Abstract
Key points
Diaphragmatic fatigue (DF) elicits a sympathetically mediated metaboreflex resulting in increased heart rate, blood pressure and limb vascular resistance.
Women may be more resistant to DF compared to men, and therefore it was hypothesised that women would experience an attenuated inspiratory muscle metaboreflex during inspiratory pressure‐threshold loading (PTL) performed to task failure.
At the time of PTL task failure, the severity of DF was not different between sexes; however, inspiratory muscle endurance time was significantly longer in women than in men.
For a given cumulative diaphragmatic force output, the severity of DF was less in women than in men.
Women exhibited a blunted cardiovascular response to inspiratory resistance (i.e. metaboreflex) that may have implications for exercise tolerance.
Abstract
Diaphragmatic fatigue (DF) elicits reflexive increases in sympathetic vasomotor outflow (i.e. metaboreflex). There is some evidence suggesting women may be more resistant to DF compared to men, and therefore may experience an attenuated inspiratory muscle metaboreflex. To this end, we sought to examine the cardiovascular response to inspiratory resistance in healthy young men (n = 9, age = 24 ± 3 years) and women (n = 9, age = 24 ± 3 years). Subjects performed isocapnic inspiratory pressure‐threshold loading (PTL, 60% maximal inspiratory mouth pressure) to task failure. Diaphragmatic fatigue was assessed by measuring transdiaphragmatic twitch pressure (P di,tw) using cervical magnetic stimulation. Heart rate (HR) and mean arterial pressure (MAP) were measured beat‐by‐beat throughout PTL via photoplethysmography, and low‐frequency systolic pressure (LFSBP; a surrogate for sympathetic vasomotor tone) calculated from arterial waveforms using power spectrum analysis. At PTL task failure, the degree of DF was similar between sexes (∼23% reduction in P di,tw; P = 0.33). However, time to task failure was significantly longer in women than in men (27 ± 11 vs. 16 ± 11 min, respectively; P = 0.02). Women exhibited less of an increase in HR (13 ± 8 vs. 19 ± 12 bpm; P = 0.02) and MAP (10 ± 8 vs. 14 ± 9 mmHg; P = 0.01), and significantly lower LFSBP (23 ± 11 vs. 34 ± 8 mmHg2; P = 0.04) during PTL compared to men. An attenuation of the inspiratory muscle metaboreflex may influence limb and respiratory muscle haemodynamics with implications for exercise performance.
Keywords: cervical magnetic stimulation, diaphragm, inspiratory muscle fatigue, metaboreflex, phrenic nerve, sex‐differences
Key points
Diaphragmatic fatigue (DF) elicits a sympathetically mediated metaboreflex resulting in increased heart rate, blood pressure and limb vascular resistance.
Women may be more resistant to DF compared to men, and therefore it was hypothesised that women would experience an attenuated inspiratory muscle metaboreflex during inspiratory pressure‐threshold loading (PTL) performed to task failure.
At the time of PTL task failure, the severity of DF was not different between sexes; however, inspiratory muscle endurance time was significantly longer in women than in men.
For a given cumulative diaphragmatic force output, the severity of DF was less in women than in men.
Women exhibited a blunted cardiovascular response to inspiratory resistance (i.e. metaboreflex) that may have implications for exercise tolerance.
Introduction
The conjecture that the diaphragm, the principle muscle of respiration, could begin to fail as a ventilatory pump due to fatigue of central or peripheral structures was first established in the early 20th Century by British physiologists Davies, Haldane and Priestley (Davies et al. 1919). Upon excessive respiratory resistance, the authors made the observation that breathing becomes shallow and more frequent, leading to inadequate alveolar ventilation and the clinical appearance of anoxaemia and hypercapnia. It was not until 60 years thereafter that a series of experiments demonstrated the relationship between respiratory muscle work/demand and capacity. It was theorised that when the work required to breathe exceeds the capacity of the respiratory muscles to perform that work, fatigue will ensue (Roussos & Macklem, 1977; Bellemare & Grassino, 1982a , b ).
Diaphragmatic fatigue (DF) is associated with a sympathetically mediated metaboreflex. As the diaphragm contracts more forcefully, mechanically (thinly myelinated group III) and metabolically (unmyelinated group IV) sensitive thin‐fibre phrenic afferents are stimulated (Hussain et al. 1990; Jammes & Balzamo, 1992; Haouzi et al. 1999; Hill, 2000; Rodman et al. 2003), causing a time‐dependent increase in muscle sympathetic nerve activity, heart rate, mean arterial blood pressure (St Croix et al. 2000) and limb vascular resistance, and a concurrent reduction in limb blood flow (Sheel et al. 2001). In the rodent, diaphragm arterioles are less sensitive to α1‐adrenergic vasoconstriction than the gastrocnemius muscle (Aaker & Laughlin, 2002). Theoretically, a global increase in sympathetic outflow would result in a preferential redistribution of blood flow away from the periphery and directed towards the respiratory muscles (Harms et al. 1997; Dominelli et al. 2017). Thus, a major consequence of DF may be considered cardiovascular in nature.
Much of the aforementioned research was conducted in animal models or men exclusively. Whilst studies investigating sex‐based differences in DF are few, there is a growing body of evidence to suggest there are important distinctions. For example, Guenette et al. (2010) found the magnitude of exercise‐induced DF to be significantly less in women than men. Recently, Smith et al. (2016) asked whether or not female resistance to DF was accompanied by an attenuation of the inspiratory muscle metaboreflex. Femoral artery blood flow ( L) and limb vascular resistance (LVR) were assessed during inspiratory resistive breathing at rest until mean arterial pressure (MAP) reached a plateau. The authors found less of an increase in MAP (7 vs. 11 mmHg) and LVR (17% vs. 47%), as well as less of a decrease in L (7% vs. 23%) in women compared to men. Crucially, Smith and colleagues (2016) did not assess DF, nor did they report any indices of respiratory muscle force output during the loading task. Hence, it is unclear if the differences observed were due to women experiencing less fatigue, performing less work, a combination of both, or other undetermined mechanisms.
To this end, the aims of the study were: (1) to determine if there are sex differences in DF when the work of breathing is increased at rest, and (2) to monitor the cardiovascular responses of men and women to imposed inspiratory resistance. We hypothesised that women would be more resistant to DF following inspiratory pressure‐threshold loading (PTL) to task failure than men. By virtue of this fatigue resistance, we further hypothesised that women would experience a blunted sympathetic response of the cardiovascular system, leading to an attenuation of the inspiratory muscle metaboreflex.
Methods
Ethical approval
Written informed consent was obtained from all subjects prior to testing. Experimental procedures were approved by the Clinical Research Ethics Board at the University of British Columbia (approval number: H15‐00801) and conformed to the Declaration of Helsinki for human experimentation, except for registration in a database.
Subjects
Eighteen subjects (nine men and nine women) were recruited for the study. Subjects were young (M, 24 ± 3 years; W, 24 ± 3 years), healthy (normal height and body mass, no history of smoking or presence of any known cardiovascular, neurological or pulmonary disease) and recreationally active. Women were tested during the early follicular phase of the menstrual cycle (determined by self‐report) to minimise any potential effect of circulating sex hormones on autonomic function.
Experimental design
Familiarisation sessions were conducted prior to the PTL trial. Subjects were afforded sufficient time to become accustomed to the breathing apparatus. Once subjects could achieve 2–3 min of uninterrupted PTL they were deemed adequately prepared. On this general familiarisation visit (which included a maximal incremental exercise test), anthropometrics were taken and basic spirometry (Spirolab II, Medical International Research; Rome, Italy) performed according to established guidelines (ATS/ERS, 2002). At least 24 h separated the familiarisation visit and PTL trial.
During the experimental trial, subjects completed a single bout of constant load isocapnic PTL to task failure. Diaphragm contractility was assessed before and immediately after the loading protocol by measuring transdiaphragmatic twitch pressure (P di,tw) in response to cervical magnetic stimulation. Force development across the muscle was estimated by calculating the difference in gastric (P ga) and oesophageal (P oes) pressure with the use of balloon‐tipped catheters (no. 47‐9005, Ackrad Laboratory; Cranford, NJ, USA). Topical anaesthetic (2% lidocaine hydrochloride, AstraZeneca; Mississauga, ON, Canada) was applied to the nasal and pharyngeal passages to minimise discomfort during catheter insertion. Catheters were directed intranasally and positioned in the stomach and lower one‐third of the oesophagus to measure P ga and P oes, respectively. Each catheter was connected to a piezoelectric pressure transducer (Raytech Instruments; Vancouver, BC, Canada), which was independently calibrated using a digital pressure manometer (2021P, Digitron; Torquay, UK). Subjects were asked to perform a Valsalva manoeuvre in order to evacuate air from the balloons. Oesophageal and gastric balloons were then filled with 1 and 2 ml of air, respectively. Correct placement was confirmed using the occlusion technique. Phrenic nerve activation was determined by inspection of the compound muscle action potential (CMAP, or M‐wave) using surface recordings of the diaphragm electromyogram (EMG). Continuous beat‐by‐beat arterial blood pressure was taken throughout PTL via finger pulse photoplethysmography for measurement of cardiovascular responses.
Inspiratory pressure‐threshold loading
Pressure‐threshold loading is an indirect means of evoking DF without the confounding effects of whole‐body exercise (e.g. acidosis). A bespoke PTL device was used for the experiment. The design was based on the weighted plunger model described by Nickerson and Keens (1982). In brief, subjects were required to generate an inspiratory pressure sufficient to overcome a threshold load in order to initiate inspiration‐expiration was unimpeded. Unlike flow‐resistive loading, PTL allows greater control of pressure/force production, as flow is pressure dependent until the threshold pressure is generated. Once pressure degrades below the threshold required to lift the weighted plunger, flow stops entirely.
Subjects were seated comfortably in the upright position. A customised two‐way non‐rebreathing valve was connected to the PTL device on the inspired side and a pneumotachograph (no. 3813, Hans Rudolph; Kansas City, MO, USA) for measurement of flow and volume on the expired side. Resistance was added to the weighted plunger such that inspiratory pressure was equal to 60% of predetermined maximal inspiratory mouth pressure (MIP). A minimum of five Mueller manoeuvres were performed from residual volume with the average of the three highest values defined as the MIP. A metronome was used to control breathing pattern. Breathing frequency (f b) was set to 15 breaths min−1 and inspiratory duty cycle (i.e. ratio of inspiratory contraction time to total respiratory cycle duration; T I/T TOT) at 0.7. Target inspiratory pressure was displayed on a computer screen to provide continuous visual feedback. Subjects were instructed to breathe diaphragmatically as natural breathing strategies during resistive inspirations can preferentially target accessory inspiratory muscles (Ramsook et al. 2016). The task was terminated when subjects failed to generate the target inspiratory pressure for four consecutive breaths or the second occasion of three missed consecutive breaths despite verbal encouragement.
Mouth pressure (P m) and end‐tidal partial pressure of CO2 () were measured via a side‐port in the mouthpiece, which was connected to a piezoelectric pressure transducer (Raytech Instruments) and CO2 gas analyser (no. 17630, VacuMed; Ventura, CA, USA), respectively. Manual adjustments to the inspired fraction of CO2 were made in the event of hypocapnia ( < 30 mmHg) using a 7% CO2 gas mixture. Prior to commencement of the PTL trial, 5–10 min of resting cardiorespiratory data were collected. Cardiovascular responses were measured throughout PTL and diaphragm contractile function was assessed before the trial began and immediately after task failure.
Cervical magnetic stimulation
A handheld 90 mm circular coil (P/N 9784‐00; peak magnetic field strength = 2.0 T, average inductance = 23.3 μH) powered by a magnetic stimulator (200‐2, Magstim; Whitland, UK) was used to stimulate the phrenic nerve roots at the level of the third–seventh cervical vertebrae, according to the original technique described by Similowski et al. (1989). The coil was held horizontal and centred over the midline of the cervical spine with the current flowing in a clockwise direction. Subjects were seated comfortably with the neck flexed. Stimuli were delivered at end‐expiration (determined by end‐expiratory P oes) with the glottis closed to preclude lung volume influence on diaphragm EMG and twitch pressure responses. The optimal site of stimulation was identified by gradually moving the coil along cervical vertebrae C3–C7 until the largest twitch pressure was observed. This location was marked and used for all subsequent stimuli.
Phrenic nerve excitability was determined by gradually increasing the intensity of the stimulator output from 60 to 100%. Three twitches were delivered at each intensity with a 30 s interval between successive stimuli to minimise the effect of twitch potentiation. A series of 1 Hz potentiated (preceded by ∼5 s maximal inspiratory effort, i.e. Mueller manoeuvre) twitches were performed at 100% of peak stimulator output at baseline and immediately after PTL to determine the presence of DF. Skeletal muscle fatigue may be defined as ‘a condition in which there is a loss in the capacity for developing force and/or velocity of a muscle, resulting from muscle activity under load and which is reversible by rest’ (NHLBI, 1990). Fatigue of the inspiratory muscles was assumed present if there was a ≥15% reduction in P di,tw relative to resting baseline levels. Twitches were excluded for the following reasons: (1) the twitch was not initiated at functional residual capacity, (2) if oesophageal peristalsis was evident at the time of the twitch, (3) if a cardiac artefact was superimposed upon a stimulus, and (4) if there was lack of diaphragmatic relaxation evidenced by noticeable diaphragm EMG. An example of the twitch potentiation protocol is provided in Fig. 1.
Figure 1. Twitch potentiation protocol.
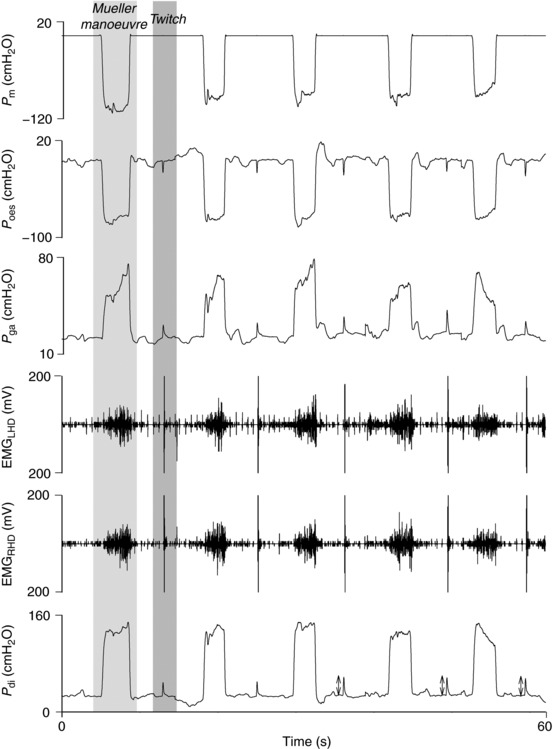
Pressure (mouth, oesophageal, gastric and transdiaphragmatic) and EMG (left and right hemi‐diaphragm) responses during maximal inspiratory manoeuvres separated by magnetic stimulation (1 Hz) of the phrenic nerve roots. First two twitches were not included in analysis (double‐sided arrows represent twitches that were used in twitch analysis). LHD, left hemi‐diaphragm; RHD, right hemi‐diaphragm.
Electromyography
To record electrical activity of the left and right costal diaphragm, self‐adhesive surface Ag/AgCl electrodes (H59P, Kendall; Mansfield, MA, USA) were placed on the chest wall in bipolar arrangement (∼2 cm apart) between the sixth and eighth intercostal spaces along the anterior‐axillary line. The ground electrode was placed on the acromion process of the scapula. The skin was lightly abraded and cleansed with alcohol to minimise electrical impedance. Electrodes were further secured using surgical tape and repositioned if necessary. Signals were amplified (×200) and band‐pass filtered (0.1 Hz to 3 kHz; P511 Series, Grass Instruments; Warwick, RI, USA). M‐waves were analysed for onset latency, duration, peak‐to‐peak amplitude and total rectified area (see below for analysis details).
Cardiovascular variables
Heart rate (HR) and arterial blood pressure were measured beat‐by‐beat using finger pulse photoplethysmography (Finometer, Finapres Medical Systems BV; Arnhem, Netherlands). An automated sphygmomanometer (BPM‐100, VSM MedTech Ltd; Vancouver, BC, Canada) was used to calibrate the Finometer during resting conditions. Physiological calibrations were made frequently during inspiratory loading (approximately once per minute). Power–frequency spectrum analysis of the raw blood pressure trace was used to calculate low‐frequency systolic blood pressure variability (LFSBP) over the PTL bout. The first 2 min of loading was not included. Low‐frequency oscillations (around 0.1 Hz) in arterial blood pressure (i.e. Meyer waves) are believed to reflect efferent sympathetic nerve activity indicative of changes in vasomotor tone (Malliani et al. 1991).
Data analysis
Transdiaphragmatic twitch pressure was determined as the change in pressure from stimulus onset to peak pressure. The ratio of twitch P oes to twitch P ga (P oes,tw/P ga,tw) was used to discriminate diaphragm and ribcage muscle fatigue. Cumulative force output of the diaphragm (i.e. pressure–time product) was calculated by integrating P di down to end‐inspiratory pressure over the periods of inspiratory flow for the entire duration of the PTL trial. Pressure was maintained in approximate square‐wave fashion during each inspiratory effort, such that P di was equal to mean P di ( di). The highest P di generated throughout loading or during Mueller manoeuvres was defined as P di,max. Diaphragm tension–time index (TTIdi) was calculated for each minute of PTL as the product of di/P di,max and T I/T TOT. M‐wave characteristics (latency, duration, amplitude and area) were calculated in MATLAB (R2015a, MathWorks; Natick, MA, USA) using a custom algorithm (Welch et al. 2017). Cardiorespiratory data were averaged over 30–60 s throughout threshold loading. Systolic peaks underwent fast Fourier transformation to produce a spectrum; the power of the systolic peaks was calculated by measuring the area under the spectra curve. Low‐frequency components of the resulting power spectrum ranged from 0.04 to 0.15 Hz. Flow, pressure and EMG signals were amplified, A/D converted (PowerLab 16SP, ADInstruments; Colorado Springs, CO, USA), sampled at 10 kHz and monitored online using LabChart data acquisition software (v8.1, ADInstruments).
Statistics
Descriptive characteristics were compared using an independent‐samples Student's t‐test. Repeated measures ANOVA was used to determine if the phrenic nerves were maximally activated by comparing P di,tw at all intensities of stimulation (60, 70, 80, 90, 95%) with the maximal stimulator output (100%). If there were significant main effects, pairwise comparisons were made using Tukey's post hoc test. On an individual basis, a plateau was considered present if the average P di,tw at submaximal and maximal stimulation intensities was separated by equal to or less than the within‐block coefficient of variation for all twitches. Diaphragmatic fatigue (P di,tw) was tested by independent samples t‐test post‐PTL. Sex‐based differences in cardiovascular responses and DF were assessed using a two‐way mixed factorial ANOVA. Time to task failure was compared using independent‐samples t‐tests. M‐wave characteristics were compared at baseline and post‐PTL by repeated measures ANOVA. For all statistical tests, normality was assessed qualitatively by visually inspecting descriptive statistics, histograms, and Q–Q plots and quantitatively using the Shapiro–Wilk test for small samples. An ANOVA on ranks was used in the event of failed normality. Significance was set at P < 0.05 for all statistical comparisons (SigmaPlot v12, Systat Software Inc.; San Jose, CA, USA). Results are expressed as mean ± SD, unless otherwise stated.
Results
Subjects
Subject characteristics, including anthropometrics and spirometry are presented in Table 1. Groups were of similar age and spirometry was within normal limits based upon predictive equations (Tan et al. 2011). Men had significantly greater maximal static inspiratory pressures than women (P = 0.011), but no differences were observed (P = 0.230) when expressed as percentages of predicted values (Black & Hyatt, 1969).
Table 1.
Subject characteristics
| Characteristic | Men (n = 9) | Women (n = 9) |
|---|---|---|
| Age (years) | 24 ± 3 | 24 ± 3 |
| Height (cm) | 181 ± 8 | 170 ± 8a |
| Mass (kg) | 76 ± 11 | 62 ± 9a |
| FVC (l) | 6.1 ± 1.1 | 4.4 ± 1.1a |
| % predicted | 113 ± 12 | 111 ± 17 |
| FEV1 (l s−1) | 4.8 ± 0.6 | 3.8 ± 0.9a |
| % predicted | 106 ± 7 | 108 ± 16 |
| FEV1/FVC | 80 ± 6 | 85 ± 6 |
| % predicted | 96 ± 8 | 101 ± 7 |
| PEF (l s−1) | 10.8 ± 1.1 | 8.1 ± 1.4a |
| % predicted | 106 ± 14 | 107 ± 18 |
| FEF25–75 (l s−1) | 4.3 ± 0.8 | 4.2 ± 1.4 |
| % predicted | 84 ± 15 | 97 ± 29 |
| MIP (cmH2O) | −138 ± 32 | −100 ± 23a |
| % predicted | 108 ± 22 | 123 ± 27 |
FEF25–75, forced expiratory flow at 25–75% of FVC; FEV1, forced expired volume in one‐second; FVC, forced vital capacity; PEF, peak expiratory flow; MIP, maximal inspiratory mouth pressure.
Significantly different from men (P < 0.05).
Diaphragmatic neuromuscular function
A plateau in P di,tw was observed at 80, 90 and 95% of peak stimulator output in both men and women (Fig. 2). Upon inspection of individual data, clear evidence of a plateau existed in 83% of all subjects (eight of nine men and seven of nine women). Coefficients of variation between subsequent stimuli were 6.8% for men and 6.6% for women. There was no change in M‐wave characteristics (spatial or temporal) pre–post threshold loading in men or women. Full twitch control measures (mechanical and electrical) are shown in Table 2.
Figure 2. Recruitment curve.
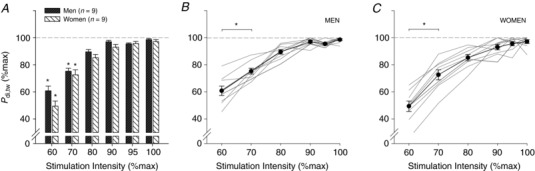
Bar graph depicting mean P di,tw ± SEM as a percentage of peak P di,tw in response to gradually increasing intensities of phrenic nerve stimulation. A plateau was observed at 80, 90 and 95% of peak power output in men and women. Group mean data is shown in A. Individual curves for men and women are displayed in B and C, respectively. *Significantly different from 100% (P < 0.05).
Table 2.
Twitch control measures
| Characteristic | Men (n = 9) | Women (n = 9) | ||
|---|---|---|---|---|
| Baseline | Post‐PTL | Baseline | Post‐PTL | |
| Twitch characteristic | ||||
| P di,tw (cmH2O) | 38.0 ± 8.2 | 28.9 ± 8.0a | 35.9 ± 7.2 | 27.8 ± 5.6a |
| CT (ms) | 133 ± 4 | 127 ± 5a | 139 ± 12 | 127 ± 5a |
| ½RT (ms) | 83 ± 11 | 73 ± 12a | 82 ± 13 | 74 ± 9a |
| M‐wave characteristic | ||||
| Right hemi‐diaphragm | ||||
| Amplitude (V) | 3.4 ± 0.8 | 3.5 ± 0.1 | 4.1 ± 0.5 | 4.3 ± 0.3 |
| Area (V ms) | 34.4 ± 6.0 | 34.3 ± 6.8 | 36.9 ± 5.2 | 37.4 ± 5.4 |
| Latency (ms) | 4.5 ± 0.5 | 4.6 ± 0.5 | 4.2 ± 0.3 | 4.4 ± 0.3 |
| Duration (ms) | 50.8 ± 1.0 | 49.1 ± 6.1 | 48.4 ± 0.8 | 39.9 ± 3.3 |
| Left hemi‐diaphragm | ||||
| Amplitude (V) | 4.2 ± 0.8 | 4.4 ± 1.4 | 4.2 ± 0.8 | 4.6 ± 1.0 |
| Area (V ms) | 38.9 ± 9.4 | 39.9 ± 8.7 | 37.5 ± 6.4 | 38.2 ± 9.7 |
| Latency (ms) | 5.1 ± 0.3 | 5.1 ± 0.4 | 4.4 ± 0.1 | 4.3 ± 0.2 |
| Duration (ms) | 46.6 ± 8.2 | 47.1 ± 3.8 | 53.4 ± 0.9 | 49.5 ± 3.2 |
Mechanical and electrical responses of the diaphragm to CMS before and immediately after inspiratory PTL to task failure. Results are given as mean ± SD. ½RT, half‐relaxation time; CT, contraction time; P di,tw, transdiaphragmatic twitch pressure.
Significantly different from baseline.
Baseline twitch pressures were 38.0 ± 8.2 and 35.9 ± 7.2 cmH2O for men and women, respectively. At task failure, twitch pressures were significantly reduced in both sexes and to a similar extent (M, 28.9 ± 8.0 cmH2O (−24.3%); W, 27.8 ± 6.3 cmH2O (−22.8%); P = 0.328; see Fig. 3). There were no sex differences in ΔP oes,tw/P ga,tw post‐PTL compared to baseline (M, −0.63 ± 0.23; W, 0.02 ± 0.36; P = 0.355). Predominant diaphragm fatigue (increase ΔP oes,tw/P ga,tw) was found in four men and six women (Fig. 4).
Figure 3. Time to task failure and severity of diaphragmatic fatigue.
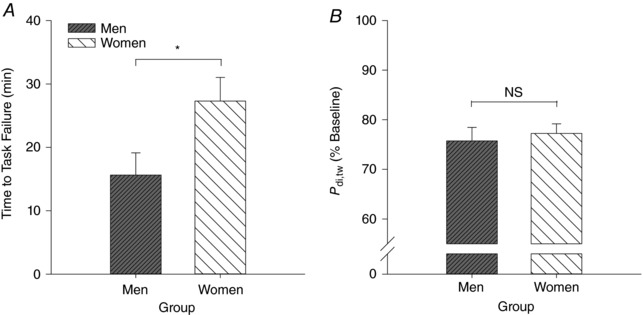
Bar graphs demonstrating (A) time to task failure (in minutes) and (B) severity of diaphragmatic fatigue (percentage change from baseline). NS, not significant (P > 0.05). *Significantly different from men (P < 0.05).
Figure 4. Relative contributions of the diaphragm and ribcage muscles to changes in transdiaphragmatic twitch pressure.
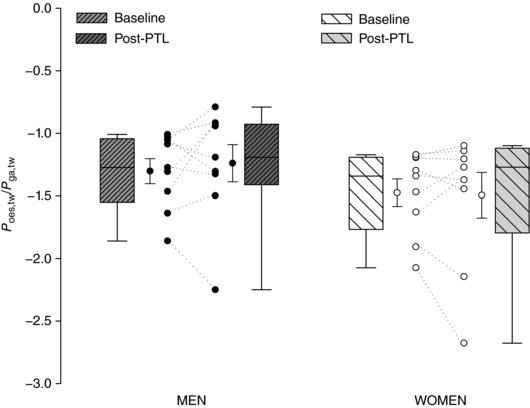
Box and whisker plot showing the ratio of P oes,tw to P ga,tw in men and women at baseline and pressure‐threshold loading task failure. An increase in P oes,tw/P ga,tw following PTL indicates predominant diaphragmatic fatigue. No sex differences were observed in ΔP oes,tw/P ga,tw (P > 0.05).
Time to task failure was significantly longer (27.3 ± 11.2 vs. 15.6 ± 10.5 min; P = 0.018) and consequently, the rate of fatigue development slower in women than men (M, −0.69 ± 0.30 cmH2O min−1; W, −0.34 ± 0.16 cmH2O min−1; P = 0.009). A main effect of time (P < 0.001) and sex (P = 0.001) was found for cumulative diaphragm force output (M, 32,288 ± 20,752 cmH2O s; W, 46,805 ± 16,723 cmH2O s; Fig. 5). Of note, at the point of task failure, two subjects (one male and one female) did not reach our definition of fatigue (i.e. ≥15% reduction in P di,tw post‐PTL).
Figure 5. Pressure–time product and cumulative diaphragmatic work.
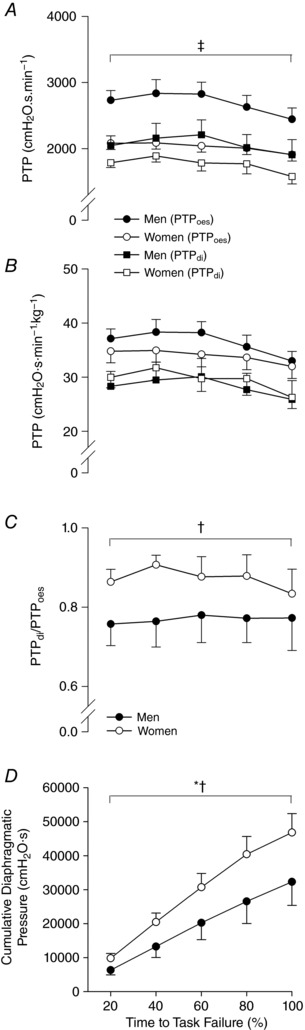
A and B, PTPoes and PTPdi in absolute units of pressure and time. C, ratio of PTPdi to PTPoes during threshold breathing. Men generated significantly greater absolute pressure; however, PTPdi/PTPoes was greater in women than men throughout. *Main effect of time; †main effect of sex; ‡main effect of sex on PTPoes and PTPdi.
Respiratory responses to PTL
The PTL protocol was identical for men and women, equating to a target TTI of 0.42. Parameters used to control breathing pattern were not different between sexes (P > 0.05) and were well maintained throughout the trial; this includes f b, T I/T TOT, target P m and TTIdi (Table 3). In the final minute, pressure generation began to decay, resulting in a small decrease in TTIdi. Oesophageal (PTPoes) and transdiaphragmatic (PTPdi) pressure–time products are shown in Fig. 5. Diaphragm contribution to total respiratory muscle pressure production (i.e. PTPdi/PTPoes) did not change during the loading protocol in men or women (P > 0.05); nevertheless, PTPdi/PTPoes was greater in women throughout (P = 0.006). There were no sex differences in PTPdi relative to body mass (P = 0.675).
Table 3.
Physiological responses during pressure‐threshold loading to task failure in men and women
| Variable | Time to task failure (min) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 | 2 | 3 | 4 | 5 | Final | |
| Men | |||||||
| f b (breaths min−1) | 10 ± 3 | 15 ± 0.1a | 15 ± 0.1a | 15 ± 0.4a | 15 ± 0.2a | 15 ± 0.8a | 14 ± 2a |
| V T (l) | 1.3 ± 0.3 | 1.6 ± 0.7 | 1.3 ± 0.4 | 1.5 ± 0.6 | 1.4 ± 0.6 | 1.5 ± 0.6 | 1.7 ± 0.6 |
| V T/T I (l s−1) | 0.69 ± 0.23 | 0.48 ± 0.20 | 0.42 ± 0.12 | 0.46 ± 0.20 | 0.45 ± 0.20 | 0.45 ± 0.19 | 0.52 ± 0.21 |
| E (l min−1) | 14 ± 4 | 24 ± 9a | 20 ± 6a | 23 ± 9a | 22 ± 8a | 22 ± 9a | 24 ± 9a |
| (mmHg) | 38 ± 5 | 32 ± 4a | 35 ± 3 | 37 ± 5b | 37 ± 4b | 38 ± 4b | 38 ± 3b |
| HR (bpm) | 59 ± 8 | 72 ± 8a | 71 ± 7a | 72 ± 9a | 73 ± 15a | 74 ± 11a | 79 ± 9a , b |
| MAP (mmHg) | 90 ± 5 | 93 ± 13 | 97 ± 14 | 99 ± 14 | 100 ± 18 | 101 ± 16a | 104 ± 12a , b |
| P m (cmH2O) | −1.1 ± 0.4 | −82 ± 16a | −80 ± 16a | −81 ± 18a | −81 ± 17a | −82 ± 17a | −81 ± 17a |
| di/P di,max (%) | 8 ± 3 | 58 ± 6a | 56 ± 9a | 59 ± 10a | 60 ± 7a | 59 ± 6a | 53 ± 9a |
| T I/T TOT | 0.34 ± 0.03 | 0.72 ± 0.02a | 0.72 ± 0.01a | 0.69 ± 0.02a | 0.70 ± 0.01a | 0.70 ± 0.02a | 0.70 ± 0.03a |
| TTIdi | 0.03 ± 0.01 | 0.42 ± 0.05a | 0.40 ± 0.06a | 0.41 ± 0.07a | 0.42 ± 0.05a | 0.41 ± 0.05a | 0.37 ± 0.07a , b |
| Women | |||||||
| f b (breaths min−1) | 14 ± 2c | 15 ± 0.1 | 15 ± 0.5 | 15 ± 0.6 | 15 ± 0.2 | 15 ± 0.0 | 15 ± 1.0 |
| V T (l) | 0.8 ± 0.3c | 1.2 ± 0.4a | 1.0 ± 0.3a | 1.0 ± 0.3a | 1.0 ± 0.3a | 1.0 ± 0.2a | 0.7 ± 0.2b , c |
| V T/T I (l s−1) | 0.50 ± 0.15 | 0.41 ± 0.18 | 0.36 ± 0.12 | 0.41 ± 0.16 | 0.38 ± 0.10 | 0.39 ± 0.09 | 0.31 ± 0.14b , c |
| E (l min−1) | 12 ± 5 | 18 ± 6 | 14 ± 4c | 15 ± 5c | 14 ± 4c | 15 ± 3c | 11 ± 3b , c |
| (mmHg) | 38 ± 3 | 32 ± 5a | 33 ± 3a | 35 ± 4a | 36 ± 3 | 36 ± 2b | 41 ± 1b |
| HR (bpm) | 65 ± 13 | 73 ± 15 | 75 ± 14 | 75 ± 15a | 75 ± 16a | 75 ± 16a | 78 ± 14a |
| MAP (mmHg) | 86 ± 5c | 87 ± 9 | 89 ± 6 | 90 ± 4 | 91 ± 4a | 92 ± 5a | 96 ± 7a |
| P m (cmH2O) | −0.9 ± 0.2 | −63 ± 10a , c | −64 ± 9a , c | −65 ± 10a , c | −64 ± 9a , c | −64 ± 10a , c | −66 ± 16a , c |
| di/P di,max (%) | 6 ± 3 | 54 ± 7a | 53 ± 9a | 54 ± 7a | 55 ± 8a | 57 ± 5a | 52 ± 10a |
| T I/T TOT | 0.37 ± 0.05 | 0.71 ± 0.02a | 0.68 ± 0.05a | 0.68 ± 0.03a | 0.69 ± 0.01a | 0.68 ± 0.04a | 0.67 ± 0.04a |
| TTIdi | 0.02 ± 0.01 | 0.38 ± 0.05a | 0.36 ± 0.05a | 0.36 ± 0.04a | 0.38 ± 0.05a | 0.39 ± 0.04a | 0.34 ± 0.06a |
Breathing pattern, heart rate, mean arterial pressure and respiratory muscle pressure generation during PTL. Values are presented as mean ± SD. f b, breathing frequency; HR, heart rate; MAP, mean arterial pressure; P di, transdiaphragmatic pressure; , end‐tidal partial pressure of CO2; P m, mouth pressure; E, minute ventilation; V T, tidal volume; V T/T I, mean inspiratory flow rate; T I/T TOT, inspiratory duty cycle; TTIdi, tension–time index of the diaphragm.
Significantly different from rest.
significantly different from first minute.
significantly different from men (P < 0.05).
Cardiovascular responses to PTL
An illustration of raw cardiorespiratory traces collected during PTL are provided in Fig. 6. There was a time‐dependent increase in HR and MAP until the final minute of the trial, whereby small decreases in both variables were observed (Fig. 7). A main effect of time was found for all variables, including HR (P < 0.001), SBP (P < 0.001), DBP (P < 0.001) and MAP (P < 0.001). At the time of task failure, HR (M, +19 ± 12 bpm; W, +13 ± 8 bpm), SBP (M, +22 ± 12 mmHg; W, +16 ± 17 mmHg), DBP (M, +10 ± 8 mmHg; W, +7 ± 4 mmHg) and MAP (M, +14 ± 9 mmHg; W, +10 ± 8 mmHg) were well above resting levels. A main effect of sex was found for HR (P = 0.009), SBP (P = 0.019) and MAP (P = 0.024), but not DBP (P = 0.095). No significant interactions existed for any dependent variable (P > 0.05). Post hoc tests did not reveal any statistically significant differences in HR, SBP or MAP at 20 (P = 0.65, 0.49 and 0.57, respectively), 40 (P = 0.19, 0.18 and 0.18, respectively), 60 (P = 0.10, 0.14 and 0.14, respectively), 80 (P = 0.08, 0.14 and 0.14, respectively) or 100% (P = 0.18, 0.42 and 0.46, respectively) of time to task failure. Finally, LFSBP was significantly lower in women compared to men (23.2 ± 11.1 vs. 33.9 ± 7.7 mmHg2; P = 0.038).
Figure 6. Raw traces of cardiorespiratory variables from one representative subject during pressure‐threshold loading.
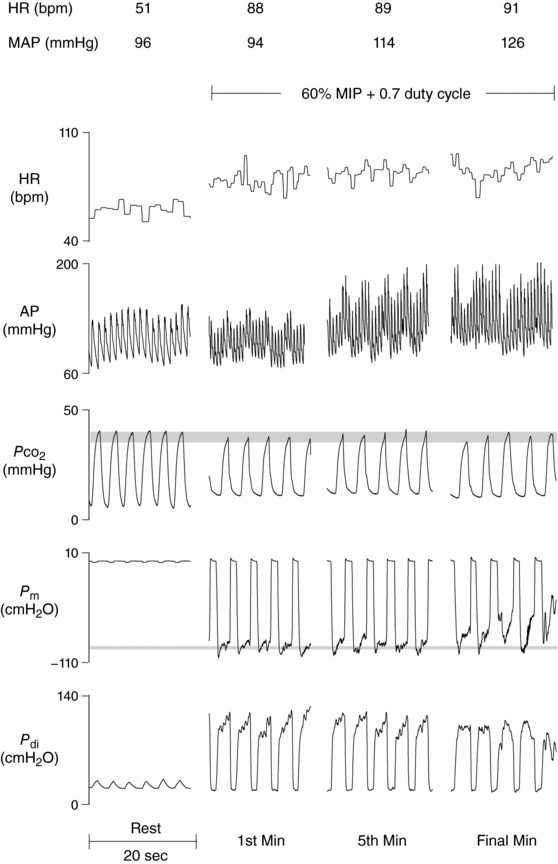
A time‐dependent increase in HR and MAP was observed. Isocapnia was maintained throughout as shown by end‐tidal Approximate square‐wave pressure generation was achieved until the final minute of loading. AP, arterial pressure; HR, heart rate; , partial pressure of CO2; P di, transdiaphragmatic pressure; P m, mouth pressure.
Figure 7. Cardiovascular responses to pressure‐threshold loading.
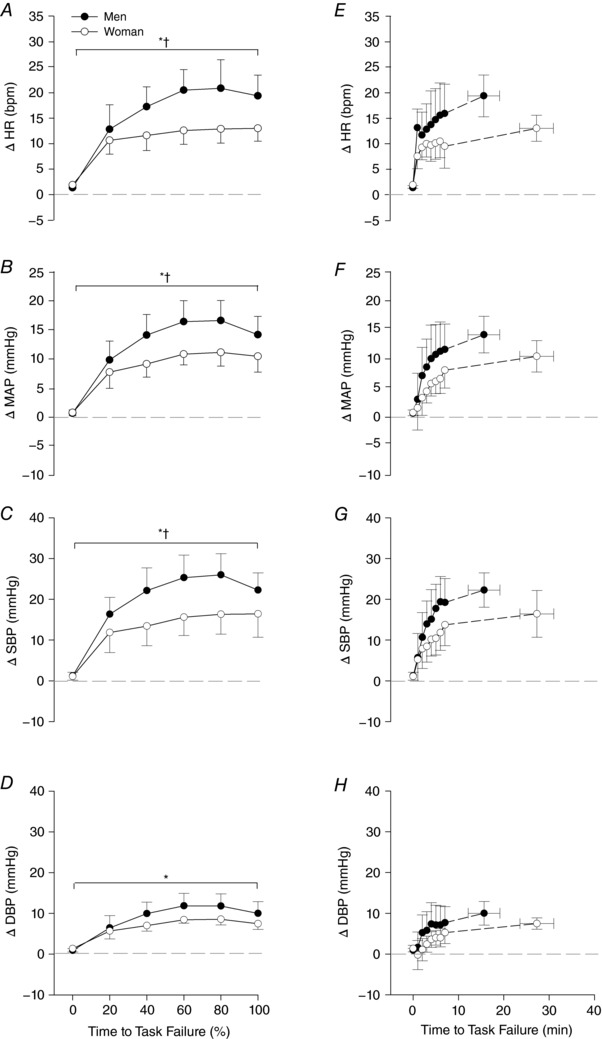
Heart rate and blood pressure responses are reported as group mean ± SEM. A–D, relative time (% of time to task failure) during a bout of inspiratory pressure‐threshold loading to task failure; E–H, the corresponding results in absolute time (minutes). A main effect of time was found for all variables and a main effect of sex for HR, SBP and MAP. Post hoc tests revealed no significant differences between men and women for any dependent variable at any time point. DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure. *Main effect of time; †main effect of sex.
Discussion
Main findings
The main findings of the present study are three‐fold. Firstly, inspiratory muscle endurance time was significantly longer in women relative to men. Secondly, the severity of PTL‐induced DF did not differ between sexes at the point of task failure. Finally, notwithstanding a similar degree of DF, women experienced a blunted cardiovascular response to inspiratory resistance, emphasised by less of an increase in HR and MAP, and significantly lower LFSBP. This is the first study to demonstrate an attenuation of the inspiratory muscle metaboreflex in women subjected to the same reflex‐evoking stimulus as men (i.e. degree of DF). We interpret our collective findings to mean that sex‐based differences in diaphragmatic function have important implications concerning sympathetic vasomotor outflow that may affect limb and respiratory muscle haemodynamics.
Task failure
On average, women were able to perform inspiratory PTL for 75% longer than men before reaching task failure. Only two previous studies have compared inspiratory muscle endurance time between men and women. Using flow‐resistive loads (70% MIP, 18 breaths min−1, 0.5 duty cycle; calculated TTI = 0.35), Gonzales and Scheuermann (2006) found no significant sex‐based difference in time to task failure despite women on average lasting 13% longer (M, 12.3 min; W, 14.1 min). Similarly, Shimizu et al. (2017) did not observe a sex‐based difference in respiratory muscle endurance time (M, 12.1 min; W, 11.6 min) with an incremental hyperpnoea challenge (30% of maximal voluntary ventilation for 3 min plus 10% every 3 min until task failure). These observations are in contrast to findings presented in the current study. While the incremental protocol employed by Shimizu and colleagues likely nullified any differences between groups in endurance time, it is unclear why our results vary from that of Gonzalez and Scheuermann. Interestingly, the authors point out that the absence of a sex‐based difference in time to task failure was surprising given what is known about sex differences in muscle fatigue and task specificity.
During intermittent isometric contractions (similar to the present study) of various muscle groups (including elbow and finger flexors, thumb adductors, and ankle dorsiflexors), women on average, are able to sustain the task for 33% longer than men (Hunter, 2009), regardless of the absolute strength exerted by the muscle (Hunter et al. 2004). Our results are the first to demonstrate a sex‐based difference in endurance time of the human inspiratory muscles. However, task failure has been shown to occur during inspiratory resistive breathing without the presence of DF (McKenzie et al. 1997).
Two of our subjects did not display peripheral DF at the time of task failure. Hence, alternative mechanisms that have been proposed include hypo/hypercapnia, central fatigue, and/or breathing discomfort. None of our subjects became hypercapnic during loading and was maintained within 3–4 mmHg of resting values. Dyspnoea, on the other hand, was not measured in the current study. Women breathed with a higher diaphragmatic contribution to total inspiratory muscle force output (i.e. the ratio of PTPdi to PTPoes, see Fig. 5) in relation to men. Thus, it is possible that by generating pressure primarily through diaphragm activation and not synergist inspiratory muscles, the disassociation between mechanical input and central respiratory motor output was minimised. As a result, women were able to sustain the task for longer without experiencing extreme dyspnoea. This notion remains speculative and warrants further enquiry. Although beyond the scope of the present investigation, it is posited that the relative influence of central and peripheral factors to task failure is contingent upon exercise intensity, as denoted by the power–duration relationship (Burnley & Jones, 2018). Anticipatory feedforward regulation of skeletal muscle recruitment to ensure organism homeostasis (St Clair Gibson & Noakes, 2004), attainment of a ‘sensory tolerance limit’ modulated by peripheral feedback (Amann et al. 2011), and psychobiological constructs such as perception of effort and potential motivation (Marcora & Staiano, 2010) are all believed to play a role in the aetiology of task failure.
Diaphragmatic fatigue
At the cessation of PTL, the magnitude of DF in men and women was within 2% of each other. Thus, despite similar reductions in diaphragm force output, women were able to sustain the task for a longer duration. As a result, women produced greater cumulative diaphragmatic pressure during the loading task. Depending on the time course of fatigue development (linear or exponential decay), it is possible that the rate of fatigue development was slower in women than in men. In this regard, Laghi et al. (1996) found the diaphragm to progressively fatigue during mechanical loading in healthy young men. To our knowledge, only one study has previously compared sex‐based differences in DF during a resistive breathing protocol at rest. In accordance with our results, Gonzales and Scheuermann (2006) did not observe any differences between men and women at the time of task failure in the severity of DF (M, 16.9%; W, 14.8%), albeit using volitional techniques (i.e. MIP). Moreover, the authors found that women fatigued at slower rates compared to men (−1.5 vs. −2.9 cmH2O min−1). We speculate that the reason for parallel reductions in diaphragm contractility between sexes in the current study was because the task was terminated before fatigue reached a so‐called ‘critical threshold’ (Amann et al. 2011).
Fatigue may occur anywhere along the brain–muscle pathway. Stimulation of the phrenic nerve roots using single 1 Hz stimuli allows for the assessment of low‐frequency peripheral DF. A reduction in muscle force output without any concurrent alteration in the M‐wave configuration implies disruption to the excitation–contraction coupling mechanism. The accumulation of metabolites within skeletal muscle inhibits the binding of calcium with troponin–tropomyosin, preventing the formation of actin–myosin crossbridges (Edwards et al. 1977). End‐organ fatigue (i.e. at the level of the diaphragm) was present in all but two subjects. Although we must reject our first hypothesis, that the magnitude of fatigue was not less in women compared to men, the rate of fatigue development was slower in women. Intracellular changes within the muscle are not independent of the central nervous system in vivo (Kent‐Braun et al. 2012). The absence of central measures in the present study prohibits our ability to comment on sex differences in neural activation. Several mechanisms have been put forth to explain female resistance to skeletal muscle fatigue, in particular, differences in muscle morphology, substrate utilisation and sex hormones (Hicks et al. 2001; Hunter, 2014).
Women have less muscle mass than men and this alone may provide some insight into the greater fatigue resistance observed in women. When performing work at the same relative intensity, smaller muscle mass translates directly into lower absolute force generation. Consequently, there is less O2 demand, a decrease in mechanical compression of the local vasculature and less intramuscular occlusion of blood flow, which averts the accumulation of metabolites that interfere with contractile machinery (Russ & Kent‐Braun, 2003). The diaphragm itself has a remarkable ability to maintain perfusion in the face of high inspiratory loads. In an animal model, blood flow to the diaphragm increased 26‐fold with a 15‐fold increase in the work of breathing (Robertson et al. 1977). However, blood flow to the diaphragm is impeded once the critical TTI of 0.20 is reached (Bellemare et al. 1983). It has been shown in anaesthetised dogs that combining a high diaphragm force output with a prolonged inspiratory duty cycle compromises diaphragmatic perfusion, rendering the muscle ischaemic (Buchler et al. 1985; Bark et al. 1987). Conversely, increasing phrenic artery blood flow partially reverses DF in situ (Supinski et al. 1988). The relationship between muscle metabolic demand and delivery of blood‐borne substrates is clearly a major determinant of DF. In our study, TTIdi was not different between men and women at any point during PTL and far exceeded the threshold for DF to occur, ranging between 0.34 and 0.42. Nonetheless, the TTI should be considered a conceptual framework rather than a precise numerical instrument. The protocol used in the present study (targeted TTI of 0.42) may have provided a disproportionate stimulus for fatigue development in men and women, and therefore, endurance time was not equal.
With regard to muscle morphological differences, there is evidence (muscle biopsy of vastus lateralis and deltoid) to suggest that women possess a greater percentage of slow‐twitch type I muscle fibres than men (Nygard, 1981). Type I fibres have a slower rate of contraction, and thus, a slower rate of metabolism. As a result, type I fibres fatigue at slower rates compared to glycolytic fast‐twitch type II fibres. Additionally, type I muscle fibres possess a greater fraction of vasodilatory β2‐adrenergic receptors, thereby improving O2 transport kinetics. Approximately 75% of diaphragm muscle fibres are oxidative (Lieberman et al. 1973). It is not known if the histochemical composition of costal or crural diaphragm regions differs between men and women. Anecdotally, in the present study, contraction time (time difference between stimulus onset and peak pressure) was slower for women than men (see Table 2), which may indicate a higher proportion of slow‐twitch muscle fibres.
Inspiratory muscle metaboreflex
Fatiguing diaphragmatic contractions elicits increased efferent sympathetic activity, producing widespread vasoconstrictor influences via supraspinal pathways (Dempsey et al. 2006). Inspiratory PTL was used to evoke reflex effects secondary to DF. Women exhibited an attenuated cardiovascular response to PTL. The changes in HR (+13 vs. +19 bpm), SBP (+16 vs. +22 mmHg) and MAP (+10 vs. +14 mmHg) were significantly lower in women versus men. Furthermore, women had lower LFSBP (23 vs. 34 mmHg2), suggesting repressed sympathetic vascular transduction and improved conductance. Recent work indicates that the interaction between pressor and carotid baroreflex control of HR and MAP may be additive and independent of central command (Hureau et al. 2018). It remains to be elucidated if attenuation of group III/IV afferent feedback compromises cardiovascular adjustments during exercise differently in men and women.
Two previous investigations have examined sex‐based differences in the cardiovascular consequences to elevated inspiratory muscle work. Smith et al. (2016) found less of an increase in HR (4 vs. 14 bpm), MAP (7.3 vs. 11.1 mmHg) and LVR (17.7% vs. 47.9%), as well as less of a decrease in L (7.5% vs. 23.3%) in women compared to men during inspiratory resistive loading (65% MIP, 20 breaths min−1, 0.5 duty cycle; calculated TTI = 0.33). Similarly, Shimizu et al. (2017) found the change in MAP (14.9 vs. 32.1 mmHg) to be lower in women during incremental isocapnic voluntary hyperpnoea. While both studies corroborate our findings, there are fundamental differences in experimental design that should be acknowledged. Firstly, DF was not assessed in either study. Furthermore, no information regarding inspiratory muscle pressure generation (including P m, P di or TTI) was provided. Inferences made are based on the premise that men and women were subjected to the same reflex‐evoking stimulus. However, without any indication of the amount of work done by the diaphragm during inspiratory loading, conclusions drawn are limited. Thus, we believe that our findings add much needed mechanistic insight into sex‐differences in DF and the associated metaboreflex. In combination with the findings of Smith and Shimizu, it appears that women develop DF at slower rates than men and experience an attenuated cardiovascular response to inspiratory resistance. This is highlighted by less of an increase in MAP and LVR, and less of a reduction in L. Sex differences in autonomic control of the circulation may assist in explaining our observations.
According to Darcy's law, MAP is equal to the product of cardiac output () and total peripheral resistance (TPR). The relationship between , TPR and MAP is dependent upon age and sex (Charkoudian et al. 2005; Hart et al. 2009, 2011, 2012). For example, the positive relationship between muscle sympathetic nerve activity (MSNA) and TPR that exists in young men does not exist in pre‐menopausal women due to the sympatho‐inhibitory effects of oestrogen upon the central nervous system and peripheral vasculature (Joyner et al. 2015; Barnes, 2017). Increased quantity and sensitivity of β2‐adrenergic receptors, heightened expression of endothelial nitric oxide synthase and enhanced functional sympatholysis promote vasodilation, which offsets α‐adrenergic vasoconstriction (Hart et al. 2011; Just & DeLorey, 2017). The attenuated blood pressure response to DF shown by women in the present study may be attributed to the physiological mechanisms outlined above, which not only work to counter vasoconstrictor influences, but preserve muscle oxygenation, and thus delay the development of fatigue.
Technical considerations
A primary limitation of our work is the use of HR and MAP to imply changes in sympathetic nerve activity. Importantly, MAP is not correlated with MSNA in healthy young men or women (Narkiewicz et al. 2005; Hart et al. 2009). Therefore, we cannot be certain that a blunted blood pressure response in women accurately reflects changes in MSNA. A clear demonstration of the relationship between DF and MSNA is needed. Furthermore, we did not record resting limb or respiratory muscle haemodynamics during threshold loading. Sonography and near‐infrared spectroscopy coupled with fluorescent tracer dye are highly informative; however, with the addition of cervical magnetic stimulation and invasive balloon catheters, we chose not to utilise the former techniques. Instead, power spectrum analysis of arterial waveforms (i.e. LFSBP) provided a non‐invasive means of gaining insight into the peripheral vasculature.
We found that women generated inspiratory pressure with a greater relative contribution of the diaphragm than did men. Respiratory inductance plethysmography would have allowed a more comprehensive assessment of diaphragmatic and ribcage muscle action on lung volume displacement. In addition, prescribing a workload based upon TTI permits comparisons with previous literature, but does withhold certain limitations (e.g. f b is not factored into calculations and P di timing intervals may be preferred over inspiratory flow) (Barnard & Levine, 1986). An incremental protocol to establish peak inspiratory muscle strength may also reduce variability between groups in target workload.
In an attempt to mitigate the effect of sex hormones of autonomic function, women were tested during the early follicular phase of the menstrual cycle, when sex hormone concentrations reach a nadir. However, self‐reported menstrual history poorly predicts circulating hormone concentrations, and therefore, it is difficult to predict the influence of this variable upon results (MacNutt et al. 2015). Axonal hyperpolorisation may have led to a depression in phrenic nerve excitability due to repeated near‐maximal contractions. A plateau in P di,tw was observed in men and women at 80% of the maximal stimulator capacity, demonstrating effective and supramaximal bilateral phrenic nerve stimulation. Thus, the potential effect of axonal hypoexcitability was minimised if not entirely removed. Lastly, we provide indirect evidence that when matched for absolute diaphragm force output and/or duration of inspiratory loading performed at equal relative intensities, the magnitude of DF is less in women than men. These postulates require further scrutiny. Differences in absolute force may affect postganglionic sympathetic discharge patterns, including differential recruitment of low‐ (rate coding) and high‐threshold (population coding) axons, which modulate neurotransmitter release and the post‐neurovascular junction response (Badrov et al. 2016).
Conclusions
In conclusion, men and women experience time‐dependent sympathoexcitation in response to fatiguing contractions of the diaphragm. These cardiorespiratory interactions are attenuated in women, despite the absence of a sex‐based difference in DF at the time of PTL task failure. Women are able to breathe against externally applied inspiratory resistance for significantly longer and generate greater cumulative diaphragmatic pressure than do men. Thus, our results indicate that the female diaphragm is highly fatigue resistant, leading to blunted increases in HR, MAP and LFSBP attendant on high levels of inspiratory muscle work. An attenuation of the inspiratory metaboreflex may influence limb and respiratory muscle haemodynamics with implications for exercise performance.
Additional information
Competing interests
None
Author contributions
J.F.W. and A.W.S. conceptualised and designed the work. J.F.W. and B.A. collected and analysed data. J.F.W., B.A., J.A.G., C.R.W. and A.W.S. contributed to interpretation of data and revisions of intellectual content. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Biography
Joseph F. Welch is a PhD candidate in the School of Kinesiology at the University of British Columbia (Canada), working under the supervision of William Sheel. He completed a BSc Hons degree and Master of Research degree in Sport and Exercise at the University of Derby (UK). His current research broadly examines how sex‐based differences in diaphragmatic fatigue affects sympathetic cardiovascular control and the integrated whole‐body response to exercise.

Edited by: Janet Taylor & Steven Segal
Linked articles This article is highlighted by a Perspective by Noakes. To read this Perspective, visit https://doi.org/10.1113/JP276411.
This is an Editor's Choice article from the 1 September 2018 issue.
References
- Aaker A & Laughlin MH (2002). Diaphragm arterioles are less responsive to α1‐adrenergic constriction than gastrocnemius arterioles. J Appl Physiol 92, 1808–1816. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2011). Implications of group III and IV muscle afferents for high‐intensity endurance exercise performance in humans. J Physiol 589, 5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATS/ERS (2002). ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med 166, 518–624. [DOI] [PubMed] [Google Scholar]
- Badrov MB, Olver TD & Shoemaker JK (2016). Central vs. peripheral determinants of sympathetic neural recruitment: insights from static handgrip exercise and postexercise circulatory occlusion. Am J Physiol Regul Integr Comp Physiol 311, R1013–R1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark H, Supinski GS, LaManna JC & Kelson SG (1987). Relationship of changes in diaphragmatic muscle blood flow to muscle contractile activity. J Appl Physiol 62, 291–299. [DOI] [PubMed] [Google Scholar]
- Barnard PA & Levine S (1986). Critique on application of diaphragmatic tension‐time index to spontaneously breathing humans. J Appl Physiol 60, 1067–1072. [DOI] [PubMed] [Google Scholar]
- Barnes JN ( 2017). Sex‐specific factors regulating pressure and flow. Exp Physiol 102, 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare F & Grassino A (1982a). Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol Respir Environ Exerc Physiol 53, 1190–1195. [DOI] [PubMed] [Google Scholar]
- Bellemare F & Grassino A (1982b). Evaluation of human diaphragm fatigue. J Appl Physiol Respir Environ Exerc Physiol 53, 1196–1206. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Wight D, Lavigne CM & Grassino A (1983). Effect of tension and timing of contraction on the blood flow of the diaphragm. J Appl Physiol Respir Environ Exerc Physiol 54, 1597–1606. [DOI] [PubMed] [Google Scholar]
- Black LF & Hyatt RE (1969). Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 99, 696–702. [DOI] [PubMed] [Google Scholar]
- Buchler B, Magder S & Roussos C (1985). Effects of contraction frequency and duty cycle on diaphragmatic blood flow. J Appl Physiol 58, 265–273. [DOI] [PubMed] [Google Scholar]
- Burnley M & Jones AM (2018). Power‐duration relationship: physiology, fatigue, and the limits of human performance. Eur J Sport Sci 18, 1–12. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM & Wallin BG (2005). Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HW, Haldane JS & Priestley JG (1919). The response to respiratory resistance. J Physiol 53, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J & Smith C (2006). Consequences of exercise‐induced respiratory muscle work. Respir Physiol Neurobiol 151, 242–250. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Archiza B, Ramsook AH, Mitchell RA, Peters CM, Molgat‐Seon Y, Henderson WR, Koehle MS, Boushel R & Sheel AW (2017). Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102, 1535–1547. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA & Merton PA (1977). Fatigue of long duration in human skeletal muscle after exercise. J Physiol 272, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales JU & Scheuermann BW (2006). Gender differences in the fatigability of the inspiratory muscles. Med Sci Sports Exerc 38, 472–479. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Romer LM, Querido JS, Chua R, Eves ND, Road JD, McKenzie DC & Sheel AW (2010). Sex differences in exercise‐induced diaphragmatic fatigue in endurance‐trained athletes. J Appl Physiol 109, 35–46. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Hill JM, Lewis BK & Kaufman MP (1999). Responses of group III and IV muscle afferents to distension of the peripheral vascular bed. J Appl Physiol 87, 545–553. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB & Dempsey JA (1997). Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82, 1573–1583. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH & Joyner MJ (2011). Sex and ageing differences in resting arterial pressure regulation: the role of β‐adrenergic receptors. J Physiol 589, 5285–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG & Charkoudian N (2012). Sex, ageing and resting blood pressure: gaining insights from the intergrated balance of neural and haemodynamic factors. J Physiol 590, 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH & Charkoudian N (2009). Age‐related differences in the sympathetic‐hemodynamic balance in men. Hypertension 54, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AL, Kent‐Braun J & Ditor DS (2001). Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev 29, 109–112. [DOI] [PubMed] [Google Scholar]
- Hill JM ( 2000). Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res 856, 240–244. [DOI] [PubMed] [Google Scholar]
- Hunter SK ( 2009). Sex differences and mechanisms of task‐specific muscle fatigue. Exerc Sport Sci Rev 37, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK ( 2014). Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol Scand 210, 768–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS & Enoka RM (2004). Men are more fatigable than strength‐matched women when performing intermittent submaximal contractions. J Appl Physiol 96, 2125–2132. [DOI] [PubMed] [Google Scholar]
- Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Walter Wray D & Amann M (2018). Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. J Physiol 596, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SNA, Magder S, Chatillon A & Roussos C (1990). Chemical activation of thin‐fiber phrenic afferents: respiratory responses. J Appl Physiol 69, 1002–1011. [DOI] [PubMed] [Google Scholar]
- Jammes Y & Balzamo E (1992). Changes in afferent and efferent phrenic activities with electrically‐induced diaphragmatic fatigue. J Appl Physiol 73, 894–902. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Barnes JN, Hart EC, Wallin BG & Charkoudian N (2015). Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol 5, 193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just TP & DeLorey DS (2017). Sex differences in sympathetic vasoconstrictor responsiveness and sympatholysis. J Appl Physiol 123, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent‐Braun J, Fitts RA & Christie A (2012). Skeletal muscle fatigue. Compr Physiol 2, 997–1044. [DOI] [PubMed] [Google Scholar]
- Laghi F, Harrison MJ & Tobin MJ (1996). Comparison of magnetic and electrical phrenic nerve stimulation in assessment of diaphragmatic contractility. J Appl Physiol 80, 1731–1742. [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Faulkner JA, Craig AB & Maxwell LC (1973). Performance and histochemical composition of guinea pig and human diaphragm. J Appl Physiol 34, 233–237. [DOI] [PubMed] [Google Scholar]
- MacNutt MJ, Peters CM, Chan C, Moore J, Shum S & Sheel AW (2015). Day‐to‐day variability in cardiorespiratory responses to hypoxic cycle exercise. Appl Physiol Nutr Metab 40, 155–161. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F & Cerutti S (1991). Cardiovascular neural regulation explored in the frequency domain. Circulation 84, 482–492. [DOI] [PubMed] [Google Scholar]
- Marcora SM & Staiano W (2010). The limit to exercise tolerance in humans: mind over muscle? Eur J Appl Physiol 109, 763–770. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Allen GM, Butler JE & Gandevia SC (1997). Task failure with lack of diaphragm fatigue during inspiratory resistive loading in human subjects. J Appl Physiol 82, 2011–2019. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L & Somers VK (2005). Gender‐selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45, 522–525. [DOI] [PubMed] [Google Scholar]
- NHLBI (1990). NHLBI workshop summary: respiratory muscle fatigue. Am Rev Respir Dis 142, 474–480. [DOI] [PubMed] [Google Scholar]
- Nickerson BG & Keens TG (1982). Measuring ventilatory muscle endurance in humans as sustainable inspiratory pressure. J Appl Physiol Respir Environ Exerc Physiol 52, 768–772. [DOI] [PubMed] [Google Scholar]
- Nygard E ( 1981). Skeletal muscle fibre characteristics in young women. Acta Physiol Scand 112, 299–304. [DOI] [PubMed] [Google Scholar]
- Ramsook AH, Koo R, Molgat‐Seon Y, Dominelli PB, Syed N, Ryerson CJ, Sheel AW & Guenette JA (2016). Diaphragm recruitment increases during a bout of targeted inspiratory muscle training. Med Sci Sports Exerc 48, 1179–1186. [DOI] [PubMed] [Google Scholar]
- Robertson CH, Foster GH & Johnson RL Jr (1977). The relationship of respiratory failure to the oxygen consumption of, lactate production by, and distribution of blood flow among respiratory muscles during increasing inspiratory resistance. J Clin Invest 59, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman JR, Henderson KS, Smith CA & Dempsey JA (2003). Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol 95, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Roussos C & Macklem PT (1977). Diaphragmatic fatigue in man. J Appl Physiol 43, 189–197. [DOI] [PubMed] [Google Scholar]
- Russ DW & Kent‐Braun J (2003). Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol 94, 2414–2422. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Derchak A, Morgan BJ, Pegelow DF, Jacques AJ & Dempsey JA (2001). Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol 537, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Goto K, Ishida K, Saito M, Akima H & Katayama K (2017). Blood pressure response during normocapnic hyperpnoea is blunted in young women compared to men. Respir Physiol Neurobiol 247, 52–56. [DOI] [PubMed] [Google Scholar]
- Similowski T, Fleury B, Launois S, Cathala HP, Bouche P & Derenne JP (1989). Cervical magnetic stimulation: a new painless method for bilateral phrenic nerve stimulation in conscious humans. J Appl Physiol 67, 1311–1318. [DOI] [PubMed] [Google Scholar]
- Smith JR, Broxterman RM, Hammer SM, Alexander AM, Didier KD, Kurti SP, Barstow TJ & Harms CA (2016). Sex differences in the cardiovascular consequences of the inspiratory muscle metaboreflex. Am J Physiol Regul Integr Comp Physiol 311, H574–H581. [DOI] [PubMed] [Google Scholar]
- St Clair Gibson A & Noakes TD (2004). Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med 38, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ & Dempsey JA ( 2000). Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Dimarco A, Ketai L, Hussein F & Altose M (1988). Reversibility of diaphragm fatigue by mechanical hyperperfusion. Am Rev Respir Dis 138, 604–609. [DOI] [PubMed] [Google Scholar]
- Tan WC, Bourbeau J, Hernandez P, Chapman K, Cowie R, FitzGerald MJ, Aaron S, Marciniuk DD, Maltais F, O'Donnell DE, Goldstein R, Sin D, Chan‐Yeung M, Manfreda J, Anthonisen NR, Tate RB, Sears MR, Siersted HC, Becklake MR, Ernst P, Bowie DM, Sweet L & Van Til L (2011). Canadian prediction equations for spirometric lung function for Caucasian adults 20–90 years of age: Results from the Canadian Obstructive Lung Disease (COLD) study and the Lung Health Canadian Environment (LHCE) study. Can Respir J 18, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JF, Mildren RL, Zaback M, Archiza B, Allen GP & Sheel AW (2017). Reliability of the diaphragmatic compound muscle action potential evoked by cervical magnetic stimulation and recorded via chest wall surface EMG. Respir Physiol Neurobiol 243, 101–106. [DOI] [PubMed] [Google Scholar]


