and prevent bone destruction due to RA in experimental animal models (37,38). It is assumed that HMGB1 stimulates the production of proinflammatory cytokines, such as tumor necrosis factor (TNF) and IL-1 (39). The inflammation of joints can promote cellular stress and lead to an increase in HSPs in the synovial tissue (40). It has been reported that the levels of HSP70 were elevated in the synovial fluid of RA patients (41), and heat shock protein gp96 was increased in the synovial fluid of RA patients; this is considered to promote inflammation by activating macrophages through TLR2 signaling (42). HSP90 also contributes to the pathogenesis of RA by inducing a tumor-like synovial overgrowth by stabilizing integrin-linked kinase (ILK), extracellular signal-regulated kinase (ERK), and protein kinase B (Akt) (43).
Recently, citrullinated histones and their immune complexes have been reported to function as DAMPs in RA (44). Citrullinated H2B was increased in the synovial fluid of RA patients and activated macrophages to produce inflammatory cytokines, which were enhanced by immune complexes with RA patient-derived IgGs. Moreover, immunization with citrullinated H2B in the presence of low-grade joint inflammation induced inflammatory arthritis in an animal model of RA (44).
Systemic lupus erythematosus (SLE) is one of the chronic autoimmune diseases that invades multiple organs (45). HMGB1 expression was enhanced in SLE patients and correlated with the SLE disease activity index (46). Furthermore, the urine HMGB1 level was elevated in lupus nephritis patients (47). However, a monoclonal anti-HMGB1 antibody has no therapeutic effect on a mouse model of lupus nephritis (48). This suggests that HMGB1 could be a good biomarker, but not a potential therapeutic target for SLE. Oxidized mitochondrial DNA (mtDNA) was found in the blood neutrophils of SLE patients, and extrusion of oxidized mtDNA could stimulate IFN production by activating plasmacytoid DCs (49). Recent evidence has suggested that neutrophil extracellular traps (NETs) are implicated in SLE, and NETs derived from the low-density granulocytes of SLE patients are enriched in oxidized mtDNA, which induces the inflammatory response (50).
DAMPs IN OSTEOARTHRITIS (OA)
OA has been regarded as a degenerative joint disease that is characterized by the destruction of cartilage (51). There are several risk factors for OA pathogenesis, which include age, physical trauma, and obesity (51). However, emerging evidence suggests that DAMPs-induced inflammation plays an important role in the pathogenesis of OA (14,52,53). Although the HMGB1 level was higher in the synovial fluid of RA patients than OA patients (35), more HMGB1-positive cells were found in the knee cartilage of high-grade OA patients compared to normal cartilage (54). Another study has provided evidence that the HMGB1 and RAGE levels are upregulated in OA knees compared to those of healthy controls (55). As demonstrated in other inflammatory diseases, extracellular HMGB1 activates the NF-κB signaling pathway to induce inflammation, and HMGB1 expression is related to the grade of cartilage destruction (16,54).
S100 proteins are also involved in the pathogenesis of OA. S100A8/A9 protein expression was elevated in the synovium of a collagenase-induced OA mouse model, and when S100A8 was intra-articularly injected into the knee joint of mice, it induced the expression of inflammatory markers, including Ly6C, F4/80, CCL2, and CCR2 in the synovium (56). Although S100A12 expression was unchanged in the serum between the OA patients and the healthy controls, the S100A12 level in the synovial fluid of OA patients was greatly increased compared to the healthy controls (57). In addition, S100A12 increased the secretion of MMP-13 and vascular endothelial growth factor (VEGF) in human OA chondrocytes, suggesting that S100A12 induces the progression of OA by increasing MMP-13 and VEGF (58).
DAMPs originated from plasma may also contribute to the pathogenesis of OA. It was reported that the levels of several inflammatory mediators, such as IL-6 and MCP-1, were higher in OA sera compared to healthy sera, suggesting the inflammatory nature of OA (14). Moreover, various plasma proteins are enriched in the synovial fluid of OA patients, and some of the plasma proteins, such as Gc-globulin, α1-microglobulin, and α2-macroglobulin, induced inflammation by functioning as DAMPs by activating TLR4 (14). This result suggests that certain plasma proteins can contribute to the low-grade inflammation observed in OA patients.
DAMPs IN CARDIOVASCULAR DISEASES
Atherosclerosis is an inflammatory disease of the arterial wall, in which the vessels narrow due to accumulating plaques of inflammatory cells and lipids (59). Although innate immunity is essential to maintain a healthy arterial wall, it also has a distinct role in stimulating the development of atherosclerosis (60). Macrophages are recruited to arterial lesions, which are rich in DAMPs, and contribute to the pathogenesis of atherosclerosis not only by the formation of lipid-filled foam cells, but also by inducing inflammation through the activation of PRRs (60,61).
HMGB1 is released from macrophages and vascular smooth muscle cells (VSMCs) in the lesions; therefore, the HMGB1 levels are highly elevated in atheromatous plaques (62). Recombinant human HMGB1 induced proinflammatory responses in endothelial cells by increasing leukocyte adhesion molecules, such as ICAM-1 and VCAM-1, and by inducing inflammatory mediators, such as IL-8, MCP-1, and TNFα (63). These results suggest that high expression of HMGB1 has the possibility to increase inflammation and accumulate atherogenesis (62,63).
S100 proteins are also involved in the pathogenesis of atherosclerosis. S100A8 and S100A9 exist in plaques, and they increase atherogenesis by activating neutrophils and monocytes in arterial lesions (64,65). S100A8, S100A9, and S100A12 have an important role in the mediation of inflammation and increase atherosclerosis in human and rodent models by interacting with RAGE, which plays an important role in endothelial dysfunction and inflammation (66,67). Consistent with the prospective population-based cohort study, S100A12 showed the strongest association with the risk of coronary heart disease (CHD), among the conventional risk factors (68). Likewise, other DAMPs are also upregulated in cardiovascular diseases. HSP70 was elevated and concentrated in the central portions of thick atheromas compared to normal arterial specimens (69). Soluble HSP60 was increased in patients with early carotid atherosclerosis (70), and HSP60 promoted atherosclerosis by inducing VSMC migration via TLR4 and ERK mitogen activated protein kinase (MAPK) activation (71). Finally, expression of α-defensin was upregulated in hyperlipidemia and CHD patients, which suggests that α-defensin can also be a potential biomarker for atherosclerosis (72).
DAMPs IN NEURODEGENERATIVE DISEASES
Alzheimer's disease (AD) is a chronic neurodegenerative disease that is characterized by several symptoms, such as amnesia, inability to manage self-care, and eventually dementia (73). Although the pathology of AD is still mostly unknown, several hypotheses have been suggested to explain it. DAMPs are also known to be involved in neuroinflammation in neurodegenerative disorders (74). The levels of HMGB1 and soluble RAGE are significantly elevated in the sera of AD patients, which was correlated with the levels of amyloid beta (75). A recent study demonstrated that HMGB1 and thrombin are triggers of inflammation and dysfunction of the blood-brain barrier (BBB) (75). In AD patients, the serum levels of S100B were intimately related to the severity of the disease (76), and the administration of pentamidine, a S100B inhibitor, reduced the levels of S100B and RAGE, thereby inhibiting neuroinflammation in the brain of an AD mouse model (77).
Parkinson's disease (PD) is a common, age-related neurodegenerative disorder, and the main symptoms of which are several cardinal motor symptoms, including bradykinesia, spasticity, and gait abnormality. The most noticeable feature of PD is chronic inflammation (78). The role of the HMGB1-TLR4 axis is very important in the pathogenesis of PD. The serum HMGB1 and TLR4 protein levels were significantly elevated in PD patients and correlated with the PD stages (79). In a rat model of PD, an anti-HMGB1 monoclonal antibody inhibited inflammation by maintaining the BBB and reducing the secretion of inflammatory cytokines, such as IL-1β and IL-6 (80). The S100B protein level was elevated in the substantia nigra and cerebrospinal fluid of PD patients, and S100B was also increased in the ventral midbrain of a mouse model treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (81). Although, the serum S100B level was similar between the PD patients and healthy individuals, it correlated with the scales for the severity of PD, such as the Hoehn and Yahr scale (82).
DAMPs IN CANCER
The role of DAMPs in the pathogenesis of cancer is still controversial. DAMPs may mediate tumor progression by inducing chronic inflammation, which is a compound risk factor for tumor progression (83,84). To our knowledge, IL-1, IL-6, and lymphotoxin (LT)-β are well known promoters of carcinogenesis (83,84,85). DAMPs, such as HMGB1, S100 proteins, and HSPs, activate inflammatory pathways and release IL-1, IL-6, LT-β, IFN-γ, TNF, and transforming growth factor (TGF)-β (83). ATP, IL-1α, adenosine, and uric acid also promote carcinogenesis by inflammation, immunosuppression, angiogenesis, and tumor cell proliferation (83). In this context, it appears that DAMPs increase tumor development in the early stages of carcinogenesis (83).
In contrast, DAMPs may inhibit tumor progression via immunogenic cell death (ICD). Calreticulin functions as an important effector of ICD by inducing the DC-mediated phagocytosis of tumor cells, which reduces the tumor growth in colon carcinoma (86). In addition, extracellular ATP, released from dying tumor cells, is a significant mediator in ICD via the activation of the NLRP3 inflammasome (87). The release of HMGB1 from dying tumor cells increased the presentation of tumor antigens and regulated the TLR4-dependent immune response (88). In summary, DAMPs may increase carcinogenesis or inhibit tumor development, like a double-edged sword. Future work will be necessary to further understand the complicated roles of DAMPs in cancer.
DAMPs AS BIOMARKERS AND POTENTIAL THERAPEUTIC TARGETS
DAMPs may be valuable biomarkers for inflammatory diseases. Many researchers have worked to identify DAMPs and understand their relationships with multiple diseases. It is well established that several DAMPs are increased or decreased in various human diseases. Increased S100A8/A9 is associated with osteophyte progression in early human OA (89), suggesting that S100 proteins can be used as biomarkers for the diagnosis of the progressive grade of OA. Furthermore, many clinical studies have assessed the prognostic and predictive value of DAMPs, such as HSPs, ATP, and HMGB1, in cancer patients (90), which has raised the possibility that DAMPs may be useful prognostic factors for cancer. These results are invaluable for the management of cancer patients. Patient classification may be improved, and a suitable therapy can be given to patients by diagnosing with DAMPs (90).
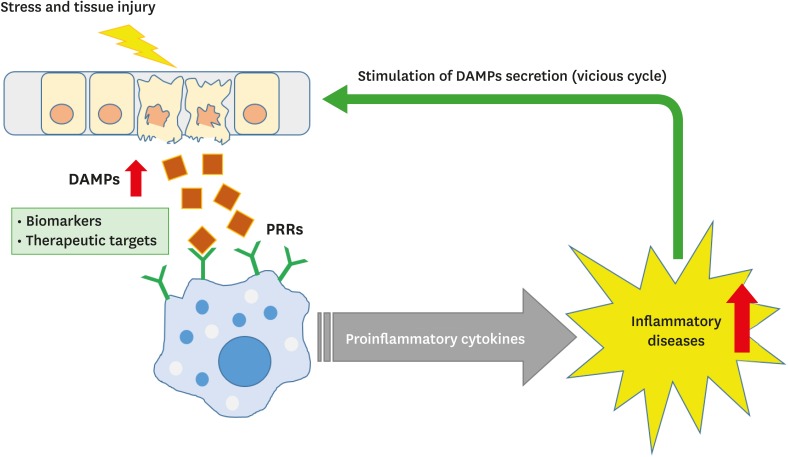
The regulation of DAMPs signaling can be a potential therapeutic target to reduce inflammation and treat diseases (Figure 1). Administration of neutralizing HMGB1 antibodies or truncated HMGB1-derived A-box protein ameliorated arthritis in collagen-induced arthritis rodent models (38). Clinical trials with HSP inhibitors have also been reported. For non-small cell lung cancer (NSCLC), HSP27, HSP70, and HSP90 inhibitors are under investigation in clinical trials (91). In addition, treatment with dnaJP1, which is a synthetic peptide derived from DnaJ (HSP40), had a curative effect in RA patients without critical side effects (92). Taken together, DAMPs can be useful therapeutic targets for various human diseases, including cancer and autoimmune diseases.
Figure 1. DAMPs as biomarkers and potential therapeutic targets.
DAMPs are released upon cellular stress or tissue injury and activate the innate immune system by interacting with PRRs to produce proinflammatory cytokines. Chronic inflammation can contribute to the development of various inflammatory diseases, which in turn stimulate the secretion of DAMPs, thus establishing a vicious cycle of DAMPs production and inflammation.
CONCLUSION
In this review, we have described the general concept of DAMPs, which play a key role in sterile inflammation, and discussed the possibility of DAMPs as biomarkers and therapeutic targets for various human inflammatory diseases. Although it is clear that DAMPs are closely related to the progress of inflammatory diseases, there are several questions that remain unclear. For example, there is little information on the interacting regions for DAMPs and their PRRs. It will be important to define the interacting regions for DAMPs and PRRs for the development of specific inhibitory molecules that can interfere with the interaction and thereby regulate inflammation. In addition, the development of medications that can inhibit the release of DAMPs will also be a promising therapeutic strategy. However, the inhibition of DAMPs should be taken into careful consideration for the treatment of human diseases, because DAMPs themselves can be effective therapeutic agents for the inhibition of tumor progression via ICD. Therefore, further research on DAMPs will be essential to significantly improve current medical problems.
ACKNOWLEDGEMENTS
This work was supported by a 2-Year Research Grant of Pusan National University.
Abbreviations
- AD
Alzheimer's disease
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- CHD
coronary heart disease
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- HMGB1
high-mobility group box 1
- HSP
heat-shock protein
- ICD
immunogenic cell death
- IFN
interferon
- LT
lymphotoxin
- MMP
matrix metalloproteinase
- mtDNA
mitochondrial DNA
- MTX
methotrexate
- NET
neutrophil extracellular trap
- NLR
NOD-like receptor
- NLRP
NLR family, pyrin domain containing
- OA
osteoarthritis
- PAMP
pathogen-associated molecular pattern
- PD
Parkinson's disease
- PRR
pattern recognition receptor
- RA
rheumatoid arthritis
- RAGE
receptor for advanced glycation end products
- RLR
RIG-like receptor
- SAA
serum amyloid A
- SLE
systemic lupus erythematosus
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Writing - original draft: Roh JS, Sohn DH; Writing - review & editing: Sohn DH.
References
- 1.Albiger B, Dahlberg S, Henriques-Normark B, Normark S. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med. 2007;261:511–528. doi: 10.1111/j.1365-2796.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee MS, Kim YJ. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol Cells. 2007;23:1–10. [PubMed] [Google Scholar]
- 3.Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev. 2015;24:29–39. doi: 10.1016/j.arr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2011;10:369–374. doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Land WG. The role of damage-associated molecular patterns (DAMPs) in human diseases: part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J. 2015;15:e157–e170. [PMC free article] [PubMed] [Google Scholar]
- 9.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 13.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye RD, Sun L. Emerging functions of serum amyloid A in inflammation. J Leukoc Biol. 2015;98:923–929. doi: 10.1189/jlb.3VMR0315-080R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertheloot D, Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14:43–64. doi: 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YJ, Binder RJ. The heat shock protein-CD91 pathway mediates tumor immunosurveillance. OncoImmunology. 2014;3:e28222. doi: 10.4161/onci.28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 21.Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 22.Jin HS, Suh HW, Kim SJ, Jo EK. Mitochondrial control of innate immunity and inflammation. Immune Netw. 2017;17:77–88. doi: 10.4110/in.2017.17.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation. 2015;12:21. doi: 10.1186/s12974-015-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll-like receptors - from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev. 2016;15:1–8. doi: 10.1016/j.autrev.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kufer TA, Banks DJ, Philpott DJ. Innate immune sensing of microbes by Nod proteins. Ann N Y Acad Sci. 2006;1072:19–27. doi: 10.1196/annals.1326.020. [DOI] [PubMed] [Google Scholar]
- 26.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.PrabhuDas MR, Baldwin CL, Bollyky PL, Bowdish DM, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, et al. A consensus definitive classification of scavenger receptors and their roles in health and disease. J Immunol. 2017;198:3775–3789. doi: 10.4049/jimmunol.1700373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 31.van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res. 2002;4:87–93. doi: 10.1186/ar395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baillet A, Trocmé C, Berthier S, Arlotto M, Grange L, Chenau J, Quétant S, Sève M, Berger F, Juvin R, et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology (Oxford) 2010;49:671–682. doi: 10.1093/rheumatology/kep452. [DOI] [PubMed] [Google Scholar]
- 33.Andrés Cerezo L, Šumová B, Prajzlerová K, Veigl D, Damgaard D, Nielsen CH, Pavelka K, Vencovský J, Šenolt L. Calgizzarin (S100A11): a novel inflammatory mediator associated with disease activity of rheumatoid arthritis. Arthritis Res Ther. 2017;19:79. doi: 10.1186/s13075-017-1288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, Patel NB, Huston BJ, Chavan S, Rosas-Ballina M, Gregersen PK, et al. Cholinergic anti-inflammatory pathway activity and high mobility group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–215. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971–981. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- 36.Li YB, Xu P, Xu K, Cai YS, Sun MY, Yang L, Sun J, Lu SM. Methotrexate affects HMGB1 expression in rheumatoid arthritis, and the downregulation of HMGB1 prevents rheumatoid arthritis progression. Mol Cell Biochem. 2016;420:161–170. doi: 10.1007/s11010-016-2783-1. [DOI] [PubMed] [Google Scholar]
- 37.Schierbeck H, Lundbäck P, Palmblad K, Klevenvall L, Erlandsson-Harris H, Andersson U, Ottosson L. Monoclonal anti-HMGB1 (high mobility group box chromosomal protein 1) antibody protection in two experimental arthritis models. Mol Med. 2011;17:1039–1044. doi: 10.2119/molmed.2010.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kokkola R, Li J, Sundberg E, Aveberger AC, Palmblad K, Yang H, Tracey KJ, Andersson U, Harris HE. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–2058. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 39.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spierings J, van Eden W. Heat shock proteins and their immunomodulatory role in inflammatory arthritis. Rheumatology (Oxford) 2017;56:198–208. doi: 10.1093/rheumatology/kew266. [DOI] [PubMed] [Google Scholar]
- 41.Martin CA, Carsons SE, Kowalewski R, Bernstein D, Valentino M, Santiago-Schwarz F. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J Immunol. 2003;171:5736–5742. doi: 10.4049/jimmunol.171.11.5736. [DOI] [PubMed] [Google Scholar]
- 42.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, 2nd, Tak PP, Haines GK, 3rd, Nicchitta CV, Pope RM. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182:4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashiramoto A, Murata M, Kawazoe T, Yoshida K, Akiyama C, Shiozawa K, Shiozawa S. Heat shock protein 90 maintains the tumour-like character of rheumatoid synovial cells by stabilizing integrin-linked kinase, extracellular signal-regulated kinase and protein kinase B. Rheumatology (Oxford) 2011;50:852–861. doi: 10.1093/rheumatology/keq385. [DOI] [PubMed] [Google Scholar]
- 44.Sohn DH, Rhodes C, Onuma K, Zhao X, Sharpe O, Gazitt T, Shiao R, Fert-Bober J, Cheng D, Lahey LJ, et al. Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis Rheumatol. 2015;67:2877–2887. doi: 10.1002/art.39283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 46.Abdulahad DA, Westra J, Bijzet J, Limburg PC, Kallenberg CG, Bijl M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jog NR, Blanco I, Lee I, Putterman C, Caricchio R. Urinary high-mobility group box-1 associates specifically with lupus nephritis class V. Lupus. 2016;25:1551–1557. doi: 10.1177/0961203316644331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaper F, Van Timmeren MM, Petersen A, Horst G, Bijl M, Limburg PC, Westra J, Heeringa P. Treatment with anti-HMGB1 monoclonal antibody does not affect lupus nephritis in MRL/lpr mice. Mol Med. 2016;22:12–21. doi: 10.2119/molmed.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, Baisch J, Phelps K, Clayton S, Gong M, et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med. 2016;213:697–713. doi: 10.1084/jem.20151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 54.Terada C, Yoshida A, Nasu Y, Mori S, Tomono Y, Tanaka M, Takahashi HK, Nishibori M, Ozaki T, Nishida K. Gene expression and localization of high-mobility group box chromosomal protein-1 (HMGB-1)in human osteoarthritic cartilage. Acta Med Okayama. 2011;65:369–377. doi: 10.18926/AMO/47262. [DOI] [PubMed] [Google Scholar]
- 55.Sun XH, Liu Y, Han Y, Wang J. Expression and Significance of high-mobility group protein B1 (HMGB1) and the receptor for advanced glycation end-product (RAGE) in knee osteoarthritis. Med Sci Monit. 2016;22:2105–2112. doi: 10.12659/MSM.895689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cremers NA, van den Bosch MH, van Dalen S, Di Ceglie I, Ascone G, van de Loo F, Koenders M, van der Kraan P, Sloetjes A, Vogl T, et al. S100A8/A9 increases the mobilization of pro-inflammatory Ly6Chigh monocytes to the synovium during experimental osteoarthritis. Arthritis Res Ther. 2017;19:217. doi: 10.1186/s13075-017-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang LC, Zhang HY, Shao L, Chen L, Liu ZH, He X, Gong WX. S100A12 levels in synovial fluid may reflect clinical severity in patients with primary knee osteoarthritis. Biomarkers. 2013;18:216–220. doi: 10.3109/1354750X.2013.766262. [DOI] [PubMed] [Google Scholar]
- 58.Nakashima M, Sakai T, Hiraiwa H, Hamada T, Omachi T, Ono Y, Inukai N, Ishizuka S, Matsukawa T, Oda T, et al. Role of S100A12 in the pathogenesis of osteoarthritis. Biochem Biophys Res Commun. 2012;422:508–514. doi: 10.1016/j.bbrc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 59.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 60.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 61.Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14:133–144. doi: 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, Maruyama I. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. 2007;16:136–143. doi: 10.1016/j.carpath.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 64.McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 65.Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013;2013:828354. doi: 10.1155/2013/828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oesterle A, Bowman MA. S100A12 and the S100/calgranulins: emerging biomarkers for atherosclerosis and possibly therapeutic targets. Arterioscler Thromb Vasc Biol. 2015;35:2496–2507. doi: 10.1161/ATVBAHA.115.302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ligthart S, Sedaghat S, Ikram MA, Hofman A, Franco OH, Dehghan A. EN-RAGE: a novel inflammatory marker for incident coronary heart disease. Arterioscler Thromb Vasc Biol. 2014;34:2695–2699. doi: 10.1161/ATVBAHA.114.304306. [DOI] [PubMed] [Google Scholar]
- 69.Berberian PA, Myers W, Tytell M, Challa V, Bond MG. Immunohistochemical localization of heat shock protein-70 in normal-appearing and atherosclerotic specimens of human arteries. Am J Pathol. 1990;136:71–80. [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao Q, Mandal K, Schett G, Mayr M, Wick G, Oberhollenzer F, Willeit J, Kiechl S, Xu Q. Association of serum-soluble heat shock protein 60 with carotid atherosclerosis: clinical significance determined in a follow-up study. Stroke. 2005;36:2571–2576. doi: 10.1161/01.STR.0000189632.98944.ab. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Zhang C, Wei X, Li P, Cui Y, Qin Y, Wei X, Jin M, Kohama K, Gao Y. Heat shock protein 60 stimulates the migration of vascular smooth muscle cells via Toll-like receptor 4 and ERK MAPK activation. Sci Rep. 2015;5:15352. doi: 10.1038/srep15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maneerat Y, Prasongsukarn K, Benjathummarak S, Dechkhajorn W, Chaisri U. Increased alpha-defensin expression is associated with risk of coronary heart disease: a feasible predictive inflammatory biomarker of coronary heart disease in hyperlipidemia patients. Lipids Health Dis. 2016;15:117. doi: 10.1186/s12944-016-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilkinson K, El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer's disease. Int J Alzheimers Dis. 2012;2012:489456. doi: 10.1155/2012/489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Venegas C, Heneka MT. Danger-associated molecular patterns in Alzheimer's disease. J Leukoc Biol. 2017;101:87–98. doi: 10.1189/jlb.3MR0416-204R. [DOI] [PubMed] [Google Scholar]
- 75.Festoff BW, Sajja RK, van Dreden P, Cucullo L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer's disease. J Neuroinflammation. 2016;13:194. doi: 10.1186/s12974-016-0670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaves ML, Camozzato AL, Ferreira ED, Piazenski I, Kochhann R, Dall’Igna O, Mazzini GS, Souza DO, Portela LV. Serum levels of S100B and NSE proteins in Alzheimer's disease patients. J Neuroinflammation. 2010;7:6. doi: 10.1186/1742-2094-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cirillo C, Capoccia E, Iuvone T, Cuomo R, Sarnelli G, Steardo L, Esposito G. S100B inhibitor pentamidine attenuates reactive gliosis and reduces neuronal loss in a mouse model of Alzheimer's disease. BioMed Res Int. 2015;2015:508342. doi: 10.1155/2015/508342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herrero MT, Estrada C, Maatouk L, Vyas S. Inflammation in Parkinson's disease: role of glucocorticoids. Front Neuroanat. 2015;9:32. doi: 10.3389/fnana.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y, Han C, Guo L, Guan Q. High expression of the HMGB1-TLR4 axis and its downstream signaling factors in patients with Parkinson's disease and the relationship of pathological staging. Brain Behav. 2018;8:e00948. doi: 10.1002/brb3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasaki T, Liu K, Agari T, Yasuhara T, Morimoto J, Okazaki M, Takeuchi H, Toyoshima A, Sasada S, Shinko A, et al. Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson's disease. Exp Neurol. 2016;275:220–231. doi: 10.1016/j.expneurol.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, Martin HL, Schulte C, Mustafa S, Synofzik M, Vukovic Z, et al. S100B is increased in Parkinson's disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-α pathway. Brain. 2012;135:3336–3347. doi: 10.1093/brain/aws250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schaf DV, Tort AB, Fricke D, Schestatsky P, Portela LV, Souza DO, Rieder CR. S100B and NSE serum levels in patients with Parkinson's disease. Parkinsonism Relat Disord. 2005;11:39–43. doi: 10.1016/j.parkreldis.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene. 2016;35:5931–5941. doi: 10.1038/onc.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 87.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 88.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 89.Schelbergen RF, de Munter W, van den Bosch MH, Lafeber FP, Sloetjes A, Vogl T, Roth J, van den Berg WB, van der Kraan PM, Blom AB, et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann Rheum Dis. 2016;75:218–225. doi: 10.1136/annrheumdis-2014-205480. [DOI] [PubMed] [Google Scholar]
- 90.Fucikova J, Moserova I, Urbanova L, Bezu L, Kepp O, Cremer I, Salek C, Strnad P, Kroemer G, Galluzzi L, et al. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front Immunol. 2015;6:402. doi: 10.3389/fimmu.2015.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hendriks LE, Dingemans AC. Heat shock protein antagonists in early stage clinical trials for NSCLC. Expert Opin Investig Drugs. 2017;26:541–550. doi: 10.1080/13543784.2017.1302428. [DOI] [PubMed] [Google Scholar]
- 92.Koffeman EC, Genovese M, Amox D, Keogh E, Santana E, Matteson EL, Kavanaugh A, Molitor JA, Schiff MH, Posever JO, et al. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60:3207–3216. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]