Abstract
Key points
Sodium nitroprusside lowers blood pressure by vasodilatation but is reported to reduce cerebral blood flow.
In healthy young men sodium nitroprusside reduced blood pressure, total peripheral resistance, and arterial CO2 tension and yet cerebral blood flow was maintained, with an increase in internal carotid artery blood flow and cerebrovascular conductance.
Sodium nitroprusside induces both systemic and cerebral vasodilatation affecting internal carotid artery more than vertebral artery flow.
Abstract
Cerebral autoregulation maintains cerebral blood flow (CBF) despite marked changes in mean arterial pressure (MAP). Sodium nitroprusside (SNP) reduces blood pressure by vasodilatation but is reported to lower CBF, probably by a reduction in its perfusion pressure. We evaluated the influence of SNP on CBF and aimed for a 20% and then 40% reduction in MAP, while keeping MAP ≥ 50 mmHg, to challenge cerebral autoregulation. In 19 healthy men (age 24 ± 4 years; mean ± SD) duplex ultrasound determined right internal carotid (ICA) and vertebral artery (VA) blood flow. The SNP reduced MAP (from 83 ± 8 to 69 ± 8 and 58 ± 4 mmHg; both P < 0.0001), total peripheral resistance, and arterial CO2 tension (; 41 ± 3 vs. 39 ± 3 and 37 ± 4 mmHg; both P < 0.01). Yet ICA flow increased with the moderate reduction in MAP but returned to the baseline value with the large reduction in MAP (336 ± 66 vs. 365 ± 69; P = 0.013 and 349 ± 82 ml min–1; n.s.), while VA flow (114 ± 34 vs. 112 ± 38 and 110 ± 42 ml min–1; both n.s.) and CBF ((ICA + VA flow) × 2; 899 ± 135 vs. 962 ± 127 and 918 ± 197 ml min–1; both n.s.) were maintained with increased cerebrovascular conductance. In conclusion, CBF is maintained during SNP‐induced reduction in MAP despite reduced and the results indicate that SNP dilates cerebral vessels and increases ICA flow.
Keywords: cerebral blood flow, Doppler ultrasound, hypotension, sodium nitroprusside
Key points
Sodium nitroprusside lowers blood pressure by vasodilatation but is reported to reduce cerebral blood flow.
In healthy young men sodium nitroprusside reduced blood pressure, total peripheral resistance, and arterial CO2 tension and yet cerebral blood flow was maintained, with an increase in internal carotid artery blood flow and cerebrovascular conductance.
Sodium nitroprusside induces both systemic and cerebral vasodilatation affecting internal carotid artery more than vertebral artery flow.
Introduction
Cerebral blood flow (CBF) is thought to be maintained by autoregulation when mean arterial pressure (MAP) ranges from approximately 60 to 150 mmHg (Lassen, 1959) following modulation of pial and large cerebral artery tone (Willie et al. 2014; Lewis et al. 2015). Yet CBF may be affected by changes in MAP within these limits, particularly by reduction in MAP (Numan et al. 2014; Willie et al. 2014). Further, the lower limit of cerebral autoregulation varies between individuals and may depend on the intervention applied to reduce MAP (Drummond, 1997). Thus, during orthostatic stress CBF decreases with a minimal reduction in MAP (Sato et al. 2012a) while, on the other hand, CBF may increase in response to vasodilators (Joshi et al. 2002; Hussain et al. 2009; Perko et al. 2011) and cerebral oxygenation is preserved even when MAP is reduced to about 40 mmHg during anaesthesia (Nissen et al. 2009).
Regarding the influence of sodium nitroprusside (SNP) on CBF, traditional assessment of CBF by 133Xe clearance has led to contradictory conclusions (Brown et al. 1977; Henriksen & Paulson, 1982; Henriksen et al. 1982). The conflicting reports on the effect of SNP on CBF may relate to a concomitant increase in ventilation that reduces arterial CO2 tension () and thereby CBF (Kety & Schmidt, 1948), albeit SNP and hypotension may also affect cerebral CO2 reactivity (Harper & Glass, 1965; Lavi et al. 2003). Even when corrected for changes in , a SNP‐induced reduction in MAP of approximately 20% has been reported to reduce CBF (Brown et al. 1977; Henriksen et al. 1982) or have no effect (Henriksen & Paulson, 1982), while a 40% reduction in MAP reduced CBF by about 15% (Henriksen & Paulson, 1982).
The brain is perfused by the internal carotid (ICA) and vertebral (VA) arteries. The brainstem is important for regulation of blood pressure and vertebro‐basilar hypoperfusion may be important in regard to development of (pre)syncopal symptoms (Shin et al. 1999). Considering that changes in flow may be more pronounced in ICA than in VA, as demonstrated during orthostatic stress (Sato et al. 2012a; Ogoh et al. 2015), and CO2 reactivity may be different in the two vessels (Deegan et al. 2010, Sato et al. 2012b; Willie et al. 2012), we evaluated the influence of SNP on CBF by determining both ICA and VA flow, as well as middle cerebral artery mean blood velocity (MCA V mean). Administration of SNP aimed for a reduction in MAP by 20% and then 40%, while keeping MAP ≥ 50 mmHg, to challenge the conventionally accepted lower limit of cerebral autoregulation. In order to account for the potential influence of hypocapnic vasoconstriction, we evaluated CO2 reactivity in ICA and VA by hyperventilation at rest. CBF is reported as measured and also with a ‘correction’ for eventual changes in , accepting that CO2 reactivity may be affected during SNP infusion. We hypothesised that a SNP‐induced reduction in MAP affects CBF, with a larger reduction in ICA flow than VA flow, and that CO2 reactivity is larger for ICA than for VA.
Methods
Subjects and ethical approval
Twenty healthy men were recruited after giving verbal and written informed consent as approved by the ethical committee of the Copenhagen Region (H‐17017045), in accordance with the Declaration of Helsinki, and the study was registered as a clinical trial (https://clinicaltrials.gov/ct2/show/NCT03317652?cond=NCT03317652&rank=1; Clinical Trials ID NCT03317652; October, 2017). Inclusion criteria were men aged 18–35 years and exclusion criteria included alcohol intake ≥ 420 g week–1; body mass index < 18 or > 25 kg m–2; smoking; heart, lung, liver, kidney, or metabolic disease that require medication; anti‐hypertensive medication and medication that may affect CBF; neurologic disease considered to affect CBF including epilepsy and sclerosis; > 15% obstruction of the ICA (Saam et al. 2008); haemoglobin < 6 mM; vitamin B12 deficiency; Leber's hereditary optic neuropathy; use of monoamine oxidase inhibitors; and intake of sildenafil or vardenafil for 24 h and tadalafil for 48 h.
Measurements
A radial (n = 3) or brachial artery catheter (n = 16) was inserted for evaluation of blood gas variables and connected to a transducer (Edwards Life Sciences, Irvine, CA, USA) placed at heart level, and a monitor (Patient Monitor M1166A model 66s, Hewlett Packard, CA, USA) reported MAP and heart rate. Invasive arterial pressure monitoring allowed for evaluation of stroke volume, cardiac output (CO), and total peripheral resistance by modified pulse contour analysis (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands) which is correlated to evaluation by thermodilution (Harms et al. 1999; Ameloot et al. 2013). A line was placed in a cubital vein for infusion of SNP (Cavafix 20G 32 cm, Braun, Melsungen, Germany).
Right ICA and VA flow and MCA V mean were determined. Duplex ultrasound evaluated ICA at least 1.5 cm distal to its bifurcation and VA between the transverse processes C2–5 at 10–12 MHz (Logiq E, GE Medical System, Jiangsu, China) in a longitudinal view with the head turned left. At a stable angle ≤ 60 deg, pulsed wave Doppler determined the angle‐corrected time averaged maximum velocity that corresponds to two times the mean velocity (Li et al. 1993) and edge‐detection software evaluated vessel diameter (Brachial Analyser for Research v. 6, Medical Imaging Applications LLC, Coralville, IA, USA). The ICA and VA flows were calculated as:
(Thomas et al. 2015), with:
To limit the influence of ventilation on CBF, two recordings over 15–20 s were conducted at each MAP and the mean reported with evaluation of the two arteries in random order.
Frictional stress on the ICA and VA caused by blood velocity was estimated as shear rate using the following formula (Parker et al. 2009):
Transcranial Doppler evaluated MCA V mean using a 2 MHz probe (Multidop X; DWL, Sipplingen, Germany) stabilised by a headband. Two pairs of electrodes were placed at the right side of the neck and in the upper left mid‐axillary line to determine changes in extracellular and total water admittance by current at 3 and 100 kHz (C‐guard, DanMeter, Odense, Denmark). The low frequency current crosses the cell membrane only with difficulty and we report the difference in electrical admittance between the two frequencies, which reflects the intracellular compartment admittance and thereby changes in central blood volume (Cai et al. 2000).
Central haemodynamics and MCA V mean were recorded at 100 Hz (Powerlab 16/35 and LabChart 7, ADInstruments, Bella Vista, Australia) and thoracic admittance every 15th second and saved on a PC. Values were averaged over 2 min and arterial blood sampled in pre‐heparinised syringes (QS50, Radiometer, Copenhagen, Denmark) for analysis of blood gas variables (ABL 725, Radiometer).
Infusion protocol
Subjects presented after at least 4 h fast and had abstained from alcohol and caffeine for 12 h and rigorous exercise for 24 h. The subjects were supine in a room kept at 22⁰C with baseline evaluation at least 30 min after instrumentation. Cerebral CO2 reactivity was evaluated by hyperventilation for 8 min to provoke a 5–9 mmHg reduction in .
Subsequently, the subjects rested for at least 15 min before administration of SNP in 5% dextrose (Nitropress Hospira, Pfizer, IL, USA) covered by aluminium foil, and infused using a pump (Perfusor Space, B. Braun, Melsungen, Germany). The aim was to achieve a 20% and then a 40% reduction in MAP, while keeping MAP ≥ 50 mmHg and infusion rate ≤ 10 μg kg–1 min–1, with the administration of SNP stopped if nausea or dizziness appeared (Henriksen & Paulson, 1982; Van Lieshout et al. 2003). When the targeted MAP was reached, MAP was maintained for 2 min before measurement and the infusion rate reduced over 5–10 min to avoid rebound hypertension (Bünemann et al. 1991).
Statistics
A power calculation indicated that 14 subjects were needed to detect a 15% change in CBF, corrected for , resulting from a SNP‐induced reduction in MAP by 40% (Henriksen & Paulson, 1982), assuming a SD of 18% (Lewis et al. 2015) with a 5% significance level and a power of 80%. The primary outcome was change in CBF resulting from a SNP‐induced 40% reduction in MAP. Secondary outcomes were change in CBF resulting from a 20% reduction in MAP, change in ICA flow compared to VA flow, and CO2 reactivity for the two arteries.
Vascular conductance was flow divided by MAP and an index was calculated similarly for MCA V mean. The CO2 reactivity for ICA and VA was calculated as:
and was calculated similarly for MCA V mean. The ICA and VA flow and thus CBF during SNP infusion was also ‘corrected’ for change in from baseline by the determined CO2 reactivity:
and similarly for MCA V mean, with the reservation that CO2 reactivity may be affected by SNP. We estimated the relative change in MCA diameter in response to SNP by reordering the equation of blood flow, assuming similar changes in ICA and MCA flow, as ICA probably supplies MCA:
whereby:
A Student's paired t test was used to compare variables at baseline and during hyperventilation and to evaluate change in flow for ICA vs. VA during infusion of SNP, and the CO2 reactivity of the two vessels. Change by SNP infusion was evaluated by a repeated measures mixed model, fitted by restricted maximum likelihood in a structured covariance model with intervention (baseline and low and high SNP infusion rate) as a fixed effect and the subject variable as a random effect, in order to take into account the correlation of data within subjects (Proc mixed; SAS 9.4, SAS Institute, Cary, NC, USA). Arterial lactate was analysed after logarithmic transformation to obtain a normal distribution. Evaluation of changes in arterial O2 saturation was by Friedman's test. Change in the estimated MCA diameter was by a one‐sample t test. Pearson correlation coefficient was used to evaluate the relation between diameter and shear rate for ICA and VA and between changes in MAP and CO and changes in shear rate. Values are presented as means ± SD or median (IQR) for non‐normally distributed data, and changes as means (95% CI). Statistical significance was set at P < 0.05.
Results
The study was not conducted for one subject as we could not place the arterial catheter. Subjects in the study were aged 24 ± 4 years, with a mean height of 182 ± 5 cm, and a mean weight of 75 ± 9 kg (body mass index 22 ± 2 kg m–2). For one subject, ICA flow was not evaluated following the moderate reduction in MAP, and three subjects became ill during the large reduction in MAP, at which point the infusion of SNP was stopped, the subjects’ legs were elevated and data obtained at that time was not included in the analysis.
Cerebral CO2 reactivity
Cerebral haemodynamic and arterial gas variables at baseline and during hyperventilation are presented in Table 1. The CO2 reactivity was similar for ICA and VA (2.7 ± 1.3 vs. 3.0 ± 1.7% mmHg–1; P = 0.5393) and thus the value for CBF was 2.8 ± 1.0% mmHg–1. For MCA V mean it was 2.1 ± 1.5% mmHg–1.
Table 1.
Cerebral haemodynamic and arterial gas variables at baseline and during hyperventilation
| Baseline (n = 19) | Hyperventilation (n = 19) | P value | |
|---|---|---|---|
| ICA TAVMAX (cm s–1] | 47 ± 9 | 38 ± 8* | < 0.0001 |
| ICA diameter (mm) | 5.5 ± 0.5 | 5.5 ± 0.6 | 0.794 |
| ICA blood flow (ml min–1) | 336 ± 66 | 270 ± 61* | < 0.0001 |
| ICA conductance (ml min–1 mmHg–1) | 4.1 ± 0.9 | 3.2 ± 0.7* | < 0.0001 |
| ICA shear rate (s–1) | 347 ± 87 | 286 ± 77* | 0.0002 |
| VA TAVMAX (cm s–1) | 29 ± 6 | 23 ± 5* | < 0.0001 |
| VA diameter (mm) | 4.0 ± 0.4 | 3.9 ± 0.5* | 0.0403 |
| VA blood flow (ml min–1) | 114 ± 34 | 88 ± 29* | < 0.0001 |
| VA conductance (ml min–1 mmHg–1) | 1.4 ± 0.4 | 1.0 ± 0.4* | < 0.0001 |
| VA shear rate (s–1) | 293 ± 64 | 240 ± 56* | < 0.0001 |
| CBF (ml min–1) | 899 ± 135 | 717 ± 127* | < 0.0001 |
| CBF conductance (ml min–1 mmHg–1) | 10.8 ± 1.7 | 8.5 ± 1.4* | < 0.0001 |
| MCA mean blood velocity (cm s–1) | 69 ± 16 | 57 ± 16* | < 0.0001 |
| MCA conductance index (cm s–1 mmHg–1) | 0.8 ± 0.2 | 0.7 ± 0.2* | < 0.0001 |
| Arterial CO2 tension (mmHg) | 41 ± 3 | 34 ± 4* | < 0.0001 |
| Arterial O2 tension (mmHg) | 106 ± 8 | 121 ± 13* | < 0.0001 |
ICA, internal carotid artery; TAVMAX, time averaged maximum blood velocity; VA, vertebral artery; CBF, cerebral blood flow; MCA, middle cerebral artery. Values are means ± SD. P values are from Student's paired t test. * P < 0.05 vs. baseline.
Infusion of sodium nitroprusside
Haemodynamics and arterial variables
Infusion of SNP (at 2.0 (1.5–2.5) and 8.6 (6.0–9.4) μg kg–1 min–1) reduced MAP by 17 ± 5% and 31 ± 6% (both P < 0.0001; Fig. 1; Table 2), with an accumulated dose of 14 (10–23) μg kg–1 over 10 (9–14) min and 71 (54–79) μg kg–1 over 20 (17–24) min and with no significant difference between values derived from the radial and brachial arteries.
Figure 1.
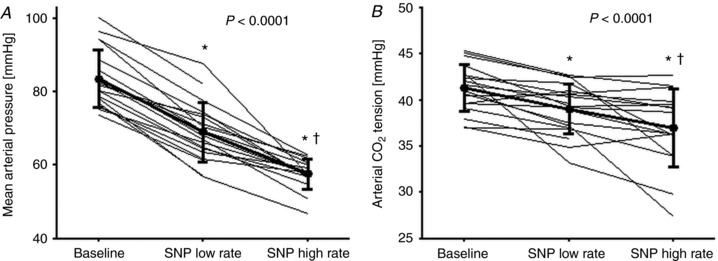
Mean arterial pressure and arterial CO2 tension at baseline and during infusion of sodium nitroprusside (SNP)
A and B, mean arterial pressure (A) and arterial CO2 tension (B) at baseline (n = 19) and during low (n = 19) and high (n = 16) SNP infusion rate. Values are individual data and means ± SD. P values represent the overall effect as estimated by a repeated measure mixed model. * P < 0.05 vs. baseline. † P < 0.05 for low vs. high SNP infusion rate.
Table 2.
Central haemodynamic and arterial variables at baseline and during infusion of sodium nitroprusside (SNP)
| Baseline (n = 19) | SNP low rate (n = 19) | SNP high rate (n = 16) | P value | |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 83 ± 8 | 69 ± 8* | 58 ± 4* † | < 0.0001 |
| Heart rate (min–1) | 55 ± 9 | 82 ± 14* | 94 ± 16* † | < 0.0001 |
| Stroke volume (ml) | 96 ± 9 | 93 ± 16 | 91 ± 18 | 0.1538 |
| Cardiac output (l min–1) | 5.3 ± 1.0 | 7.4 ± 1.5* | 8.4 ± 2.0* † | < 0.0001 |
| TPR (mmHg min l–1) | 17 ± 4 | 10 ± 2* | 7 ± 1* † | < 0.0001 |
| Thoracic admittance index (S × 104) | 102 ± 22 | 98 ± 20* | 97 ± 20* | 0.0042 |
| Arterial CO2 tension (mmHg) | 41 ± 3 | 39 ± 3* | 37 ± 4* † | < 0.0001 |
| Arterial O2 tension (mmHg) | 106 ± 8 | 117 ± 14* | 126 ± 12* † | < 0.0001 |
| Arterial O2 saturation (%) | 98 (98–98) | 99 (98–99)* | 99 (99–99)* † | 0.0003 |
| Haemoglobin concentration (mM) | 8.8 ± 0.7 | 8.9 ± 0.7* | 8.9 ± 0.7* | 0.0002 |
| Arterial O2 content (mM) | 8.6 ± 0.7 | 8.8 ± 0.7* | 8.8 ± 0.7* | < 0.0001 |
| Arterial glucose (mM) | 5.3 ± 0.2 | 5.6 ± 0.4* | 6.1 ± 0.5* † | < 0.0001 |
| Arterial lactate (mM) | 0.5 (0.4–0.6) | 0.7 (0.6–0.8)* | 0.6 (0.5–0.7)* † | 0.0001 |
TPR, total peripheral resistance. Values are means ± SD or median (IQR). P values represent the overall effect as estimated by a repeated measure mixed model. * P < 0.05 vs. baseline. † P < 0.05 for low vs. high SNP infusion rate.
Heart rate and CO increased, while total peripheral resistance was reduced with increasing SNP infusion, whereas stroke volume was maintained despite a reduction in thoracic electrical admittance (Table 2). The decreased with the infusion rate (by 2.3 mmHg (95% CI: 0.7–3.8; P = 0.0051) and 4.5 mmHg (95% CI: 2.9–6.1; P < 0.0001)), while arterial O2 tension and saturation, haemoglobin, O2 content, glucose, and lactate increased marginally.
Cerebral haemodynamics
The SNP infusion did not affect ICA blood velocity significantly but its diameter increased at the low infusion rate and also tended to increase at the high rate (P = 0.0525; Table 3). In contrast, VA blood velocity decreased, but its diameter also increased. Thus, ICA flow increased at the low rate (by 27 ml min–1; 95% CI: 6–48; P = 0.013), but returned to the baseline value at the high rate, while there was no significant change in VA flow and CBF (by 52 ml min–1 (95% CI: –1 to 106; P = 0.0563) and 24 ml min–1 (95% CI: –32 to 80; P = 0.3937); Fig. 2). Hence, the change in ICA flow was larger than that for VA at the low rate (9 ± 13% vs. –1 ± 20%; P = 0.0321), but similar at the high rate (5 ± 15% vs. –3 ± 26%; P = 0.2382), and both ICA and VA conductance increased. Shear rate for ICA was unaffected by SNP, whereas it decreased for VA. For both ICA and VA, shear rate was unrelated to change in diameter at both infusion rates. Further, changes in MAP and CO were unrelated to change in shear rate for both vessels.
Table 3.
Cerebral haemodynamic variables at baseline and during infusion of sodium nitroprusside (SNP) without and with ‘correction’ for changes in arterial CO2 tension by the determined CO2 reactivity
| Baseline (n = 19) | SNP low rate (n = 19) | SNP high rate (n = 16) | P value | |
|---|---|---|---|---|
| Without ‘correction’ for changes in arterial CO2 tension | ||||
| ICA TAVMAX (cm s–1) | 47 ± 9 | 47 ± 10 | 48 ± 12 | 0.9823 |
| ICA diameter (mm) | 5.5 ± 0.5 | 5.8 ± 0.5* | 5.6 ± 0.3 | 0.0018 |
| ICA blood flow (ml min–1) | 336 ± 66 | 365 ± 69* | 349 ± 82 | 0.0417 |
| ICA conductance (ml min–1 mmHg–1) | 4.0 ± 0.8 | 5.3 ± 0.8* | 6.1 ± 1.3* † | < 0.0001 |
| ICA shear rate (s–1) | 347 ± 87 | 332 ± 90 | 348 ± 94 | 0.4222 |
| VA TAVMAX (cm s–1) | 29 ± 6 | 25 ± 5* | 26 ± 8* | 0.0017 |
| VA diameter (mm) | 4.0 ± 0.4 | 4.3 ± 0.6* | 4.3 ± 0.6* | 0.0002 |
| VA blood flow (ml min–1) | 114 ± 34 | 112 ± 38 | 110 ± 42 | 0.7963 |
| VA conductance (ml min–1 mmHg–1) | 1.4 ± 0.4 | 1.6 ± 0.6* | 1.9 ± 0.7* † | < 0.0001 |
| VA shear rate (s–1) | 293 ± 64 | 242 ± 52* | 247 ± 95* | 0.0001 |
| CBF (ml min–1) | 899 ± 135 | 962 ± 127 | 918 ± 197 | 0.157 |
| CBF conductance (ml min–1 mmHg–1) | 10.8 ± 1.7 | 14.1 ± 2.3* | 15.9 ± 3.1* † | < 0.0001 |
| MCA mean blood velocity (cm s–1) | 69 ± 16 | 59 ± 14* | 52 ± 14* † | < 0.0001 |
| MCA conductance index (cm s–1 mmHg–1) | 0.82 ± 0.17 | 0.86 ± 0.18 | 0.91 ± 0.23* † | 0.0014 |
| With ‘correction’ for changes in arterial CO2 tension | ||||
| ICA blood flow (ml min–1) | 336 ± 66 | 388 ± 74* | 382 ± 78* | < 0.0001 |
| ICA conductance (ml min–1 mmHg–1) | 4.0 ± 0.8 | 5.7 ± 1.2* | 6.6 ± 1.2* † | < 0.0001 |
| VA blood flow (ml min–1) | 114 ± 34 | 119 ± 40 | 125 ± 46 | 0.2608 |
| VA conductance (ml min–1 mmHg–1) | 1.4 ± 0.4 | 1.7 ± 0.6* | 2.2 ± 0.8* † | < 0.0001 |
| CBF (ml min–1) | 899 ± 135 | 1023 ± 131* | 1013 ± 183* | 0.0002 |
| CBF conductance (ml min–1 mmHg–1) | 10.8 ± 1.7 | 15.0 ± 2.5* | 17.6 ± 2.8* † | < 0.0001 |
| MCA mean blood velocity (cm s–1) | 69 ± 16 | 61 ± 15* | 56 ± 15* † | < 0.0001 |
| MCA conductance index (cm s–1 mmHg–1) | 0.82 ± 0.17 | 0.89 ± 0.19* | 0.98 ± 0.27* † | < 0.0001 |
ICA, internal carotid artery; TAVMAX, time averaged maximum blood velocity; VA, vertebral artery; CBF, cerebral blood flow; MCA, middle cerebral artery. n = 18 for ICA and CBF at the low SNP rate. Values are means ± SD. P values represent the overall effect as estimated by a repeated measure mixed model. * P < 0.05 vs. baseline. † P < 0.05 for low vs. high SNP infusion rate.
Figure 2.
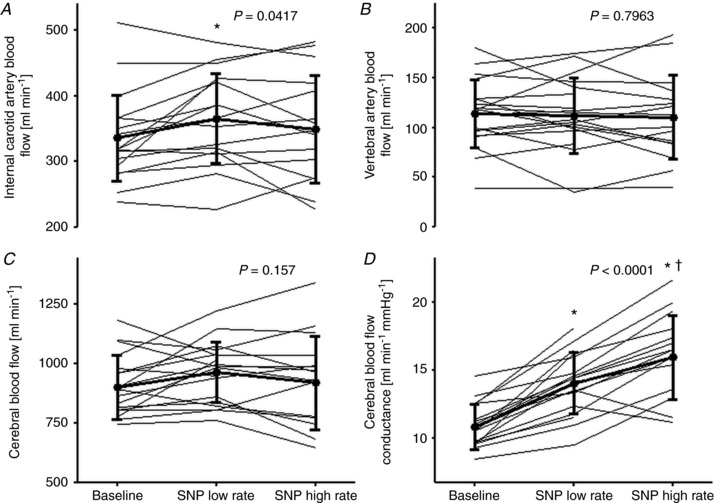
Cerebral haemodynamic variables at baseline and during infusion of sodium nitroprusside (SNP) without ‘correction’ for changes in arterial CO2 tension
A–D, internal carotid artery blood flow (A), vertebral artery blood flow (B), cerebral blood flow (C), and cerebral blood flow conductance (D) at baseline (n = 19) and during low (n = 18; n = 19 for VA flow) and high (n = 16) SNP infusion rate. Values are individual data and means ± SD. P values represent the overall effect as estimated by a repeated measure mixed model. * P < 0.05 vs. baseline. † P < 0.05 for low vs. high SNP infusion rate.
In contrast to ICA flow, which probably supplies the MCA, MCA V mean decreased with infusion rate (by 14 ± 7% and 25 ± 10%; both P < 0.0001) and the difference from changes in ICA flow was significant at both infusion rates (by 23 ± 14% and 29 ± 14%; both P < 0.0001), while MCA V mean conductance index decreased at the high infusion rate. Thus, the results suggest that MCA diameter increased at both infusion rates (by 12 ± 7% and 18 ± 8%; both P < 0.0001). At the time (pre)syncopal symptoms developed in three subjects, as identified by nausea and dizziness, MAP was reduced by 20%, 42%, and 26%, CBF by 27%, 8%, and 16%, and by 7, 9, and 9 mmHg, while CO was increased by 4% and 66% or reduced by 8%.
Correction for change in
After ‘correction’ using the determined CO2 reactivity at rest, infusion of SNP at the two rates increased ICA flow similarly (by 50 ml min–1 (95% CI: 27–72; P < 0.0001) and 50 ml min–1 (95% CI: 26–73; P = 0.0001), respectively), whereas VA flow was unaffected (Table 3). Thus, the change in ICA flow was larger than for VA at the low infusion rate (16 ± 14% vs. 5 ± 21%; P = 0.0234), but similar at the high infusion rate (15 ± 15% vs. 10 ± 28%; P = 0.4617) and conductance increased for both arteries. Thereby CBF increased similarly at the two rates (by 112 ml min–1 (95% CI: 55–170; P = 0.0004) and 120 ml min–1 (95% CI: 60–180; P = 0.0003)).
In contrast, MCA V mean decreased with increasing SNP rate (by 10 ± 9% and 19 ± 10%; both P < 0.0001), while its conductance index increased. Thus change in MCA Vmean was still different from that of ICA flow (by 26 ± 15% and 35 ± 15%; both P < 0.0001) suggesting that MCA diameter increased (by 14 ± 8% and 20 ± 8%; both P < 0.0001).
Discussion
The focus of the study was on whether CBF is influenced by a sodium nitroprusside (SNP)‐induced reduction in MAP that challenges the lower limit of cerebral autoregulation. In healthy young men, a SNP‐induced reduction in MAP did not affect VA flow and CBF, although decreased and ICA flow increased with a moderate reduction in MAP. Further, when taking the reduction in into account, SNP increased CBF, albeit CO2 reactivity may be attenuated by SNP. SNP lowers blood pressure by vasodilatation, as total peripheral resistance decreased despite a reduced central blood volume as indicated by thoracic electrical admittance. Here we demonstrate that SNP dilates large cerebral vessels and increases ICA flow.
In apparent contrast to its effect on CBF, SNP reduced MCA V mean, as reported also by Lucas et al. (2010). We take that observation to indicate that SNP dilates large cerebral arteries, as demonstrated for both ICA and VA and confirming the results of Liu et al. (2013) for ICA. Thus, MCA V mean underestimates changes in CBF in response to SNP and here the results suggest that SNP increased MCA diameter by approximately 15%, as ICA flow increased while MCA V mean was reduced. Indeed, MCA diameter is reported to increase by about 18% in response to nitroglycerin (White et al. (2000).
A concern is that three subjects became ill in response to administration of SNP and presented a reduction in CBF by 8–27%, similar to the reduction in CBF and symptoms of incipient fainting following a SNP‐induced 40% reduction in MAP reported by Henriksen & Paulson (1982). Thus, reduction in MAP should be kept within 20–30%, although Lucas et al. (2010) reported no (pre)syncopal symptoms when MAP was reduced by 40% in response to SNP in healthy young men.
Vasodilators and cerebral blood flow
The lower limit of cerebral autoregulation varies between individuals and appears to depend on whether the intervention implies cerebral vasodilatation (Drummond, 1997). The CBF increases in response to vasodilatation by, for example, L‐arginine (Perko et al. 2011), adenosine (Hussain et al. 2009), and verapamil (Joshi et al. 2002), while nitroglycerin may (Bednarczyk et al. 2002) or may not affect CBF (White et al. 2000). SNP dilates pial vessels (Auer 1978) and we take the results to illustrate the influence of nitric oxide‐mediated vasodilatation on CBF. A reduction in CO may limit CBF (Meng et al. 2015), but as CO increased with administration of SNP there was no indication for a central limitation to control of CBF. Changes in MAP within the conventional limits of cerebral autoregulation may affect CBF (Numan et al. 2014; Willie et al. 2014) and we interpret the results of maintained CBF despite reductions in MAP and to indicate that SNP reduces the lower limit of cerebral autoregulation.
SNP and 133Xe clearance‐determined CBF
Taken together, there seems to be a distinct difference in the effect of SNP on CBF when evaluated by 133Xe clearance or by duplex ultrasound. With 133Xe clearance, SNP reduces or does not influence CBF (Brown et al. 1977; Henriksen & Paulson, 1982; Henriksen et al. 1982), while with duplex ultrasound evaluation of ICA and VA flow, the results indicate an increase in CBF. Some considerations seem relevant in regard to the 133Xe clearance method. First, 133Xe clearance determines not only CBF but also scalp blood flow, although during exercise increased skin blood flow appears not to be significant in evaluation of CBF (Thomas et al. 1989). More importantly, MCA V mean and VA flow analysis seems to provide an explanation for the discrepancy between results obtained when recording of CBF by 133Xe clearance or duplex ultrasound during administration of SNP. Cerebral vasodilatation in response to SNP is so large that not only MCA V mean but also VA blood velocity decreases and thus transient time for blood decreases and clearance of 133Xe is slowed, and this is interpreted as a reduction in CBF.
Regional distribution of flow
Vasomotor control appears to differ for ICA and VA (Sato et al. 2012a; Willie et al. 2012; Ogoh et al. 2015), which may be related to more prominent sympathetic innervation of arteries originating from the ICA than those supplied by VA (Edvinsson & Owman, 1977). Sympathetic activity appears to limit surges in CBF during transient hypertension and hypercapnia but may have only a modest effect during hypotension and hypocapnia (Cassaglia et al. 2008; ter Laan et al. 2013; Brassard et al. 2017). As evaluated by administration of L‐arginine, transcranial Doppler‐determined endothelial function seems more important for the posterior than for the middle cerebral artery (Perko et al. 2011). Yet the effect on CBF by SNP appeared more prominent for ICA than for VA.
Cerebral CO2 reactivity
Cerebral CO2 reactivity for ICA has been found to be larger (Sato et al. 2012b), similar (Deegan et al. 2010), or lower than for VA (Willie et al. 2012, Lewis et al. 2015) and in this study it was similar for the two vessels. We took changes in in response to SNP into account, but in another study cerebral CO2 reactivity was attenuated when MAP was reduced (Harper & Glass, 1965). In anaesthetised patients, a SNP‐induced reduction in MAP by some 30% approximately halved cerebral CO2 reactivity (Matta et al. 1995), while in healthy young subjects a minor reduction in MAP by SNP reduced CO2 reactivity by about 15% (Lavi et al. 2003). Thus, ‘correction’ using the CO2 reactivity determined at rest may overestimate CBF during SNP infusion. The was reduced in response to SNP, possibly by increased ventilation mediated by unloading of the arterial baroreflex (Stewart et al. 2011). Evaluation of CO2 reactivity for MCA V mean may underestimate changes in CBF, as a reduction in by approximately 13 mmHg reduces MCA diameter by about 5% (Coverdale et al. 2014), while a reduction by about 10 mmHg seems not to affect its diameter (Verbree et al. 2014).
Limitations
In this study, evaluation of cerebral haemodynamics was on the right side and we can only assume the responses on the left side would be similar, as shown during administration of nitroglycerin (Bednarczyk et al. 2002) and L‐arginine (Pretnar‐Oblak et al. 2006) and in response to hypoxia (Mikhail Kellawan et al. 2017). We did not evaluate whether SNP affects CBF when MAP is preserved, but as CO increased with administration of SNP there was no indication for a central limitation to CBF.
The study could have clamped as SNP probably affects cerebral CO2 reactivity, and the evaluation encompassed only Caucasian men. We aimed for a 40% reduction in MAP, maintaining MAP ≥ 50 mmHg, but only attained a 31% reduction in MAP because some subjects demonstrated a low MAP at baseline, developed (pre)syncopal symptoms, or because the maximal SNP infusion rate was reached. The arterial O2 tension was not controlled but we take the minor increase induced by SNP to have limited effect on CBF (Ainslie & Duffin, 2009).
Conclusion
Sodium nitroprusside induces systemic and cerebral vasodilatation and increased cerebrovascular conductance. The CBF was maintained in response to SNP‐induced reduction in MAP despite reduced and the results indicate differences in regional regulation of CBF as SNP increased ICA but not VA flow. Future studies could evaluate the effect of SNP on CBF when is maintained and whether SNP affects CBF when MAP is preserved.
Additional information
Competing interests
The authors declare that they have no conflict of interest.
Author contributions
N.D.O.: conception and design of the study, acquisition, analysis, and interpretation of data and writing the first draft of the manuscript. M.F.: acquisition of data and revision of the manuscript. N.H.S.: conception and design of the study, interpretation of data, and revision of the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by the University of Copenhagen.
Acknowledgements
We thank the subjects for their time and commitment to the study.
Biography
Niels D. Olesen is doing his PhD under the supervision of Dr Niels H. Secher. He is studying the influence of changes in blood pressure and anaesthesia on cerebral blood flow. Outside of the lab he enjoys spending time with his family and reading. In the future, he plans to become an anaesthesiologist and continue his scientific pursuits.

Edited by: Laura Bennet & Philip Ainslie
References
- Ainslie PN & Duffin J (2009). Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296, R1473–R1495. [DOI] [PubMed] [Google Scholar]
- Ameloot K, Van De Vijver K, Broch O, Van Regenmortel N, De Laet I, Schoonheydt K, Dits H, Bein B & Malbrain ML (2013). Nexfin noninvasive continuous hemodynamic monitoring: validation against continuous pulse contour and intermittent transpulmonary thermodilution derived cardiac output in critically ill patients. ScientificWorldJournal 2013, 519080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer L (1978). The action of sodium nitroprusside on the pial vessels. Acta Neurochir (Wien) 43, 297–306. [DOI] [PubMed] [Google Scholar]
- Bednarczyk EM, Wack DS, Kassab MY, Burch K, Trinidad K, Haka M & Gona J (2002). Brain blood flow in the nitroglycerin (GTN) model of migraine: measurement using positron emission tomography and transcranial Doppler. Cephalalgia 22, 749–757. [DOI] [PubMed] [Google Scholar]
- Brassard P, Ferland‐Dutil H, Smirl JD, Paquette M, Le Blanc O, Malenfant S & Ainslie PN (2017). Evidence for hysteresis in the cerebral pressure‐flow relationship in healthy men. Am J Physiol Heart Circ Physiol 312, H701–H704. [DOI] [PubMed] [Google Scholar]
- Brown FD, Hanlon K, Crockard HA & Mullan S (1977). Effect of sodium nitroprusside on cerebral blood flow in conscious human beings. Surg Neurol 7, 67–70. [PubMed] [Google Scholar]
- Bünemann L, Jensen KA, Riisager S & Thomsen LJ (1991). Cerebral blood flow and metabolism during hypotension induced with sodium nitroprusside and metoprolol. Eur J Anaesthesiol 8, 197–201. [PubMed] [Google Scholar]
- Cai Y, Holm S, Jenstrup M, Stromstad M, Eigtved A, Warberg J, Højgaard L, Friberg L & Secher NH (2000). Electrical admittance for filling of the heart during lower body negative pressure in humans. J Appl Physiol (1985) 89, 1569–1576. [DOI] [PubMed] [Google Scholar]
- Cassaglia PA, Griffiths RI & Walker AM (2008). Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 294, R1255–R1261. [DOI] [PubMed] [Google Scholar]
- Coverdale NS, Gati JS, Opalevych O, Perrotta A & Shoemaker JK (2014). Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117, 1090–1096. [DOI] [PubMed] [Google Scholar]
- Deegan BM, Cooke JP, Lyons D, Olaighin G & Serrador JM (2010). Cerebral autoregulation in the vertebral and middle cerebral arteries during combine head upright tilt and lower body negative pressure in healthy humans. Conf Proc IEEE Eng Med Biol Soc 2010, 2505–2508. [DOI] [PubMed] [Google Scholar]
- Drummond JC (1997). The lower limit of autoregulation: time to revise our thinking? Anesthesiology 86, 1431–1433. [DOI] [PubMed] [Google Scholar]
- Edvinsson L & Owman C (1977). Sympathetic innervation and adrenergic receptors in intraparenchymal cerebral arterioles of baboon. Acta Neurol Scand Suppl 64, 304–305. [PubMed] [Google Scholar]
- Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH & Van Lieshout JJ (1999). Continuous stroke volume monitoring by modelling flow from non‐invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97, 291–301. [PubMed] [Google Scholar]
- Harper AM & Glass HI (1965). Effects of alterations in the arterial CO2 tension on the blood flow through the cerebral cortec at normal and low arterial blood pressures. J Neurol Neurosurg Psychiat 28, 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen L & Paulson OB (1982). The effects of sodium nitroprusside on cerebral blood flow and cerebral venous blood gases. II. Observations in awake man during successive blood pressure reduction. Eur J Clin Invest 12, 389–393. [DOI] [PubMed] [Google Scholar]
- Henriksen L, Paulson OB & Lauritzen M (1982). The effects of sodium nitroprusside on cerebral blood flow and cerebral venous blood gases. I. Observations in awake man during and following moderate blood pressure reduction. Eur J Clin Invest 12, 383–387. [DOI] [PubMed] [Google Scholar]
- Hussain R, Tsuchida T, Kudo T, Kobayashi M, Tsujikawa T, Kiyono Y, Fujibayashi Y & Okazawa H (2009). Vasodilatory effect of adenosine triphosphate does not change cerebral blood flow: a PET study with 15O‐water. Ann Nucl Med 23, 717–723. [DOI] [PubMed] [Google Scholar]
- Joshi S, Young WL, Duong H, Aagaard BA, Ostapkovich ND, Connolly ES & Pile‐Spellman J (2002). Intracarotid nitroprusside does not augment cerebral blood flow in human subjects. Anesthesiology 96, 60–66. [DOI] [PubMed] [Google Scholar]
- Kety SS & Schmidt CF (1948). The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27, 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA (1959). Cerebral blood flow and oxygen consumption in man. Physiol Rev 39, 183–238. [DOI] [PubMed] [Google Scholar]
- Lavi S, Egbarya R, Lavi R & Jacob G (2003). Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation 107, 1901–1905. [DOI] [PubMed] [Google Scholar]
- Lewis NC, Smith KJ, Bain AR, Wildfong KW, Numan T & Ainslie PN (2015). Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond) 129, 169–178. [DOI] [PubMed] [Google Scholar]
- Li S, Hoskins PR, Anderson T & McDicken WN (1993). Measurement of mean velocity during pulsatile flow using time‐averaged maximum frequency of Doppler ultrasound waveforms. Ultrasound Med Biol 19, 105–113. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhu YS, Hill C, Armstrong K, Tarumi T, Hodics T, Hynan LS & Zhang R (2013). Cerebral autoregulation of blood velocity and volumetric flow during steady‐state changes in arterial pressure. Hypertension 62, 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S & Ainslie PN (2010). Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension 55, 698–705. [DOI] [PubMed] [Google Scholar]
- Matta BF, Lam AM, Mayberg TS, Eng CC & Strebel S (1995). Cerebrovascular response to carbon dioxide during sodium nitroprusside‐ and isoflurane‐induced hypotension. Br J Anaesth 74, 296–300. [DOI] [PubMed] [Google Scholar]
- Meng L, Hou W, Chui J, Han R & Gelb AW (2015). Cardiac output and cerebral blood flow: the integrated regulation of brain perfusion in adult humans. Anesthesiology 123, 1198–1208. [DOI] [PubMed] [Google Scholar]
- Mikhail Kellawan J, Harrell JW, Roldan‐Alzate A, Wieben O & Schrage WG (2017). Regional hypoxic cerebral vasodilation facilitated by diameter changes primarily in anterior versus posterior circulation. J Cereb Blood Flow Metab 37, 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen P, van Lieshout JJ, Nielsen HB & Secher NH (2009). Frontal lobe oxygenation is maintained during hypotension following propofol‐fentanyl anesthesia. AANA Journal 77, 271–276. [PubMed] [Google Scholar]
- Numan T, Bain AR, Hoiland RL, Smirl JD, Lewis NC & Ainslie PN (2014). Static autoregulation in humans: a review and reanalysis. Med Eng Phys 36, 1487–1495. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Sadamoto T & Shibasaki M (2015). Blood flow in internal carotid and vertebral arteries during graded lower body negative pressure in humans. Exp Physiol 100, 259–266. [DOI] [PubMed] [Google Scholar]
- Parker BA, Trehearn TL & Meendering JR (2009). Pick your Poiseuille: normalizing the shear stimulus in studies of flow‐mediated dilation. J Appl Physiol (1985) 107, 1357–1359. [DOI] [PubMed] [Google Scholar]
- Perko D, Pretnar‐Oblak J, Sabovic M, Zvan B & Zaletel M (2011). Differences between cerebrovascular reactivity to L‐arginine in the anterior and posterior cerebral circulation. Cerebrovasc Dis 31, 358–364. [DOI] [PubMed] [Google Scholar]
- Pretnar‐Oblak J, Zaletel M, Zvan B, Sabovic M & Pogacnik T (2006). Cerebrovascular reactivity to L‐arginine in patients with lacunar infarctions. Cerebrovasc dis 21, 180–186. [DOI] [PubMed] [Google Scholar]
- Saam T, Underhill HR, Chu B, Takaya N, Cai J, Polissar NL, Yuan C & Hatsukami TS (2008). Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol 51, 1014–1021. [DOI] [PubMed] [Google Scholar]
- Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH & Ogoh S (2012a). Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol 97, 1272–1280. [DOI] [PubMed] [Google Scholar]
- Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T & Ogoh S (2012b). Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol 590, 3277–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Yoo KM, Chang HM & Caplan LR (1999). Bilateral intracranial vertebral artery disease in the New England Medical Center, Posterior Circulation Registry. Arch Neurol 56, 1353–1358. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C & Medow MS (2011). Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am J Physiol Heart Circ Physiol 300, H1492–H1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Laan M, van Dijk JM, Elting JW, Staal MJ & Absalom AR (2013). Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 111, 361–7. [DOI] [PubMed] [Google Scholar]
- Thomas KN, Lewis NC, Hill BG & Ainslie PN (2015). Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am J Physiol Regul Integr Comp Physiol 309, R707–R720. [DOI] [PubMed] [Google Scholar]
- Thomas SN, Schroeder T, Secher NH & Mitchell JH (1989). Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J Appl Physiol (1985) 67, 744–748. [DOI] [PubMed] [Google Scholar]
- Van Lieshout JJ, Wieling W, Karemaker JM & Secher NH (2003). Syncope, cerebral perfusion, and oxygenation. J Appl Physiol (1985) 94, 833–848. [DOI] [PubMed] [Google Scholar]
- Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ & van Osch MJ (2014). Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra‐high‐field MRI. J Appl Physiol (1985) 117, 1084–1089. [DOI] [PubMed] [Google Scholar]
- White RP, Deane C, Hindley C, Bloomfield PM, Cunningham VJ, Vallance P, Brooks DJ & Markus HS (2000). The effect of the nitric oxide donor glyceryl trinitrate on global and regional cerebral blood flow in man. J Neurol Sci 178, 23–28. [DOI] [PubMed] [Google Scholar]
- Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA & Ainslie PN (2012). Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590, 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, Tzeng YC, Fisher JA & Ainslie PN (2014). Integrative regulation of human brain blood flow. J Physiol 592, 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]


