Abstract
Key points
Animal models have shown that beta2‐adrenoceptor stimulation increases protein synthesis and attenuates breakdown processes in skeletal muscle. Thus, the beta2‐adrenoceptor is a potential target in the treatment of disuse‐, disease‐ and age‐related muscle atrophy.
In the present study, we show that a few days of oral treatment with the commonly prescribed beta2‐adrenoceptor agonist, salbutamol, increased skeletal muscle protein synthesis and breakdown during the first 5 h after resistance exercise in young men.
Salbutamol also counteracted a negative net protein balance in skeletal muscle after resistance exercise.
Changes in protein turnover rates induced by salbutamol were associated with protein kinase A‐signalling, activation of Akt2 and modulation of mRNA levels of growth‐regulating proteins in skeletal muscle.
These findings indicate that protein turnover rates can be augmented by beta2‐adrenoceptor agonist treatment during recovery from resistance exercise in humans.
Abstract
The effect of beta2‐adrenoceptor stimulation on skeletal muscle protein turnover and intracellular signalling is insufficiently explored in humans, particularly in association with exercise. In a randomized, placebo‐controlled, cross‐over study investigating 12 trained men, the effects of beta2‐agonist (6 × 4 mg oral salbutamol) on protein turnover rates, intracellular signalling and mRNA response in skeletal muscle were investigated 0.5–5 h after quadriceps resistance exercise. Each trial was preceded by a 4‐day lead‐in treatment period. Leg protein turnover rates were assessed by infusion of [13C6]‐phenylalanine and sampling of arterial and venous blood, as well as vastus lateralis muscle biopsies 0.5 and 5 h after exercise. Furthermore, myofibrillar fractional synthesis rate, intracellular signalling and mRNA response were measured in muscle biopsies. The mean (95% confidence interval) myofibrillar fractional synthesis rate was higher for salbutamol than placebo [0.079 (95% CI, 0.064 to 0.093) vs. 0.066 (95% CI, 0.056 to 0.075%) × h−1] (P < 0.05). Mean net leg phenylalanine balance 0.5–5 h after exercise was higher for salbutamol than placebo [3.6 (95% CI, 1.0 to 6.2 nmol) × min−1 × 100 gLeg Lean Mass −1] (P < 0.01). Phosphorylation of Akt2, cAMP response element binding protein and PKA substrate 0.5 and 5 h after exercise, as well as phosphorylation of eEF2 5 h after exercise, was higher (P < 0.05) for salbutamol than placebo. Calpain‐1, Forkhead box protein O1, myostatin and Smad3 mRNA content was higher (P < 0.01) for salbutamol than placebo 0.5 h after exercise, as well as Forkhead box protein O1 and myostatin mRNA content 5 h after exercise, whereas ActivinRIIB mRNA content was lower (P < 0.01) for salbutamol 5 h after exercise. These observations suggest that beta2‐agonist increases protein turnover rates in skeletal muscle after resistance exercise in humans, with concomitant cAMP/PKA and Akt2 signalling, as well as modulation of mRNA response of growth‐regulating proteins.
Keywords: adrenergic, adrenoceptor, beta‐agonists, metabolism, hypertrophy, strength, training, doping, SABA, LABA, albuterol
Key points
Animal models have shown that beta2‐adrenoceptor stimulation increases protein synthesis and attenuates breakdown processes in skeletal muscle. Thus, the beta2‐adrenoceptor is a potential target in the treatment of disuse‐, disease‐ and age‐related muscle atrophy.
In the present study, we show that a few days of oral treatment with the commonly prescribed beta2‐adrenoceptor agonist, salbutamol, increased skeletal muscle protein synthesis and breakdown during the first 5 h after resistance exercise in young men.
Salbutamol also counteracted a negative net protein balance in skeletal muscle after resistance exercise.
Changes in protein turnover rates induced by salbutamol were associated with protein kinase A‐signalling, activation of Akt2 and modulation of mRNA levels of growth‐regulating proteins in skeletal muscle.
These findings indicate that protein turnover rates can be augmented by beta2‐adrenoceptor agonist treatment during recovery from resistance exercise in humans.
Introduction
Skeletal muscle encompasses ∼40% of body mass in lean individuals, making it the largest organ of the human body (Zurlo et al. 1990). Loss of muscle mass (muscle atrophy) can have critical consequences for contractile function and exercise capacity (Ryall et al. 2007) and may reduce quality of life and life expectancy (McLeod et al. 2016). During muscle atrophic conditions, muscle protein breakdown exceeds synthesis, resulting in a negative protein balance (Goldspink & Goldspink, 1977). Strategies that reduce muscle protein breakdown and/or increase synthesis are therefore an important area of research. Although resistance exercise training effectively promotes muscle hypertrophy and decelerates disuse‐ and age‐related muscle atrophy and weakness (Macaluso & De Vito, 2004), pharmacological compounds may be used to augment the response to exercise (Kumar et al. 2009; Egan & Zierath, 2013).
Skeletal muscle beta2‐adrenoceptors are among potential therapeutic targets that have attracted interest in treatment of muscle atrophy and weakness (Lynch & Ryall, 2008; Joassard et al. 2013). Beta2‐adrenoceptors are the most predominant subtype of adrenoceptors in skeletal muscle (Williams et al. 1984; Jensen et al. 2002), where they serve a crucial role in the adrenergic fight‐or‐flight response (Emrick et al. 2010; Andersson et al. 2012; Hostrup et al. 2014b b). Furthermore, beta2‐adrenoceptors play a role in regulation of muscle protein turnover. Beta2‐adrenoceptor knockout mice display lower muscle mass than their wild‐type peers (Hinkle et al. 2002) and beta2‐adrenoceptor stimulation with selective agonists (beta2‐agonists) increases muscle mass in mammals (Lynch & Ryall, 2008), including humans (Hostrup et al. 2015; Jessen et al. 2018). In rodents, beta2‐agonists have also been shown to accelerate muscle recovery from injury (Beitzel et al. 2004; Church et al. 2014) and to reverse muscle atrophy associated with ageing (Ryall et al. 2007), cancer cachexia (Busquets et al. 2004) and muscular dystrophies (Harcourt et al. 2007; Gehrig et al. 2010). Moreover, studies in humans have shown that a few weeks of beta2‐agonist treatment enhances muscle strength (Martineau et al. 1992; Hostrup et al. 2015, 2016) and preserves muscle function during disuse conditions when combined with resistance exercise (Caruso et al. 2004, 2005). Accordingly, beta2‐agonists have been proposed as pharmacotherapy to prevent muscle atrophy and loss of muscle function in muscle wasting conditions (Signorile et al. 1995; Lynch & Ryall, 2008; Atherton & Szewczyk, 2011; Joassard et al. 2013) and to augment muscle adaptations to exercise training (Caruso et al. 2004, 2005; Hostrup et al. 2018; Jessen et al. 2018).
Beta2‐agonists are widely used because of their application as first‐line treatment of the bronchoconstriction associated with asthma, exercise‐induced bronchoconstriction (Price et al. 2014) and chronic obstructive pulmonary disease (Barnes, 2005). Although beta2‐agonists have been marketed for decades, information on the effect of these substances on skeletal muscle protein turnover in humans is lacking. Contradictory findings exist in that Robinson et al. (2010) found no effect of the non‐selective beta‐agonist isoproterenol on whole‐body and muscle protein synthesis, whereas a recent study showed that 7 days of treatment with selective beta2‐agonist formoterol increased whole‐body protein synthesis (Lee et al. 2015). In mice, however, Koopman et al. (2010) observed that beta2‐agonist only increased muscle protein synthesis after consecutive days of treatment and not after the first day. Thus, the therapeutic application of beta2‐agonists in humans may involve several days of treatment before a net positive protein balance incurs (Koopman et al. 2010; Atherton & Szewczyk, 2011). Nevertheless, despite the advances made, no studies have investigated the potential of consecutive days of beta2‐agonist treatment to improve muscle protein balance after exercise in humans.
In rodents, the muscle hypertrophic effect of beta2‐agonist is mediated by increased protein synthesis (Maltin et al. 1989; Hesketh et al. 1992) and/or reduced breakdown (Busquets et al. 2004; Yimlamai et al. 2005), resulting in an overall net positive protein balance. The mechanisms underlying the growth‐promoting actions of beta2‐agonists, however, are not entirely clear, although they involve modulation of various signalling pathways and gene programs that regulate muscle protein synthesis and proteolysis in rodents (Spurlock et al. 2006; Pearen et al. 2009; Koopman et al. 2010). Beta2‐adrenergic signalling induces cAMP‐dependent activation of protein kinase A (PKA), Epac and extracellular signal‐regulated kinases 1/2 (Shi et al. 2007; Ohnuki et al. 2014), which have a wide range of downstream targets that regulate ribosomal translation processes and transcription of growth‐modulating genes (Spurlock et al. 2006; Pearen et al. 2009), including cAMP response element binding protein (CREB) (Hinkle et al. 2002), Akt, mammalian target of rapamycin (mTOR) and mitogen‐activated protein kinase (MAPK) (Kline et al. 2007; Koopman et al. 2010). In addition, several regulators of protein synthesis may be modulated by beta2‐adrenergic signalling, including Akt‐effector Forkhead box protein O1 (FoxO1), a regulator of the atrophy‐related genes atrogin and MurF (Bodine & Baehr, 2014), and eEF2, a regulator of translational elongation. However, the myocellular signalling and mRNA response to beta2‐agonists in relation to muscle protein synthesis and breakdown after exercise remain unexplored in humans.
The present study aimed to investigate the effect of 5 days of beta2‐adrenoceptor stimulation with the selective beta2‐agonist salbutamol on protein turnover of skeletal muscle following resistance exercise in young men. Secondary purposes were to elucidate associated changes in intracellular signalling and mRNA content of selected canonical beta2‐adrenergic targets in skeletal muscle. We hypothesized that beta2‐agonist treatment would increase protein synthesis and reduce breakdown, resulting in an overall net positive protein balance compared to placebo during recovery from resistance exercise.
Methods
Human subjects and ethics
Thirteen healthy trained young men volunteered to participate in the present study. Before inclusion in the study, subjects underwent a medical examination where resting blood pressure, heart rate and ECG of the subjects were measured. Furthermore, body composition was measured by dual‐energy X‐ray absorptiometry (Lunar DPX‐IQ, GE Healthcare, Chalfont St Giles, UK). Inclusion criteria were age 18–40 years and an active life‐style, defined as more than 3 h of physical activity per week. Exclusion criteria were smoking, chronic disease, allergy towards medication and the use of beta2‐agonist or other prescription medication. Subjects were informed about risks and discomforts related to the different tests and procedures of the study. Each subject provided their written and oral informed consent prior to inclusion in the study. The study was approved by the Committee on Health Research Ethics of the Capital Region of Denmark (H‐1‐2012‐119) and performed in accordance with the standards set by the Declaration of Helsinki. The study was registered in ClinicalTrials.gov (NCT02551276).
Of the 13 subjects who were screened, 12 were included in the study and completed it (Fig. 1). The characteristics of the 12 subjects who completed the study are presented in Table 1.
Figure 1. Flow diagram.
Flow diagram providing details of the present study.
Table 1.
Subject characteristics (n = 12)
| Age (years) | 23.4 ± 3.8 |
| Height (cm) | 181.3 ± 5.6 |
| Body mass (kg) | 74.4 ± 9.2 |
| Lean body mass (kg) | 61.0 ± 5.4 |
| Leg lean mass (kg) | 17.8 ± 1.8 |
Values are the mean ± SD.
Study design
The study was designed as a randomized, double‐blinded, placebo‐controlled, cross‐over study. During two identical trials, subjects received either oral salbutamol or placebo. Each trial was preceded by a 4‐day lead‐in period with oral salbutamol (4 × 4 mg × day−1) or placebo treatment because animal studies have shown that the effect of beta2‐agonist on protein synthesis is evident after a few days of treatment (Koopman et al. 2010). The two trials were separated by 3–6 weeks to minimize potential confounding carry‐over effects of salbutamol (Le Panse et al., 2005). Prior to the first experimental trial, subjects met at the laboratory for two familiarizations to the resistance exercise protocol of the experimental trials.
Experimental protocol
An overview of the experimental protocol is illustrated in Fig. 2. After the 4 days of lead‐in treatment, subjects met in the morning after an overnight fast and received either oral salbutamol (6 × 4 mg) or placebo (same treatment as during lead‐in) with a standardized light meal low on protein and fat consisting of white bread with jam (energy: 369 kcal; protein: 12 g; carbohydrate: 67 g; fat: 3 g) and 400 mL of water. Subjects then rested in a bed in the supine position and catheters were inserted: one in the dorsal hand vein for tracer infusion, one in the brachial artery and one in the femoral vein during local anaesthesia (lidocaine without epinephrine, Xylocaine; AstraZeneca, Cambridge, UK) for arterial and venous blood sampling. A primed, continuous infusion of stable amino acid isotope [13C6]‐phenylalanine (l‐phenylalanine, ring‐13C6, 99%, CLM‐1055‐MPT; Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA) was used for measurement of amino acid kinetics across the limb and incorporation of labelled phenylalanine into muscle. [13C6]‐phenylalanine was dissolved in isotonic saline (0.9%) using a sterile procedure, filtered through disposable, sterile, non‐pyrogenic filters with 0.2 μm pore size (Minisart; Sartorius Stedim Biotech, Aubagne, France) and kept at 5°C until infusion. The priming dose of 8 μmol × kg−1 lean body mass (LBM) labelled phenylalanine was dissolved in 20 mL saline and infused at once (1 min). The continuous infusion rate of labelled phenylalanine was 7 μmol × kgLBM −1 × h−1 dissolved in saline and infused with a constant rate throughout the trial.
Figure 2. Overview of the experimental protocol.
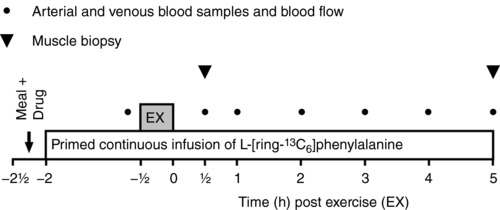
In a randomized placebo‐controlled, double‐blinded, cross‐over design, the study participants conducted two experimental trials (salbutamol vs. placebo) that were separated by 3–6 weeks. Filled circles indicate when arterial and venous blood samples were drawn. Filled triangles indicate when muscle biopsies of the vastus lateralis were collected. EX, resistance exercise.
After 90 min of [13C6]‐phenylalanine infusion (i.e. to reach tracer steady‐state), subjects moved to a knee extensor resistance exercise model. Subjects then performed two sets of 10 repetition knee extensor exercise at an intensity corresponding to 50% of three repetition maximum, followed by eight sets of 12 repetitions of knee‐extensor exercise at an intensity corresponding to 12 repetition maximum (75 ± 11 kg) (mean ± SD) with 2 min of recovery between each set. If subjects failed to perform 12 repetitions in a given set, load was decreased for the following set. The mean load performed during the final set was 69 ± 12 kg (mean ± SD). Intensity and recovery time were duplicated for each subject during the two trials. After exercise, subjects remained inactive in a supine position for 5 h. Biopsies were obtained from the vastus lateralis muscle 0.5 and 5 h after resistance exercise. Brachial arterial and femoral venous blood samples were drawn in EDTA tubes (9 mL) prior to exercise as well as 0.5, 1, 2, 3, 4 and 5 h following exercise. Blood samples were kept at 5°C for 30 min before centrifugation at 5°C and 3200 g for 10 min, after which plasma was collected and stored at –80°C until analyses. Furthermore, 1 mL of arterial and venous blood were sampled in heparin tubes at same time points for determination of haematocrit using an ABL 800 flex (Radiometer, Copenhagen, Denmark). Prior to exercise as well as 0.5, 1, 2, 3, 4 and 5 h following exercise, femoral arterial blood flow was measured with ultrasound Doppler (Vivid E9; GE Healthcare, Brøndbyvester, Denmark) equipped with a linear probe operating at an imaging frequency of 8 MHz and Doppler frequency of 3.1 MHz, as described previously (Nyberg et al. 2014).
Subjects were asked to refrain from caffeine, nicotine and alcohol 24 h before each trial, as well as from exercise 48 h before each trial.
Study drugs
Salbutamol (Ventolin, 4 mg tablets; GlaxoSmithKline, London, UK) and identically looking placebo (lactose monohydrate/starch) were delivered by the hospital pharmacy of Copenhagen. Beta2‐adrenoceptors were stimulated with the highly selective beta2‐agonist salbutamol (Baker, 2010), which has a duration of action of 6–8 h and a plasma elimination half‐life of 3–4 h (Rosen et al. 1986; Jacobson et al. 2015). Salbutamol concentrations peak systemically 1 h 30 min to 3 h after oral administration (Hostrup et al. 2014a). The dose administered during the lead‐in period (16 mg × day−1) was based on studies showing significant effect of daily treatment with oral salbutamol (16 mg × day−1) on muscle strength (Martineau et al. 1992; Caruso et al. 2004). The increase in dose to 24 mg of oral salbutamol during the experimental day was because of potential desensitization of the beta2‐adrenoceptors during the lead‐in treatment period. Drugs were administered in a double‐blinded manner. Randomization was conducted in SPSS, version 24 (IBM Corp., Armonk, NY, USA) by personnel who did not take part in any of the experimental procedures or data analyses. To ensure a drug compliance of 100% during the 4‐day lead‐in period, subjects met at the laboratory in the morning or noon and ingested the study drugs during supervision. Eight of the 12 subjects experienced common side effects of salbutamol during the first 2 days of treatment, including tremors (n = 7) and palpitations (n = 6).
Dual‐energy X‐ray absorptiometry
Subjects laid in the scanner in supine position undressed for 20 min before the scan. To reduce variation, two scans at medium speed were performed in accordance with the manufacturer's guidelines. The scanner was calibrated before the scan using daily calibration procedures (Lunar ‘System Quality Assurance’). All scans were conducted by the same hospital technician.
Muscle biopsies
Muscle biopsies were obtained from the vastus lateralis using a 4 mm Bergström biopsy needle (Stille, Stockholm, Sweden) with suction (Bergström, 1975). Before biopsies were sampled, two incisions were made in the skin at the belly of the vastus lateralis muscle during local anaesthesia (2 mL of lidocaine without epinephrine, Xylocaine 20 mg × mL−1; Astra Zeneca). After sampling, the muscle biopsy was cleaned from visible blood, connective tissue and fat and immediately frozen in liquid nitrogen. Biopsies were stored in cryo tubes at –80°C until analyses.
Leg and muscle protein turnover rates
The influence of beta2‐agonist on muscle protein turnover and myofibrillar protein fractional synthesis rates (FSR) were measured 0.5 to 5 h during recovery from exercise by infusion of stable isotope‐labelled phenylalanine, collection of arteriovenous blood samples and muscle biopsies from the vastus lateralis muscle, and measurement of femoral blood flow (Fig. 2).
Femoral arteriovenous plasma phenylalanine enrichment and concentration were measured using 400 μL of plasma with a known amount of [U‐13C9]‐phenylalanine added as internal standard. Samples were derivatized using N‐methyl‐N‐(tert‐butyldimethylsilyl) trifluoroacetamide + 1% tert‐butyl‐dimethylchlorosilane (Regis Technologies, Morton Grove, IL, USA) and analysed on a triple‐stage quadrupole‐mass spectrometer via gas chromatography‐tandem mass spectrometry (GC‐MS/MS) (TSQ Quantum; Thermo Scientific, San Jose, CA, USA), as described previously (Holm et al. 2014).
Muscle specimens of ∼20 mg wet weight were homogenized using a FastPrep 120A‐230 homogenizer (Thermo Savant, Holbrook, NY, USA) in 1.5 mL of ice‐cold Milli‐Q saline water (Merck‐Millipore, Burlington, MA, USA) and after a spin (5500 g for 10 min at 5°C), the supernatant containing the muscle free amino acids was transferred to a new vial. Muscle free phenylalanine enrichment was then measured by GC‐MS/MS in the same way as described for the plasma phenylalanine enrichment. The pellet from the spin was added a Tris‐buffer (pH 7.4, containing 2 mm EGTA chelating agent, 0.5% Triton‐X100 and 0.25 m sucrose), homogenized once again, left 3 h at 5°C and spun (800 g for 20 min at 5°C) to pellet all structural proteins. Subsequently, myofibrillar proteins were dissolved in a 0.7 m KCl and 0.1 m Na4P2O7‐buffer and, after overnight incubation at 5°C and a subsequent spin (1600 g for 20 min at 5°C), the supernatant containing the myofibrillar proteins were transferred to other vials. The myofibrillar proteins were denatured by adding 2.3 volumes of ethanol. After 2 h of incubation at 5°C, vials were spun (1600 g for 20 min at 5°C) to pellet the myofibrillar proteins. After a wash with 70% ethanol, proteins were hydrolysed in 6 m HCl at 110°C overnight, and the ratio of 13CO2 and 12CO2 from N‐acetyl n‐propyl (NAP)‐derivatized phenylalanine was analysed using a GC‐combustion‐isotope ratio mass spectrometer (Finnigan Delta Plus, Bremen, Germany), as described previously (Holm et al. 2014).
Calculations of phenylalanine kinetics across the leg and muscle, as well as muscle protein turnover parameters, were based on two‐ and three‐pool modelling and myofibrillar FSR on a direct incorporation model (Wolfe & Chinkes, 2005; Smith et al. 2015). All calculations are based on phenylalanine enrichment as mole percent excess (MPE) or atomic percent excess, and phenylalanine concentration is the total concentration (i.e. unlabelled and labelled phenylalanine). Plasma flow, derived from blood flow and haematocrit, were used in the calculations. All phenylalanine kinetic values are expressed as nmol × min−1 × 100 g leg lean mass (LLM)−1. LLM was derived from the dual‐energy X‐ray absorptiometry scan. Models and calculations applied in the present study are in accordance with those described previously (Biolo et al. 1995; Wolfe & Chinkes, 2005; Smith et al. 2015):
Two‐ and three‐pool models shared calculations
where C A and C V are arterial and venous phenylalanine concentration, respectively.
Two‐pool model calculations
where E A and E V are arterial and venous phenylalanine enrichment, respectively.
Three‐pool model calculations
where E M is muscle intracellular phenylalanine enrichment.
Direct incorporation model calculations for myofibrillar protein synthesis
where E P1 and E P2 are the myofibrillar product enrichment at time point 0.5 and 5 h, respectively, E precursor is the muscle free phenylalanine enrichment (or venous or arterial plasma phenylalanine enrichment), and T 1 and T 2 are the specific time points at 0.5 and 5 h, respectively. Myofibrillar FSR is expressed as % × h−1.
Protein phosphorylation in muscle homogenate lysates
Protein phosphorylation was determined by western blotting, as described previously (Thomassen et al. 2016). In short, ∼1.5 mg freeze dried muscle tissue was homogenized (Qiagen Tissuelyser II; Retsch GmbH, Haan, Germany) in a fresh batch of buffer containing (in mm): 10% glycerol, 20 Na‐pyrophosphate, 150 NaCl, 50 Hepes (pH 7.5), 1% NP‐40, 20 β‐glycerophosphate, 2 Na3VO4, 10 NaF, 2 phenylmethylsulphonyl fluoride, 1 EDTA (pH 8), 1 EGTA (pH 8), 10 μg × mL−1 Aprotinin, 10 μg × mL−1 Leupeptin and 3 benzamidine. Samples were rotated end‐over‐end for 1 h at 4°C and centrifuged at 18,320 g for 20 min at 4°C to exclude non‐dissolved structures and the supernatant (lysate) was used for further analyses. Total protein concentration in each sample was determined by a BSA standard kit (Thermo Fisher Scientific, Hvidovre, Denmark) and samples were mixed with 6 × Laemmli buffer (7 mL of 0.5 m Tris‐base, 3 mL of glycerol, 0.93 g of dithiothreitol, 1 g of SDS and 1.2 mg of bromophenol blue) and ddH20 to reach equal protein concentration before protein content was determined by western blotting.
An equal amount of total protein was loaded in each well of precast gels (Bio‐Rad, Hercules, CA, USA). All samples from each subject were loaded on the same gel with a mixed human muscle standard lysate loaded in two different wells used for normalization. Analysis of phosphorylated proteins and corresponding total protein were performed on separate gels. Proteins were separated according to their molecular weight by SDS page gel electrophoresis and semi‐dry transferred to a polyvinylidene fluoride membrane (Bio‐Rad). The membranes were blocked in either 2% skimmed milk or 3% BSA in Tris‐buffered saline including 0.1% Tween‐20 before overnight incubation in primary antibody at 4°C and a subsequent 1 h incubation in HRP conjugated secondary antibody at room temperature. The bands were visualized with ECL (Merck‐Millipore) and recorded with a digital camera (ChemiDoc MP Imaging System; Bio‐Rad). Densitometry quantification of the western blot band intensity was performed using Image Lab, version 4.0 (Bio‐Rad) and determined as the total band intensity adjusted for background intensity. Primary antibodies used are presented in Table 2. Primary antibodies were optimized by use of mixed human muscle standard lysates. Two mixed study samples containing tissue from biopsies were used to ensure that the protein amount loaded would result in band signal intensities localized on the steep and linear part of a standard curve. Secondary antibodies used were HRP conjugated rabbit anti‐sheep (P‐0163), goat anti‐mouse (P‐0447; Dako, Glostrup, Denmark) and goat anti‐rabbit IgM/IgG (4010‐05; SouthernBiotech, Birmingham, AL, USA).
Table 2.
Primary antibodies used for western blotting
| Target protein | Manufacturer | Number | Molecular weight (kDa) |
|---|---|---|---|
| 4E‐BP1 | Cell Signaling | 9452 | 15–20 |
| p‐4E‐BP1Thr37/46 | Cell Signaling | 2855 | 15–20 |
| Akt2 | Cell Signaling | 3063 | 60 |
| p‐Akt2Ser473 | Cell Signaling | 9271 | 60 |
| CREB | Cell Signaling | 9197 | 43 |
| p‐CREBSer129/Ser133 | Abcam | ab10564 | 37–43 |
| eEF2 | Abcam | ab130187 | 95 |
| p‐eEF2Thr56 | Cell Signaling | 2331 | 95 |
| mTOR | Cell Signaling | 2972 | 289 |
| p‐mTORSer2448 | Cell Signaling | 2971 | 289 |
| p38MAPK | Cell Signaling | 9212 | 37–43 |
| p‐p38MAPKThr180/Tyr182 | Cell Signaling | 9211 | 37–43 |
| p70S6K | Cell Signaling | 2708 | 70 |
| p‐p70S6KThr389 | Cell Signaling | 9234 | 70 |
Suppliers: Abcam, Cambridge, MA, USA; Cell Signaling, Beverly, MA, USA.
RNA isolation, reverse transcription, and real‐time PCR
The method for RNA isolation, reverse transcription and real‐time PCR has been described previously (Pilegaard et al. 2000; Brandt et al. 2016). Total RNA was isolated from ∼5 mg wet weight muscle tissue using a modified guanidinium thiocyanate–phenol–chloroform extraction method from Chomczynski and Sacchi (1987) as described by Pilegaard et al. (2000), except for the use of a TissueLyser (TissueLyser II; Qiagen, Valencia, CA, USA) for homogenization. Superscript II RNase H‐ and Oligo dT (Invitrogen, Carlsbad, CA, USA) were used to reverse transcribe mRNA to cDNA (Pilegaard et al. 2000). Quantification of cDNA as a measure of mRNA content of a given gene was performed by real‐time PCR using an ABI 7900 sequence‐detection system (Applied Biosystems, Foster City, CA, USA). Probes and primers were either self‐designed (Table 3) or pre‐developed gene expression assays (ActivinRIIB Hs00609603_m1, calpain‐1 Hs00559804_m1, Smad3 Hs00969210_m1) (Applied Biosystems). Self‐designed probes and 5′‐6‐carboxyfluorescein (FAM)/3′‐6‐carboxy‐N,N,N′,N′‐tetramethylrhodamine labelled TaqMan probes were designed from human specific databases from ensemble (www.ensembl.org/homo_sapiens/info/index) using Primer Express, version 3.0 (Applied Biosystems) and were obtained from TAG Copenhagen (Copenhagen, Denmark).
Table 3.
Primers used for real‐time PCR
| Target gene | Sense primer | Antisense primer | TaqMan probe |
|---|---|---|---|
| Atrogin | 5'‐GATGTTACCCAAGGAAAGAGCAGTAT‐3' | 5'‐ACGGATGGTCAGTGCCCTT‐3' | 5'‐CCCTTCAGCTCTGCAAACACTGTCACAT‐3' |
| FoxO1 | 5'‐ACCGAACAGGATGATCTTGGA‐3' | 5'‐CCATCTGCCGCAAAGATGGCCTCTA‐3' | 5'‐CCATCTGCCGCAAAGATGGCCTCTA‐3' |
| MurF | 5'‐GGAGCCACCTTCCTCTTGACT‐3' | 5'‐CTCAAAGCCCTGCTCTGTCTTC‐3' | 5'‐AACTCATCAAAAGCATTGTGGAAGCTTCCAA‐3' |
| Myostatin | 5'‐ACCAGGAGAAGATGGGCTGAA‐3' | 5'‐GTCAAGACCAAAATCCCTTCTGGA‐3' | 5'‐CCGTTTTTAGAGGTCAAGGTAACAGACACACCA‐3' |
| PGC‐1α | 5'‐CAAGCCAAACCAACAACTTTATCTCT‐3' | 5'‐CACACTTAAGGTGCGTTCAATAGTC‐3' | 5'‐AGTCACCAAATGACCCCAAGGGTTCC‐3' |
Real‐time PCR was performed in triplicates in a total reaction volume of 10 μL using Universal Mastermix with UNG (Applied Biosystems). The obtained cycle threshold values reflecting the initial content of the specific transcript in the samples were converted to a relative amount by using standard curves constructed from serial dilution of a pooled sample made from all samples. Target mRNA content was normalized to single‐stranded DNA content in each sample determined by using OliGreen reagent (Molecular Probes, Leiden, The Netherlands) as described previously (Lundby et al. 2005).
Plasma concentrations of salbutamol
Plasma concentrations of salbutamol were measured by ultra high performance liquid chromatography (UPLC)‐MS/MS using deuterated internal standard based on methods described previously (Jacobson et al. 2015). In brief, calibration samples were prepared using unlabelled salbutamol in drug free plasma over a concentration range of 2–200 ng × mL−1 and internal standard salbutamol‐D3 (3‐hydroxymethyl‐D2, α‐D1; Medical Isotopes, Inc., Pelham, NH, USA) was added to each plasma sample (200 μL) or calibration sample equivalent to 20 ng × mL−1. Ammonia solution (200 μL, pH 9) was then added to each sample and vortex mixed before the addition of 1000 μL of HPLC grade ethyl acetate. This was vortex mixed for 1 min and then centrifuged at 15,000 g for 5 min. The organic supernatant was then transferred to a glass autosampler vial, from which the solvent was evaporated under nitrogen at 40°C. The residue was reconstituted using 100 μL of methanol and vortex mixed prior to analysis via UPLC‐MS/MS consisting of a Waters Acquity® H‐class UPLC system (Waters Corporation, Milford, MA, USA) with chromatography performed using an Astec® CHIROBIOTIC™ T2 chiral column (4.6 × 250 mm × 5 μm particles) (Sigma‐Aldrich) coupled to a Waters Xevo® triple quadrupole mass spectrometer (Waters Corporation) with analyses undertaken using multiple reaction monitoring with conditions as described previously (Jacobson et al. 2015). Assay performance data were within acceptance criteria, with accuracy and precision (% relative SD; n = 5 at 5 ng × mL−1) both less than 5% and calibration r 2 > 0.9998. Total salbutamol levels were calculated from the sum of individual enantiomers.
Statistical analysis
Statistical analyses were performed in SPSS, version 24 (IBM Corp., Armonk, US). Sample size was determined for the primary outcome measure (myofibrillar FSR) and was estimated from the effect of beta2‐agonist treatment on protein synthesis in animals (Koopman et al. 2010) and between‐subject SD from resistance exercise studies in humans (Kumar et al., 2009). Data were tested for normality using the Shapiro–Wilks test and Q‐Q plots. Variables that violated normality were log‐transformed (i.e. phosphorylation‐ratio and mRNA level data). To estimate differences between treatments, two‐tailed linear mixed modelling was used with treatment as a fixed effect and a random effect for subjects. In addition, age and LBM were included in the model as time invariant covariates because they may confound the effect of beta2‐agonist (White & Leenen, 1994; Cheymol, 2000). Area under the phenylalanine leg net balance–time curve (AUC) was analysed using the trapezoidal rule with inclusion of baseline net balance as a covariate in the mixed model. For mRNA content, technical replicates were nested within the fixed effects (Acharya & Zhu, 2011). In the case of repeated measures, sampling point was included in the model as a fixed effect for a full factorial design. Within‐sampling point P values were adjusted using the Bonferroni method. Data are presented as the mean with the 95% confidence interval (CI), unless otherwise stated, and exact P values (unless <0.01 or >0.50) to represent probability for treatment fixed effects.
Results
Plasma concentrations of salbutamol
Arterial plasma concentrations of salbutamol were 46.5 (95% CI, 39.1 to 53.8) and 52.0 (95% CI, 42.8 to 61.3) ng × mL−1 0.5 and 5 h after exercise (2 h 30 min and 7 h after drug administration, respectively). No salbutamol was detected in the blood during the placebo trial.
Circulating phenylalanine availability
Average arterial plasma [13C6]‐phenylalanine enrichment was lower (P < 0.01) for salbutamol than placebo, whereas femoral venous enrichment was higher (P < 0.01) for salbutamol than placebo (Fig. 3 A and B). Average arterial and femoral venous plasma concentrations of phenylalanine were lower (P < 0.01) for salbutamol than placebo (Fig. 3 C and D).
Figure 3. Circulating phenylalanine availability for salbutamol (black) and placebo (white) before (–0.5 h) and 0.5–5 h following resistance exercise.
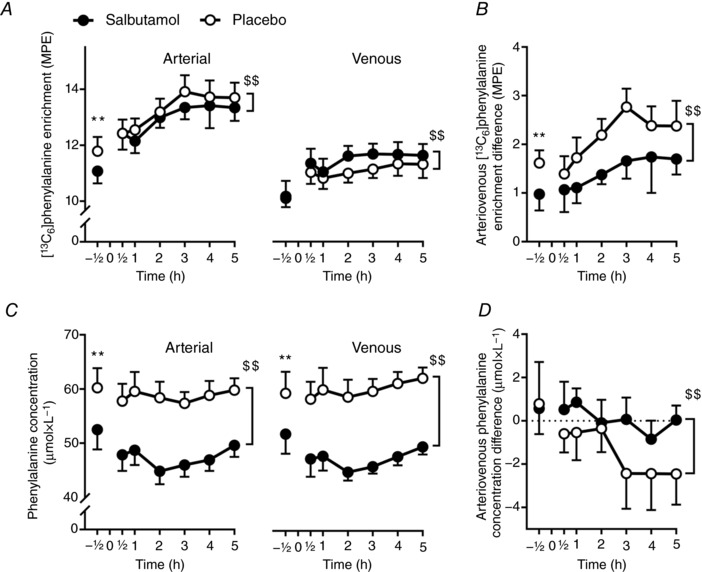
A, arterial and venous [13C6]‐phenylalanine enrichment. B, arteriovenous difference in [13C6]‐phenylalanine enrichment. C, femoral arterial and venous phenylalanine concentration. D, arteriovenous difference in phenylalanine concentration. Values are the mean (n = 12). Error bars represent upper or lower bound of the 95% CI. **Treatment difference (P < 0.01) at same point. $$Overall treatment main effect (P < 0.01).
Leg phenylalanine kinetics based on the two‐pool model
Average femoral arterial plasma flow was more than two‐fold higher (P < 0.01) for salbutamol than placebo (Fig. 4 A). No differences were observed in [13C6]‐phenylalanine leg net balance between salbutamol and placebo before exercise (Fig. 4 B). In the period 0.5–5 h after exercise, mean phenylalanine leg net balance was 0.6 (95% CI, −2.4 to 3.7) and –3.0 (95% CI, −0.7 to −5.2) nmol × min−1 × 100 g LLM −1 for salbutamol and placebo, respectively (P < 0.01) (Fig. 4 B). Phenylalanine leg net balance AUC was higher (P = 0.05) for salbutamol than placebo (Fig. 4 C). Average rate of disappearance and appearance of [13C6]‐phenylalanine was higher (P < 0.01) for salbutamol than placebo (Fig. 4 D and E).
Figure 4. Leg phenylalanine kinetics based on the two‐pool model for salbutamol (black) and placebo (white) before (–0.5 h) and 0.5–5 h following resistance exercise.
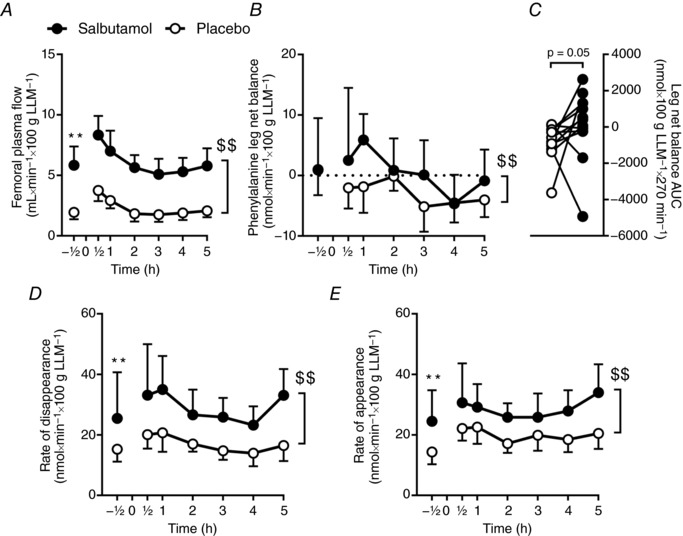
A, femoral arterial plasma flow. B, leg net phenylalanine balance curve. C, area under the leg net phenylalanine balance–time curve. C and D, rate of disappearance. E, rate of appearance. Values are the mean (n = 12). Error bars represent upper or lower bound of the 95% CI.**Treatment difference (P < 0.01) at same point. $$Overall treatment main effect (P < 0.01).
Muscle protein synthesis rate and leg phenylalanine kinetics based on 3‐pool model
Myofibrillar FSR was determined as the incorporation of tracer into myofibrillar proteins using the i.m. tracer enrichment (MPE) as precursor [salbutamol 0.5 h: 7.0 (95% CI, 6.4 to 7.6%) and 5 h: 7.6 (95% CI, 7.0 to 8.2%) and placebo 0.5 h: 7.6 (95% CI, 6.9 to 8.3%) and 5 h: 7.5 (95% CI, 6.8 to 8.2%)]. Myofibrillar FSR was 0.013 (95% CI, 0.001 to 0.025%) × h−1 higher (P = 0.03) for salbutamol than placebo (Fig. 5 A). LBM was a significant negative confounder (P = 0.03) of the salbutamol‐induced change in myofibrillar FSR, whereas age did not confound the response (P > 0.50). The effect of salbutamol on myofibrillar FSR was moderate (Cohen's d = 0.78).
Figure 5. Muscle protein synthesis rate and leg phenylalanine kinetics based on the three‐pool model for salbutamol (black) and placebo (white) 0.5–5 h following resistance exercise.
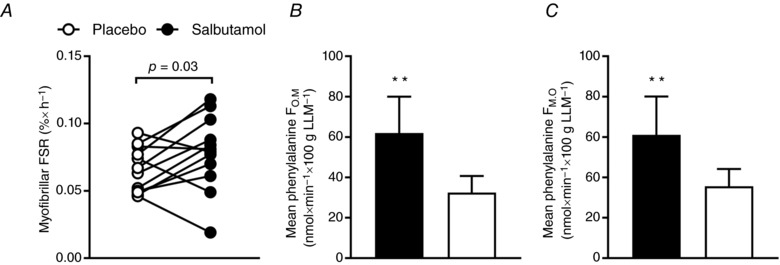
A, myofibrillar FSR. B, average protein synthesis (F O,M) (three‐pool model). C, average protein breakdown (F M,O) (three‐pool model). Values are the mean (n = 12). Error bars represent upper or lower bound of the 95% CI. **Treatment difference (P < 0.01).
Based on the three‐pool model, salbutamol had higher estimates of protein synthesis (F O,M) (P < 0.01) and breakdown (F M,O) (P < 0.01) than placebo (Fig. 5 B and C).
Inward and outward muscle transmembrane transport of phenylalanine was not significantly different between treatments 0.5 h after exercise, although it was higher (P < 0.01) for salbutamol than placebo 5 h after exercise (Table 4). Arteriovenous shunting was more than two‐fold higher (P < 0.01) for salbutamol than placebo 0.5 and 5 h after exercise (Table 4).
Table 4.
Selected phenylalanine kinetics parameters based on the three‐pool model
| Placebo | Salbutamol | Tests of fixed effects | |||||
|---|---|---|---|---|---|---|---|
| 0.5 h | 5 h | 0.5 h | 5 h | Treatment | Time | Treatment by Time | |
| Inward muscle transmembrane transport (PHE nmol × min−1 × 100 g LLM−1) | 57 | 41 | 72 | 78 | <0.01 | 0.58 | 0.43 |
| (40 to 73) | (32 to 49) | (39 to 105) | (62 to 94)** | ||||
| Outward muscle transmembrane transport (PHE nmol × min−1 × 100 g LLM−1) | 59 | 45 | 69 | 79 | 0.03 | 0.79 | 0.21 |
| (45 to 72) | (36 to 53) | (37 to 101) | (58 to 99)** | ||||
| Arteriovenous shunting (PHE nmol × min−1 × 100 g LLM−1) | 166 | 83 | 324 | 209 | <0.01 | <0.01 | 0.87 |
| (107 to 224) | (54 to 112) | (249 to 399)** | (131 to 286)** | ||||
Values are the mean (95% CI) (n = 12). **Statistically significant difference (P ≤ 0.01) compared to placebo at same time point.
Muscle signalling
PKA substrate intensity (P = 0.01) and phosphorylation of Akt2 (P = 0.02) and CREB (P < 0.01) were higher for salbutamol than placebo 0.5 h after exercise, whereas no relevant differences were observed between the treatments in phosphorylation of 4E‐BP1 (P = 0.23), eEF2 (P = 0.17), MAPK (P > 0.50), mTOR (P = 0.17) and p70S6K (P = 0.39) (Fig. 6 A). PKA substrate intensity (P < 0.01) and phosphorylation of Akt2 (P < 0.01), CREB (P < 0.01) and eEF2 (P = 0.01) were higher for salbutamol than placebo 5 h following exercise, whereas phosphorylation of 4E‐BP1 (P = 0.38), MAPK (P > 0.50), mTOR (P > 0.50) and p70S6K (P = 0.13) was not different between treatments (Fig. 6 B).
Figure 6. Phosphorylation‐ratio induced by salbutamol (SAL) compared to placebo (PLA).
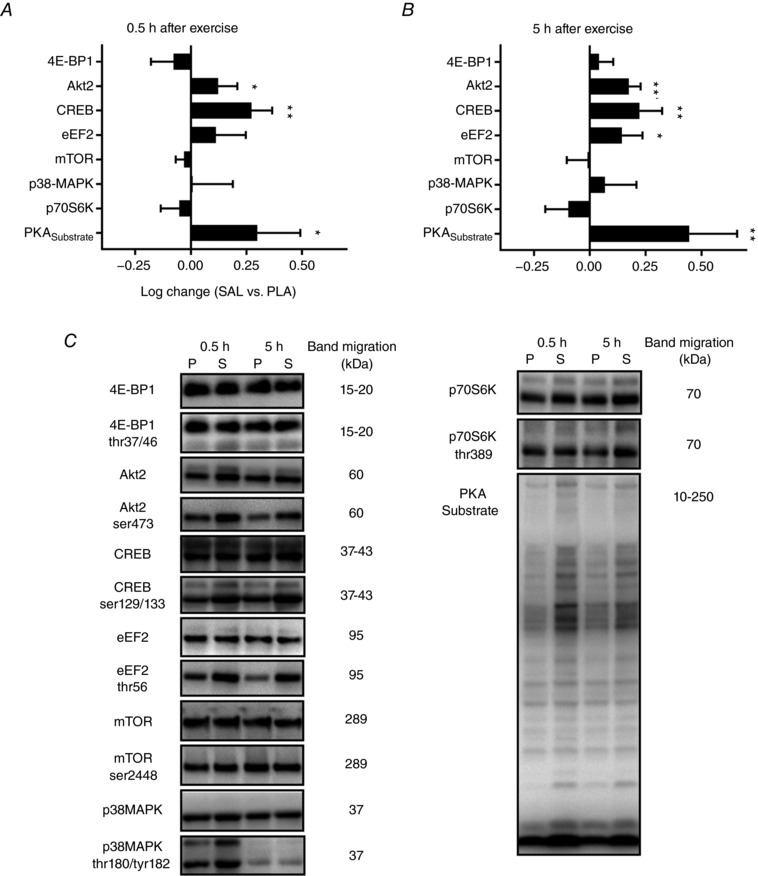
Phosphorylation‐ratio induced by SAL compared to PLA in biopsies sampled from the vastus lateralis muscle 0.5 (A) and 5 h (B) after resistance exercise. Values are the mean log‐change (n = 12). Error bars represent upper or lower bound of the 95% CI. *Treatment difference (P < 0.05). **Treatment difference (P < 0.01). C, representative blots for salbutamol (S) and placebo (P).
mRNA content
The mRNA content of calpain‐1 (P < 0.01), FoxO1 (P < 0.01), myostatin (P < 0.01) and Smad3 (P < 0.01) was higher for salbutamol than placebo 0.5 h after exercise, whereas no significant changes were induced by salbutamol in content of ActivinRIIB (P = 0.25), atrogin (P > 0.50), MuRF (P > 0.50) and PGC‐1α (P = 0.33) compared to placebo (Fig. 7 A). The mRNA content of ActivinRIIB was lower (P < 0.01) for salbutamol than placebo 5 h after exercise, whereas FoxO1 (P < 0.01) and myostatin (P < 0.01) mRNA content was higher for salbutamol than placebo (Fig. 7 B). No treatment differences were observed in the mRNA content of atrogin (P = 0.48), calpain‐1 (P > 0.50), PGC‐1α (P = 0.09), MuRF (P = 0.24) and Smad3 (P > 0.50) 5 h after exercise (Fig. 7 B).
Figure 7. mRNA response induced by salbutamol (SAL) compared to placebo (PLA).
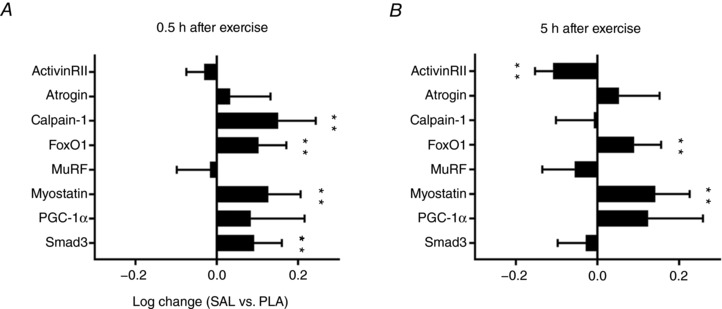
mRNA response induced by SAL compared to PLA in biopsies sampled from the vastus lateralis muscle 0.5 (A) and 5 h (B) after resistance exercise. Values are the mean log‐change (n = 12). Error bars represent upper or lower bound of the 95% CI. **Treatment difference (P < 0.01).
Discussion
In the present study, we have reported the beta2‐adrenergically‐induced changes in protein turnover rates and associated changes in intracellular signalling and mRNA content in skeletal muscle after resistance exercise in trained young men. The most important findings are that beta2‐adrenergic stimulation with the commonly prescribed selective beta2‐agonist, salbutamol, increased myofibrillar FSR and protein turnover rates, thus favouring an improved net protein balance in skeletal muscle following resistance exercise. Changes in protein turnover induced by salbutamol were associated with PKA‐signalling, activation of Akt2 and modulation of mRNA response of growth‐regulating proteins in skeletal muscle.
Although beta2‐agonists have been marketed for more than 50 years, the present study is first to show that a few days of beta2‐agonist treatment increases myofibrillar FSR and leg protein turnover rates, resulting in an improved leg net protein balance after resistance exercise in humans. The higher myofibrillar FSR induced by salbutamol was in agreement with our working hypothesis and consistent with reported observations in rodents (Maltin et al. 1989; Hesketh et al. 1992; Koopman et al. 2010). Contrary to our observations, isoproterenol was shown to have no effect on whole‐body and muscle protein synthesis in young men (Robinson et al. 2010). The type of beta2‐agonist and dosing regimen applied may explain this discrepancy. In the present study, we administered salbutamol, which has superior selectivity for the beta2‐adrenoceptor than isoproterenol (Baker, 2010). Furthermore, we chose to administer salbutamol in supratherapeutic doses and daily for four days prior to the experiments because studies in rodents have shown that the stimulatory effect of beta2‐agonist on anabolism is dose‐dependent and that the beta2‐agonist‐induced increase in protein synthesis requires consecutive days of treatment (Koopman et al. 2010). Consistent with this, Lee et al. (2015) observed that 7 days of oral treatment with formoterol increased whole‐body protein synthesis. In addition, we investigated the effect of beta2‐agonist on 5 h of protein turnover following resistance exercise and not during resting conditions as in the previous human studies (Robinson et al. 2010; Lee et al. 2015). Accordingly, the present study indicates that a few days of beta2‐agonist treatment increase protein turnover rates in the first 5 h period following resistance exercise, which is in agreement with the augmenting effect of daily salbutamol treatment on muscle adaptations to resistance training observed in previous studies (Caruso et al. 2004, 2005).
We observed that LBM confounded the effect of salbutamol on myofibrillar FSR, whereas no relevant confounding effect was observed for body mass (Pearson's r = –0.096, P = 0.32; data not shown). Distribution of drugs, including beta2‐agonists, is influenced by body composition (Cheymol, 2000), and LBM has been shown to be a superior predictor of the response to drugs than body mass (Morgan & Bray, 1994; Han et al. 2007). The influence of LBM on the response to salbutamol is probably related to the distribution kinetics of salbutamol, exhibiting extensive disposition in skeletal muscle (Jacobson et al. 2014). The beta‐adrenoceptor cardiac response has also been shown to decline with age (White & Leenen, 1994), although we observed no relevant impact of subject age on the salbutamol‐induced change in myofibrillar FSR. This may be explained by the relatively low heterogeneity in the present study population (range 19–32 years) and the different target tissue (cardiac vs. skeletal muscle). Nevertheless, the present observations suggest that LBM may be taken into consideration when investigating effects of beta2‐agonists. In this context, it has been speculated that the effect of beta2‐agonist on exercise performance and muscle excitation–contraction coupling depends on the training level of the subject (van Baak et al. 2004; Decorte et al. 2013).
Although studies in rodents have indicated that the hypertrophic effect of beta2‐agonist may involve both attenuation of muscle proteolytic processes (Busquets et al. 2004; Yimlamai et al. 2005) and an increase in protein synthesis (Maltin et al. 1989; Hesketh et al. 1992), we observed that salbutamol markedly increased leg protein turnover rates by almost doubling the rate of protein breakdown and synthesis during the 5 h period following exercise. Given that the resistance exercise undertaken was matched between the salbutamol and placebo trial, the higher rate of protein breakdown induced by salbutamol is related to factors other than the total work performed. Although a putative mechanism could be the pronounced increase in arterial femoral plasma flow (Biolo et al. 1997) induced by salbutamol, we observed no apparent association between femoral plasma flow and myofibrillar FSR (r = 0.11, P = 0.60) or leg net balance AUC (r = 0.05, P = 0.80). Furthermore, despite a higher femoral plasma flow and lower arterial plasma concentration of phenylalanine, the arteriovenous phenylalanine difference was more positive for salbutamol than placebo, in which there was a net release of phenylalanine in the 0.5–5 h following exercise. As such, our observations suggest that the greater protein turnover rates for salbutamol is related to myocellular mechanisms.
Despite the increase in rate of protein breakdown, salbutamol counteracted a net negative protein balance following resistance exercise, which was evident in the placebo condition. The negative protein balance observed for placebo is consistent with previous studies, where the balance is negative in the post‐absorptive state following resistance exercise (Biolo et al. 1995; Phillips et al. 1997). In this context, it is important to emphasize that subjects in the present study consumed a standardized low‐protein meal 2 h prior to the resistance exercise to provide some energy to be available for the exercise session, at the same time as being sufficiently low in protein to affect metabolism at the post‐exercise measurements. Studies have shown that there is a graded response of muscle FSR to dietary protein or amino acid infusion (Bohé et al. 2003; Moore et al. 2009), and whether the effect of beta2‐agonist on protein turnover rates would have been different in completely fasting conditions or in conditions where subjects had consumed higher amounts of essential amino acids remains to be determined. Nonetheless, the beneficial effect of salbutamol in enhancing net balance to more positive levels than with placebo underpins the efficacy of beta2‐agonist in stimulating muscle anabolism.
We observed that salbutamol induced significant beta2‐adrenergic signalling in skeletal muscle 0.5 and 5 h following resistance exercise, as indicated by a higher phosphorylation of PKA substrates and downstream activation of cAMP/PKA‐dependent target CREB. In rodents, the growth‐promoting mechanisms of beta2‐adrenergic signalling involves Akt, mTOR and MAPK pathways (Kline et al. 2007; Sato et al. 2013), which are predominant in the regulation of translation initiation (Goodman, 2014) and cell proliferation and differentiation (Pearson et al. 2001). Despite the induced PKA‐signalling and higher phosphorylation of Akt2 with salbutamol 0.5 and 5 h following exercise, we observed no changes in phosphorylation of mTORSer2448 and downstream effectors of translation initiation, p70S6K and 4E‐BP1, or in phosphorylation of p38‐MAPK. Although the latter observations may appear to be inconsistent with reports in rodents (Sato et al. 2013), beta2‐adrenergic signalling may be muscle fibre‐type specific (Gonçalves et al. 2012) and some studies report no effect of beta2‐adrenergic stimulation on p38‐MAPK phosphorylation (Kim et al. 2013). Reports in mice also indicate that beta2‐adrenergic stimulation does not phosphorylate mTORSer2448, although it may phosphorylate mTORSer2481 (Sato et al. 2014), which could be a possible explanation of the observed increase in phosphorylation of AktSer473 (Copp et al. 2010). Indeed, mTORSer2448 may not be a target of Akt (Figueiredo et al. 2017). We also observed that salbutamol increased the phosphorylation of eEF2, which acts to reduce ribosomal elongation activity (Ryazanov et al. 1988). Although this may appear to be unexpected considering the higher protein synthesis rate and phosphorylation of Akt2 with salbutamol, studies have shown that cAMP‐PKA‐dependent signalling induces phosphorylation of eEF2 and inhibition of peptide elongation in vitro (Redpath & Proud, 1993).
Aside from the induced changes in signalling, we observed that salbutamol modulated mRNA levels of ActivinRIIB, calpain‐1, FoxO1, myostatin and Smad3 following exercise. Most noteworthy was the upregulation of mRNA levels of the negative regulator of growth, myostatin. Although increased mRNA levels of myostatin may appear counterintuitive given the anabolic properties of beta2‐agonists, Abo et al. (2012) observed that hypertrophy induced by beta2‐agonist was associated with increased protein levels of myostatin in rats. Importantly, we also observed that salbutamol induced a downregulation of the mRNA level of the receptor target of myostatin, ActivinRIIB, 5 h after exercise. Thus, a potential upregulation of myostatin induced by beta2‐agonist may be counteracted by a concurrent downregulation of ActivinRIIB, which is consistent with that observed in rat tibialis anterior muscle following beta2‐agonist treatment (Pearen et al. 2009). In addition, we observed that salbutamol upregulated mRNA levels of Smad3 and FoxO1. Given that Smad3‐null mice display loss of satellite cells and muscle atrophy (Ge et al. 2011), it may be speculated that a beta2‐agonist‐induced upregulation of Smad3 plays a role in growth‐promotion. FoxO1, a regulator of the atrophy‐related genes atrogin and MuRF (Bodine & Baehr, 2014), is among the targets that are regulated by the ActivinRIIB‐myostatin system and Akt signalling. However, despite significant Akt activation and upregulation of FoxO1 mRNA levels with salbutamol, we observed no changes in the mRNA level of atrogin and MuRF with salbutamol compared to placebo. Furthermore, although a potential effect of CREB activation is increased transcription of PGC‐1α, we observed no effect of salbutamol on mRNA levels of PGC‐1α compared to placebo. The latter observation is consistent with observations in rats, where beta2‐agonist treatment with clenbuterol did not necessarily affect PGC‐1α mRNA levels (Kim et al. 2013; Shimamoto et al. 2017). We also observed that the calpain1 mRNA content was increased by salbutamol 0.5 h following exercise, which potentially may have contributed to Ca2+‐dependent proteolysis and thus the higher protein breakdown for salbutamol than placebo. The observation that salbutamol increased calpain mRNA levels is consistent with rodent studies, where beta2‐agonist treatment with formoterol increased calpain mRNA levels (Koopman et al. 2010).
The effect of beta2‐agonist on gene transcription and signalling possibly depends on timing of sampling and the biological samples (e.g. cells vs. tissue), as well as on type and dose of beta2‐agonist used (Baker, 2010; Wannenes et al. 2012). For example, although clenbuterol repressed mRNA levels of atrogin and MuRF in C2C12 muscle cell lines (Wannenes et al. 2012), no effect was found in rat soleus muscle after 3 days of treatment with clenbuterol (Gonçalves et al. 2012). Furthermore, unlike clenbuterol, salbutamol was shown to have no apparent effect on mRNA levels of atrogin and MuRF in C2C12 muscle cell lines (Wannenes et al. 2012). Based on the present study, along with studies in rodents, changes in muscle protein turnover induced by beta2‐agonists are possibly multifactorial, involving complex regulation of gene transcription and ribosomal translation (Pearen et al. 2009; Koopman et al. 2010).
In summary, the present observations show that selective activation of beta2‐adrenoceptors with salbutamol increases myofibrillar FSR and protein turnover rates in skeletal muscle following resistance exercise in trained young men. Furthermore, our observations indicate that LBM confounds the salbutamol‐induced change in myofibrillar FSR. The effect of salbutamol in protein turnover rates was associated with pronounced PKA‐signalling and phosphorylation of CREB and Akt2, as well as a concurrent mRNA response for growth‐regulating genes, including ActivinRII, FoxO1 and myostatin.
Methodological considerations
We observed that the arterial phenylalanine enrichment rose from ∼12% to 13.5% MPE during the period of which the tracer measures were performed and therefore whether isotopic steady‐state was achieved in the present study could be considered. Nonetheless, we observed a constant venous enrichment of ∼11.5% MPE and no difference in the i.m. enrichment at 0.5 and 5 h in recovery from exercise (0.079 ± 0.014 and 0.082 ± 0.013, respectively, P = 0.32) (mean ± SD), demonstrating that close to the actual site of protein turnover, tracer enrichments were not changing significantly. In clinical trials where homeostasis may be affected by drugs, exercise or other factors, minor fluctuations in circulating tracer enrichments may also be expected. In the present study, as well as in some other protocols (Rahbek et al. 2014; Mikkelsen et al. 2015), we applied a rather high tracer infusion rate (7 μmol × kg LBM−1 × h−1) compared to 3.6 μmol × kg whole body weight−1 × h−1 used in other studies (Wilkinson et al. 2015; Wall et al. 2016). Our rationale for this infusion rate was to improve analytical sensitivity to allow detection of expectedly small intervention differences. However, with the precision of modern mass spectrometers, the relative high tracer infusion rate was probably not necessary and it is recommended to use a lower infusion rate to limit costs and reduce potential impact of the tracer on metabolism. It was shown, however, that the myofibrillar FSR was unaffected by flooding with 1665 mg phenylalanine (>10,000 μmol), increasing the blood (and most probably also intracellular) concentrations several‐fold (Holm et al. 2014). For comparison, the infusion in the present study equalled a total amount of ∼500–600 mg phenylalanine over a 7 h time period, which probably did not affect phenylalanine metabolism and the muscle protein synthesis rate. In addition, in the present study, we used different tracer principles to investigate the effect of salbutamol vs. placebo on protein turnover rates. Although some variability was observed within the different estimates, consistency appeared across the findings when evaluated over the entire post‐exercise period and, for the primary outcome measure (myofibrillar FSR determined by the direct incorporation method), we observed consistent findings with the three‐pool tracer dilution estimate of protein synthesis rate (F O,M) (Pearson's r = 0.52, P = 0.009). It should also be highlighted that the effect of beta2‐agonist vs. placebo on protein turnover rates observed in the present study was in a postprandial setting where the subjects also performed exercise. Therefore, any interpretation of the sole beta2‐adrenergic effect based on the present study should be made with caution because nutritional intake and exercise may confound the effect of beta2‐agonist.
Translational perspectives
The present study adds to animal studies showing that beta2‐agonist can alter protein turnover in skeletal muscle following resistance exercise in humans. The practical implications of beta2‐agonist‐induced changes in protein turnover rates remain to be determined. Although studies in rodents have provided support of beta2‐agonists as treatment of muscle atrophy, concerns were raised because of concurrent adverse ventricular remodelling and collagen filtration (Gregorevic et al. 2005; Burniston et al. 2007). However, given the markedly lower relative doses prescribed to humans, such effects are possibly not a major concern. Furthermore, the most commonly used beta2‐agonists in humans, such as salbutamol and formoterol, have superior selectivity for the beta2‐adrenoceptor compared to clenbuterol and fenoterol (Baker, 2010), thus reducing or avoiding potential adverse activation of cardiac beta1‐adrenoceptors. Recent human studies also show that beta2‐agonists may hold some promise as anabolic agents with few minor side effects (Hostrup et al. 2015; Lee et al. 2015; Jessen et al. 2018). The beta2‐agonist‐induced increase in protein turnover may also have implications for proteome signature remodelling of various components in skeletal muscle. Indeed, beta2‐agonist treatment has been shown to modulate proteome signature adaptations to endurance training in humans (Hostrup et al. 2018). Furthermore, given that remodelling and recycling of myocellular proteins are important adaptive processes with respect to stress and exercise (Camera et al. 2017), it may be that beta2‐agonists augment post‐exercise recovery processes after resistance exercise. The observation that supratherapeutic oral doses of salbutamol increase protein turnover rates in association with resistance exercise provides support for the anti‐doping regulatory restrictions regarding the supratherapeutic use of beta2‐agonists in competitive sport.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
MH designed the study and participated in the human experiments. LH and SR contributed to the conception of design and phenylalanine analyses. AK, JB, JE, MK, MN, SJ and VB participated in the human experiments. MT performed the immunoblotting. CMK and HP performed the RNA isolation, reverse transcription and real‐time PCR. GAJ developed the salbutamol assay and performed the analysis of salbutamol in plasma. All authors contributed to the analysis and interpretation of data and the drafting of the manuscript. All authors approved the final version of the manuscript submitted for publication.
Funding
The study was supported by grants from the Danish Ministry of Culture and the World Anti‐Doping Agency.
Biography
Morten Hostrup is Associate Professor in the Section of Integrative Physiology, Department of Nutrition, Exercise and Sports, University of Copenhagen. He obtained his PhD degree in integrative physiology from the University of Copenhagen in 2015, covering the effects of beta2‐adrenoceptor agonists in relation to skeletal muscle physiology and exercise performance. His current research areas are the biomedical basis of exercise performance and experimental pharmacology in humans, with an emphasis on the adrenergic system, metabolism, ion transport and skeletal muscle remodelling induced by exercise.

Edited by: Scott Powers & Troy Hornberger
References
- Abo T, Iida RH, Kaneko S, Suga T, Yamada H, Hamada Y & Yamane A (2012). IGF and myostatin pathways are respectively induced during the earlier and the later stages of skeletal muscle hypertrophy induced by clenbuterol, a β2‐adrenergic agonist. Cell Biochem Funct 30, 671–676. [DOI] [PubMed] [Google Scholar]
- Acharya LR & Zhu D (2011). Multivariate models and algorithms for learning correlation structures from replicated molecular profiling data In Advanced Biomedical Engineering, ed. Garglulo G. InTech, Rijeka, Croatia. [Google Scholar]
- Andersson DC, Betzenhauser MJ, Reiken S, Umanskaya A, Shiomi T & Marks AR (2012). Stress‐induced increase in skeletal muscle force requires protein kinase A phosphorylation of the ryanodine receptor. J Physiol 590, 6381–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton PJ & Szewczyk NJ (2011). Don't ‘agonise' over the mechanisms underlying beta‐agonist‐induced muscle hypertrophy! J Physiol 589, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG (2010). The selectivity of β‐adrenoceptor agonists at human β1‐, β2‐ and β3‐adrenoceptors. Br J Pharmacol 160, 1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ (2005). Drugs for the treatment of asthma and COPD In Principles of Immunopharmacology, 2nd edn., ed. Nijkamp FP. & Parnham MJ, pp. 281–344. Birkhäuser Basel, Basel. [Google Scholar]
- Beitzel F, Gregorevic P, Ryall JG, Plant DR, Sillence MN & Lynch GS (2004). β2‐adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J Appl Physiol 96, 1385–1392. [DOI] [PubMed] [Google Scholar]
- Bergström J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD & Wolfe RR (1995). Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 268, E514–E520. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S & Wolfe RR (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 273, 122–129. [DOI] [PubMed] [Google Scholar]
- Bodine SC & Baehr LM (2014). Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin‐1. AJP Endocrinol Metab 307, 469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohé J, Low A, Wolfe RR & Rennie MJ (2003). Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose‐response study. J Physiol 552, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Gunnarsson TP, Hostrup M, Tybirk J, Nybo L, Pilegaard H & Bangsbo J (2016). Impact of adrenaline and metabolic stress on exercise‐induced intracellular signaling and PGC‐1α mRNA response in human skeletal muscle. Physiol Rep 4, e12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burniston JG, Wa C, Tan L & Goldspink DF (2007). Dose‐dependent separation of the hypertrophic and myotoxic effects of the β2‐adrenergic receptor agonist clenbuterol in rat striated muscles. Muscle Nerve 33, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets S, Figueras MT, Fuster G, Almendro V, Moore‐Carrasco R, Ametller E, Argilés JM & López‐Soriano FJ (2004). Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res 64, 6725–6731. [DOI] [PubMed] [Google Scholar]
- Camera DM, Burniston JG, Pogson MA, Smiles WJ & Hawley JA (2017). Dynamic proteome profiling of individual proteins in human skeletal muscle after a high‐fat diet and resistance exercise. FASEB J 31, 5478–5494. [DOI] [PubMed] [Google Scholar]
- Caruso JF, Hamill J, Yamauchi M, Mercado D, Cook T, Higginson B, O'Meara S, Elias J & Siconolfi S (2005). Albuterol aids resistance exercise in reducing unloading‐induced ankle extensor strength losses. J Appl Physiol 98, 1705–1711. [DOI] [PubMed] [Google Scholar]
- Caruso JF, Hamill JL, Yamauchi M, Mercado DR, Cook TD, Keller CP, Montgomery AG & Elias J (2004). Albuterol helps resistance exercise attenuate unloading‐induced knee extensor losses. Aviat Space Environ Med 75, 505–511. [PubMed] [Google Scholar]
- Cheymol G (2000). Effects of obesity on pharmacokinetics. Clin Pharmacokinet 39, 215–231. [DOI] [PubMed] [Google Scholar]
- Chomczynski P & Sacchi N (1987). Single‐step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Church JE, Trieu J, Sheorey R, Chee AY‐M, Naim T, Baum DM, Ryall JG, Gregorevic P & Lynch GS (2014). Functional β‐adrenoceptors are important for early muscle regeneration in mice through effects on myoblast proliferation and differentiation. PLoS ONE 9, e101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp J, Manning G & Hunter T (2010). TORC‐specific phosphorylation of mTOR: phospho‐Ser2481 is a marker for intact mTORC2. Cancer Res 69, 1821–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decorte N, Bachasson D, Guinot M, Flore P, Levy P, Verges S & Wuyam B (2013). Effect of salbutamol on neuromuscular function in endurance athletes. Med Sci Sports Exerc 45, 1925–1932. [DOI] [PubMed] [Google Scholar]
- Egan B & Zierath JR (2013). Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17, 162–184. [DOI] [PubMed] [Google Scholar]
- Emrick MA, Sadilek M, Konoki K & Catterall WA (2010). Beta‐adrenergic‐regulated phosphorylation of the skeletal muscle CaV1.1 channel in the fight‐or‐flight response. Proc Natl Acad Sci U S A 107, 18712–18717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo VC, Markworth JF & Cameron‐Smith D (2017). Considerations on mTOR regulation at serine 2448: Implications for muscle metabolism studies. Cell Mol Life Sci 74, 2537–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, McFarlane C, Vajjala A, Lokireddy S, Ng ZH, Tan CK, Tan NS, Wahli W, Sharma M & Kambadur R (2011). Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res 21, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig SM, Koopman R, Naim T, Tjoakarfa C & Lynch GS (2010). Making fast‐twitch dystrophic muscles bigger protects them from contraction injury and attenuates the dystrophic pathology. Am J Pathol 176, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink DF & Goldspink G (1977). Age‐related changes in protein turnover and ribonucleic acid of the diaphragm muscle of normal and dystrophic hamsters. Biochem J 162, 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves DAP, Silveira WA, Lira EC, Graça FA, Paula‐Gomes S, Zanon NM, Kettelhut IC & Navegantes LCC (2012). Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy‐related genes in denervated rat soleus muscles independently of Akt. Am J Physiol Endocrinol Metab 302, E123–E133. [DOI] [PubMed] [Google Scholar]
- Goodman CA (2014). The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev Physiol Biochem Pharmacol 166, 43–95. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Ryall JG, Plant DR, Sillence MN, Lynch GS, Ryall JG, Plant DR, Martin N & Lynch GS (2005). Chronic β‐agonist administration affects cardiac function of adult but not old rats, independent of β‐adrenoceptor density. Am J Physiol Heart Circ Physiol 289, H344–H349. [DOI] [PubMed] [Google Scholar]
- Han PY, Duffull SB, Kirkpatrick CM & Green B (2007). Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther 82, 505–508. [DOI] [PubMed] [Google Scholar]
- Harcourt LJ, Schertzer JD, Ryall JG & Lynch GS (2007). Low dose formoterol administration improves muscle function in dystrophic mdx mice without increasing fatigue. Neuromuscul Disord 17, 47–55. [DOI] [PubMed] [Google Scholar]
- Hesketh JE, Campbell GP, Lobley GE, Maltin CA, Acamovic F & Palmer RM (1992). Stimulation of actin and myosin synthesis in rat gastrocnemius muscle by clenbuterol; evidence for translational control. Comp Biochem Physiol C 102, 23–27. [DOI] [PubMed] [Google Scholar]
- Hinkle RT, Hodge KMB, Cody DB, Sheldon RJ, Kobilka BK & Isfort RJ (2002). Skeletal muscle hypertrophy and anti‐atrophy effects of clenbuterol are mediated by the β2‐adrenergic receptor. Muscle Nerve 25, 729–734. [DOI] [PubMed] [Google Scholar]
- Holm L, Reitelseder S, Dideriksen K, Nielsen RH, Bülow J & Kjaer M (2014). The single‐biopsy approach in determining protein synthesis in human slow‐turning‐over tissue: use of flood‐primed, continuous infusion of amino acid tracers. Am J Physiol Endocrinol Metab 306, E1330–E1339. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Auchenberg M, Bangsbo J & Backer V (2016). Effects of acute and 2‐week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scand J Med Sci Sports 26, 8–16. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Auchenberg M, Rzeppa S, Hemmersbach P, Bangsbo J & Backer V (2014a). Urine concentrations of oral salbutamol in samples collected after intense exercise in endurance athletes. Drug Test Anal 6, 528–532. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Onslev J, Jessen S, Haase C, Habib S, Ørtenblad N, Backer V & Bangsbo J (2015). Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2‐adrenergic stimulation in men. J Appl Physiol 119, 475–486. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Ørtenblad N, Juel C, Mørch K, Rzeppa S, Karlsson S, Backer V & Bangsbo J (2014b). β2‐Adrenergic stimulation enhances Ca2+ release and contractile properties of skeletal muscles, and counteracts exercise‐induced reductions in Na+‐K+‐ATPase Vmax in trained men. J Physiol 592, 5445–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostrup M, Onslev J, Jacobson GA, Wilson R & Bangsbo J (2018). Chronic β2 ‐adrenoceptor agonist treatment alters muscle proteome and functional adaptations induced by high intensity training in young men. J Physiol 596, 231–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson GA, Yee KC, Wood‐Baker R & Walters EH (2015). SULT 1A3 single‐nucleotide polymorphism and the single dose pharmacokinetics of inhaled salbutamol enantiomers: are some athletes at risk of higher urine levels? Drug Test Anal 7, 109–113. [DOI] [PubMed] [Google Scholar]
- Jacobson GA, Yee KC, Premilovac D & Rattigan S (2014). Enantioselective disposition of (R/S)‐albuterol in skeletal and cardiac muscle. Drug Test Anal 6, 563–567. [DOI] [PubMed] [Google Scholar]
- Jensen J, Brennesvik EO, Bergersen H, Oseland H, Jebens E & Brørs O (2002). Quantitative determination of cell surface beta‐adrenoceptors in different rat skeletal muscles. Pflugers Arch 444, 213–219. [DOI] [PubMed] [Google Scholar]
- Jessen S, Onslev J, Lemminger A, Backer V, Bangsbo J & Hostrup M (2018). Hypertrophic effect of inhaled beta2‐agonist with and without concurrent exercise training: a randomized controlled trial. Scand J Med Sci Sports. 10.1111/sms.13221. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Joassard OR, Durieux AC & Freyssenet DG (2013). β2‐adrenergic agonists and the treatment of skeletal muscle wasting disorders. Int J Biochem Cell Biol 45, 2309–2321. [DOI] [PubMed] [Google Scholar]
- Kim SH, Asaka M, Higashida K, Takahashi Y, Holloszy JO & Han D‐H (2013). β‐Adrenergic stimulation does not activate p38 MAP kinase or induce PGC‐1 in skeletal muscle. AJP Endocrinol Metab 304, E844–E852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline WO, Panaro FJ, Yang H & Bodine SC (2007). Rapamycin inhibits the growth and muscle‐sparing effects of clenbuterol. J Appl Physiol 102, 740–747. [DOI] [PubMed] [Google Scholar]
- Koopman R, Gehrig SM, Léger B, Trieu J, Walrand S, Murphy KT & Lynch GS (2010). Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β‐adrenoceptor stimulation in mice. J Physiol 588, 4811–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Atherton P, Smith K & Rennie MJ (2009). Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 106, 2026–2039. [DOI] [PubMed] [Google Scholar]
- Le Panse B, Collomp K, Portier H, Lecoq A‐M, Jaffre C, Beaupied H, Richard O, Benhamou L, De Ceaurriz J & Courteix D (2005). Effects of short‐term salbutamol ingestion during a wingate test. Int J Sports Med 26, 518–523. [DOI] [PubMed] [Google Scholar]
- Lee P, Birzniece V, Umpleby AM, Poljak A & Ho KKY (2015). Formoterol, a highly β2‐selective agonist, induces gender‐dimorphic whole body leucine metabolism in humans. Metabolism 64, 506–512. [DOI] [PubMed] [Google Scholar]
- Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD & Pilegaard H (2005). Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol 95, 351–360. [DOI] [PubMed] [Google Scholar]
- Lynch GS & Ryall JG (2008). Role of β‐adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88, 729–767. [DOI] [PubMed] [Google Scholar]
- Macaluso A & De Vito G (2004). Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 91, 450–472. [DOI] [PubMed] [Google Scholar]
- Maltin CA, Hay SM, Delday MI, Lobley GE & Reeds PJ (1989). The action of the beta‐agonist clenbuterol on protein metabolism in innervated and denervated phasic muscles. Biochem J 261, 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau L, Horan MA, Rothwell NJ & Little RA (1992). Salbutamol, a β2‐adrenoceptor agonist, increases skeletal muscle strength in young men. Clin Sci 83, 615–621. [DOI] [PubMed] [Google Scholar]
- McLeod M, Breen L, Hamilton DL & Philp A (2016). Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology 17, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen UR, Dideriksen K, Andersen MB, Boesen A, Malmgaard‐Clausen NM, Sørensen IJ, Schjerling P, Kjær M & Holm L (2015). Preserved skeletal muscle protein anabolic response to acute exercise and protein intake in well‐treated rheumatoid arthritis patients. Arthritis Res Ther 17, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA & Phillips SM (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89, 161–168. [DOI] [PubMed] [Google Scholar]
- Morgan DJ & Bray KM (1994). Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet 26, 292–307. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Christensen PM, Mortensen SP, Hellsten Y & Bangsbo J (2014). Infusion of ATP increases leg oxygen delivery but not oxygen uptake in the initial phase of intense knee‐extensor exercise in humans. Exp Physiol 99, 1399–1408. [DOI] [PubMed] [Google Scholar]
- Ohnuki Y, Umeki D, Mototani Y, Jin H, Cai W, Shiozawa K, Suita K, Saeki Y, Fujita T, Ishikawa Y & Okumura S (2014). Role of cyclic AMP sensor Epac1 in masseter muscle hypertrophy and myosin heavy chain transition induced by β2‐adrenoceptor stimulation. J Physiol 592, 5461–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearen MA, Ryall JG, Lynch GS & Muscat GE (2009). Expression profiling of skeletal muscle following acute and chronic β2‐adrenergic stimulation: implications for hypertrophy, metabolism and circadian rhythm. BMC Genomics 10, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K & Cobb MH (2001). Mitogen‐activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22, 153–183. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE & Wolfe RR (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273, E99–E107. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B & Neufer PD (2000). Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 279, E806–E814. [DOI] [PubMed] [Google Scholar]
- Price OJ, Hull JH, Backer V, Hostrup M & Ansley L (2014). The impact of exercise‐induced bronchoconstriction on athletic performance: a systematic review. Sports Med 44, 1749–1761. [DOI] [PubMed] [Google Scholar]
- Rahbek SK, Farup J, Møller AB, Vendelbo MH, Holm L, Jessen N & Vissing K (2014). Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids 46, 2377–2392. [DOI] [PubMed] [Google Scholar]
- Redpath NT & Proud CG (1993). Cyclic AMP‐dependent protein kinase phosphorylates rabbit reticulocyte elongation factor‐2 kinase and induces calcium‐independent activity. Biochem J 293, 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MM, Richards JC, Hickey MS, Moore DR, Phillips SM, Bell C & Miller BF (2010). Acute β‐adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. AJP Regul Integr Comp Physiol 298, R25–R33. [DOI] [PubMed] [Google Scholar]
- Rosen JP, Chervinsky P, Renard RL, Kemp JP, Mendelson LM, Selcow JE, Noyes JH, Meltzer EO, Welch MJ & Orgel HA (1986). Duration of action of oral albuterol in an asthmatic population. Ann Allergy 56, 28–33. [PubMed] [Google Scholar]
- Ryall JG, Schertzer JD & Lynch GS (2007). Attenuation of age‐related muscle wasting and weakness in rats after formoterol treatment: therapeutic implications for sarcopenia. J Gerontol A Biol Sci Med Sci 62, 813–823. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Shestakova EA & Natapov PG (1988). Phosphorylation of elongation factor 2 by EF‐2 kinase affects rate of translation. Nature 334, 170–173. [DOI] [PubMed] [Google Scholar]
- Sato M, Dehvari N, Öberg AI, Dallner OS, Sandström AL, Olsen JM, Csikasz RI, Summers RJ, Hutchinson DS & Bengtsson T (2014). Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes 63, 4115–4129. [DOI] [PubMed] [Google Scholar]
- Sato S, Shirato K, Mitsuhashi R, Inoue D, Kizaki T, Ohno H, Tachiyashiki K & Imaizumi K (2013). Intracellular β2‐adrenergic receptor signaling specificity in mouse skeletal muscle in response to single‐dose β2‐agonist clenbuterol treatment and acute exercise. J Physiol Sci 63, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Zeng C, Ricome A, Hannon KM, Grant AL & Gerrard DE (2007). Extracellular signal‐regulated kinase pathway is differentially involved in beta‐agonist‐induced hypertrophy in slow and fast muscles. Am J Physiol Cell Physiol 292, C1681–C1689. [DOI] [PubMed] [Google Scholar]
- Shimamoto S, Ijiri D, Kawaguchi M, Nakashima K, Tada O, Inoue H & Ohtsuka A (2017). β1‐ and β2‐adrenergic receptor stimulation differ in their effects on PGC‐1α and atrogin‐1/MAFbx gene expression in chick skeletal muscle. Comp Biochem Physiol A Mol Integr Physiol 211, 1–6. [DOI] [PubMed] [Google Scholar]
- Signorile JF, Banovac K, Gomez M, Flipse D, Caruso JF & Lowensteyn I (1995). Increased muscle strength in paralyzed patients after spinal cord injury: effect of beta‐2 adrenergic agonist. Arch Phys Med Rehabil 76, 55–58. [DOI] [PubMed] [Google Scholar]
- Smith GI, Patterson BW, Klein SJ & Mittendorfer B (2015). Effect of hyperinsulinaemia‐hyperaminoacidaemia on leg muscle protein synthesis and breakdown: reassessment of the two‐pool arterio‐venous balance model. J Physiol 593, 4245–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlock DM, McDaneld TG & McIntyre LM (2006). Changes in skeletal muscle gene expression following clenbuterol administration. BMC Genomics 7, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M, Gunnarsson TP, Christensen PM, Pavlovic D, Shattock MJ & Bangsbo J (2016). Intensive training and reduced volume increases muscle FXYD1 expression and phosphorylation at rest and during exercise in athletes. Am J Physiol Regul Integr Comp Physiol 310, R659–R669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baak MA, de Hon OM, Hartgens F & Kuipers H (2004). Inhaled salbutamol and endurance cycling performance in non‐asthmatic athletes. Int J Sports Med 25, 533–538. [DOI] [PubMed] [Google Scholar]
- Wall BT, Burd NA, Franssen R, Gorissen SH, Snijders T, Senden JM, Gijsen AP & van Loon LJ (2016). Presleep protein ingestion does not compromise the muscle protein synthetic response to protein ingested the following morning. Am J Physiol Endocrinol Metab 311, E964–E973. [DOI] [PubMed] [Google Scholar]
- Wannenes F, Magni L, Bonini M, Dimauro I, Caporossi D, Moretti C & Bonini S (2012). In vitro effects of beta‐2 agonists on skeletal muscle differentiation, hypertrophy, and atrophy. World Allergy Organ J 5, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M & Leenen FH (1994). Aging and cardiovascular responsiveness to beta‐agonist in humans: role of changes in beta‐receptor responses versus baroreflex activity. Clin Pharmacol Ther 56, 543–553. [DOI] [PubMed] [Google Scholar]
- Wilkinson DJ, Cegielski J, Phillips BE, Boereboom C, Lund JN, Atherton PJ & Smith K (2015). Internal comparison between deuterium oxide (D2O) and L‐[ring‐13C6] phenylalanine for acute measurement of muscle protein synthesis in humans. Physiol Rep 3, pii: e12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Caron MG & Daniel K (1984). Skeletal muscle beta‐adrenergic receptors: variations due to fiber type and training. Am J Physiol 246, E160–E167. [DOI] [PubMed] [Google Scholar]
- Wolfe RR & Chinkes DL (2005). Isotope Tracers in Metabolic Research, 2nd edn John Wiley & Sons, Hoboken NJ. [Google Scholar]
- Yimlamai T, Dodd SL, Borst SE & Park S (2005). Clenbuterol induces muscle‐specific attenuation of atrophy through effects on the ubiquitin‐proteasome pathway. J Appl Physiol 99, 71–80. [DOI] [PubMed] [Google Scholar]
- Zurlo F, Larson K, Bogardus C & Ravussin E (1990). Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86, 1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]


