Abstract
Key Points
Changes in CO2 result in corresponding changes in both H+ and HCO3 − and despite evidence that HCO3 − can function as an independent signalling molecule, there is little evidence suggesting HCO3 − contributes to respiratory chemoreception.
We show that HCO3 − directly activates chemosensitive retrotrapezoid nucleus (RTN) neurons.
Identifying all relevant signalling molecules is essential for understanding how chemoreceptors function, and because HCO3 − and H+ are buffered by separate cellular mechanisms, having the ability to sense both modalities adds additional information regarding changes in CO2 that are not necessarily reflected by pH alone.
HCO3 − may be particularly important for regulating activity of RTN chemoreceptors during sustained intracellular acidifications when TASK‐2 channels, which appear to be the sole intracellular pH sensor, are minimally active.
Abstract
Central chemoreception is the mechanism by which the brain regulates breathing in response to changes in tissue CO2/H+. The retrotrapezoid nucleus (RTN) is an important site of respiratory chemoreception. Mechanisms underlying RTN chemoreception involve H+‐mediated activation of chemosensitive neurons and CO2/H+‐evoked ATP‐purinergic signalling by local astrocytes, which activates chemosensitive neurons directly and indirectly by maintaining vascular tone when CO2/H+ levels are high. Although changes in CO2 result in corresponding changes in both H+ and HCO3 − and despite evidence that HCO3 − can function as an independent signalling molecule, there is little evidence suggesting HCO3 − contributes to respiratory chemoreception. Therefore, the goal of this study was to determine whether HCO3 − regulates activity of chemosensitive RTN neurons independent of pH. Cell‐attached recordings were used to monitor activity of chemosensitive RTN neurons in brainstem slices (300 μm thick) isolated from rat pups (postnatal days 7–11) during exposure to low or high concentrations of HCO3 −. In a subset of experiments, we also included 2′,7′‐bis(2carboxyethyl)‐5‐(and 6)‐carboxyfluorescein (BCECF) in the internal solution to measure pHi under each experimental condition. We found that HCO3 − activates chemosensitive RTN neurons by mechanisms independent of intracellular or extracellular pH, glutamate, GABA, glycine or purinergic signalling, soluble adenylyl cyclase activity, nitric oxide or KCNQ channels. These results establish HCO3 − as a novel independent modulator of chemoreceptor activity, and because the levels of HCO3 − along with H+ are buffered by independent cellular mechanisms, these results suggest HCO3 − chemoreception adds additional information regarding changes in CO2 that are not necessarily reflected by pH.
Keywords: chemoreception, HCO3, pHi, pH‐independent, brain slice
Key Points
Changes in CO2 result in corresponding changes in both H+ and HCO3 − and despite evidence that HCO3 − can function as an independent signalling molecule, there is little evidence suggesting HCO3 − contributes to respiratory chemoreception.
We show that HCO3 − directly activates chemosensitive retrotrapezoid nucleus (RTN) neurons.
Identifying all relevant signalling molecules is essential for understanding how chemoreceptors function, and because HCO3 − and H+ are buffered by separate cellular mechanisms, having the ability to sense both modalities adds additional information regarding changes in CO2 that are not necessarily reflected by pH alone.
HCO3 − may be particularly important for regulating activity of RTN chemoreceptors during sustained intracellular acidifications when TASK‐2 channels, which appear to be the sole intracellular pH sensor, are minimally active.
Introduction
Breathing is maintained unconsciously by the ability of discrete subsets of cells (neurons and astrocytes) to sense the accumulation of tissue CO2 (i.e. respiratory chemoreceptors) as would occur during hypoventilation or apnoeic events and relay this information to downstream components of the respiratory circuit to increase rate and depth of breathing (Nattie & Li, 2012). A brainstem region called the retrotrapezoid nucleus (RTN) is important for multiple aspects of breathing including respiratory chemoreception (Guyenet et al. 2012; Guyenet & Bayliss, 2015). RTN neurons are thought to primarily respond to CO2 via the proxy of H+ by inhibition of TASK‐2 potassium channels (Wang et al. 2013) and activation of GPR4 (Kumar et al. 2015). However, dissociated chemosensitive RTN neurons show differential firing responses to pH in the absence and presence of CO2 /HCO3 −; RTN chemoreceptors show a pH sensitivity of ∼5 Hz/pH in HCO3 −‐free Hepes buffer, whereas in HCO3 − solution their sensitivity increased to ∼8 Hz/pH (Wang et al. 2013). Furthermore, in rat brain slices chemosensitive RTN neurons showed similar firing responses to 10% CO2 under control conditions (HCO3 − = 26 mM) and when the concentration of HCO3 − was increased (HCO3 − = 52 mM) to maintain extracellular pH (pHo) and dampen changes in pHi (isohydric hypercapnia) (Ritucci et al. 2005). Together, these results suggest pH per se is not the sole transducer for CO2 detection by RTN neurons. Given that changes in CO2 result in corresponding changes in H+ and HCO3 −, and because HCO3 − can function as a signalling molecule independent of pH (Chen et al. 2000), we investigated whether HCO3 − contributes to RTN chemoreception.
Bicarbonate can modulate neuronal activity in a potentially pH‐independent manner by activation of cyclic nucleotide signalling (Chen et al. 2000) or by influencing membrane potential by flux through Cl− channels (Bonnet & Bingmann, 1993; Duran et al. 2010; Hamidi & Avoli, 2015) or electrogenic transporters (Romero & Boron, 1999). For example, HCO3 − is a positive allosteric modulator of soluble adenylyl cyclase (Steegborn et al. 2005) that may contribute to central chemoreception including at the level of the RTN (Ritucci et al. 2005) or peripheral chemoreception (Summers et al. 2002) by activation of cAMP and protein kinase A (PKA) signalling. Interestingly, recent evidence showed that HCO3 − stimulated hippocampal pyramidal neurons in a pH‐independent manner by inhibition of KCNQ channels (Jones et al. 2014), possibly by PKA‐dependent depletion of phosphatidylinositol 4,5‐bisphosphate (PIP2; a requisite cofactor for channel function; Suh & Hille, 2008). Given that KCNQ channels are potent modulators of RTN chemoreceptor activity (Hawryluk et al. 2012; Hawkins et al. 2015; Mulkey et al. 2015), we therefore considered the possibility that HCO3 − modulates RTN chemoreceptors by inhibition of KCNQ channels.
Here, we use a combination of slice‐patch electrophysiology, pharmacology and intracellular pH imaging to show that HCO3 −, in addition to H+, can modulate activity of chemosensitive RTN neurons by mechanisms independent of pH, fast excitatory or inhibitory transmission, purinergic or nitric oxide signalling, soluble adenylyl cyclase (sAC) activity, or KCNQ channels. Conversely, CO2/H+‐insensitive RTN neurons also did not respond to high HCO3 −, suggesting HCO3 − sensitivity is specific to respiratory chemoreceptors. These results establish HCO3 − as a novel independent modulator of chemoreceptor activity.
Methods
Ethical approval
Animal use was in accordance with guidelines approved by the University of Connecticut Institutional Animal Care and Use Committee. Brain slices were isolated from neonatal Sprague‐Dawley rat pups (7–12 days old; n = 63) (Charles River Laboratories, Kingston, NY, USA). All efforts were made to minimize the number of animals used.
Electrophysiological recordings in brainstem slices
Slices containing the RTN were prepared as previously described (Mulkey et al. 2004; Wenker et al. 2012). In short, rats were anaesthetized by administration of ketamine (375 mg/kg, i.p.) and xylazine (25 mg/kg, i.p.) and rapidly decapitated; brainstems were removed and transverse brain stem slices (300 μm) were cut using a microslicer (DSK 1500E; Dosaka, Kyoto, Japan) in ice‐cold substituted Ringer's solution containing the following (in mM): 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose and 1 kynurenic acid. Slices were incubated for 30 min at 37°C and subsequently at room temperature in a normal Ringer's solution containing (in mM): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose. Both substituted and normal Ringer's solutions were bubbled with 95% O2 and 5% CO2 (pH 7.30).
Individual slices containing the RTN were transferred to a recording chamber mounted on a fixed‐stage microscope with infrared Nomarski optics (Zeiss Axioskop FS); slices were perfused continuously (∼2 ml/min) with a bath solution containing (in mM): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 Hepes and 10 glucose (equilibrated with 5% CO2; pH 7.3). All recordings were made with an Axopatch 200B patch‐clamp amplifier, digitized with a Digidata 1322A A/D converter, and recorded using pCLAMP 10.0 software (Molecular Devices, San Jose, CA, USA). Recordings were obtained at room temperature (∼22°C) with patch electrodes pulled from borosilicate glass capillaries (Harvard Apparatus, Molliston, MA, USA) on a two‐stage puller (P‐97; Sutter Instrument, Novato, CA, USA) to a DC resistance of 5–7 MΩ when filled with a pipette solution containing the following (in mM): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 Hepes, 10 EGTA, 3 Mg‐ATP and 0.3 GTP‐Tris (pH 7.20). Electrode tips were coated with Sylgard 184 (Dow Corning, Midland, MI, USA). Spontaneous neuronal activity was measured in the cell‐attached voltage‐clamp configuration with holding potential matched to the resting membrane potential of RTN neurons (V hold = −60 mV) and with no current generated by the amplifier (I amp = 0 pA) (Perkins, 2006). Firing rate histograms were generated by integrating action potential discharge in 10‐ to 20‐s bins using Spike 5.0 software (Cambridge Electronic Design, CED, Cambridge, UK).
Intracellular pH imaging
In a subset of experiments, we measured intracellular pH (pHi) of chemosensitive neurons during exposure to high CO2 or HCO3 −. After functionally identifying chemosensitive RTN neurons in the cell‐attached configuration as described above, we obtained whole‐cell access to dialyse cells with the pH‐sensitive fluorescent dye BCECF free acid (Invitrogen, Carlsbad, CA, USA; dissolved in pipette solution at 100 μM). We waited ∼15 min for BCECF to load the cell and establish stable fluorescence values before measuring pHi. To activate the dye we toggled between (< 1 s transition) excitation wavelengths of 440 (pH‐insensitive) and 490 nm (pH‐sensitive) (xenon arc lamp), and the fluorescence emitted at 530 nm was collected for both excitation wavelengths. Images were acquired at an interval of 60 s. The background fluorescence intensity was subtracted, and an intensity ratio for each cell was calculated. The ratio of fluorescence intensities at 490 nm to that at 440 nm (B 490/B 440) was used to estimate pHi. We normalized pHi values measured in RTN neurons during incubation in 5% and 10% CO2 to previously reported values (Ritucci et al. 2005). We also confirmed that all measured pH values are in line with expected ΔpHi based on the following relationship:
where βint is intrinsic buffer power of 9.8 meq/l/pH unit (Nottingham et al. 2001) and Δ[CO2] is the concentration difference in 5% (1.38 mM) and 10% (2.77 mM), determined using a CO2 solubility coefficient of 0.03 mM/mmHg (Nottingham et al. 2001) and CO2 partial pressures of 35.6 and 71.3 mmHg, respectively.
Solutions
Normal ACSF contained (in mM): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4 and 26 NaHCO3, equilibrated with 10 glucose. To expose slices to hypercapnia, we equilibrated normal Ringer's solution with 10% CO2 (pH 7.0). Isohydric HCO3 − (which may also be considered isohydric hypercapnia) solution contained (in mM): 104 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 52 NaHCO3 and 10 glucose, and was bubbled with 10% CO2 (pH 7.3). HCO3 −‐free Hepes buffer contained (in mM): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 Hepes and 10 glucose. For experiments where 26 mM NaHCO3 was added to Hepes buffer, we lowered NaCl by an equimolar amount. The osmolarity of all solutions was maintained at ∼300 mOsm.
Drugs
All chemicals were obtained from Sigma‐Aldrich (St. Louis, MO, USA), unless otherwise stated. All drugs were bath applied at the following concentrations: XE991 (10 μM; Tocris Bioscience, Bristol, UK) was used to block KCNQ channels, 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX, 10 μM) was used to block AMPA/kainite receptors, strychnine (2 μM) was used to block glycine receptors, gabazine (10 μM) was used to block GABAA receptors, pyridoxalphosphate‐6‐azophenyl‐2′,4′‐disulfonic acid (PPADS, 5 μM) was used to block P2 receptors, acetazolamide (1 mM) was used to inhibit carbonic anhydrase activity, KH7 (10 μM; Tocris) was used to inhibit soluble adenylyl cyclase activity, and N‐nitro‐l‐arginine methyl ester (l‐NAME, 1 mM) was used to inhibit nitric oxide synthase activity.
Data analysis
Data are reported as mean ± SE. All statistical analysis was performed in GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons were made using a t‐test or one‐way ANOVA followed by Tukey or Dunnett multiple comparison tests as appropriate. The relevant values used for statistical analysis are provided in the Results.
Results
Chemosensitive RTN neurons were initially identified in slices incubated in normal Ringer's solution (HCO3 − = 26 mM) based on their characteristic firing response to CO2. Neurons that were spontaneously active in 5% CO2 (pHo 7.3) and responded to 10% CO2 (pHo∼7.0) with at least a 1.0 Hz increase in firing rate were considered chemosensitive. This level of CO2/H+ sensitivity is similar to what we (Wenker et al. 2012, Hawkins et al. 2015) and others (Ritucci et al. 2005) have previously reported for chemosensitive RTN neurons. Neurons that showed < 1 Hz firing response to 10% CO2 or pH 7.0 were considered non‐chemosensitive.
The goal of this study was to determine whether HCO3 − can modulate activity of chemosensitive RTN neurons, and considering that these cells sense extracellular H+ in part by activation of GPR4 (Kumar et al. 2015), we initially tested firing responses to low and high concentrations of HCO3 − while maintaining pHo. We found that exposure to HCO3 −‐free Hepes‐buffered solution (pHo = 7.3 equilibrated with room air or 100% O2) decreased activity of chemosensitive RTN neurons from 0.95 ± 0.01 to 0.20 ± 0.06 Hz (F 2,17 = 161.7, P < 0.0001) (Fig. 1 A, B). Conversely, exposure to isohydric (pHo = 7.3) HCO3 − increased activity of chemosensitive RTN neurons from 0.48 ± 0.1 to 1.15 ± 0.2 Hz (F 2,18 = 186.1, P < 0.0001) (Fig. 1 C, D). Note that basal activity in HCO3 −‐free Hepes‐buffered solution (0.1 ± 0.04 Hz) is less than basal activity in HCO3 −‐buffered solution (26 mM; pH 7.3) (0.71 ± 0.08 Hz; T 49 = 4.834, P < 0.0001), consistent with the possibility that HCO3 − activates chemosensitive RTN neurons in a manner that contributes to baseline activity and CO2/H+ sensitivity. Bicarbonate sensitivity also appears specific to chemosensitive RTN neurons because CO2/H+‐insensitive cells showed no measurable change in activity in response to isohydric HCO3 − (P = 0.991) (Fig. 1 E, F). The response of chemosensitive neurons to changes in HCO3 − was retained in the presence of gabazine (10 μM) to block GABAA receptors, strychnine (2 μM) to block glycine receptors and CNQX (10 μM) to block AMPA/kainite receptors (Fig. 2 A, F, G). These results confirm that neuronal firing responses to changes in HCO3 − are not dependent on synaptic transmission or HCO3 − flux through GABAA receptors (Bormann et al. 1987).
Figure 1. HCO3 −‐dependent modulation of chemosensitive RTN neurons.
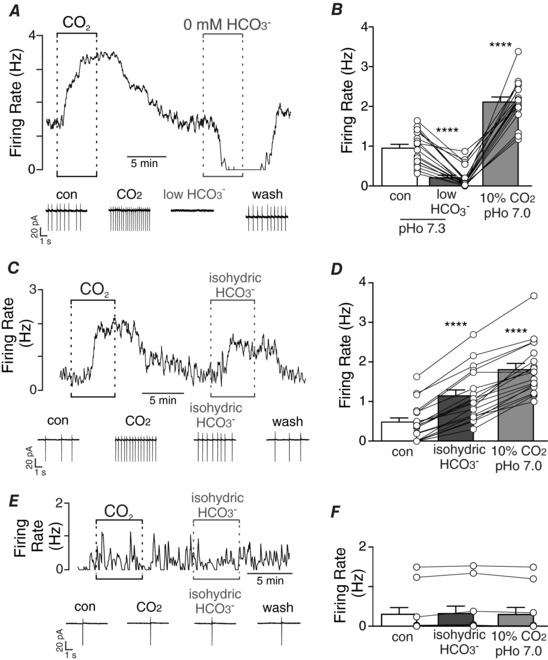
A, trace of firing rate and segments of holding current shows a typical response of an RTN neuron in a slice incubated in normal Ringer's solution containing 26 mM HCO3 − to an increase in CO2 from 5% to 10%. After returning to control conditions, exposure to HCO3 −‐free Hepes buffer (pH 7.3) strongly inhibited baseline activity. B, summary data (n = 18) show average firing rate under control conditions and during exposure to HCO3 −‐free Hepes buffer (pHo 7.3) and 10% CO2 (pHo 7.0). C, trace of firing rate and segments of holding current from a chemosensitive RTN neuron shows that exposure to isohydric HCO3 − (10% CO2/52 mM HCO3 −) caused a robust increase in activity. D, summary data (n = 19) show average firing rate of chemosensitive RTN neurons under control conditions and during exposure to isohydric HCO3 − and 10% CO2 (pHo 7.0). E, trace of firing rate and segments of holding current from a CO2/H+‐insensitive RTN neuron shows that exposure to isohydric HCO3 − (10% CO2/52 mM HCO3 −) minimally affected firing behaviour. F, summary data (n = 10) show average firing activity of CO2/H+‐insensitive RTN neurons under control conditions and during exposure to isohydric HCO3 − and 10% CO2 (pHo 7.0). One‐way ANOVA with Tukey multiple comparison test. **** P < 0.0001. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2. HCO3 − directly modulates activity of RTN neurons.
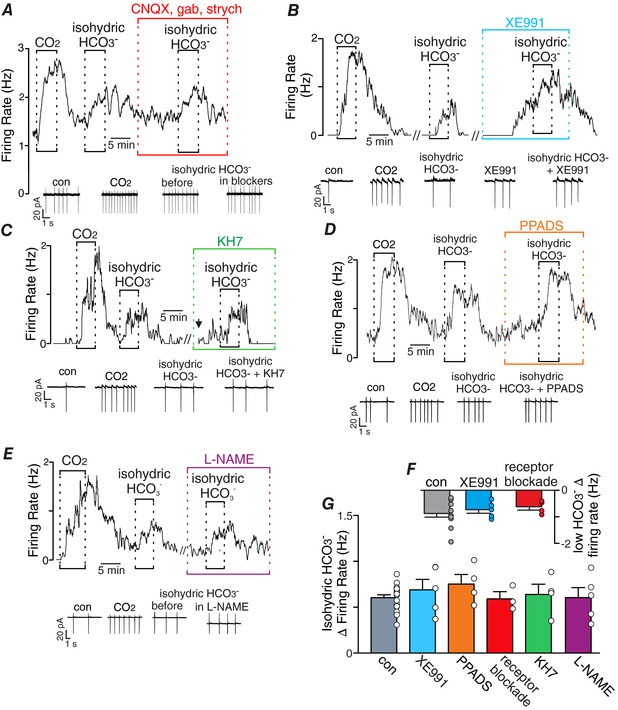
A–E, traces of firing rate and segments of holding current from chemosensitive RTN neurons show that exposure to isohydric HCO3 − (10% CO2/52 mM HCO3 −) stimulated neural activity under control conditions and after 10 min of incubation in a transmitter receptor blocker cocktail containing CNQX (10 μM), gabazine (10 μM) and strychnine (2 μM) (A), KCNQ channels were blocked with XE991 (10 μM) (B), sAC activity was inhibited with KH7 (10 μM) (C), purinergic receptors were blocked with PPADS (5 μM) (D), and when nitric oxide synthase was inhibited with l‐NAME (1 mM). F, summary data show that exposure to HCO3 −‐free Hepes buffer inhibited activity under control conditions (n = 10), and in XE991 (n = 5) or the blocker cocktail (n = 4). G, summary data plotted as isohydric HCO3 −‐induced change in activity under control conditions (n = 13) and in the presence of XE991 (n = 5), PPADS (n = 4), blocker cocktail (n = 3), KH7 (n = 4), or l‐NAME (n = 5). //, a 10–20 min break in the recording. ↓, injection of a positive DC current to adjust baseline activity to near control levels. One‐way ANOVA (F 5,33 = 0.5909, P > 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Based on evidence that KCNQ channels are key determinants of RTN chemoreceptor activity (Hawryluk et al. 2012) and in other brain regions HCO3 − has been shown to activate sAC (Chen et al. 2000) and inhibit KCNQ by a mechanism that may involve PKA‐dependent depletion of PIP2 (Jones et al. 2014), we also explored the possibility that KCNQ or sAC contribute to HCO3 − modulation of RTN neurons. Contrary to our expectations, firing responses to changes in HCO3 − were retained when KCNQ channels were blocked with XE991 (10 μM) (Fig. 2 B, F, G) or when sAC activity was blocked with KH7 (10 μM) (Fig. 2 C, G). For example, exposure to isohydric HCO3 − increased chemoreceptor activity by 0.60 ± 0.03 Hz under control conditions, 0.69 ± 0.11 Hz in the presence of XE991 (Fig. 2B) and by 0.64 ± 0.11 after 10 min incubation in KH7 (Fig. 2 C). It is also possible that exposure to high HCO3 − in the presence of nitric oxide can facilitate the formation of peroxynitrite (Lymar et al. 1996), which can regulate neural activity by reduction–oxidation modulation of various ion channels and second messenger pathways. Therefore, we also tested the effects of isohydric HCO3 − on RTN chemoreceptor activity when the activity of nitric oxide synthase was blocked with l‐NAME. We found that incubation (20 min) in l‐NAME (1 mM) had negligible effects on basal activity (Δ firing rate 0.20 ± 0.2 Hz; P = 0.281) and did not blunt the firing response to isohydric HCO3 − (Δ firing rate 0.60 ± 0.1 Hz; P = 0.005) (Fig. 2 E, G).
Considering that astrocytes contribute to RTN chemoreception by providing a CO2/H+‐dependent purinergic drive to activate local neurons (Gourine et al. 2010; Huckstepp et al. 2010; Wenker et al. 2012), and because astrocytes express high levels of the Na+/HCO3 − cotransporter (NBC) (Erlichman & Leiter, 2010) and activation of the NBC has been shown to contribute to astrocyte chemoreception (Turovsky et al. 2016), we tested the possibility that HCO3 −‐dependent activation of RTN neurons involves ATP release by RTN astrocytes. We found that exposure to isohydric HCO3 − increases activity of RTN neurons by similar amounts under control conditions and during purinergic receptor blockade; exposure to a solution containing high CO2 (10%) plus HCO3 − (52 mM) with a pHo of 7.3 increased chemoreceptor activity by 0.60 ± 0.03 Hz under control conditions and by 0.75 ± 0.10 Hz in the presence of PPADS (5 μM) (Fig. 2 D, G).
A caveat to the above experiments is that manipulations of HCO3 − may also affect pHi (Table 1). For example, the transition from normal HCO3 −‐buffered solution to a HCO3 −‐free Hepes‐buffered solution increased pHi by 0.08 ± 0.02 pH units, presumably due to the rapid diffusion of CO2 out of RTN neurons, whereas exposure to isohydric HCO3 − decreased pHi by 0.06 ± 0.01 pH units probably as a result of CO2 influx. This is relevant because RTN neurons also express TASK‐2 channels (Wang et al. 2013), which are activated by changes in intracellular or extracellular pH with a pK1/2 of ∼8.6 (i.e. the pH that achieves half maximum channel activation) (Reyes et al. 1998; Cid et al. 2013). Our experimental conditions result in pHi changes ∼1 pH unit lower than the effective pHi sensing range of TASK‐2. However, evidence also suggests TASK‐2 channels contribute to RTN chemoreceptor activity at physiological pH of 7.3 and so even slight increases in pHi, as observed during exposure to Hepes, may enhance TASK‐2 activity, and thus inhibit neuronal firing. Consistent with this, in voltage clamp (I hold = −60 mV, in tetrodotoxin to block neuronal action potentials), we found that exposure to HCO3 −‐free Hepes buffer elicited a modest increase in outward current by activation of a relatively voltage‐independent TASK‐like current (data not shown). Because exposure to this experimental condition also results in a 0.08 ± 0.02 alkalization (Table 1), we suspect that pH rather than HCO3 − activates this outward current. These results further support the possibility that activation of TASK‐2 by intracellular alkalization can regulate activity of RTN neurons. However, these results also underline the need to independently control pHi and HCO3 −.
Table 1.
pHi and firing responses of RTN neurons under all experimental conditions
| Test condition | ΔpHi | Δ firing rate (Hz) |
|---|---|---|
| 10% CO2 | 0.12 acidification ‡ | 1.25 ± 0.1 * |
| HCO3 −‐free Hepes | 0.08 ± 0.02 alkalization * | −0.75 ± 0.1 * |
| high HCO3 − (52 mM) Ringer's solution | 0.06 ± 0.01 acidification * | 0.66 ± 0.1 * |
| Hepes + HCO3 − (26 mM) | 0.07 ± 0.01 acidification * | 0.73 ± 0.1 * |
| Hepes + HCO3 − (26 mM) + acetazolamide | 0.09 ± 0.01 alkalization * | 0.72 ± 0.1 * |
‡5 and 10% CO2 were used as pHi calibration points.*Significant differences from control (P < 0.05)
To differentiate between pHi and HCO3 −‐dependent modulation of neuronal activity, we tested effects of HCO3 − (26 mM) in Hepes‐buffered solution under control conditions and when carbonic anhydrase activity was blocked with acetazolamide (1 mM). Carbonic anhydrase catalyses the hydration/dehydration of CO2 and so application of acetazolamide to a slice perfused with HCO3 −‐ree Hepes solution is expected slow the formation of CO2 during exposure to high HCO3 −, and thus minimize changes in pHi. To confirm this possibility, we included BCECF in our pipette internal solution to measure pHi in chemosensitive RTN neurons during exposure to high HCO3 − in Hepes alone and in Hepes solution supplemented with acetazolamide. Exposure to high HCO3 − alone caused a modest acidification of 0.07 ± 0.01 pH units (T 3 = 12.63, P < 0.01) that persisted for the 5 min duration of the exposure (Fig. 3 A, B). Application of acetazolamide (1 mM) also decreased pHi by 0.40 ± 0.085 pH units (T 2 = 4.714, P<0.05) (Fig. 3 A), probably by favouring the intracellular accumulation of metabolically generated carbonic acid (Erlichman et al. 1994). In the continued presence of acetazolamide, a second exposure to high HCO3 − this time increased pHi by 0.09 ± 0.01 pH units (T 2 = 5.883, P < 0.05), suggesting that in this condition CO2 transport is reduced and HCO3 − is the primary molecule transported into the cell (Fig. 3 A, B).
Figure 3. HCO3 − modulates activity of chemosensitive RTN neurons by a mechanism independent of pHi .
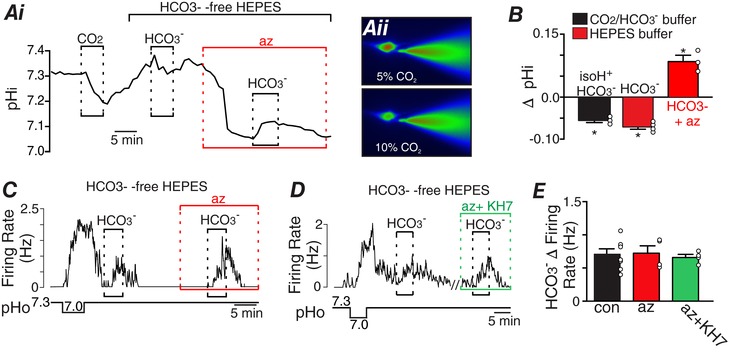
BCECF (100 μM) was included in our pipette internal solution to measure pHi of chemosensitive RTN neurons incubated in Hepes buffer during exposure to high HCO3 − (26 mM) alone and in the presence of acetazolamide (az; 1 mM). A, trace of pHi (Ai) and fluorescence images (490 nm excitation) (Aii) from a chemosensitive RTN neuron in normal Ringer's solution (26 mM HCO3 −) equilibrated with 5% CO2 (pHo 7.3) shows that exposure to 10% CO2 decreased pHi ∼0.1 pH units. After returning to control conditions exposure to HCO3 −‐free Hepes buffer (pHo 7.3) increased pHi ∼0.08 pH units. In the continued presence of Hepes buffer, exposure to HCO3 − (26 mM) reversibly decreased pHi by ∼0.07 pH units under control conditions. Under these conditions exposure to acetazolamide (1 mM) also decreased pHi by ∼0.4 pH units. However, in acetazolamide subsequent exposure to HCO3 − (26 mM) this time increased pHi ∼0.1 pH units. B, summary data plotted as change in pHi during exposure to isohydric HCO3 – (10% CO2 + 52 mM HCO3 −) in normal Ringer's solution (n = 4), and HCO3 − (26 mM) alone (n = 3) or together with acetazolamide under Hepes buffer conditions (n = 3). C, trace of firing rate shows a typical H+ response of an RTN neuron in a slice incubated in Hepes buffer. After returning to control conditions (pH– 7.3), exposure to HCO3 − (26 mM; pHo 7.3) alone or in the presence of acetazolamide (1 mM) increased ∼0.75 Hz. Note that bath application of acetazolamide minimally affected neuronal activity despite resulting in a strong intracellular acidification. D, trace of firing rate from a chemosensitive RTN neuron in a slice incubated in Hepes buffer shows that exposure to HCO3 − (26 mM; pHo 7.3) increased neural activity by similar amounts under control conditions and in the presence of acetazolamide (1 mM) plus KH7 (10 μM). E, summary data show that the firing response to HCO3 − was similar under control conditions (n = 7) and in acetazolamide alone (n = 4) or in combination with KH7 (n = 4) (one‐way ANOVA; F 2,13 = 0.115, P > 0.05). *Paired t test, P < 0.05. [Color figure can be viewed at http://wileyonlinelibrary.com]
These results show that exposure to HCO3 − alone and in the presence of acetazolamide have opposite effects of pHi, and assuming TASK‐2 channels are the sole pHi sensor in these cells, we expect these changes in pHi to also have opposite effects on neural activity. Therefore, by comparing neuronal responses to HCO3 − under these conditions, we will be able to determine whether HCO3 − signalling affects chemoreceptor function independent of pHi. We found that chemosensitive RTN neurons in slices incubated in HCO3 −‐free Hepes buffer at pH 7.3 showed lower baseline activity (0.14 ± 0.06 Hz) compared to RTN neurons in slices incubated in HCO3 −‐buffered conditions at the same pH (0.79 ± 0.09 Hz) (T 39 = 3.864, P < 0.001), suggesting factors other than pH influence chemoreceptor activity. Consistent with this, we found under Hepes buffer conditions that exposure to HCO3 − (26 mM) alone increased activity of chemosensitive RTN neurons by 0.71 ± 0.08 Hz (T 7 = 9.1, P < 0.0001) (Fig. 3 C). However, bath application of acetazolamide (1 mM) minimally affected chemoreceptor activity (Fig. 3 C) despite causing a large intracellular acidification (Fig. 3 A). These results suggest that TASK‐2 channels are minimally active at pH values less than 7.3. Furthermore, in the continued presence of acetazolamide, a second exposure to 26 mM HCO3 − increased chemoreceptor activity by 0.72 ± 0.11 Hz (T 3 = 14.98, P < 0.001) (Fig. 3 C–E), despite occurring in conjunction with an 0.09 ± 0.01 increase in pHi, which is expected to limit chemoreceptor activity by TASK‐2 channel activation. These results suggest HCO3 − can regulate activity of RTN neurons independent of pHi. Additionally, the combined application of acetazolamide plus KH7 (10 μM) minimally affected HCO3 −‐mediated excitation (Fig. 3 D, E), suggesting sAC activity does not contribute to HCO3 − modulation of RTN neurons.
Discussion
The main finding of this study is that HCO3 −, in addition to H+, can selectively activate chemosensitive RTN neurons. This is important because identifying all relevant signalling molecules is essential for understanding how chemoreceptors function, and as the levels of HCO3 − along with other chemosensory modalities (namely H+) are buffered by independent cellular mechanisms (Chesler, 2003), these results suggest HCO3 − chemoreception adds additional information regarding changes in CO2 that are not necessarily reflected by pH. Furthermore, HCO3 − may be particularly important for regulating activity of RTN chemoreceptors during sustained intracellular acidifications when TASK‐2 channels, which appear to be the sole intracellular pH sensor, are minimally active. In addition, considering CO2/H+‐induced changes in HCO3 − may be small relative to the background HCO3 − (26 mM), it is also possible that the role of HCO3 − is to provide a tonic enhancement of baseline activity and CO2/H+ sensitivity.
Mechanisms of RTN chemoreception
Chemosensitive RTN neurons primarily sense extracellular H+ by inhibition of TASK‐2 channels (Wang et al. 2013) and activation of GPR4 (Kumar et al. 2015). Although TASK‐2 channels also sense pHi, their capacity to respond to pHi changes in the lower range is limited because TASK‐2 channels have a pKa of 7.8 (Reyes et al. 1998). Consistent with this, our results show that intracellular acidification by bath application of acetazolamide minimally affected neural activity. However, under these conditions RTN neurons are still able to respond to HCO3 −. These results show that HCO3 − chemoreception expands the functional pHi sensing capacity of RTN neurons.
The CO2/H+‐dependent output of RTN neurons is further enhanced by CO2/H+‐evoked ATP‐purinergic signalling by local astrocytes (Gourine et al. 2010; Wenker et al. 2010, 2012), which activates chemosensitive neurons directly (Gourine et al. 2010; Wenker et al. 2012) and indirectly by maintaining vascular tone when CO2/H+ levels are high (Hawkins et al. 2017). Interestingly, the contribution of astrocytes to RTN chemoreception requires both H+ and molecular CO2; exposure to H+ has been shown to inhibit astrocyte Kir4.1 potassium K+ channels (Wenker et al. 2010) and activate the NBC which together favour Ca2+ influx by reverse mode operation of the sodium calcium exchanger (NCX) (Turovsky et al. 2016). However, CO2‐dependent gating of connexin 26 hemichannels also appears to be required for ATP release (Huckstepp et al. 2010). Because HCO3 − did not facilitate purinergic modulation of RTN neurons, we do not think this signalling pathway contributes to HCO3 − chemoreception.
Possible mechanisms of HCO3 − chemoreception
In comparison to pH, HCO3 − has been shown to target only a limited number of effectors. For example, olfactory neurons sense CO2 in part by HCO3 − activation of guanylyl cyclase‐D (GC‐D) (EC50 ∼20 mM; Tresguerres et al. 2010) which stimulates neuronal activity by activation of cyclic nucleotide‐gated channels (Hu et al. 2007). Although, GC‐D has only been found in neurons localized to the olfactory bulb (Fulle et al. 1995), because the olfactory and respiratory systems are functionally coupled and may share information regarding timing of inspiration and expiration (Perez de Los Cobos Pallares et al. 2016; Short et al. 2016), it is conceivable that chemosensitive RTN neurons share common HCO3 − sensing mechanisms including GC‐D. However, this interesting possibly requires further investigation. Furthermore, despite evidence that HCO3 − can enhance the production of peroxynitrite (Lymar et al. 1996), which can modulate neural function by oxidizing nucleophilic residues on target proteins including various ion channels, blocking the production of nitric oxide did not affect HCO3 − chemosensitivity. These results argue against the possible involvement of peroxynitrite in HCO3 − signalling. In addition, our evidence that HCO3 − modulation of RTN neurons was retained when GABAA receptors were blocked rules out involvement of this Cl− channel. However, involvement of other Cl− channels remains an open possibility. It is also possible that HCO3 − directly interacts with other unidentified ion channels.
In summary, we show that variations in HCO3 − above and below normal physiological levels (26 mM) increase and decrease activity of chemosensitive RTN neurons by mechanisms independent of pH, fast excitatory or inhibitory transmission, purinergic or nitric oxide signalling, sAC activity, or KCNQ channels. Although the mechanism(s) of HCO3 − chemoreception remain unknown, these results establish HCO3 − as a novel independent modulator of chemoreceptor activity.
Additional Information
Competing interests
We have no competing interests.
Author contributions
CMG: experimental design; collection and analysis of in vitro data; revising the manuscript; final approval of the manuscript. DKM: experimental design; data analysis; revising the manuscript; drafting the manuscript; final approval of the manuscript.
Funding
This work was supported by funds from the National Institutes of Health Grants HL104101 and R01HL137094. Additional funds were also provided by the Dravet Foundation.
Biography
Christopher Gonçalves received a BS in Physiology and Neurobiology from the University of Connecticut in 2013 and is currently a graduate student in the laboratory of Daniel K. Mulkey at the University of Connecticut. His research focuses on understanding the ion channel basis for how astrocytes and neurons contribute to control of breathing, and how disruption of these mechanisms contributes to respiratory dysfunction in certain diseases states including Rett syndrome.

Edited by Harold Schultz and Benedito Machado
Linked articles This article is highlighted by a Perspective by Garcia III & Sullere. To read this Perspective, visit https://doi.org/10.1113/JP276558.
References
- Bonnet U & Bingmann D (1993). GABA‐responses of CA3 neurones at epileptogenic threshold concentrations of convulsants. Neuroreport 4, 715–718. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP & Sakmann B (1987). Mechanism of anion permeation through channels gated by glycine and γ‐aminobutyric acid in mouse cultured spinal neurones. J Physiol 385, 243–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR & Buck J (2000). Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628. [DOI] [PubMed] [Google Scholar]
- Chesler M (2003). Regulation and modulation of pH in the brain. Physiol Rev 83, 1183–1221. [DOI] [PubMed] [Google Scholar]
- Cid LP, Roa‐Rojas HA, Niemeyer MI, Gonzalez W, Araki M, Araki K & Sepulveda FV (2013). TASK‐2: a K2P K+ channel with complex regulation and diverse physiological functions. Front Physiol 4, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran C, Thompson CH, Xiao Q & Hartzell HC (2010). Chloride channels: often enigmatic, rarely predictable. Annu Rev Physiol 72, 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Coates EL & Leiter JC (1994). Carbonic anhydrase and CO2 chemoreception in the pulmonate snail Helix aspersa . Respir Physiol 98, 27–41. [DOI] [PubMed] [Google Scholar]
- Erlichman JS & Leiter JC (2010). Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol (1985) 108, 1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulle HJ, Vassar R, Foster DC, Yang RB, Axel R & Garbers DL (1995). A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci U S A 92, 3571–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K & Kasparov S (2010). Astrocytes control breathing through pH‐dependent release of ATP. Science 329, 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG & Bayliss DA (2015). Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Abbott SB, Depuy SD & Kanbar R (2012). The retrotrapezoid nucleus and breathing. Adv Exp Med Biol 758, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi S & Avoli M (2015). Carbonic anhydrase inhibition by acetazolamide reduces in vitro epileptiform synchronization. Neuropharmacology 95, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS & Mulkey DK (2015). HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J Neurophysiol 113, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins VE, Takakura AC, Trinh A, Malheiros‐Lima MR, Cleary CM, Wenker IC, Dubreuil T, Rodriguez EM, Nelson MT, Moreira TS & Mulkey DK (2017). Purinergic regulation of vascular tone in the retrotrapezoid nucleus is specialized to support the drive to breathe. Elife 6, e25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV & Mulkey DK (2012). KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci 32, 16943–16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H & Luo M (2007). Detection of near‐atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 317, 953–957. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV & Dale N (2010). Connexin hemichannel‐mediated CO2‐dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588, 3901–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT, Faas GC & Mody I (2014). Intracellular bicarbonate regulates action potential generation via KCNQ channel modulation. J Neurosci 34, 4409–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez‐Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA & Bayliss DA (2015). Regulation of breathing by CO2 requires the proton‐activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymar SV, Jiang Q & Hurst JK (1996). Mechanism of carbon dioxide‐catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry 35, 7855–7861. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Hawkins VE, Hawryluk JM, Takakura AC, Moreira TS & Tzingounis AV (2015). Molecular underpinnings of ventral surface chemoreceptor function: focus on KCNQ channels. J Physiol 593, 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA & Guyenet PG (2004). Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7, 1360–1369. [DOI] [PubMed] [Google Scholar]
- Nattie E & Li A (2012). Central chemoreceptors: locations and functions. Compr Physiol 2, 221–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham S, Leiter JC, Wages P, Buhay S & Erlichman JS (2001). Developmental changes in intracellular pH regulation in medullary neurons of the rat. Am J Physiol Regul Integr Comp Physiol 281, R1940–1951. [DOI] [PubMed] [Google Scholar]
- Perez de Los Cobos Pallares F, Bautista TG, Stanic D, Egger V & Dutschmann M (2016). Brainstem‐mediated sniffing and respiratory modulation during odor stimulation. Respir Physiol Neurobiol 233, 17–24. [DOI] [PubMed] [Google Scholar]
- Perkins KL (2006). Cell‐attached voltage‐clamp and current‐clamp recording and stimulation techniques in brain slices. J Neurosci Methods 154, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N & Lazdunski M (1998) Cloning and expression of a novel pH‐sensitive two pore domain K+ channel from human kidney. J Biol Chem 273, 30863–9. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC & Putnam RW (2005). Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol 289, R851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF & Boron WF (1999). Electrogenic Na+/HCO3 − cotransporters: cloning and physiology. Annu Rev Physiol 61, 699–723. [DOI] [PubMed] [Google Scholar]
- Short SM, Morse TM, McTavish TS, Shepherd GM & Verhagen JV (2016). Respiration gates sensory input responses in the mitral cell layer of the olfactory bulb. PLoS One 11, e0168356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegborn C, Litvin TN, Levin LR, Buck J & Wu H (2005). Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol 12, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC & Hille B (2008). PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37, 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BA, Overholt JL & Prabhakar NR (2002). CO2 and pH independently modulate L‐type Ca2+ current in rabbit carotid body glomus cells. J Neurophysiol 88, 604–612. [DOI] [PubMed] [Google Scholar]
- Tresguerres M, Buck J & Levin LR (2010). Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Arch 460, 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turovsky E, Theparambil SM, Kasymov V, Deitmer JW, Del Arroyo AG, Ackland GL, Corneveaux JJ, Allen AN, Huentelman MJ, Kasparov S, Marina N & Gourine AV (2016). Mechanisms of CO2/H+ sensitivity of astrocytes. J Neurosci 36, 10750–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J & Bayliss DA (2013). TASK‐2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci 33, 16033–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A & Mulkey DK (2010). Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1‐Kir5.1‐like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104, 3042–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS & Mulkey DK (2012). Regulation of ventral surface CO2/H+‐sensitive neurons by purinergic signalling. J Physiol 590, 2137–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]


