Abstract
To study the effect of alloy composition on phase selection in the CoCrCu0.1FeMoNi high-entropy alloy (HEA), Mo was partially replaced by Co, Cr, Fe, and Ni. The microstructures and phase selection behaviors of the CoCrCu0.1FeMoNi HEA system were investigated. Dendritic, inter-dendritic, and eutectic microstructures were observed in the as-solidified HEAs. A simple face centered cubic (FCC) single-phase solid solution was obtained when the molar ratio of Fe, Co, and Ni was increased to 1.7 at the expense of Mo, indicating that Fe, Co, and Ni stabilized the FCC structure. The FCC structure was favored at the atomic radius ratio δ ≤ 2.8, valence electron concentration (VEC) ≥ 8.27, mixing entropy ΔS ≤ 13.037, local lattice distortion parameter α2 ≤ 0.0051, and ΔS/δ2 > 1.7. Mixed FCC + body centered cubic (BCC) structures occurred for 4.1 ≤ δ ≤ 4.3 and 7.71 ≤ VEC ≤ 7.86; FCC or/and BCC + intermetallic (IM) mixtures were favored at 2.8 ≤ δ ≤ 4.1 or δ > 4.3 and 7.39 < VEC ≤ 8.27. The IM phase is favored at electronegativity differences greater than 0.133. However, ΔS, α2, and ΔS/δ2 were inefficient in identifying the (FCC or/and BCC + IM)/(FCC + BCC) transition. Moreover, the mixing enthalpy cannot predict phase structures in this system.
Keywords: high-entropy alloy, microstructure, eutectic structure, phase selection
1. Introduction
High-entropy alloys (HEAs) represent a new class of materials that have attracted extensive attention since 2004 [1,2,3,4,5,6,7,8]. HEAs are defined as alloys containing at least five major elements wherein every major element has an atomic fraction between 5% and 35% [9]. Various studies on HEAs, including composition, processing, crystal structure and microstructure, and physical and mechanical properties, have been performed in the past years [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23].
The microstructures and crystalline phases present in HEAs are very sensitive to the alloy composition. An α phase appears when the molar ratio of Mo in AlCoCrFeNiMox alloys exceeds 0.1 [17]. Changes in the molar ratio of Fe, Co, and Cr in AlCoCrFeMo0.5Ni alloys affect the crystalline phases and mechanical properties. As the Mo content increases, the volume fraction of the σ phase increases in Ni2CrFeMox alloys [18]. However, the volume fraction of the σ phase is increased with increasing Cr content. Eventually, the dendritic matrix of AlCoCrxFeMo0.5Ni HEAs is changed from the ordered B2 phase to the σ phase [19]. As the Co content in AlCoxCrFeMo0.5Ni HEAs changes from x = 0.5 to x = 2.0, the phase changes from BCC to BCC + FCC + σ, respectively. Recently, it was found that the precipitation of intermetallic (IM) compounds of σ and μ phases could strengthen CoCrFeNiMo0.3 alloy without causing serious embrittlement [21]. Moreover, the ordered B2 solid-solution and σ phases were presented in FeAlCrNiMox HEAs with increasing Mo content [22]. Most previous investigations have focused on the effects of the addition or content change of one or two elements on the microstructure and properties of the HEA. However, studies on the effect of each individual element on the microstructure and phase selection have not yet been reported. Additional systematic research will be necessary in the near future to guide the exploration of HEAs.
Many parameters are related to phase selection in HEAs, including the atomic radii differences (δ), differences in electronegativity (ΔX), the valence electron concentration (VEC), the enthalpy of mixing (ΔHmix), and the mixing entropy (ΔSmix) [24,25,26,27,28,29,30,31]. Based on these parameters, many criteria have been proposed for phase prediction in HEAs. Zhang et al. summarized a solid-solution phase-forming rule using δ, ΔHmix, and ΔSmix with δ ≤ 6.6%, −22 ≤ ΔHmix ≤ 7 kJ/mol, and 11 ≤ ΔSmix ≤ 19.5 J/(K·mol) [24]. To limit the target of discussion to simple disordered phases, the conditions are more strict: δ ≤ 4.3%, −15 ≤ ΔHmix ≤ 5 kJ/mol, and 12 ≤ ΔSmix ≤ 17.5 J/(K·mol). Guo proposed that the phase stability of the FCC and BCC solid solution was correlated with VEC; for VEC < 6.87 the BCC phase was stable; for VEC > 8, FCC was [25]. Later, the stability of the σ phase was studied and it was predicted that alloys with 6.88 ≤ VEC ≤ 7.84 were prone to σ phase formation [30]. However, this criterion works well only for Cr- and V-containing HEAs. Recently, complex ordered phases were found to be stable for ΔX > 0.133, except for HEAs containing a large amount of Al [31]. More recently, Wang et al. [32] proposed a new parameter, α2, to address the local lattice distortion of crystalline lattices in HEAs. This parameter effectively explained the lattice distortion, intrinsic strain energy, and excess entropy in HEAs.
As mentioned above, the phases present in HEAs are remarkably dependent on the alloy composition. In a previous work, the CoCrCu0.1FeMoNi alloy exhibited the duplex microstructure of BCC + FCC [33]. Under compositional change, the microstructural behavior is uncertain: the HEA could retain this simple solid solution mixture or intermetallic (IM) phases could appear. In order to address this question, it is necessary to study the effect of alloy composition on the phase selection of CoCrCu0.1FeMoNi alloys. Hence, the present work investigates the partial substitution of Mo by Cr, Co, Ni, and Fe. The effect of the relative contents of Cr, Co, Ni, Fe, and Mo on the microstructure and crystal structures of CoCrCu0.1FeMoNi-based HEAs was studied in this work in order to understand the phase selection mechanism in this alloy system.
2. Materials and Methods
The proposed HEAs were prepared via vacuum arc melting in a Ti-gettered Ar atmosphere with subsequent melt solidification in a water-cooled Cu crucible. A mixture of the appropriate amounts of the constituent elements with purities > 99.9 wt % for each alloy was flipped and melted at least four times to ensure thorough chemical homogeneity. Table 1 shows the compositions prepared in this study. As-cast samples were then sectioned and polished for microstructural and compositional characterization using scanning electron microscopy (SEM, JEOL-5410, JEOL Ltd., Tokyo, Japan), energy dispersive X-ray spectrometry (EDS, JEOL Ltd., Tokyo, Japan), and an X-ray diffractometer (XRD, Rigaku ME510-FM2, Rigaku Ltd., Tokyo, Japan) at a scanning speed of 4°/min and a scanning range from 30° to 100° using a Cu target and an applied voltage and current of 30 kV and 20 mA, respectively.
Table 1.
A list of chemical composition of CoCrCu0.1FeMoNi-based alloys (at %).
| Alloys | Co | Cr | Cu | Fe | Mo | Ni |
|---|---|---|---|---|---|---|
| Co1.2CrCu0.1FeMo0.8Ni | 23.53 | 19.61 | 1.96 | 19.61 | 15.69 | 19.61 |
| Co1.5CrCu0.1FeMo0.5Ni | 29.41 | 19.61 | 1.96 | 19.61 | 9.8 | 19.61 |
| Co1.7CrCu0.1FeMo0.3Ni | 33.33 | 19.61 | 1.96 | 19.61 | 5.88 | 19.61 |
| CoCr1.2Cu0.1FeMo0.8Ni | 19.61 | 23.53 | 1.96 | 19.61 | 15.69 | 19.61 |
| CoCr1.5Cu0.1FeMo0.5Ni | 19.61 | 29.41 | 1.96 | 19.61 | 9.8 | 19.61 |
| CoCrCu0.1Fe1.2Mo0.8Ni | 19.61 | 19.61 | 1.96 | 23.53 | 15.69 | 19.61 |
| CoCrCu0.1Fe1.5Mo0.5Ni | 19.61 | 19.61 | 1.96 | 29.41 | 9.8 | 19.61 |
| CoCrCu0.1Fe1.7Mo0.3Ni | 19.61 | 19.61 | 1.96 | 33.33 | 5.88 | 19.61 |
| CoCrCu0.1Fe Mo0.8Ni1.2 | 19.61 | 19.61 | 1.96 | 19.61 | 15.69 | 23.53 |
| CoCrCu0.1Fe Mo0.5Ni1.5 | 19.61 | 19.61 | 1.96 | 19.61 | 9.8 | 29.41 |
| CoCrCu0.1Fe Mo0.3Ni1.7 | 19.61 | 19.61 | 1.96 | 19.61 | 5.88 | 33.33 |
3. Results
3.1. CoaCrCu0.1FeMo2−aNi Alloys
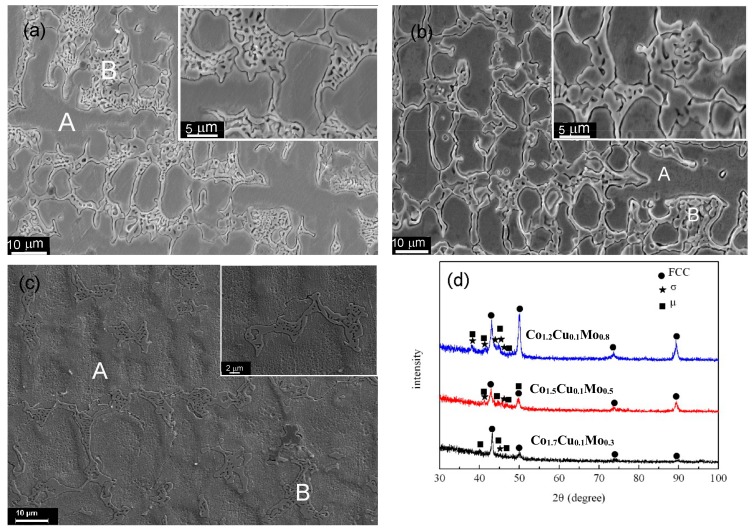
The microstructures of the CoaCrCu0.1FeMo2−aNi (a = 1.2, 1.5 and 1.7) alloys are shown in Figure 1. A typical eutectic structure is found in the inter-dendritic region, and the volume fraction of the eutectic mixture is decreased with increasing Co and decreasing Mo. Table 2 shows the actual composition and contents of different regions in the microstructures, as detected by EDS. The dendrites are enriched in Co, Cu, Fe, and Ni, while the contents of Cr and Mo are higher in the inter-dendritic region B. The composition of the inter-dendritic eutectic region B is approximately (CrMo)54(CoCuFeNi)46 according to EDS. This means that the content of Cr and Mo is 54% and that of Co, Cu, Fe, and Ni is 46%. Furthermore, the Cu content in the inter-dendritic region is increased with decreasing Mo content. This is related to the positive ΔHmix between Cu and Mo (+19 kJ/mol).
Figure 1.
The microstructures and phases of CoaCrCu0.1FeMo2−aNi HEAs. (a) Co1.2CrCu0.1FeMo0.8Ni; (b) Co1.5CrCu0.1FeMo0.5Ni; (c) Co1.7CrCu0.1FeMo0.3Ni; (d) X-ray diffractometer (XRD) patterns.
Table 2.
The components of different regions in microstructures of CoCrCu0.1FeMoNi-based high-entropy alloys (HEAs).
| Alloy | Value | Region | Co | Cr | Cu | Fe | Mo | Ni |
|---|---|---|---|---|---|---|---|---|
| CoaCrCu0.1FeMo2−aNi | a = 1.2 | Content | 23.55 | 18.37 | 1.84 | 19.73 | 17.93 | 18.58 |
| A | 24.17 | 18.46 | 1.85 | 20.45 | 14.32 | 20.75 | ||
| B | 19.32 | 18.01 | 0.43 | 14.57 | 36.10 | 11.57 | ||
| a = 1.5 | Content | 27.92 | 20.60 | 1.8 | 19.63 | 9.5 | 19.55 | |
| A | 20.45 | 27.40 | 1.68 | 21.25 | 8.93 | 20.29 | ||
| B | 17.03 | 33.13 | 0.82 | 16.70 | 19.91 | 12.41 | ||
| a = 1.7 | Content | 33.63 | 19.57 | 1.75 | 19.8 | 6.00 | 19.25 | |
| A | 34.86 | 17.83 | 1.61 | 20.30 | 6.09 | 19.31 | ||
| B | 30.34 | 20.33 | 1.90 | 19.79 | 8.98 | 18.66 | ||
| CoCrbCu0.1FeMo2−bNi | b = 1.2 | Content | 19.32 | 25.69 | 1.85 | 20.44 | 12.36 | 20.34 |
| A | 18.68 | 30.11 | - | 20.20 | 9.53 | 21.47 | ||
| B | 22.51 | 22.99 | 1.77 | 17.02 | 17.53 | 18.17 | ||
| b = 1.5 | Content | 20.02 | 29.45 | 1.51 | 19.23 | 10.36 | 19.43 | |
| A | 15.50 | 26.95 | - | 18.31 | 24.98 | 14.25 | ||
| B | 16.76 | 24.25 | - | 19.83 | 19.13 | 20.03 | ||
| CoCrCu0.1FecMo2−cNi | c = 1.2 | Content | 15.33 | 21.76 | 1.83 | 23.53 | 17.92 | 19.63 |
| A | 15.70 | 21.66 | - | 20.57 | 29.21 | 12.86 | ||
| B | 14.95 | 22.12 | 0.40 | 20.93 | 29.95 | 11.65 | ||
| c = 1.5 | Content | 17.71 | 21.65 | 1.94 | 29.41 | 10.82 | 18.47 | |
| A | 17.63 | 19.24 | 2.25 | 31.42 | 10.49 | 18.95 | ||
| B | 15.51 | 21.98 | 0.34 | 23.16 | 27.19 | 11.83 | ||
| C | 15.94 | 22.96 | 0.62 | 22.66 | 24.96 | 12.85 | ||
| c = 1.7 | Content | 20.8 | 19.98 | 1.64 | 33.30 | 6.05 | 18.23 | |
| A | 18.95 | 19.89 | 1.58 | 32.19 | 8.45 | 18.94 | ||
| B | 22.54 | 20.36 | 1.89 | 34.23 | 2.81 | 18.16 | ||
| CoCrCu0.1FeMo2−dNid | d = 1.2 | Content | 19.37 | 19.63 | 1.36 | 19.47 | 16.64 | 23.53 |
| A | 19.49 | 18.93 | 1.20 | 21.82 | 13.50 | 25.06 | ||
| B | 16.80 | 22.33 | 0.67 | 16.58 | 27.68 | 15.95 | ||
| C | 17.03 | 21.79 | 0.45 | 13.97 | 32.56 | 14.20 | ||
| d = 1.5 | Content | 19.79 | 19.68 | 1.96 | 19.16 | 10.37 | 29.04 | |
| A | 19.09 | 19.13 | 2.15 | 17.77 | 13.22 | 28.65 | ||
| B | 21.82 | 20.07 | 1.71 | 21.02 | 3.28 | 32.10 | ||
| C | 18.28 | 22.86 | 0.67 | 15.67 | 21.82 | 20.70 | ||
| d = 1.7 | Content | 20.82 | 19.67 | 1.53 | 19.74 | 6.25 | 31.89 | |
| A | 17.80 | 21.90 | 1.84 | 18.78 | 8.06 | 31.62 | ||
| B | 21.38 | 19.25 | 1.65 | 19.93 | 4.60 | 33.18 | ||
| C | 2.44 | 88.74 | - | 3.85 | 0.68 | 4.29 |
Figure 1d shows the XRD patterns of the CoaCrCu0.1FeMo2−aNi HEAs. The FCC, σ, and μ phases are detected. The crystal structure of the σ phase is tetragonal with the lattice constants of a = 0.885 nm and c = 0.459 nm, and the σ phase is similar to the binary Co2Mo3 phase. The μ phase is tetragonal with lattice constants of a = 0.7381 nm and c = 1.8504 nm, and probably Co7Mo3 or Fe7Mo6. Both σ and μ are topologically close-packed (TCP) phases. Obviously, the volume fractions of the σ and μ phases, represented by peaks in the range of 40–50°, are decreased as the Co content increases and Mo decreases. According to the EDS and XRD results, we can identify the dendrites as the FCC phase, while σ and μ are eutectic structures.
3.2. CoCrbCu0.1FeMo2−bNi Alloys
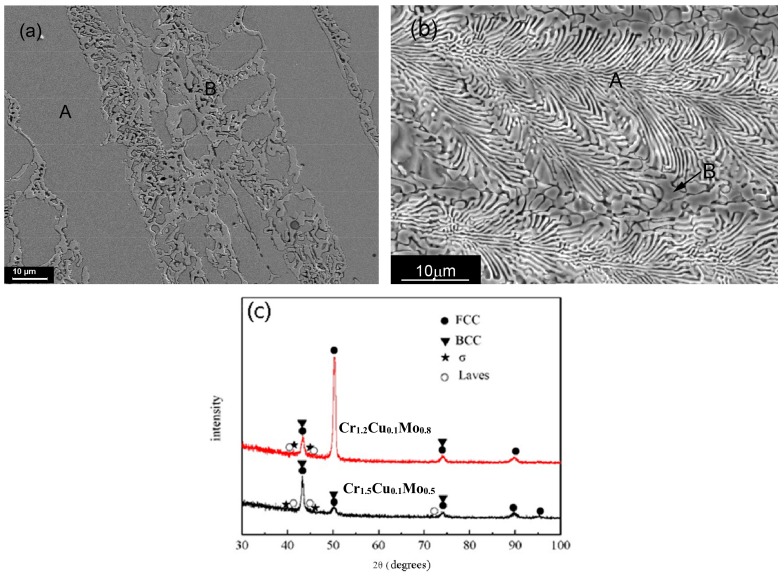
The microstructures of CoCrbCu0.1FeMo2−bNi (b = 1.2 and 1.5) alloys are shown in Figure 2. Dendrites and inter-dendritic regions remain in the CoCr1.2Cu0.1FeMo0.8Ni alloy (referred to as Cr1.2Cu0.1Mo0.8). The dendrites are enriched in Fe and Ni, and the Cr content in the dendrites is increased (A in Figure 2a), while the inter-dendritic region (B) is enriched with Co and Mo. However, for the CoCr1.5Cu0.1FeMo0.5Ni alloy (referred to as Cr1.5Cu0.1Mo0.5), a fully eutectic structure is found, indicating that the alloy has a eutectic composition, probably of (CrMo)52(CoCuFeNi)48, according to region A in Table 2 as detected by EDS. This means that the content of Cr and Mo is 52%, and that of Co, Cu, Fe, and Ni is 48%.
Figure 2.
The SEM micrographs and phase structures of CoCrbCu0.1FeMo2−bNi alloys. (a) CoCr1.2Cu0.1FeMo0.8Ni; (b) CoCr1.5Cu0.1FeMo0.5Ni; (c) XRD patterns.
It is apparent that CoCrbCu0.1FeMo2−bNi (b = 1.2 and 1.5) alloys contain FCC, BCC, and TCP phases according to the XRD patterns, as shown in Figure 2c. The TCP phases correspond to the tetragonal σ phase (a = 0.917 nm, c = 0.474 nm) and a hexagonal close-packed (HCP) Laves phase (a = 0.473 nm, c = 0.772 nm). As can be seen in Figure 2c, the volume fraction of the BCC phase increases with increasing Cr, which enhances the formation of BCC phase in CoCrbCu0.1FeMo2−bNi alloys (b = 1.2 and 1.5). With increasing Cr and decreasing Mo, the BCC phase appears, and the volume fraction of both TCP phases decreases. According to the EDS and XRD results, the dendrites should be FCC, while the eutectic structures include BCC, σ, and Laves phases.
3.3. CoCrCu0.1FecMo2−cNi Alloys
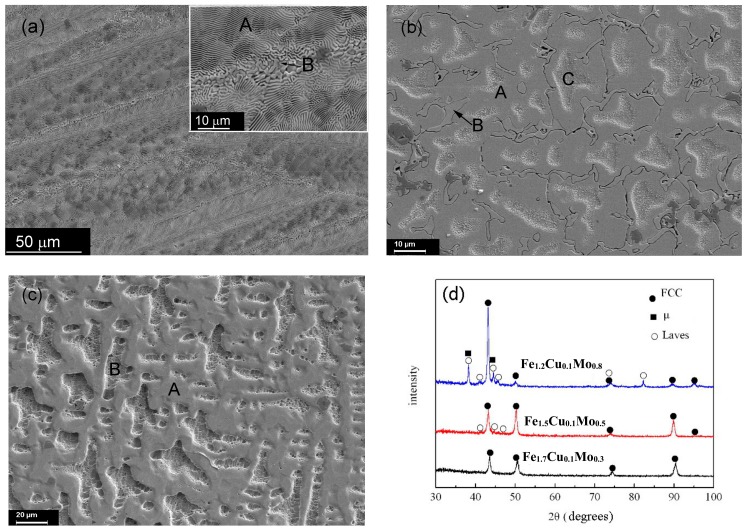
Figure 3 presents the microstructures of the CoCrCu0.1FecMo2−cNi (c = 1.2, 1.5 and 1.7) HEAs. A fully eutectic structure is obtained in the CoCrCu0.1Fe1.2Mo0.8Ni alloy (referred to as Cu0.1Fe1.2Mo0.8). As shown in Table 2, the composition of the eutectic region is approximately (CrMo)51(CoCuFeNi)49. Similar to the Co1.5Cu0.1Mo0.5 and Co1.7Cu0.1Mo0.3 alloys, the microstructures of the CoCrCu0.1Fe1.5Mo0.5Ni (referred to as Cu0.1Fe1.5Mo0.5) and CoCrCu0.1Fe1.7Mo0.3Ni (referred to as Cu0.1Fe1.7Mo0.3) alloys comprise dendritic and inter-dendritic regions. The volume fraction of the inter-dendritic eutectic region decreases dramatically with increasing Fe and decreasing Mo contents. The dendrites (region A) of the Cu0.1Fe1.5Mo0.5 alloy is enriched in Co, Cu, Fe, and Ni; the content of Cr and Mo is higher in the inter-dendritic regions B and C. Region A of the Cu0.1Fe1.7Mo0.3 alloy is enriched in Mo; the content of Cu, Co, and Fe is higher in region B; and the contents of Cr and Ni are almost the same.
Figure 3.
Micrographs and XRD patterns of CoCrCu0.1FecMo2−cNi HEAs: (a) Cu0.1Fe1.2Mo0.8; (b) Cu0.1Fe1.5Mo0.5; (c) Cu0.1Fe1.7Mo0.3; (d) XRD patterns.
The XRD patterns of the CoCrCu0.1FecMo2−cNi (c = 1.2, 1.5, 1.7) HEAs are shown in Figure 3d. FCC, μ (Fe7Mo3), and Laves phases are found in these alloys. The μ phase is trigonal (a = 0.7381 nm, c = 18.504 nm) and the Laves phase is HCP with a = 0.473 nm and c = 0.772 nm). Based on the intensities of the diffraction peaks, decreased Mo and increased Fe contents yield decreases in the volume fractions of the μ and Laves phases and increases in that of the FCC phase. For the Cu0.1Fe1.2Mo0.8 alloy, the FCC, μ, and Laves phases form a eutectic structure. For the Cu0.1Fe1.5Mo0.5 alloy, region A is FCC, region B should be the μ (Fe7Mo3) phase, and region C is probably FCC. For the Cu0.1Fe1.7Mo0.3 alloy, both region A and B are FCC structures with different contents.
3.4. CoCrCu0.1FeMo2−dNid Alloys
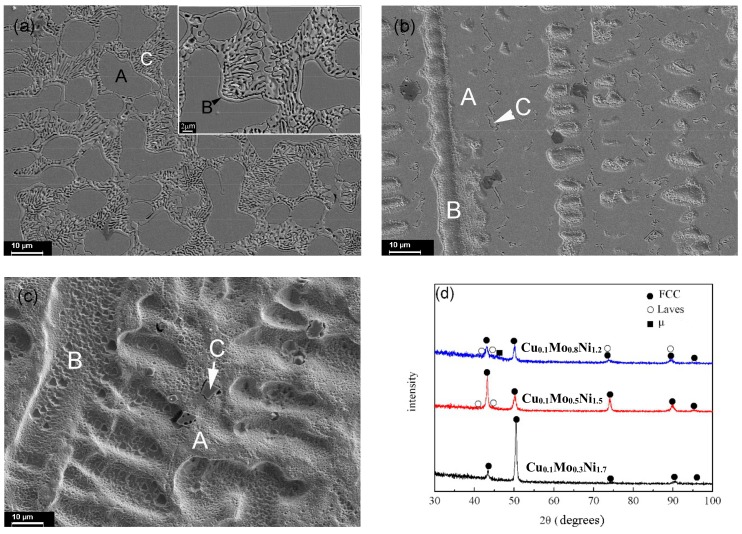
A similar microstructure, consisting of a dendritic matrix and inter-dendritic regions, is found in CoCrCu0.1FeMo2−dNid (d = 1.2, 1.5 and 1.7) HEAs, as shown in Figure 4. For the Cu0.1Mo0.8Ni1.2 alloy, the dendrites are enriched in Co, Cu, Fe, and Ni (Region A in Figure 4a), while the inter-dendritic region (B) is enriched with Cr and Mo. The composition of the eutectic region is found to be approximately (CrMo)54(CoCuFeNi)46, as shown in Table 2. For the Cu0.1Mo0.5Ni1.5 alloy, region A is enriched in Cu and Mo, region B has a higher content of Cr, Co, Fe, and Ni, and region C is enriched with Cr and Mo. Many flower-like structures with four petals (labeled C) are observed in the Cu0.1Mo0.3Ni1.7 alloy; these structures are enriched with Cr.
Figure 4.
Microstructures and XRD patterns of the CoCrCu0.1FeMo2−dNid HEAs. (a) Cu0.1Mo0.8Ni1.2; (b) Cu0.1Mo0.5Ni1.5; (c) Cu0.1Mo0.3Ni1.7; (d) XRD patterns.
The XRD results demonstrate that the alloys contain a trigonal μ phase (a = 0.7381 nm, c = 18.504 nm), FCC phase, and a small amount of an HCP Laves phase (a = 0.473 nm, c = 0.772 nm), as can be seen in Figure 4d. When the molar ratio of Ni is increased to 1.7, only the FCC phase is found in the solidified microstructure. Thus, it is demonstrated that Ni promotes the formation of the FCC phase. For the Cu0.1Mo0.8Ni1.2 alloy, FCC is the dendritic phase, while the μ and Laves phases form a eutectic structure. For the Cu0.1Mo0.5Ni1.5 alloy, both regions A and B are FCC structures, and region C should be the μ phase. For the Cu0.1Mo0.3Ni1.7 alloy, both regions A and B are FCC structures with different contents, while region C is an unknown Cr-rich phase that cannot be detected because of its small amount.
4. Discussion
Two eutectic phases are found in the Cr1.5Cu0.1Mo0.5 and Cu0.1Fe1.2Mo0.8 HEAs, with probable eutectic compositions of (CrMo)51–54(CoCuFeNi)46–49. Similarly, fully eutectic structures have been obtained in CoFeNixVMoy HEAs at both CoFeNi1.4VMo and CoFeNiVMo0.6 [14]. Recently, Lu et al. [34] have proposed a strategy to design eutectic high-entropy alloys (EHEAs) based on ΔHmix. They selected Zr, Nb, Hf, and Ta to replace Al in a previous AlCoCrFeNi2.1 EHEA, based on the relationship of ΔHmix for various atomic pairs. Unfortunately, no regularities have yet been found in the current HEA system. Further research is ongoing to clarify this relationship in the future.
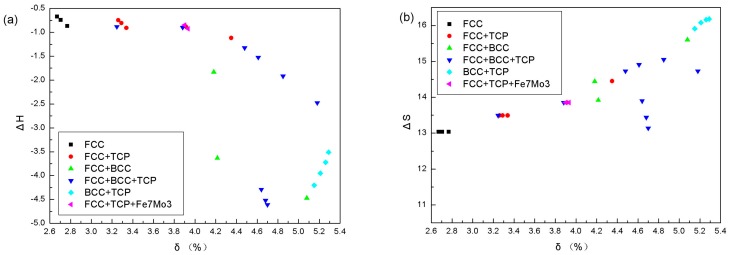
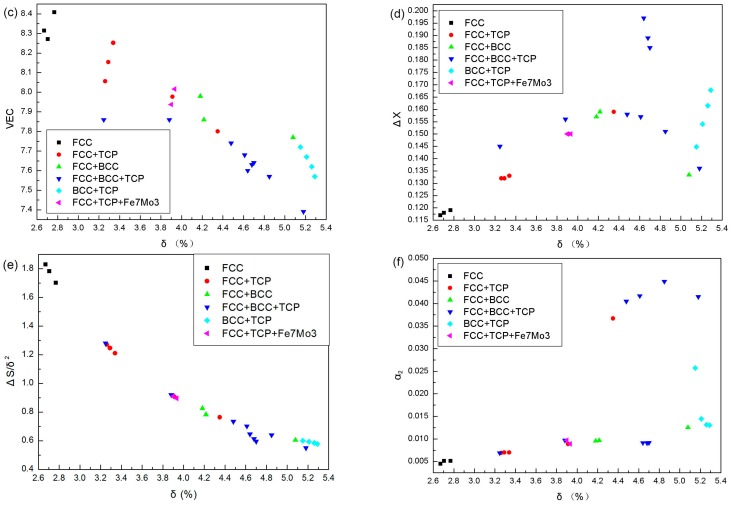
The phase selection mechanism in the CoCrCu0.1FeMoNi-based HEAs can be understood using the parameters listed in Table 3. Based on alloy composition, a simple FCC structure is obtained only when the CoCrCu0.1FeMoNi-based HEAs contain higher contents of principal elements, such as Fe/Co/Ni. This suggests that Fe, Co, and Ni are FCC stabilizers in the CoCrCu0.1FeMoNi-based alloys. It can be found that a simple FCC structure is favorable for alloys with the smallest δ, ΔX, and ΔS. Conversely, alloys with large VEC values favor the formation of simple FCC structures, while TCP phases develop in alloys with smaller VEC values. TCP phases are found when the ΔH of the alloys is largely negative, with the exception of Cu0.1Mo0.3Ni1.7. Furthermore, alloys with small α2 favor the formation of a single-phase FCC structure. In the current work, the FCC structure is stable when δ ≤ 2.8, FCC+BCC is favored when 4.1 ≤ δ ≤ 4.3, and FCC or/and BCC + IM is found when 2.8 ≤ δ ≤ 4.1 or δ > 4.3, with the only exception of AlCoCrCuFeNiMo0.2. As shown in Figure 5c, the FCC structure is stable when VEC ≥ 8.27, but there is an overlap between the mixture types of FCC+BCC and FCC or/and BCC + IM. The IM phase is favored when ΔX > 0.133 only with the exceptions of the CoCrCu0.1FeMoNi and CoCrCu0.3FeMoNi alloys.
Table 3.
Phases and parameters of CoCrCu0.1FeMoNi-based HEAs.
| Alloy | δ (%) | VEC | ΔX | ΔH (kJ·mol−1) | ΔS (J·K−1·mol−1) | α2 | ΔS/δ2 | Phases | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Co1.2CrCu0.1FeMo0.8Ni | 3.912 | 7.977 | 0.150 | −0.878 | 13.853 | 0.0089 | 0.9052 | FCC + σ + μ | |
| Co1.5CrCu0.1FeMo0.5Ni | 3.291 | 8.154 | 0.132 | −0.807 | 13.492 | 0.0070 | 1.2457 | FCC + σ + μ | |
| Co1.7CrCu0.1FeMo0.3Ni | 2.705 | 8.271 | 0.118 | −0.740 | 13.037 | 0.0051 | 1.7817 | FCC | |
| CoCr1.2Cu0.1FeMo0.8Ni | 3.882 | 7.860 | 0.156 | −0.897 | 13.853 | 0.0097 | 0.9192 | FCC + BCC + Laves | |
| CoCr1.5Cu0.1FeMo0.5Ni | 3.247 | 7.860 | 0.145 | −0.882 | 13.492 | 0.0069 | 1.2797 | FCC + BCC + σ + Laves | |
| CoCrCu0.1Fe1.2Mo0.8Ni | 3.900 | 7.938 | 0.150 | −0.847 | 13.853 | 0.0097 | 0.9108 | FCC + Laves + Fe7Mo3 | |
| CoCrCu0.1Fe1.5Mo0.5Ni | 3.260 | 8.056 | 0.132 | −0.745 | 13.492 | 0.0069 | 1.2695 | FCC + Laves | |
| CoCrCu0.1Fe1.7Mo0.3Ni | 2.669 | 8.314 | 0.117 | −0.670 | 13.037 | 0.0045 | 1.8301 | FCC | |
| CoCrCu0.1FeMo0.8Ni1.2 | 3.933 | 8.016 | 0.150 | −0.923 | 13.853 | 0.0089 | 0.8956 | FCC + Laves + Fe7Mo3 | |
| CoCrCu0.1FeMo0.5Ni1.5 | 3.339 | 8.252 | 0.133 | −0.907 | 13.492 | 0.0070 | 1.2102 | FCC + Laves | |
| CoCrCu0.1FeMo0.3Ni1.7 | 2.768 | 8.408 | 0.119 | −0.869 | 13.037 | 0.0051 | 1.7016 | FCC | |
| Al0.1CoCrCu0.1FeMo0.9Ni | 4.35 | 7.80 | 0.159 | −1.116 | 14.45 | 0.0367 | 0.7636 | FCC + Laves | [35] |
| Al0.2CoCrCu0.1FeMo0.8Ni | 4.48 | 7.74 | 0.158 | −1.322 | 14.73 | 0.0405 | 0.7339 | FCC + BCC + Laves + σ | [35] |
| Al0.3CoCrCu0.1FeMo0.7Ni | 4.61 | 7.68 | 0.157 | −1.523 | 14.91 | 0.0417 | 0.7016 | FCC + BCC + Laves + σ | [35] |
| Al0.5CoCrCu0.1FeMo0.5Ni | 4.85 | 7.57 | 0.151 | −1.915 | 15.05 | 0.0449 | 0.6398 | FCC + BCC+ Laves + σ | [35] |
| Al0.8CoCrCu0.1FeMo0.2Ni | 5.18 | 7.39 | 0.136 | −2.474 | 14.73 | 0.0415 | 0.5490 | FCC + BCC + σ | [35] |
| AlCoCrCuFeNiMo0.2 | 5.08 | 7.77 | 0.133 | −4.47 | 15.6 | 0.0125 | 0.6045 | FCC + BCC | [16] |
| AlCoCrCuFeNiMo0.4 | 5.15 | 7.72 | 0.145 | −4.2 | 15.91 | 0.0257 | 0.5999 | BCC + α | [16] |
| AlCoCrCuFeNiMo0.6 | 5.21 | 7.67 | 0.154 | −3.95 | 16.08 | 0.0144 | 0.5924 | BCC + α | [16] |
| AlCoCrCuFeNiMo0.8 | 5.26 | 7.62 | 0.162 | −3.72 | 16.16 | 0.0131 | 0.5841 | BCC + α | [16] |
| AlCoCrCuFeNiMo | 5.29 | 7.57 | 0.168 | −3.51 | 16.18 | 0.0130 | 0.5782 | BCC +α | [16] |
| CoCrCu0.1Fe0.15NiMo1.5Mn0.05 | 4.70 | 7.64 | 0.185 | −4.61 | 13.14 | 0.0092 | 0.5948 | FCC + BCC + μ | [13] |
| CoCrCu0.1Fe0.15NiMo1.5Mn0.12 | 4.68 | 7.63 | 0.189 | −4.52 | 13.44 | 0.0091 | 0.6136 | FCC + BCC + μ | [13] |
| CoCrCu0.1Fe0.15NiMo1.5Mn0.3 | 4.64 | 7.60 | 0.197 | −4.29 | 13.90 | 0.0091 | 0.6456 | FCC + BCC + μ | [13] |
| CoCrCu0.1FeNiMo | 4.216 | 7.86 | 0.159 | −3.63 | 13.92 | 0.0097 | 0.7831 | FCC + BCC | [33] |
| CoCrCu0.3FeNiMo | 4.181 | 7.98 | 0.157 | −1.83 | 14.44 | 0.0096 | 0.8260 | FCC + BCC | [33] |
Figure 5.
The relationships between parameters and phase structures.
The results are well fitted with the criterion proposed by Lu et al. As shown in Figure 5b,f, the FCC structure is stable when ΔS ≤ 13.037 and α2 ≤ 0.0051; however, the (FCC+BCC)-type phase-forming ΔS and α2 ranges show overlaps with those of the (FCC or/and BCC + IM)-type. All the calculated values of ΔH are in the range −15 ≤ ΔHmix ≤ 5 kJ/mol (Figure 5a), and except for FCC and BCC, IM phases are still found, indicating that the phase structures of the listed alloys cannot be distinguished by ΔH.
Singh demonstrated that a simple solid solution as obtained when ΔSmix/δ2 > 0.96, IM compounds when ΔSmix/δ2 < 0.24, and a mixture thereof when 0.24 < ΔSmix/δ2 < 0.96 [36]. As can be seen in Figure 5e, a large ΔS/δ2 value favors the formation of a single FCC phase. As the value of ΔS/δ2 decreases, more phases appear, and smaller ΔS/δ2 values favor the BCC phase. For CoCrCu0.1FeMoNi-based alloys, the simple FCC phase structure is favored when ΔS/δ2 > 1.7, while multiphase structures containing (FCC or/and BCC + IM) are found when 0.549 ≤ ΔS/δ2 ≤ 1.28, and the (FCC+BCC)-type phase-forming ΔS/δ2 range shows an overlap with that of the (FCC or/and BCC + IM)-type. The former famous criterion for phase-forming in HEAs cannot be used effectively in this system. Thus, new rules or parameters must be considered in the future.
5. Conclusions
Eutectic or dendritic microstructures were observed in as-solidified CoCrCu0.1FeMoNi-based HEAs. A fully eutectic microstructure was found in CoCr1.5Cu0.1FeMo0.5Ni and CoCrCu0.1Fe1.2Mo0.8Ni alloys. TCP phases were detected in most of the CoCrCu0.1FeMoNi-based HEAs except for the Co1.7CrCu0.1FeMo0.3Ni, CoCrCu0.1Fe1.7Mo0.3Ni, and CoCrCu0.1FeMo0.3Ni1.7 alloys. A simple FCC single-phase solid solution was obtained when the molar ratio of Fe, Co, and Ni was increased to 1.7 at the expense of Mo. This indicated that Fe, Co, and Ni are FCC stabilizers in the CoCrCu0.1FeMoNi-based alloy system.
Moreover, a simple FCC structure was found in the alloys with the smallest δ, ΔX, and ΔS values. Conversely, alloys with higher VEC were simple FCC structures, while TCP phases appeared to develop in alloys with decreased VEC. TCP phases were found with large negative ΔH values, with the exception of the Cu0.1Mo0.3Ni0.7 alloy. Furthermore, the value of α2 is smaller when a simple FCC structure is obtained.
For CoCrCu0.1FeMoNi-based alloys, the FCC structure was stable when δ ≤ 2.8, VEC ≥ 8.27, ΔS ≤ 13.037, α2 ≤ 0.0051, and ΔS/δ2 > 1.7; the mixture of FCC+BCC is favored when 4.1 ≤ δ ≤ 4.3 while the (FCC or/and BCC + IM) mixture is found when 2.8 ≤ δ ≤ 4.1 or δ > 4.3. IM phases are favored when ΔX > 0.133. However, some overlap remained in parameters including VEC, ΔS, α2, and ΔS/δ2. This indicated that these parameters are not sufficient to distinguish (FCC or/and BCC + IM) from (FCC+BCC) phase formation behaviors, and new rules or parameters must be considered for the described system. Moreover, ΔH could not predict phase structures in the current work. In summary, the phase selection behaviors in CoCrCu0.1FeMoNi-based HEAs can be well delineated by δ and ΔX.
Author Contributions
Conceptualization, N.L.; Methodology, C.C. and P.Z.; Software, C.C. and X.W; Validation, N.L., C.C., I.C., P.Z. and X.W.; Formal Analysis, X.W.; Investigation, N.L. and C.C.; Resources, N.L.; Data Curation, C.C.; Writing-Original Draft Preparation, N.L. and C.C.; Writing-Review & Editing, I.C.; Project Administration, N.L.; Funding Acquisition, N.L.
Funding
This research was funded by the National Natural Science Foundation of China grant number [51201072, 51471079] and the Graduate Student Innovation Projects of Jiangsu Province (KYCX18-2318).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cantor B., Chang I.T.H., Knight P., Vincent A.J.B. Microstructure development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 2004;375:213–218. doi: 10.1016/j.msea.2003.10.257. [DOI] [Google Scholar]
- 2.Miracle D.B., Senkov O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017;122:488–511. doi: 10.1016/j.actamat.2016.08.081. [DOI] [Google Scholar]
- 3.Gorsse S., Miracle D.B., Senkov O.N. Mapping the world of complex concentrated alloys. Acta Mater. 2017;135:177–187. doi: 10.1016/j.actamat.2017.06.027. [DOI] [Google Scholar]
- 4.Senkov O.N., Rao S., Chaput K.J., Woodward C. Compositional effect on microstructure and properties of NbTiZr-based complex concentrated alloys. Acta Mater. 2017;122:488–511. doi: 10.1016/j.actamat.2018.03.065. [DOI] [Google Scholar]
- 5.Yurchenko N.Y., Stepanov N.D., Zherebtsov S.V., Tikhonovsky M.A., Salishchev G.A. Structure and mechanical properties of B2 ordered refractory AlNbTiVZrx (x = 0–1.5) high-entropy alloys. Mater. Sci. Eng. A. 2017;704:82–90. doi: 10.1016/j.msea.2017.08.019. [DOI] [Google Scholar]
- 6.Stepanov N.D., Shaysultanov D.G., Chernichenko R.S., Ikornikov D.M., Zherebtsov S.V. Mechanical properties of a new high entropy alloy with a duplex ultra-fine grained structure. Mater. Sci. Eng. A. 2018;728:82–90. doi: 10.1016/j.msea.2018.04.118. [DOI] [Google Scholar]
- 7.Yurchenko N.Y., Stepanov N.D., Gridneva A.O., Mishunin M.V., Salishchev G.A., Zherebtsov S.V. Effect of Cr and Zr on phase stability of refractory Al-Cr-Nb-Ti-V-Zr high-entropy alloys. J. Alloys Compd. 2018;757:403–414. doi: 10.1016/j.jallcom.2018.05.099. [DOI] [Google Scholar]
- 8.Wang Z.J., Guo S., Liu C.T. Phase Selection in High-Entropy Alloys: From Nonequilibrium to Equilibrium. JOM. 2014;66:1966–1972. doi: 10.1007/s11837-014-0953-8. [DOI] [Google Scholar]
- 9.Lu Z.P., Wang H., Chen M.W., Baker I., Yeh J.W., Liu C.T., Nieh T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics. 2015;66:67–76. doi: 10.1016/j.intermet.2015.06.021. [DOI] [Google Scholar]
- 10.Lu Y., Dong Y., Guo S., Jiang L., Kang H., Wang T., Wen B., Wang Z., Jie J., Cao Z., et al. A promising new class of high-entropy alloys: Eutectic high-entropy alloys. Sci. Rep. 2014;4:6200. doi: 10.1038/srep06200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo S., Ng C., Liu C.T. Anomalous solidification microstructures in Co-free AlxCrCuFeNi2 High-Entropy Alloys. J. Alloys Compd. 2013;557:77–81. doi: 10.1016/j.jallcom.2013.01.007. [DOI] [Google Scholar]
- 12.Liu N., Wu P.H., Peng Z., Xiang H.F., Chen C., Wang X.J., Zhang J. Microstructure, phase stability and properties of CoCr0.5CuxFeyMoNi compositionally complex alloys. Mater. Sci. Technol. 2017;133:210–214. doi: 10.1080/02670836.2016.1177985. [DOI] [Google Scholar]
- 13.Wu P.H., Peng Z., Liu N., Niu M., Zhu Z.X., Wang X.J. The Effect of Mn Content on the Microstructure and Properties of CoCrCu0.1Fe0.15Mo1.5MnxNi near Equiatomic Alloys. Mater. Trans. 2016;57:5–8. doi: 10.2320/matertrans.M2015295. [DOI] [Google Scholar]
- 14.Jiang L., Cao Z.Q., Jie J.C., Zhang J.J., Lu Y.P., Wang T.M., Li T.J. Effect of Mo and Ni elements on microstructure evolution and mechanism properties of the CoFeNixVMoy high entropy alloys. J. Alloys Compd. 2015;649:585–590. doi: 10.1016/j.jallcom.2015.07.185. [DOI] [Google Scholar]
- 15.He F., Wang Z.J., Cheng P., Wang Q., Li J.J., Dang Y.Y., Wang J.C., Liu C.T. Designing eutectic high entropy alloys of CoCrFeNiNbx. J. Alloys Compd. 2015;656:284–289. doi: 10.1016/j.jallcom.2015.09.153. [DOI] [Google Scholar]
- 16.Zhu J.M., Zhang H.F., Fu H.M., Wang A.M., Li H., Hu Z.Q. Microstructures and compressive properties of multicomponent AlCoCrFeNiMox alloys. Mater. Sci. Eng. A. 2010;527:6975–6979. doi: 10.1016/j.msea.2010.07.028. [DOI] [Google Scholar]
- 17.Zhu J.M., Fu H.M., Zhang H.F., Wang A.M., Li H., Hu Z.Q. Microstructures and compressive properties of multicomponent AlCoCrCuFeNiMox alloys. J. Alloys Compd. 2010;497:52–56. doi: 10.1016/j.jallcom.2010.03.074. [DOI] [Google Scholar]
- 18.He F., Wang Z.J., Zhu M., Li J.J., Dang Y.Y., Wang J.C. The phase stability of Ni2CrFeMox multi-principal-component alloys with medium configurational entropy. Mater. Des. 2015;85:1–6. doi: 10.1016/j.matdes.2015.06.174. [DOI] [Google Scholar]
- 19.Hsu C.Y., Juan C.C., Wang W.R., Sheu T.S., Chen S.K., Yeh J.W. On the superior hot hardness and softening resistance of AlCoCrxFeMo0.5Ni high-entropy alloys. Mater. Sci. Eng. A. 2011;528:3581–3588. doi: 10.1016/j.msea.2011.01.072. [DOI] [Google Scholar]
- 20.Hsu C.Y., Sheu T.S., Wang W.R., Tang W.Y., Chen S.K., Yeh J.W. Microstructure and Mechanical Properties of New AlCoxCrFeMo0.5Ni High-Entropy Alloys. Adv. Eng. Mater. 2010;12:44–49. doi: 10.1002/adem.200900171. [DOI] [Google Scholar]
- 21.Liu W.H., Lu Z.P., He J.Y., Luan J.H., Wang Z.J., Liu B., Liu Y., Chen M.W., Liu C.T. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016;116:332–342. doi: 10.1016/j.actamat.2016.06.063. [DOI] [Google Scholar]
- 22.Li X.C., Dou D., Zheng Z.Y., Li J.C. Microstructure and Properties of FeAlCrNiMox High-Entropy Alloys. J. Mater. Eng. Perform. 2016;25:2164–2169. doi: 10.1007/s11665-016-2060-1. [DOI] [Google Scholar]
- 23.Tian L.H., Xiong W., Liu C., Lu S., Fu M. Microstructure and wear behavior of atmospheric plasma-sprayed AlCoCrFeNiTi high-entropy alloy coating. J. Mater. Eng. Perform. 2016;25:5513–5521. doi: 10.1007/s11665-016-2396-6. [DOI] [Google Scholar]
- 24.Zhang Y., Zhou Y.J., Lin J.P., Chen G.L., Liaw P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008;10:534–538. doi: 10.1002/adem.200700240. [DOI] [Google Scholar]
- 25.Guo S., Liu C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. 2011;21:433–446. doi: 10.1016/S1002-0071(12)60080-X. [DOI] [Google Scholar]
- 26.Zhang Y., Yang X., Liaw P.K. Alloy Design and Properties Optimization of High-Entropy Alloys. JOM. 2012;64:830–838. doi: 10.1007/s11837-012-0366-5. [DOI] [Google Scholar]
- 27.Yang X., Zhang Y. Prediction of high-entropy stabilized solid-solution in multicomponent alloys. Mater. Chem. Phys. 2012;132:233–238. doi: 10.1016/j.matchemphys.2011.11.021. [DOI] [Google Scholar]
- 28.Yang X., Chen S.Y., Cotton J.D., Zhang Y. Phase stability of low-density, multiprincipal component alloys containing aluminum, magnesium, and lithium. JOM. 2014;10:2009–2020. doi: 10.1007/s11837-014-1059-z. [DOI] [Google Scholar]
- 29.Wang Z.J., Huang Y.H., Yang Y., Wang J.C., Liu C.T. Atomic-size effect and solid solubility of multicomponent alloys. Scr. Mater. 2015;94:28–31. doi: 10.1016/j.scriptamat.2014.09.010. [DOI] [Google Scholar]
- 30.Tsai M.H., Tsai K.Y., Tsai C.W., Lee C., Juan C.C., Yeh J.W. Criterion for sigma phase formation in Cr- and V-containing high-entropy alloys. Mater. Res. Lett. 2013;1:207–212. doi: 10.1080/21663831.2013.831382. [DOI] [Google Scholar]
- 31.Dong Y., Lu Y., Jiang L., Wang T., Li T. Effect of electro-negativity on the stability of topologically close-packed phase in high entropy alloys. Intermetallics. 2014;52:105–109. doi: 10.1016/j.intermet.2014.04.001. [DOI] [Google Scholar]
- 32.Wang Z.J., Qiu W.F., Yang Y., Liu C.T. Atomic-size and lattice-distortion effects in newly developed high-entropy alloys with multiple principal elements. Intermetallics. 2015;64:63–69. doi: 10.1016/j.intermet.2015.04.014. [DOI] [Google Scholar]
- 33.Wu P.H., Liu N., Yang W., Zhu Z.X., Lu Y.P., Wang X.J. Microstructure and Solidification Behavior of Multi-component CoCrCuxFeMoNi High-entropy alloys. Mater. Sci. Eng. A. 2015;642:142–149. doi: 10.1016/j.msea.2015.06.061. [DOI] [Google Scholar]
- 34.Lu Y., Jiang H., Guo S., Wang T., Cao Z., Li T. A new strategy to design eutectic high-entropy alloys using mixing enthalpy. Intermetallics. 2017;9:124–128. doi: 10.1016/j.intermet.2017.09.001. [DOI] [Google Scholar]
- 35.Wu P.H. Master’s Thesis. Jiangsu University of Science and Technology; Zhenjiang, China: Jun 15, 2016. Microstructure and Solidification Behavior of Multi-Component CoCrCuxFeMoNi High-Entropy Alloys. [Google Scholar]
- 36.Singh A.K., Kumar N., Dwivedi A., Subramaniam A. A geometrical parameter for the formation of disordered solid solutions in multi-component alloys. Intermetallics. 2014;52:105–109. doi: 10.1016/j.intermet.2014.04.019. [DOI] [Google Scholar]