Abstract
The hypoxia‐inducible factor (HIF) co‐ordinates the adaptive transcriptional response to hypoxia in metazoan cells. The hypoxic sensitivity of HIF is conferred by a family of oxygen‐sensing enzymes termed HIF hydroxylases. This family consists of three prolyl hydroxylases (PHD1–3) and a single asparagine hydroxylase termed factor inhibiting HIF (FIH). It has recently become clear that HIF hydroxylases are functionally non‐redundant and have discrete but overlapping physiological roles. Furthermore, altered abundance or activity of these enzymes is associated with a number of pathologies. Pharmacological HIF‐hydroxylase inhibitors have recently proven to be both tolerated and therapeutically effective in patients. In this review, we focus on the physiology, pathophysiology and therapeutic potential of the PHD1 isoform, which has recently been implicated in diseases including inflammatory bowel disease, ischaemia and cancer.
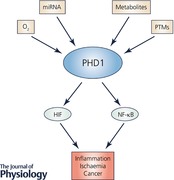
Keywords: Prolyl‐hydroxylase 1, PHD1, PHD inhibitor, hypoxia‐inducible factor, HIF, NF‐κB, apoptosis, inflammatory bowel disease, colitis, ischemia, cancer
Molecular oxygen sensing in metazoan cells
Most animals, including humans, require molecular oxygen (O2) to perform oxidative phosphorylation and thereby maintain a state of internal bioenergetic homeostasis. Oxygen‐dependent cells must therefore be capable of adapting to circumstances in which oxygen demand exceeds supply, a condition termed hypoxia. Hypoxia can occur in physiological states such as during ascent to high altitude or extreme physical exertion. Alternatively, localized tissue hypoxia may occur in pathological conditions such as inflammation, ischaemia and cancer (Bertout et al. 2008; Schneider et al. 2010; Eltzschig & Carmeliet, 2011). On a cellular level, the transcriptional response to hypoxia is orchestrated by the hypoxia‐inducible factor (HIF) (Prabhakar & Semenza, 2015). HIF is a heterodimeric transcription factor that consists of an oxygen‐dependent α‐subunit (HIF1α, HIF2α or HIF3α) and a constitutively expressed β‐subunit (HIF1β). The stability and transcriptional activity of HIF is determined by a family of 2‐oxoglutarate‐dependent dioxygenases which consist of the prolyl hydroxylases PHD1, PHD2 and PHD3 (also termed EGLN2, EGLN1 and EGLN3, respectively) and the asparaginyl hydroxylase termed factor inhibiting HIF (FIH) (Epstein et al. 2001). Under normoxic conditions, PHDs hydroxylate HIFα at two highly conserved proline residues utilizing molecular oxygen as well as 2‐oxoglutarate, iron (Fe2+) and ascorbate as co‐factors. Through proline hydroxylation, HIFα subunits are marked for ubiquitin‐dependent proteosomal degradation by an E3 ubiquitin ligase termed the Von Hippel–Lindau protein (Ivan et al. 2001; Jaakkola et al. 2001). Separately, factor inhibiting HIF (FIH) hydroxylates HIFα at an asparagine residue and prevents its interaction with the transcriptional co‐activator p300/CBP. In hypoxia, a lack of O2 inhibits the catalytic activity of the PHDs and FIH, which leads to HIFα stabilization, dimerization with HIF1β and subsequently the increased expression of target genes including erythropoietin, vascular endothelial growth factor and key glycolytic enzymes that help cells to adapt to O2 deprivation.
Although all HIF‐hydroxylase isoforms regulate the HIF pathway, a growing body of evidence suggests that they also possess isoform‐specific functions and can regulate non‐overlapping biological processes depending on the cell type and physiological context (Chan et al. 2009; Mazzone et al. 2009). These non‐redundant functions became evident through the generation of isoform‐specific knockout mouse models that display different phenotypes. While global homozygous PHD1 knockout mice are viable and exhibit no overt phenotype, mouse embryos with a biallelic loss of PHD2 (PHD2−/−) die prematurely as a consequence of placental vascular defects and homozygous PHD3−/− mice have impaired development of the sympathetic nervous system as well as systemic hypotension (Takeda et al. 2006; Bishop et al. 2008). FIH‐deficient mice display a catabolic phenotype including reduced body weight, hyperventilation, alkalosis and resistance to hepatic steatosis (Zhang et al. 2010).
Mutations in the PHD1 and PHD2 genes in humans are linked to erythrocytosis, pheochromocytoma and recurrent paraganglioma (Ladroue et al. 2008; Yang et al. 2015). In addition, a recent report suggests an association of a PHD2 mutation with a cardiopulmonary phenotype that includes elevated basal ventilation rate and pulmonary artery pressure (Talbot et al. 2017). This further corroborates the notion that HIF hydroxylases have isoform‐specific physiological functions.
One reason for this lack of redundancy may reside in the distinct cell and tissue expression profiles of PHD isoforms. While PHD2 is expressed at a moderate to high level in almost all tissues, PHD1 is markedly expressed in the testis and PHD3 shows its strongest expression in heart muscle and skin (Lieb et al. 2002; Appelhoff et al. 2004; Uhlen et al. 2015, Human Protein Atlas available from https://doi.org/www.proteinatlas.org). Furthermore, PHD isoforms possess different affinities for the three HIFα subunits with PHD2 being the main regulator of HIF1α under normoxic conditions and PHD3 being the most relevant HIF1α‐regulating isoform in hypoxia (Appelhoff et al. 2004).
Finally, PHDs as well as FIH have been shown to have isoform‐selective influence on non‐HIF pathways. For instance, it has been demonstrated that all three PHD isoforms can regulate nuclear factor‐κB (NF‐κB) signalling (Cummins et al. 2006; Xue et al. 2010). In addition, PHDs and FIH can interact with other factors critical for cellular proliferation and metabolism such as the p53 tumour suppressor protein, elements of Wnt/β‐catenin signalling and members of the ubiquitin–proteasome system (Mazzone et al. 2009; Rodriguez et al. 2016; Scholz et al. 2016).
Importantly, the clinical relevance of the PHD enzymes has recently been demonstrated by the application of pharmacological PHD inhibitors that are currently under clinical investigation for the treatment of anaemia (Gupta & Wish, 2017). Given the lack of overt phenotype in the PHD1 knockout mouse and recent evidence that suggests the involvement of PHD1 in several human diseases, specific targeting of the PHD1 isoform may be an attractive therapeutic option. Therefore, in this review, we will focus on describing the physiology, pathophysiology and therapeutic potential of PHD1.
Regulation of intracellular PHD1 quantity and activity
As the HIF hydroxylases play an important physiological role, their expression levels and activity need to be regulated. PHD1 expression is regulated at the transcriptional, post‐transcriptional and post‐translational level (Fig. 1). Furthermore, the abundance of metabolites that act as co‐factors in the PHD1‐dependent hydroxylation of target proteins can determine PHD1 activity.
Figure 1. Regulators and targets of PHD1.
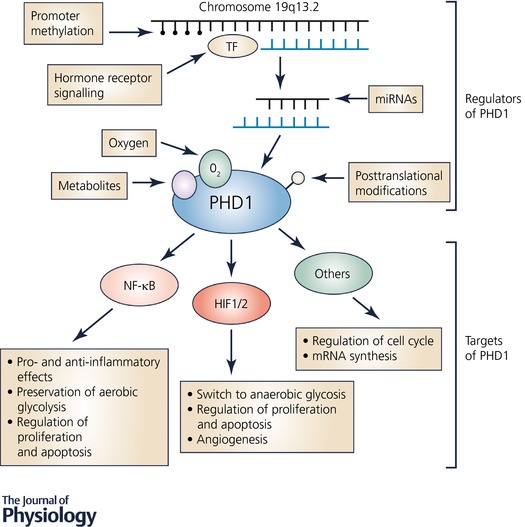
PHD1 is highly regulated at the transcriptional and translational level as well as by its co‐substrates. Apart from HIF, PHD1 targets comprise members of NF‐κB signalling and other factors influencing cellular metabolism, proliferation and apoptosis.
Transcriptional regulation of PHD1 expression
PHD1 expression is altered in a subset of renal cancers as a consequence of somatic pairing, a joining of homologous chromosomes in somatic cells with unknown functional consequences (Koeman et al. 2008). Joining of the homologous chromosomes 19 (which carry the genetic loci of PHD1) up‐regulates PHD1 transcription, which, in turn, is associated with a decrease in the expression of HIF target genes (Koeman et al. 2008).
Further studies investigated the relevance of PHD1 promoter methylation as another mechanism of controlled PHD1 expression. Hypermethylation of the PHD1 promoter region in a subset of human hepatocellular cancer specimens leads to diminished PHD1 expression and poor patient prognosis (Calvisi et al. 2007). Other studies did not find changes in PHD1 promoter methylation in two colorectal cancer cell lines and breast cancer tissue (Huang et al. 2010; Rawluszko et al. 2013). Therefore, genomic modifications resulting in an altered transcription of the PHD1 gene may be of importance in the development and progression of a subset of tumours.
Transcriptional regulation of PHD1 expression is also under the influence of oestrogen and androgen receptor signalling, especially in the setting of estrogen receptor (ER)‐positive breast cancer. Breast cancer cell lines that were stimulated with oestrogen display a transcriptional upregulation of PHD1 (Seth et al. 2002; Appelhoff et al. 2004; Zhang et al. 2017).
A major transcriptional regulator of PHD expression is HIF itself with PHD2 and PHD3 isoforms demonstrating HIF‐dependent upregulation in hypoxia suggesting an inhibitory feedback loop in HIF signalling (Cioffi et al. 2003; Aprelikova et al. 2004; Marxsen et al. 2004). Specifically, it has been demonstrated that PHD3 expression is increased by induction of HIF1α and HIF2α under both normoxic and hypoxic conditions (Aprelikova et al. 2004). An increase of PHD2 expression mediated by HIF1α has also been demonstrated in chronic hypoxia (Aprelikova et al. 2004). Intriguingly however, PHD1 abundance is not regulated by HIF (Ivan & Kaelin, 2017).
Post‐transcriptional regulation of PHD1
PHD1 is a target of non‐coding RNAs including miR‐205, a member of the microRNA family, which can bind to the 3′‐UTR of the PHD1 transcript and thereby induce its degradation. miR‐23a also suppresses PHD1 activity in lung cancer endothelial cells (Muratsu‐Ikeda et al. 2012; Hsu et al. 2017). Interestingly, parts of the PHD1 and the adjacent RAB4B gene locus together form the template sequence for the transcription of a long non‐coding RNA termed RERT (Zhu et al. 2012). In turn, RERT has been shown to positively regulate PHD1 expression (Zhu et al. 2012).
Post‐translational regulation of PHD1
Another mechanism by which PHD1 expression and activity is regulated is through post‐translational modification. For instance, PHD1 is marked for ubiquitin‐dependent proteosomal degradation by the E3 ubiquitin ligase Siah2 (Nakayama et al. 2004). Also, PHD1 has been recently demonstrated to be targeted for degradation by an E3 ubiquitin ligase called speckle‐type POZ protein (SPOP), which is commonly mutated in prostate cancer (Zhang et al. 2017). In SPOP‐deficient prostate cancer cells, knockdown of PHD1 was sufficient to dampen increased cell colony formation introduced by loss of SPOP, thus implying an oncogenic role of PHD1 in that setting (Zhang et al. 2017). In addition, FBW7, another E3 ubiquitin ligase, negatively regulates PHD1 protein expression in an in vitro model of triple receptor‐negative breast cancer (Takada et al. 2017).
Ortmann and colleagues demonstrated that PHD1 can also be also affected by protein phosphorylation and that, in particular, the S130 residue of PHD1 is targeted by cyclin‐dependent kinases (CDKs) 2, 4 and 6 (Ortmann et al. 2016). This results in a shift towards diminished interaction of PHD1 and HIF1α but a raised affinity of PHD1 towards Cep192 (a protein involved in cell cycle progression, see below), thus directly connecting PHD1 and regulation of the cell cycle (Ortmann et al. 2016).
Regulation of PHD1 activity
As well as molecular oxygen, PHDs require several co‐factors to efficiently hydroxylate target proteins. Therefore, quantitative or qualitative alterations of these co‐factors can affect PHD activity. Indeed, two studies from 2005 revealed that intermediates of the tricarboxylic acid (TCA) cycle such as succinate and fumarate regulate PHD activity and HIF stabilization (Isaacs et al. 2005; Selak et al. 2005). Intracellular accumulation of succinate through inhibition of succinate dehydrogenase is linked to a decreased activity of the PHDs via a mechanism of product inhibition (Selak et al. 2005). In addition, fumarate as well as pyruvate and oxaloacetate stabilized HIF in vitro (Dalgard et al. 2004; Isaacs et al. 2005; Lu et al. 2005). Koivunen and colleagues investigated the inhibitory effect of TCA cycle intermediates on different PHD isoforms, showing that these metabolites are distinguishable with respect to PHD isoform‐specific half‐maximal inhibitory concentration (IC50) values (Koivunen et al. 2007). For example, citrate inhibits PHD3 more effectively than PHD1 or PHD2 (Koivunen et al. 2007). Beyond that, more recent publications demonstrated that certain mutations of isocitrate dehydrogenase isoenzymes 1 and 2 (IDH1, 2) can lead to both a decrease of cytosolic 2‐oxoglutarate amounts and production of the oncometabolite R‐2‐hydroxyglutarate (also known as d‐2‐hydroxyglutarate), with the latter being synthesized instead of 2‐oxoglutarate by defective IDH (Zhao et al. 2009; Xu et al. 2011). These studies demonstrated that both low 2‐oxoglutarate concentrations and R‐2‐hydroxyglutarate can negatively affect PHD activity and therefore stabilize HIF, and hence are likely to contribute to tumourigenesis in a subset of human malignancies (Zhao et al. 2009; Xu et al. 2011). Intriguingly, an opposite effect of R‐2‐hydroxyglutarate on PHD activity has been reported. PHD1 and PHD2 activity was significantly increased in vitro following treatment with R‐2‐hydroxyglutarate, hence weakening HIF activity, while R‐2‐hydroxyglutarate's enantiomer, S‐2‐hydroxyglutarate, rather than R‐2‐hydroxyglutarate exerted inhibitory effects on PHD enzyme function (Koivunen et al. 2012; Losman et al. 2013). Although most of the evidence regarding the manipulation of PHD function by metabolic intermediates is not immediately connected to PHD1 per se, it is likely that most of these factors influence PHD1 since all of the three PHDs catalyse the same biochemical reaction.
Influence of PHD1 on cellular functions
PHD isoenzymes regulate diverse cellular processes including inflammation, metabolism, proliferation and apoptosis (Mikhaylova et al. 2008; Takeda et al. 2009; Zhang et al. 2009; Tambuwala et al. 2010). These functional roles can be mediated through either HIF‐dependent or HIF‐independent pathways (see Fig. 1). Here, we will briefly review the role of PHD1 in these processes.
PHD1 regulates inflammation
It has recently become appreciated that tissue hypoxia is a common microenvironmental feature at sites of inflammation (Colgan & Taylor, 2010). Furthermore, the HIF pathway is now recognized as a key regulator of immune cell function (Taylor et al. 2016). Subsequently, altered hydroxylase activity at sites of inflammation regulates immune cell function through altered activity of PHDs (reviewed in Taylor & Colgan, 2017). Therefore, it is highly likely that some of the impact of altered PHD1 activity on inflammatory processes is dependent upon HIF activity in immune cells.
A second target pathway of PHD1 is nuclear factor‐κB (NF‐κB), a transcription factor that controls inflammatory mechanisms in both immune and non‐immune cells. Hypoxia profoundly affects the inflammatory response and altered NF‐κB activity is likely important in this. Hypoxia modestly increases basal NF‐κB activity in multiple cell types. This is supported by evidence that IκB kinase (IKK), a key regulator of NF‐κB, is negatively regulated by PHD1 (and PHD2), thus linking oxygen‐sensing by the PHDs to altered NF‐κB activity (Cummins & Taylor, 2005; Cummins et al. 2006). The role of PHD1 in the control of this pathway has since been confirmed. For example, it has been shown that both pharmacological inhibition of PHDs with dimethyloxalylglycine (DMOG) and siRNA‐mediated knockdown of PHD1 lead to increased basal and TNF‐α‐triggered activation of NF‐κB and are able to consequently induce the expression of NF‐κB‐dependent genes (Cummins et al. 2006, 2008; Winning et al. 2010; Xue et al. 2010; Xie et al. 2014; Quaegebeur et al. 2016). In addition, global homozygous PHD1 deficiency was linked to upregulated NF‐κB in an in vivo model of ischaemia–reperfusion (I/R) injury and in livers of PHD1−/− mice (Adluri et al. 2011; Fitzpatrick et al. 2016). However, the control of NF‐κB by PHD1 is complex. For example, DMOG treatment and transient knockdown of PHD1 reduced NF‐κB activity in macrophages stimulated with lipopolysaccharide (LPS) and, furthermore, downregulated TNF‐α expression, which implies that the effect of PHD1 on the NF‐κB pathway is stimulus‐dependent (Takeda et al. 2009). These results were supported by a study demonstrating that PHD inhibition attenuates NF‐κB activity, thus weakening the proinflammatory signature of peritoneal macrophages in an LPS‐induced model of abdominal sepsis and by work showing that LPS‐stimulated PHD1‐deficient bone marrow‐derived macrophages are polarized towards an M2‐phenotype with decreased proinflammatory cytokine secretion (Hams et al. 2011; Van Welden et al. 2017a). Furthermore, IL‐1β‐induced NF‐κB activity is also significantly decreased upon combined deletion of PHD1 and FIH (Scholz et al. 2013; Ullah et al. 2017).
The relationship between PHD1 and NF‐κB activity is complex and it appears that while hypoxia moderately increases basal signalling in multiple cell types, it inhibits LPS‐ and IL‐1β‐stimulated NF‐κB activity and the nature of its effects appears to be dependent on the cell type and stimulus. For a more comprehensive summary of the complex relationship between HIF‐PHDs and NF‐κB, see Scholz and Taylor (2013).
PHD1 regulates cellular metabolism
PHD1−/− mice exhibit reduced basal oxygen consumption in comparison to control animals (Aragones et al. 2008). However, when these mice were subjected to skeletal muscle ischaemia, loss of PHD1 was protective (Aragones et al. 2008). This could be explained by a HIF2α‐mediated induction of pyruvate dehydrogenase kinase isoforms 1 and 4, which skew cellular metabolism towards lowered mitochondrial respiration and elevated glycolytic ATP production, resulting in increased hypoxic tolerance in skeletal muscle of PHD1−/− mice (Aragones et al. 2008). A recent study revealed that brains of PHD1‐deficient animals are also protected against ischaemia. In contrast to the previous study, this protective phenotype was attributed to impaired glycolysis and preserved mitochondrial respiration in PHD1−/− neurons leading to decreased generation of reactive oxygen species (ROS), which, in turn, conferred neuroprotection (Quaegebeur et al. 2016). This neuronal effect seemed to rely on NF‐κB rather than on HIF activation (Quaegebeur et al. 2016). In a separate study, PHD1‐depleted breast cancer cells also exhibit decreased O2 consumption due to impaired mitochondrial function, an effect that was likely HIF‐independent (Zhang et al. 2015).
PHD1 influences cellular proliferation and apoptosis
PHD1‐defective breast cancer cells show impaired proliferation through decreased abundance of cyclin D1 mRNA and protein (Zhang et al. 2009). One potential mechanism underlying this phenotype is increased stability of forkhead box O3a (FOXO3a) in PHD1‐defective cells. FOXO3a is hydroxylated by PHD1 leading to proteosomal degradation (Zheng et al. 2014). Therefore, loss of PHD1 results in increased levels of FOXO3a, which, in turn, suppresses cyclin D1 activity (Zheng et al. 2014). Furthermore, it has been demonstrated that PHD1 is necessary for the regulation of centrosome duplication and is capable of hydroxylating centrosome protein 192 (Cep192), which promotes mitosis (Moser et al. 2013). However, global loss of PHD1 in vivo is associated with unchanged intestinal epithelial proliferation and increased expression of cyclin D2 and mitosis in regenerating hepatocytes, suggesting that the role of PHD1 in regulating cellular proliferation might be dependent on the biological context (Tambuwala et al. 2010; Mollenhauer et al. 2012).
Loss of PHD1 in mice decreases apoptosis of hepatocytes during hepatic I/R injury. This has been confirmed for intestinal epithelial cells during experimental colitis, for cardiomyocytes in myocardial I/R injury and for retinal cells in the context of ischaemic retinopathy (Schneider et al. 2010; Tambuwala et al. 2010; Adluri et al. 2011; Huang et al. 2011). PHD1 positively correlates with caspase 3, a marker of apoptosis, in human inflammatory bowel disease (Van Welden et al. 2013). HIF1α, HIF2α as well as NF‐κB have been proposed to link PHD1 deficiency to reduced apoptosis (Schneider et al. 2010; Adluri et al. 2011; Fitzpatrick et al. 2016). Conversely, PHD1 has antiapoptotic properties in chemotherapeutically treated cancer cells where loss of PHD1 renders cells highly susceptible to 5‐fluorouracil‐induced cell death via p53‐dependent mechanisms (Deschoemaeker et al. 2015). Taken together, PHD1 plays an important and cell type‐specific role in the control of proliferation and apoptosis.
PHD1 influences mRNA synthesis
Another cellular function influenced by PHD1 is mRNA biogenesis. It has been described that PHD1 can hydroxylate the large subunit of RNA polymerase II and thereby increase mRNA synthesis and fuel oncogenesis in human renal clear cell carcinoma (Mikhaylova et al. 2008; Yi et al. 2010).
PHD1 in disease
Because PHD1 is associated with the regulation of multiple physiological functions, if its abundance or activity is altered, it has the potential to be a causative factor in disease. Below, we will give an overview on the limited current literature pertaining to the importance of PHD1 in pathological conditions.
PHD1 in intestinal inflammation
Following the discovery that pharmacological PHD inhibition alleviates intestinal inflammation in mice, subsequent studies investigated the target PHD isoform responsible (Cummins et al. 2008; Robinson et al. 2008). Interestingly, homozygous PHD1−/− mice phenocopy the protective effects of pharmacological PHD inhibition in colitis (Tambuwala et al. 2010). Homozygous PHD1 deficiency resulted in decreased intestinal epithelial apoptosis and an improved intestinal barrier function (Tambuwala et al. 2010). A more recent study supports a role for PHD1 in colitis by showing that loss of PHD1 in the haematopoietic system is sufficient to protect against colitis (Van Welden et al. 2017a). This effect is in part mediated by an anti‐inflammatory M2‐polarization of PHD1−/− macrophages. Interestingly, PHD1 (but not PHD2 or PHD3) is altered in human colitis at both the transcript and protein levels further strengthening a specific link between PHD1 and colitis and promoting the possibility of specific PHD1 inhibition as a new therapeutic approach (Tambuwala et al. 2010; Van Welden et al. 2013).
PHD1 in cancer
Multiple studies have established a link between PHD1 expression/activity and oncogenesis. While some were able to define molecular interactions between PHD1 and factors known to be vital for cancer development and progression, others described associations of PHD1 abundance with clinical parameters (Table 1).
Table 1.
Expressional status of PHD1 in various cancer entities and association with patient survival
| Cancer entity | Analysed tissue (analysed biomolecule) | Method | Expressional status | Association with survival | Reference |
|---|---|---|---|---|---|
| Lung (NSCLC) | |||||
| 1. | Cancerous tissue (protein) | IHC (TMA) | High expression of PHD1 in 45% of specimens | High expression unfavourable (reduced 5‐YS) | Andersen et al. (2011) |
| 2. | Cancerous tissue (transcript) | Microarray | Not evaluated | High expression unfavourable (reduced OS) | Hsu et al. (2009) |
| Breast | |||||
| 1. | Cancerous tissue (protein) | IHC (whole sections) | PHD1 detectable in 33% of specimens | No association | Peurala et al. (2012) |
| 2. | Cancerous tissue (protein) | IHC (TMA) | PHD1 detectable in 26.7% of specimens | No association | Fox et al. (2011) |
| 3. | Cancerous tissue (protein) | IHC (TMA) | Cytoplasmic PHD1 detectable in 47% of familial (BRCA‐associated) breast cancer specimens | No association | Yan et al. (2009) |
| Colorectal | |||||
| 1. | Cancerous and normal tissue (matched pairs; transcript) | qPCR | Unaltered in majority of tumours | No association | Radhakrishnan et al. (2016) |
| 2. | Cancerous and normal tissue (unmatched; transcript and protein) | qPCR and western blot | Underexpressed in majority of tumours | No association | Rawluszko et al. (2013) |
| 3. | Cancerous and normal tissue (matched pairs; transcript) | qPCR | Overexpressed in majority of tumours | Not evaluated | Xue et al. (2010) |
| 4. | Cancerous tissue (protein) | IHC (TMA) | High expression of PHD1 in 49% of specimens | No association | Xie et al. (2012) |
| Pancreato‐biliary | |||||
| 1. | Cancerous and normal tissue (unmatched; protein) and cancerous tissue only | IHC (TMA) | PHD1 detectable in 64% of cancer specimens and 38% of normal tissue | No association | Gossage et al. (2010) |
| 2. | Cancerous tissue (protein) | IHC (TMA) | High expression in 57% of endocrine pancreas tumour specimens | High expression unfavourable (reduced DFS and OS) | Couvelard et al. (2008) |
| Renal | |||||
| 1. | Cancerous tissue (protein) | IHC (TMA) | PHD1 expression in 39% of all nuclei | No association | Kroeze et al. (2010) |
Abbreviations: 5‐YS, 5‐year survival; BRCA, breast cancer gene; DFS, disease‐free survival; IHC, immunohistochemistry; NSCLC, non‐small‐cell lung carcinoma; TMA, tissue microarray; OS, overall survival; qPCR, quantitative real‐time polymerase chain reaction.
Overexpression of PHD1 in oestrogen‐dependent breast cancer cell lines stimulates proliferation, suggesting that PHD1 might have oncogenic properties (Seth et al. 2002). Since then, a potential deleterious effect of PHD1 in breast (and also prostate) cancer has been supported by various studies (Zheng et al. 2014; Zhang et al. 2015; Takada et al. 2017; Zhang et al. 2017). It has been shown that loss of PHD1 in breast cancer cells decreases proliferation via the induction of cyclin D1 (Zhang et al. 2009). In renal oncocytoma, suppression of pro‐apoptotic HIF target genes through induction of PHD1 participates in disease development (Koeman et al. 2008). Overexpression of PHD1 in lung cancer cell lines correlates with an invasive phenotype with poorer outcome in non‐small‐cell lung cancer patients. An insertion/deletion polymorphism (rs10680577) that increases PHD1 expression in hepatocellular carcinoma is associated with increased susceptibility to various cancers (Hsu et al. 2009; Zhu et al. 2012; Che et al. 2014; Wang et al. 2014; Li et al. 2017). The association of the rs10680577 polymorphism with cancer was more pronounced in smokers than in non‐smokers, which is in line with results from genome‐wide association studies demonstrating that diverse genetic polymorphisms affecting the PHD1 locus are linked to smoking and the probability of developing chronic obstructive pulmonary disease (Tobacco & Genetics, 2010; Cho et al. 2012; Bloom et al. 2014; Che et al. 2014; Wang et al. 2014; Loukola et al. 2015). Hence, increased risk of developing malignancies in individuals bearing certain alterations in the PHD1 gene locus may be at least in part attributed to smoking.
Conversely, a study from 2003 revealed that PHD1 might possess tumour‐suppressive properties by demonstrating that overexpression of PHD1 leads to diminished tumour growth in a murine xenograft model of colorectal cancer (Erez et al. 2003). HIF1α‐induced vascular endothelial growth factor was found to be responsible for this effect, leading to less vascularized and consequently more necrotic tumours (Erez et al. 2003). Similarly, PHD1 inactivation by miR‐23a stabilized HIF1α in lung cancer endothelial cells and promoted angiogenesis and tumour growth (Hsu et al. 2017). Furthermore, PHD1 has been shown to negatively regulate NF‐κB‐induced cyclin D1, which decreases proliferation in lung carcinoma cells (Xie et al. 2014). In gliomas, PHD1 stabilizes dual specificity tyrosine‐phosphorylation‐regulated kinase 1A (DYRK1A, a kinase) via prolyl hydroxylation, leading to increased phosphorylation of inhibitor of DNA binding 2 (ID2), which, in turn, destabilizes HIF2α and is beneficial with regard to glioma progression in both mice and humans (Lee et al. 2016). Also, a study assessing gene promoter methylation in hepatocellular carcinoma proved that hypermethylation of the PHD1 gene was associated with a poor prognosis (Calvisi et al. 2007).
PHD1 expression also influences chemotherapeutic responsiveness. Deschoemaeker et al. (2015) reported that loss of PHD1 sensitizes colorectal cancer cells to 5‐fluorouracil treatment through direct inhibition of chemotherapy‐induced p53 activity. Moreover, docetaxel‐caused apoptosis of cancer cells is at least partly mediated by activation of PHD1 and degradation of HIF1α (Oh et al. 2016). Similarly, PHD1 abundance is increased in breast cancer tissue after treatment with epirubicin and tamoxifen (Fox et al. 2011).
In summary, the functions of PHD1 during oncogenesis remain incompletely understood and seem to be determined by the type of malignancy. This is reflected by the variable expression of PHD1 in different cancers (Table 1). Further mechanistic insights are needed in order to more precisely estimate the potential value of PHD1 as a target in cancer therapy.
PHD1 in ischaemia and cardiovascular disease
PHD1−/− mice are protected against hypoxic necrosis caused by short‐term vascular occlusion (Aragones et al. 2008). This has been supported by subsequent studies that indicate a beneficial effect of PHD1 deficiency in ischaemia (Schneider et al. 2010; Adluri et al. 2011; Huang et al. 2011; Chen et al. 2012; Rishi et al. 2015; Quaegebeur et al. 2016). It has been demonstrated that global loss of PHD1 is protective in an ex vivo model of myocardial I/R injury and in an in vivo model of hepatic I/R injury through reduced apoptosis of cardiomyocytes and diminished oxidative stress in hepatocytes, respectively (Schneider et al. 2010; Adluri et al. 2011). A reduction in ROS in PHD1−/− animals has also been described in cerebral ischaemia where mice lacking PHD1 were protected from neuronal death through a raised ROS scavenging capacity (Quaegebeur et al. 2016). In murine hyperoxia‐triggered ischaemic retinopathy, PHD1 deficiency supports retinal angiogenesis and ameliorates retinal apoptosis (Huang et al. 2011).
PHD1 deficiency has recently been linked to lowered serum cholesterol levels, resistance to hyperglycaemia and an impaired development of atherosclerotic plaques in mice (Marsch et al. 2016). Intriguingly, in a rat model of gestational hypertension, PHD1 was associated with a reduction in blood pressure after physical exercise (Gilbert et al. 2012).
Taken together, PHD1 is suggested to primarily mediate detrimental effects on the cardiovascular system and pharmacological inhibition of PHD1 might therefore represent a potential treatment strategy in ischaemic disease.
PHD1 in liver disease
Several studies highlight a role of PHD1 in liver pathologies. PHD1 deficiency promotes liver regeneration after partial hepatectomy via increased hepatocellular proliferation (Schneider et al. 2010; Mollenhauer et al. 2012). In addition, combined knockdown of PHD1 and KEAP (an intracellular oxygen stress sensor) confers partial resistance to hypoxia‐induced damage in hepatocytes through a reduction of apoptosis, which is in line with results showing that hepatocyte apoptosis is regulated by PHD1 in an NF‐κB‐dependent fashion (Fitzpatrick et al. 2016; Liu et al. 2017). In hepatic steatosis, however, loss of PHD1 seems to have disease‐promoting qualities with increased liver‐specific insulin resistance and a higher body weight gain in PHD1−/− mice (Thomas et al. 2016).
PHD1 inhibition: a novel therapeutic strategy
To date, isoform‐specific PHD inhibitors have not been generated, although multiple pan‐hydroxylase inhibitors are available. Potential clinical applications of prolyl hydroxylase inhibitors (PHIs) include inflammatory disease, ischaemia and anaemia (see Fig. 2). The clinical potential of PHIs has recently increased since some PHIs have now reached stage III trials for the treatment of chronic kidney disease‐related anaemia and stage I for ulcerative colitis (Van Welden et al. 2017b) and appear to be well tolerated and clinically effective.
Figure 2. PHD1 inhibition as a therapeutic strategy.
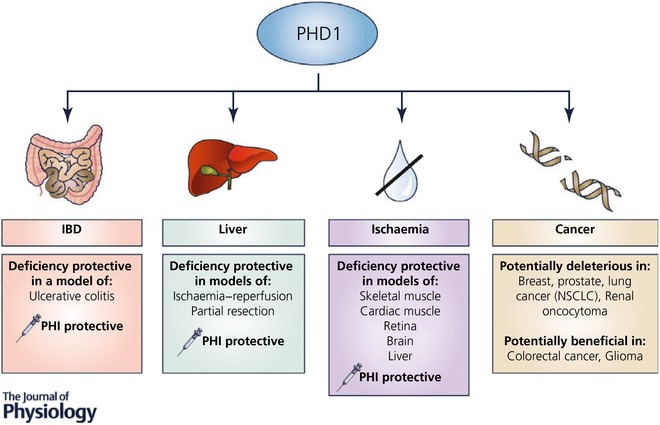
Genetic ablation of PHD1 or treatment with pan‐hydroxylase inhibitors shows protective effects in various diseases. NSCLC, non‐small‐cell lung carcinoma.
Pan‐PHD inhibition has proven protective in multiple models of murine inflammatory bowel disease (IBD) (Cummins et al. 2008, 2013; Robinson et al. 2008). This protective effect has been linked to the inhibition of PHD1 leading to decreased epithelial cell apoptosis (Tambuwala et al. 2010) and the promotion of an anti‐inflammatory M2 phenotype in macrophages (Van Welden et al. 2017a). However, to advance the use of hydroxylase inhibitors for human IBD, potential unwanted adverse side effects of PHIs need to be considered. These include HIF‐mediated induction of erythropoiesis as well as potentially pro‐tumourigenic effects related to HIF activation (Manresa & Taylor, 2017). A viable strategy to circumvent systemic exposure has been provided by developing a targeted colonic release formulation (Tambuwala et al. 2015). Furthermore, oral delivery of the inhibitor AKB‐4924 has also been reported to diminish extraintestinal off‐target effects compared to systemic administration (Marks et al. 2015).
PHD1 loss also conveys hypoxia tolerance in different settings of ischaemia. In this regard, pretreatment with ethyl‐3,4‐dihydroxybenzoate (EDHB) was shown to be hepatoprotective in a model of mouse liver I/R injury by rendering hepatocytes resistant to oxidative stress (Zhong et al. 2008), which was later also reported in PHD1‐deficient mice (Schneider et al. 2010). Thus PHIs could be of value in liver transplantation to protect donor organs from hypoxic damage (Harnoss et al. 2015). Moreover, deletion as well as inhibition of PHD1 via antisense oligonucleotides provides neuroprotection in a model of ischaemic stroke (Quaegebeur et al. 2016). Additionally, targeting PHD1 has been shown to be a potential new strategy in preventing the progression of ischaemic retinopathies like retinopathy of prematurity (Huang et al. 2011).
Recently, surgical liver resection as a treatment of colorectal liver metastases has been identified as another possible indication for PHD1 inhibition, since PHD1 deficiency leads to increased liver regeneration in mice (Mollenhauer et al. 2012). Notably, this effect has now also been achieved by pharmacological inhibition (Harnoss et al. 2017). In this study pretreatment with EDHB selectively promoted liver regeneration after partial resection without inducing metastatic tumour growth. (Harnoss et al. 2017). Although administration was systemic, EDHB accumulated in the liver, which could be a desirable advantage of this drug in terms of limiting systemic side effects (Harnoss et al. 2017).
Perspective
PHD1 is a functionally important cellular oxygen sensor with a high degree of potential as a therapeutic target in multiple clinical scenarios ranging from chronic inflammation to ischaemia. The development of effective PHD1 isoform‐specific inhibitors and effective drug targeting will enhance the possibility of realizing the potential of targeting PHD1 for therapeutic utility.
Additional information
Competing interests
C.T.T. is a member of the scientific advisory board of Akebia Therapeutics.
Author contributions
C.T.T., K.B.K., M.S. and J.B. all contributed to the writing and editing of this review. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Work from the authors’ labs is funded by Science Foundation Ireland (C.T.T.), German Cancer Aid (K.B.K.) and the European Union (ERACoSysMED grant OxyUC).
Biographies
Kilian Kennel is a medical student working at the Department of General, Visceral and Transplantation Surgery, University Hospital Heidelberg, Germany. He currently investigates mechanisms by which HIF prolyl‐hydroxylases influence inflammation‐associated tumour growth.

Julius Burmeister is a medical student at the University of Heidelberg. His work focuses on the impact of pharmacological HIF prolyl‐hydroxylase inhibition on the development and progression of inflammation‐associated cancers.
Martin Schneider received his MD degree in 2002 and pursued clinical training in general and gastrointestinal surgery at the University Hospital Heidelberg (Germany). From 2004 onwards, he spent 3 years as a postdoctoral research fellow at the Vesalius Research Centre in Leuven (Belgium). In 2013 he became attending surgeon at the Clinic for General, Visceral and Transplantation Surgery in Heidelberg. In 2015, he was appointed full professor of translational surgical oncology at the University of Heidelberg. His clinical focus is on gastrointestinal tumours. His current research focus is on hypoxia signalling in visceral disease, and on molecular mechanisms underlying colorectal cancer spread.
Cormac Taylor currently holds an appointment as Professor of Cellular Physiology at the School of Medicine and Medical Science and the Conway Institute, University College Dublin. Current research in the Taylor Lab is directed towards expanding our understanding of the physiological and pathophysiological mechanisms by which changes in micro‐environmental oxygen levels regulate gene transcription in eukaryotic cells. He was elected as a member of the Royal Irish Academy. He was awarded the 2014 Nature mid‐career mentorship award and the 2017 Takeda Distinguished Researcher Award from the American Physiological Society, GI and Liver section.
Edited by: Kim Barrett & Ole Petersen
This review was presented at the symposium ‘Gastrointestinal Tract XVII: Current Biology of the GI Tract, Mucosa, Microbiota, and Beyond’, which took place at FASEB 2017, Steamboat Springs, CO, USA, 30 July–4 August 2017.
References
- Adluri RS, Thirunavukkarasu M, Dunna NR, Zhan L, Oriowo B, Takeda K, Sanchez JA, Otani H, Maulik G, Fong GH & Maulik N (2011). Disruption of hypoxia‐inducible transcription factor‐prolyl hydroxylase domain‐1 (PHD‐1− / −) attenuates ex vivo myocardial ischemia/reperfusion injury through hypoxia‐inducible factor‐1α transcription factor and its target genes in mice. Antioxid Redox Signal 15, 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S, Donnem T, Stenvold H, Al‐Saad S, Al‐Shibli K, Busund LT & Bremnes RM (2011). Overexpression of the HIF hydroxylases PHD1, PHD2, PHD3 and FIH are individually and collectively unfavorable prognosticators for NSCLC survival. PLoS One 6, e23847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ & Gleadle JM (2004). Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia‐inducible factor. J Biol Chem 279, 38458–38465. [DOI] [PubMed] [Google Scholar]
- Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM & Barrett JC (2004). Regulation of HIF prolyl hydroxylases by hypoxia‐inducible factors. J Cell Biochem 92, 491–501. [DOI] [PubMed] [Google Scholar]
- Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez‐Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi‐Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P & Carmeliet P (2008). Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40, 170–180. [DOI] [PubMed] [Google Scholar]
- Bertout JA, Patel SA & Simon MC (2008). The impact of O2 availability on human cancer. Nat Rev Cancer 8, 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega‐Saenz P, Oster H, Wijeyekoon B, Sutherland AI, Grosfeld A, Aragones J, Schneider M, van Geyte K, Teixeira D, Diez‐Juan A, Lopez‐Barneo J, Channon KM, Maxwell PH, Pugh CW, Davies AM, Carmeliet P & Ratcliffe PJ (2008). Abnormal sympathoadrenal development and systemic hypotension in PHD3 − / − mice. Mol Cell Biol 28, 3386–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Baker TB, Chen LS, Breslau N, Hatsukami D, Bierut LJ & Goate A (2014). Variants in two adjacent genes, EGLN2 and CYP2A6, influence smoking behavior related to disease risk via different mechanisms. Hum Mol Genet 23, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, Schroeder I, Factor VM & Thorgeirsson SS (2007). Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest 117, 2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT & Giaccia AJ (2009). Tumor vasculature is regulated by PHD2‐mediated angiogenesis and bone marrow‐derived cell recruitment. Cancer Cell 15, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che J, Jiang D, Zheng Y, Zhu B, Zhang P, Lu D, Zhang J, Xiao J, Wang J, Gao Y, Yan X & Wang M (2014). Polymorphism in PHD1 gene and risk of non‐small cell lung cancer in a Chinese population. Tumour Biol 35, 8921–8925. [DOI] [PubMed] [Google Scholar]
- Chen RL, Nagel S, Papadakis M, Bishop T, Pollard P, Ratcliffe PJ, Pugh CW & Buchan AM (2012). Roles of individual prolyl‐4‐hydroxylase isoforms in the first 24 hours following transient focal cerebral ischaemia: insights from genetically modified mice. J Physiol 590, 4079–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, Himes BE, Sylvia JS, Klanderman BJ, Ziniti JP, Lange C, Litonjua AA, Sparrow D, Regan EA, Make BJ, Hokanson JE, Murray T, Hetmanski JB, Pillai SG, Kong X, Anderson WH, Tal‐Singer R, Lomas DA, Coxson HO, Edwards LD, MacNee W, Vestbo J, Yates JC, Agusti A, Calverley PM, Celli B, Crim C, Rennard S, Wouters E, Bakke P, Gulsvik A, Crapo JD, Beaty TH, Silverman EK, Investigators I, Investigators E & Investigators CO (2012). A genome‐wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet 21, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi CL, Liu XQ, Kosinski PA, Garay M & Bowen BR (2003). Differential regulation of HIF‐1α prolyl‐4‐hydroxylase genes by hypoxia in human cardiovascular cells. Biochem Biophys Res Commun 303, 947–953. [DOI] [PubMed] [Google Scholar]
- Colgan SP & Taylor CT (2010). Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvelard A, Deschamps L, Rebours V, Sauvanet A, Gatter K, Pezzella F, Ruszniewski P & Bedossa P (2008). Overexpression of the oxygen sensors PHD‐1, PHD‐2, PHD‐3, and FIH is associated with tumor aggressiveness in pancreatic endocrine tumors. Clin Cancer Res 14, 6634–6639. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J & Taylor CT (2006). Prolyl hydroxylase‐1 negatively regulates IκB kinase‐β, giving insight into hypoxia‐induced NFκB activity. Proc Natl Acad Sci USA 103, 18154–18159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Doherty GA & Taylor CT (2013). Hydroxylases as therapeutic targets in inflammatory bowel disease. Lab Invest 93, 378–383. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG & Taylor CT (2008). The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165. [DOI] [PubMed] [Google Scholar]
- Cummins EP & Taylor CT (2005). Hypoxia‐responsive transcription factors. Pflugers Arch 450, 363–371. [DOI] [PubMed] [Google Scholar]
- Dalgard CL, Lu H, Mohyeldin A & Verma A (2004). Endogenous 2‐oxoacids differentially regulate expression of oxygen sensors. Biochem J 380, 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschoemaeker S, Di Conza G, Lilla S, Martin‐Perez R, Mennerich D, Boon L, Hendrikx S, Maddocks OD, Marx C, Radhakrishnan P, Prenen H, Schneider M, Myllyharju J, Kietzmann T, Vousden KH, Zanivan S & Mazzone M (2015). PHD1 regulates p53‐mediated colorectal cancer chemoresistance. EMBO Mol Med 7, 1350–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK & Carmeliet P (2011). Hypoxia and inflammation. N Engl J Med 364, 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ & Ratcliffe PJ (2001). C. elegans EGL‐9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54. [DOI] [PubMed] [Google Scholar]
- Erez N, Milyavsky M, Eilam R, Shats I, Goldfinger N & Rotter V (2003). Expression of prolyl‐hydroxylase‐1 (PHD1/EGLN2) suppresses hypoxia inducible factor‐1α activation and inhibits tumor growth. Cancer Res 63, 8777–8783. [PubMed] [Google Scholar]
- Fitzpatrick SF, Fabian Z, Schaible B, Lenihan CR, Schwarzl T, Rodriguez J, Zheng X, Li Z, Tambuwala MM, Higgins DG, O'Meara Y, Slattery C, Manresa MC, Fraisl P, Bruning U, Baes M, Carmeliet P, Doherty G, von Kriegsheim A, Cummins EP & Taylor CT (2016). Prolyl hydroxylase‐1 regulates hepatocyte apoptosis in an NF‐κB‐dependent manner. Biochem Biophys Res Commun 474, 579–586. [DOI] [PubMed] [Google Scholar]
- Fox SB, Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Bersiga A, Allevi G, Milani M, Aguggini S, Mele T, Dogliotti L, Bottini A & Harris AL (2011). The prolyl hydroxylase enzymes are positively associated with hypoxia‐inducible factor‐1α and vascular endothelial growth factor in human breast cancer and alter in response to primary systemic treatment with epirubicin and tamoxifen. Breast Cancer Res 13, R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS, Banek CT, Bauer AJ, Gingery A & Needham K (2012). Exercise training attenuates placental ischemia‐induced hypertension and angiogenic imbalance in the rat. Hypertension 60, 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossage L, Zaitoun A, Fareed KR, Turley H, Aloysius M, Lobo DN, Harris AL & Madhusudan S (2010). Expression of key hypoxia sensing prolyl‐hydroxylases PHD1, ‐2 and ‐3 in pancreaticobiliary cancer. Histopathology 56, 908–920. [DOI] [PubMed] [Google Scholar]
- Gupta N & Wish JB (2017). Hypoxia‐inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis 69, 815–826. [DOI] [PubMed] [Google Scholar]
- Hams E, Saunders SP, Cummins EP, O'Connor A, Tambuwala MT, Gallagher WM, Byrne A, Campos‐Torres A, Moynagh PM, Jobin C, Taylor CT & Fallon PG (2011). The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL‐10 production by B1 cells. Shock 36, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnoss JM, Platzer LK, Burhenne J, Radhakrishnan P, Cai J, Strowitzki MJ, Weiss J, Ritter AS, Mollenhauer M, Schmidt T, Ulrich A, Haefeli WE & Schneider M (2017). Prolyl hydroxylase inhibition enhances liver regeneration without induction of tumor growth. Ann Surg 265, 782–791. [DOI] [PubMed] [Google Scholar]
- Harnoss JM, Strowitzki MJ, Radhakrishnan P, Platzer LK, Harnoss JC, Hank T, Cai J, Ulrich A & Schneider M (2015). Therapeutic inhibition of prolyl hydroxylase domain‐containing enzymes in surgery: putative applications and challenges. Hypoxia (Auckl) 3, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Yuan S, Chen HY, Yu SL, Liu CH, Hsu PY, Wu G, Lin CH, Chang GC, Li KC & Yang PC (2009). A four‐gene signature from NCI‐60 cell line for survival prediction in non‐small cell lung cancer. Clin Cancer Res 15, 7309–7315. [DOI] [PubMed] [Google Scholar]
- Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY & Kuo PL (2017). Hypoxic lung cancer‐secreted exosomal miR‐23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO‐1. Oncogene 36, 4929–4942. [DOI] [PubMed] [Google Scholar]
- Huang H, Van de Veire S, Dalal M, Parlier R, Semba RD, Carmeliet P & Vinores SA (2011). Reduced retinal neovascularization, vascular permeability, and apoptosis in ischemic retinopathy in the absence of prolyl hydroxylase‐1 due to the prevention of hyperoxia‐induced vascular obliteration. Invest Ophthalmol Vis Sci 52, 7565–7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KT, Mikeska T, Dobrovic A & Fox SB (2010). DNA methylation analysis of the HIF‐1α prolyl hydroxylase domain genes PHD1, PHD2, PHD3 and the factor inhibiting HIF gene FIH in invasive breast carcinomas. Histopathology 57, 451–460. [DOI] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mole DR, Lee S, Torres‐Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM & Neckers L (2005). HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153. [DOI] [PubMed] [Google Scholar]
- Ivan M & Kaelin WG Jr (2017). The EGLN‐HIF O2‐sensing system: multiple inputs and feedbacks. Mol Cell 66, 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS & Kaelin WG Jr (2001). HIFα targeted for VHL‐mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW & Ratcliffe PJ (2001). Targeting of HIF‐α to the von Hippel‐Lindau ubiquitylation complex by O2‐regulated prolyl hydroxylation. Science 292, 468–472. [DOI] [PubMed] [Google Scholar]
- Koeman JM, Russell RC, Tan MH, Petillo D, Westphal M, Koelzer K, Metcalf JL, Zhang Z, Matsuda D, Dykema KJ, Houseman HL, Kort EJ, Furge LL, Kahnoski RJ, Richard S, Vieillefond A, Swiatek PJ, Teh BT, Ohh M & Furge KA (2008). Somatic pairing of chromosome 19 in renal oncocytoma is associated with deregulated EGLN2‐mediated [corrected] oxygen‐sensing response. PLoS Genet 4, e1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen P, Hirsila M, Remes AM, Hassinen IE, Kivirikko KI & Myllyharju J (2007). Inhibition of hypoxia‐inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem 282, 4524–4532. [DOI] [PubMed] [Google Scholar]
- Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, Losman JA, Joensuu P, Bergmann U, Gross S, Travins J, Weiss S, Looper R, Ligon KL, Verhaak RG, Yan H & Kaelin WG Jr (2012). Transformation by the (R)‐enantiomer of 2‐hydroxyglutarate linked to EGLN activation. Nature 483, 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze SG, Vermaat JS, van Brussel A, van Melick HH, Voest EE, Jonges TG, van Diest PJ, Hinrichs J, Bosch JL & Jans JJ (2010). Expression of nuclear FIH independently predicts overall survival of clear cell renal cell carcinoma patients. Eur J Cancer 46, 3375–3382. [DOI] [PubMed] [Google Scholar]
- Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau‐Salle F, Feunteun J, Pouyssegur J, Richard S & Gardie B (2008). PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med 359, 2685–2692. [DOI] [PubMed] [Google Scholar]
- Lee SB, Frattini V, Bansal M, Castano AM, Sherman D, Hutchinson K, Bruce JN, Califano A, Liu G, Cardozo T, Iavarone A & Lasorella A (2016). An ID2‐dependent mechanism for VHL inactivation in cancer. Nature 529, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Feng L, Niu L, Teng Li T, Zhang B, Wan H, Zhu Z, Liu H, Wang K, Fu H & Fu W (2017). An insertion/deletion polymorphism within the promoter of EGLN2 is associated with susceptibility to colorectal cancer. Int J Biol Markers 32, e274–e277. [DOI] [PubMed] [Google Scholar]
- Lieb ME, Menzies K, Moschella MC, Ni R & Taubman MB (2002). Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochem Cell Biol 80, 421–426. [DOI] [PubMed] [Google Scholar]
- Liu J, Li Y, Liu L, Wang Z, Shi C, Cheng Z, Zhang X, Ding F & Chen PS (2017). Double knockdown of PHD1 and Keap1 attenuated hypoxia‐induced injuries in hepatocytes. Front Physiol 8, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, Cowley GS, Root DE, Ebert BL & Kaelin WG Jr (2013). ( R)‐2‐Hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 339, 1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukola A, Buchwald J, Gupta R, Palviainen T, Hallfors J, Tikkanen E, Korhonen T, Ollikainen M, Sarin AP, Ripatti S, Lehtimaki T, Raitakari O, Salomaa V, Rose RJ, Tyndale RF & Kaprio J (2015). A genome‐wide association study of a biomarker of nicotine metabolism. PLoS Genet 11, e1005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS & Verma A (2005). Reversible inactivation of HIF‐1 prolyl hydroxylases allows cell metabolism to control basal HIF‐1. J Biol Chem 280, 41928–41939. [DOI] [PubMed] [Google Scholar]
- Manresa MC & Taylor CT (2017). Hypoxia inducible factor (HIF) hydroxylases as regulators of intestinal epithelial barrier function. Cell Mol Gastroenterol Hepatol 3, 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks E, Goggins BJ, Cardona J, Cole S, Minahan K, Mateer S, Walker MM, Shalwitz R & Keely S (2015). Oral delivery of prolyl hydroxylase inhibitor: AKB‐4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm Bowel Dis 21, 267–275. [DOI] [PubMed] [Google Scholar]
- Marsch E, Demandt JA, Theelen TL, Tullemans BM, Wouters K, Boon MR, van Dijk TH, Gijbels MJ, Dubois LJ, Meex SJ, Mazzone M, Hung G, Fisher EA, Biessen EA, Daemen MJ, Rensen PC, Carmeliet P, Groen AK & Sluimer JC (2016). Deficiency of the oxygen sensor prolyl hydroxylase 1 attenuates hypercholesterolaemia, atherosclerosis, and hyperglycaemia. Eur Heart J 37, 2993–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, Jelkmann W, Jaakkola P & Metzen E (2004). Hypoxia‐inducible factor‐1 (HIF‐1) promotes its degradation by induction of HIF‐α‐prolyl‐4‐hydroxylases. Biochem J 381, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P & Carmeliet P (2009). Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova O, Ignacak ML, Barankiewicz TJ, Harbaugh SV, Yi Y, Maxwell PH, Schneider M, Van Geyte K, Carmeliet P, Revelo MP, Wyder M, Greis KD, Meller J & Czyzyk‐Krzeska MF (2008). The von Hippel‐Lindau tumor suppressor protein and Egl‐9‐type proline hydroxylases regulate the large subunit of RNA polymerase II in response to oxidative stress. Mol Cell Biol 28, 2701–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer M, Kiss J, Dudda J, Kirchberg J, Rahbari N, Radhakrishnan P, Niemietz T, Rausch V, Weitz J & Schneider M (2012). Deficiency of the oxygen sensor PHD1 augments liver regeneration after partial hepatectomy. Langenbecks Arch Surg 397, 1313–1322. [DOI] [PubMed] [Google Scholar]
- Moser SC, Bensaddek D, Ortmann B, Maure JF, Mudie S, Blow JJ, Lamond AI, Swedlow JR & Rocha S (2013). PHD1 links cell‐cycle progression to oxygen sensing through hydroxylation of the centrosomal protein Cep192. Dev Cell 26, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratsu‐Ikeda S, Nangaku M, Ikeda Y, Tanaka T, Wada T & Inagi R (2012). Downregulation of miR‐205 modulates cell susceptibility to oxidative and endoplasmic reticulum stresses in renal tubular cells. PLoS One 7, e41462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument‐Bromage H, Tempst P, Frappell PB, Bowtell DD & Ronai Z (2004). Siah2 regulates stability of prolyl‐hydroxylases, controls HIF1α abundance, and modulates physiological responses to hypoxia. Cell 117, 941–952. [DOI] [PubMed] [Google Scholar]
- Oh ET, Kim CW, Kim SJ, Lee JS, Hong SS & Park HJ (2016). Docetaxel induced‐JNK2/PHD1 signaling pathway increases degradation of HIF‐1α and causes cancer cell death under hypoxia. Sci Rep 6, 27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann B, Bensaddek D, Carvalhal S, Moser SC, Mudie S, Griffis ER, Swedlow JR, Lamond AI & Rocha S (2016). CDK‐dependent phosphorylation of PHD1 on serine 130 alters its substrate preference in cells. J Cell Sci 129, 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurala E, Koivunen P, Bloigu R, Haapasaari KM & Jukkola‐Vuorinen A (2012). Expressions of individual PHDs associate with good prognostic factors and increased proliferation in breast cancer patients. Breast Cancer Res Treat 133, 179–188. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR & Semenza GL (2015). Oxygen sensing and homeostasis. Physiology (Bethesda) 30, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaegebeur A, Segura I, Schmieder R, Verdegem D, Decimo I, Bifari F, Dresselaers T, Eelen G, Ghosh D, Davidson SM, Schoors S, Broekaert D, Cruys B, Govaerts K, De Legher C, Bouche A, Schoonjans L, Ramer MS, Hung G, Bossaert G, Cleveland DW, Himmelreich U, Voets T, Lemmens R, Bennett CF, Robberecht W, De Bock K, Dewerchin M, Ghesquiere B, Fendt SM & Carmeliet P (2016). Deletion or inhibition of the oxygen sensor PHD1 protects against ischemic stroke via reprogramming of neuronal metabolism. Cell metabolism 23, 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan P, Ruh N, Harnoss JM, Kiss J, Mollenhauer M, Scherr AL, Platzer LK, Schmidt T, Podar K, Opferman JT, Weitz J, Schulze‐Bergkamen H, Koehler BC, Ulrich A & Schneider M (2016). Prolyl hydroxylase 3 attenuates MCL‐1‐mediated ATP production to suppress the metastatic potential of colorectal cancer cells. Cancer Res 76, 2219–2230. [DOI] [PubMed] [Google Scholar]
- Rawluszko AA, Bujnicka KE, Horbacka K, Krokowicz P & Jagodzinski PP (2013). Expression and DNA methylation levels of prolyl hydroxylases PHD1, PHD2, PHD3 and asparaginyl hydroxylase FIH in colorectal cancer. BMC Cancer 13, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishi MT, Selvaraju V, Thirunavukkarasu M, Shaikh IA, Takeda K, Fong GH, Palesty JA, Sanchez JA & Maulik N (2015). Deletion of prolyl hydroxylase domain proteins (PHD1, PHD3) stabilizes hypoxia inducible factor‐1 alpha, promotes neovascularization, and improves perfusion in a murine model of hind‐limb ischemia. Microvasc Res 97, 181–188. [DOI] [PubMed] [Google Scholar]
- Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT & Colgan SP (2008). Mucosal protection by hypoxia‐inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Pilkington R, Garcia Munoz A, Nguyen LK, Rauch N, Kennedy S, Monsefi N, Herrero A, Taylor CT & von Kriegsheim A (2016). Substrate‐trapped interactors of PHD3 and FIH cluster in distinct signaling pathways. Cell Rep 14, 2745–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Van Geyte K, Fraisl P, Kiss J, Aragones J, Mazzone M, Mairbaurl H, De Bock K, Jeoung NH, Mollenhauer M, Georgiadou M, Bishop T, Roncal C, Sutherland A, Jordan B, Gallez B, Weitz J, Harris RA, Maxwell P, Baes M, Ratcliffe P & Carmeliet P (2010). Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology 138, 1143–1154.e2. [DOI] [PubMed] [Google Scholar]
- Scholz CC, Cavadas MA, Tambuwala MM, Hams E, Rodriguez J, von Kriegsheim A, Cotter P, Bruning U, Fallon PG, Cheong A, Cummins EP & Taylor CT (2013). Regulation of IL‐1β‐induced NF‐κB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci USA 110, 18490–18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz CC, Rodriguez J, Pickel C, Burr S, Fabrizio JA, Nolan KA, Spielmann P, Cavadas MA, Crifo B, Halligan DN, Nathan JA, Peet DJ, Wenger RH, Von Kriegsheim A, Cummins EP & Taylor CT (2016). FIH regulates cellular metabolism through hydroxylation of the deubiquitinase OTUB1. PLoS Biol 14, e1002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz CC & Taylor CT (2013). Hydroxylase‐dependent regulation of the NF‐κB pathway. Biol Chem 394, 479–493. [DOI] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB & Gottlieb E (2005). Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF‐α prolyl hydroxylase. Cancer Cell 7, 77–85. [DOI] [PubMed] [Google Scholar]
- Seth P, Krop I, Porter D & Polyak K (2002). Novel estrogen and tamoxifen induced genes identified by SAGE (Serial Analysis of Gene Expression). Oncogene 21, 836–843. [DOI] [PubMed] [Google Scholar]
- Takada M, Zhuang M, Inuzuka H, Zhang J, Zurlo G, Zhang J & Zhang Q (2017). EglN2 contributes to triple negative breast tumorigenesis by functioning as a substrate for the FBW7 tumor suppressor. Oncotarget 8, 6787–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A & Fong GH (2006). Placental but not heart defects are associated with elevated hypoxia‐inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol 26, 8336–8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Ichiki T, Narabayashi E, Inanaga K, Miyazaki R, Hashimoto T, Matsuura H, Ikeda J, Miyata T & Sunagawa K (2009). Inhibition of prolyl hydroxylase domain‐containing protein suppressed lipopolysaccharide‐induced TNF‐α expression. Arterioscler Thromb Vasc Biol 29, 2132–2137. [DOI] [PubMed] [Google Scholar]
- Talbot NP, Smith TG, Balanos GM, Dorrington KL, Maxwell PH & Robbins PA (2017). Cardiopulmonary phenotype associated with human PHD2 mutation. Physiol Rep 5, e13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M & Taylor CT (2010). Loss of prolyl hydroxylase‐1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 139, 2093–2101. [DOI] [PubMed] [Google Scholar]
- Tambuwala MM, Manresa MC, Cummins EP, Aversa V, Coulter IS & Taylor CT (2015). Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J Control Release 217, 221–227. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Colgan SP (2017). Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol 7, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Doherty G, Fallon PG, Cummins EP (2016). Hypoxia‐dependent regulation of inflammatory pathways in immune cells. J Clin Invest 126, 3716–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Belaidi E, Aron‐Wisnewsky J, van der Zon GC, Levy P, Clement K, Pepin JL, Godin‐Ribuot D & Guigas B (2016). Hypoxia‐inducible factor prolyl hydroxylase 1 (PHD1) deficiency promotes hepatic steatosis and liver‐specific insulin resistance in mice. Sci Rep 6, 24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco & Genetics C (2010). Genome‐wide meta‐analyses identify multiple loci associated with smoking behavior. Nat Genet 42, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J & Ponten F (2015). Proteomics. Tissue‐based map of the human proteome. Science 347, 1260419. [DOI] [PubMed] [Google Scholar]
- Ullah K, Rosendahl AH, Izzi V, Bergmann U, Pihlajaniemi T, Mäki JM, Myllyharju J (2017). Hypoxia‐inducible factor prolyl‐4‐hydroxylase‐1 is a convergent point in the reciprocal negative regulation of NF‐κB and p53 signaling pathways. Sci Rep 7, 17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Welden S, De Vos M, Wielockx B, Tavernier SJ, Dullaers M, Neyt S, Descamps B, Devisscher L, Devriese S, Van den Bossche L, Holvoet T, Baeyens A, Correale C, D'Alessio S, Vanhove C, De Vos F, Verhasselt B, Breier G, Lambrecht BN, Janssens S, Carmeliet P, Danese S, Elewaut D, Laukens D & Hindryckx P (2017a). Haematopoietic prolyl hydroxylase‐1 deficiency promotes M2 macrophage polarization and is both necessary and sufficient to protect against experimental colitis. J Pathol 241, 547–558. [DOI] [PubMed] [Google Scholar]
- Van Welden S, Laukens D, Ferdinande L, De Vos M & Hindryckx P (2013). Differential expression of prolyl hydroxylase 1 in patients with ulcerative colitis versus patients with Crohn's disease/infectious colitis and healthy controls. J Inflamm (Lond) 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Welden S, Selfridge AC & Hindryckx P (2017b). Intestinal hypoxia and hypoxia‐induced signalling as therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 14, 596–611. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang J, Zhou C, Chen L & Yu Q (2014). An insertion/deletion polymorphism within the proximal promoter of EGLN2 is associated with susceptibility for gastric cancer in the Chinese population. Genet Test Mol Biomarkers 18, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winning S, Splettstoesser F, Fandrey J & Frede S (2010). Acute hypoxia induces HIF‐independent monocyte adhesion to endothelial cells through increased intercellular adhesion molecule‐1 expression: the role of hypoxic inhibition of prolyl hydroxylase activity for the induction of NF‐κB. J Immunol 185, 1786–1793. [DOI] [PubMed] [Google Scholar]
- Xie G, Zheng L, Ou J, Huang H, He J, Li J, Pan F & Liang H (2012). Low expression of prolyl hydroxylase 2 is associated with tumor grade and poor prognosis in patients with colorectal cancer. Exp Biol Med (Maywood) 237, 860–866. [DOI] [PubMed] [Google Scholar]
- Xie X, Xiao H, Ding F, Zhong H, Zhu J, Ma N & Mei J (2014). Over‐expression of prolyl hydroxylase‐1 blocks NF‐κB‐mediated cyclin D1 expression and proliferation in lung carcinoma cells. Cancer Genet 207, 188–194. [DOI] [PubMed] [Google Scholar]
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM & Xiong Y (2011). Oncometabolite 2‐hydroxyglutarate is a competitive inhibitor of α‐ketoglutarate‐dependent dioxygenases. Cancer Cell 19, 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Li X, Jiao S, Wei Y, Wu G & Fang J (2010). Prolyl hydroxylase‐3 is down‐regulated in colorectal cancer cells and inhibits IKKβ independent of hydroxylase activity. Gastroenterology 138, 606–615. [DOI] [PubMed] [Google Scholar]
- Yan M, Rayoo M, Takano EA, Investigators KC & Fox SB (2009). BRCA1 tumours correlate with a HIF‐1α phenotype and have a poor prognosis through modulation of hydroxylase enzyme profile expression. Br J Cancer 101, 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhuang Z, Fliedner SM, Shankavaram U, Sun MG, Bullova P, Zhu R, Elkahloun AG, Kourlas PJ, Merino M, Kebebew E & Pacak K (2015). Germ‐line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma‐polycythemia. J Mol Med (Berl) 93, 93–104. [DOI] [PubMed] [Google Scholar]
- Yi Y, Mikhaylova O, Mamedova A, Bastola P, Biesiada J, Alshaikh E, Levin L, Sheridan RM, Meller J & Czyzyk‐Krzeska MF (2010). von Hippel‐Lindau‐dependent patterns of RNA polymerase II hydroxylation in human renal clear cell carcinomas. Clin Cancer Res 16, 5142–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang C, Chen X, Takada M, Fan C, Zheng X, Wen H, Liu Y, Wang C, Pestell RG, Aird KM, Kaelin WG Jr, Liu XS & Zhang Q (2015). EglN2 associates with the NRF1‐PGC1α complex and controls mitochondrial function in breast cancer. EMBO J 34, 2953–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Peng S, Dai X, Gan W, Nie X, Wei W, Hu G & Guo J (2017). Tumor suppressor SPOP ubiquitinates and degrades EglN2 to compromise growth of prostate cancer cells. Cancer Lett 390, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, Haddad GG, Poellinger L, Peet DJ, Powell F & Johnson RS (2010). The asparaginyl hydroxylase factor inhibiting HIF‐1α is an essential regulator of metabolism. Cell Metabolism 11, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Gu J, Li L, Liu J, Luo B, Cheung HW, Boehm JS, Ni M, Geisen C, Root DE, Polyak K, Brown M, Richardson AL, Hahn WC, Kaelin WG Jr & Bommi‐Reddy A (2009). Control of cyclin D1 and breast tumorigenesis by the EglN2 prolyl hydroxylase. Cancer Cell 16, 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, Ding J, Lei Q, Guan KL & Xiong Y (2009). Glioma‐derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF‐1α. Science 324, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Zhai B, Koivunen P, Shin SJ, Lu G, Liu J, Geisen C, Chakraborty AA, Moslehi JJ, Smalley DM, Wei X, Chen X, Chen Z, Beres JM, Zhang J, Tsao JL, Brenner MC, Zhang Y, Fan C, DePinho RA, Paik J, Gygi SP, Kaelin WG Jr & Zhang Q (2014). Prolyl hydroxylation by EglN2 destabilizes FOXO3a by blocking its interaction with the USP9x deubiquitinase. Genes Dev 28, 1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Ramshesh VK, Rehman H, Currin RT, Sridharan V, Theruvath TP, Kim I, Wright GL & Lemasters JJ (2008). Activation of the oxygen‐sensing signal cascade prevents mitochondrial injury after mouse liver ischemia‐reperfusion. Am J Physiol Gastrointest Liver Physiol 295, G823–G832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZS, Gao XR, He Y, Zhao H, Yu Q, Jiang D, Zhang PZ, Ma XP, Huang HX, Dong D, Wan J, Gu ZY, Jiang XH, Yu L & Gao YZ (2012). An insertion/deletion polymorphism within RERT‐lncRNA modulates hepatocellular carcinoma risk. Cancer Research 72, 6163–6172. [DOI] [PubMed] [Google Scholar]


