Abstract
Background
Mitochondrial pyruvate import via mitochondrial pyruvate carrier (MPC) is a central step in hepatic gluconeogenesis. Berberine inhibits hepatic gluconeogenesis, but the mechanism is incompletely understood. This study aims to investigate whether berberine could reduce excessive hepatic glucose production (HGP) by limiting mitochondrial import of pyruvate through MPC1.
Methods
High-fat diet (HFD) feeding augmented HGP. The effects of berberine on hepatic fatty acid oxidation, sirtuin3 (SIRT3) induction and mitochondrial pyruvate carrier 1 (MPC1) function were examined.
Findings
HFD feeding increased hepatic acetyl coenzyme A (acetyl CoA) accumulation with impaired pyruvate dehydrogenase (PDH) activity and increased pyruvate carboxylase (PC) induction. Berberine reduced acetyl CoA accumulation by limiting fatty acid oxidation and prevented mitochondrial pyruvate shift from oxidation to gluconeogenesis through carboxylation. Upon pyruvate response, SIRT3 binded to MPC1 and stabilized MPC1 protein via deacetylation modification, facilitating mitochondrial import of pyruvate. Berberine preserved the acetylation of MPC1 by suppression of SIRT3 induction and impaired MPC1 protein stabilization via protein degradation, resultantly limiting mitochondrial pyruvate supply for gluconeogenesis.
Interpretation
Berberine reduced acetyl CoA contents by limiting fatty acid oxidation and increased MPC1 degradation via preserving acetylation, thereby restraining HGP by blocking mitochondrial import of pyruvate. These findings suggest that limitation of mitochondrial pyruvate import might be a therapeutic strategy to prevent excessive hepatic glucose production.
Abbreviations: AMPK, AMP-activated protein kinase; DCA, Dichloroacetate; FACD, Fatty acyl CoA dehydrogenase; G-6-Pase, Glucose-6-phosphatase; HFD, High-fat diet; HGP, Hepatic glucose production; KCT, β-ketoacyl CoA thiolase; LDH, Lactate dehydrogenase; MPC, Mitochondrial pyruvate carrier; NAM, Nicotinamide; NEFAs, Non-esterified free fatty acids; PC, Pyruvic carboxylase; PDH, Pyruvate dehydrogenase; PDK, Pyruvate dehydrogenase kinase; PEPCK, Phosphoenolpyruvate carboxykinase; PGC-1α, Peroxisome proliferator-activated receptor-γ coactivator-1α; SIRT3, Sirtuin3; TC, Total cholesterol; TAC, Tricarboxylic acid cycle; TG, Triglyceride; THP, 3-(2,2,2-Trimethylhydrazine) propionate (also known as mildronate); TMZ, Trimetazidine.
Keywords: Berberine, Gluconeogenesis, Sirtuin3, Mitochondrial pyruvate carrier 1
Research in Context.
Evidence before this Study
Mitochondrial pyruvate carrier is composed of MPC1 and MPC2, and the increased MPC1 expression in diabetes is responsible for hyperglycemia. Hepatic gluconeogenesis is accompanied with altered metabolism and redox state, and MPC1 emerges as a key control to regulated pyruvate-driven glucose production in the liver.
Added Value of This Study
Fatty acid load enhanced hepatic fatty acid oxidation with altered redox state. SIRT3 activation improved MPC1 protein stability via deacetylation, and thus increased MPC1 protein function to import pyruvate into mitochondria, ensuring mitochondrial pyruvate’ availability for hepatic gluconeogenesis. Berberine limited hepatic fatty acid oxidation via mitochondrial complex I inhibition and suppressed SIRT3 activation via reducing NAD+/NADH ratio. Berberine impaired MPC1 stability by preserving acetylation, and thereby reduced MPC1 expression through protein degradation. Although other gluconeogenic substrates also increased hepatic glucose production, berberine mainly restrained pyruvate-driven glucose production in a manner dependent on MPC1 inhibition.
Implications of All Available Evidence
Our work suggests that pharmacological inhibition of MPC1 induction by metabolic regulation could reduce excessive hepatic glucose output in diabetes.
Alt-text: Unlabelled Box
1. Introduction
Blood glucose levels fluctuate during the feeding and fasting cycles and the glycemic control is maintained within a physiological range by the regulation of glucose production and disposal [1]. While insulin prevents postprandial hyperglycemia by promoting glucose disposal, the glucose homeostasis during fasting is mainly maintained by hepatic gluconeogenesis. However, inappropriate hepatic glucose production (HGP) is a major contributor that results in fasting hyperglycemia, especially in diabetes [2]. The vast majority of gluconeogenic carbon flux is routed through the mitochondrial matrix, while pyruvate is thought to be the major mitochondrially imported substrate for hepatic glucose production [3]. Gluconeogenesis via pyruvate/lactate predominates during prolonged food deprivation and this regulation is deranged in the diabetic liver, contributing to excessive HGP [4]. Therefore, limitation of mitochondrial pyruvate availability for substrate supply should prevent fasting hyperglycemia in diabetes.
Cytoplasmic pyruvate is mainly derived from glycolysis, while systemically produced lactate and alanine are also the sources from different mechanisms. The import of pyruvate into mitochondria is mediated by mitochondrial pyruvate carrier (MPC). MPC is composed of MPC1 and MPC2, which form a hetero-oligomeric complex in the inner mitochondrial membrane, and both proteins are required for the complex stabilize and full activity [5, 6]. Once in mitochondria, pyruvate is either channeled toward carboxylation by the enzyme pyruvate carboxylase (PC) for gluconeogenesis or oxidation by the enzyme pyruvate dehydrogenase (PDH). The role of MPC in mitochondrial pyruvate import is responsible for pancreatic β cell glucose sensing [7]. Thiazolidinediones (TZDs) are the most effective agents for preventing hyperglycemia in type 2 diabetes and unexpectedly found to inhibit MPC in muscle cells in a manner independent of PPARγ [8]. Recently, it is documented that pyruvate-driven hepatic gluconeogenesis is MPC dependent, and liver-specific loss of MPC activity impairs gluconeogenesis and decreases hyperglycemia in obesity and diabetes [9, 10]. Although other substrates, such as alanine and glutamine, can serves as gluconeogenic carbon flux in mitochondrial matrix, independent of MPC transportation [9, 10], it is now generally accepted that mitochondrial pyruvate import via the MPC is a central step in hepatic gluconeogenesis.
Hepatic gluconeogenesis is an anabolic process and needs fatty acid oxidation to supply energy [11]. Fatty acid mobilization increases hepatic fatty acid oxidation, and abnormal fatty acid metabolism has a profound impact on hepatic metabolism, leading to lipid deposition, hepatic insulin resistance and glucose overproduction [12, 13]. However, the potential implication of hepatic fatty acid oxidation in disturbed gluconeogenesis is little known. Adipose lipolysis-derived non-esterified fatty acids (NEFAs) enter into the liver and increase hepatic acetyl CoA to impair insulin sensitivity [12, 13]. More than a metabolic intermediate, acetyl CoA acts as a second messenger to inhibit pyruvate dehydrogenase (PDH) activity [14] and allosterically activate pyruvate carboxylase (PC) which catalyzes the irreversible carboxylation of pyruvate to oxaloacetate [15]. Logically, this regulation should facilitate mitochondrial pyruvate to be rerouted away from oxidation toward carboxylation for gluconeogenesis, establishing the functional link between hepatic fatty acid oxidation and hepatic gluconeogenesis.
Protein lysine acetylation is a key post-translational modification in fuel metabolism. Acetyl CoA is the major donor of the acetyl groups for acetylation and lysine acetyltransferases use acetyl CoA as an essential cofactor to donate an acetyl group to the target lysine residue [16]. In contrast, acetylated proteins could be deacetylated by sirtuins (SIRTs), a family of nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylases. Sirtuin 3 (SIRT3) is predominantly localized in the mitochondrial matrix and involved in the metabolic regulation of obesity and diabetes [17, 18]. Although SIRT3 is also shown to deacetylate MPC1 to enhance its activity in cancer cells [19], its action in the regulation of hepatic MPC1 and gluconeogenesis is unknown.
Berberine is an isoquinoline alkaloid isolated from Coptis chinensis and exerts the ability to lower blood glucose in diabetes. Similar to metformin, berberine regulates adenosine monophosphate-activated protein kinase (AMPK) activity, and this action is proposed to inhibit hepatic gluconeogenesis [[20], [21], [22]]. But this concept has been challenged by recently published studies which showed that AMPK is dispensable for the regulation of hepatic gluconeogenesis [23, 24]. Berberine shows anti-hyperlipidemia with reduced hepatic lipid deposition, which has been observed in both animals and human [25, 26], suggesting the possibility that regulation of lipid metabolism by berberine in the liver contributes to restraining HGP. Fatty acid mobilization during fasting and lipid disorders in obese and diabetes increase hepatic fatty acid oxidation to drive gluconeogenesis by raising mitochondrial levels of reducing equivalents and acetyl CoA [27]. Therefore, we hypothesized that improved lipid metabolism and redox homeostasis by berberine should contribute to reducing HGP. In this context, we investigated the effects of berberine on hepatic energy state with focus on the regulation of MPC1 function. We showed that berberine limited fatty acid oxidation and reduced mitochondrial SIRT3 induction, resultantly promoting MPC1 degradation by preserving acetylation. These findings not only identify an unrecognized role of berberine in the regulation of hepatic gluconeogenesis, but also suggest that limitation of mitochondrial pyruvate import might be a therapeutic strategy to reduce excessive hepatic glucose production.
2. Materials and Methods
2.1. Reagents
Berberine hydrochloride (purity ≥ 98%) was obtained from Nantong Jingwei Biological Technology Co, Ltd. Metformin hydrochloride (purity >98%) was purchased from Shanghai Sangon biological engineering Co, Ltd. Resveratrol (purity >98%) was from Nanjing Zelang medical technology co., LTD. Trimetazidine (TMZ) was from British Kinase Chemicals LTD. Mildronate (also known as 3-(2,2,2-Trimethylhydrazine) propionate, THP) was supplied by Dalian Meilun Biological Technology Co, Ltd. Dichloroacetate (DCA) was purchased from Sigma-Aldrich. UK-5099, CPI-613 and nicotinamide (NAM) were from ApexBio Technology. Cycloheximide and MG-132 were from MedChem Express (Shanghai, China). Lactate, alanine, glutamine and methyl pyruvate were from Aladdin (Shanghai, China). Palmitate (PA) was from Sinopharm Chemical Reagent Co, Ltd. (Shanghai, China) and dissolved in ethanol, and then diluted with 10% FFA-free bovine serum albumin (BSA) at the ratio of 1:19 before use.
2.2. Animals
ICR male mice (18–20 g) were purchased from Beijing Vital River Laboratory Animal Technology Co, Ltd. After adaptive feed in 12 h dark-light cycles ad libitum with free access to water and food for a week, mice were randomized to groups to receive berberine (100 mg kg−1 day−1) or metformin (200 mg kg−1 day−1) by gavage and to receive either a normal chow diet or a high-fat diet (HFD) (10% lard, 10% yolk, 1% cholesterol, 0.2% cholate and 78.8% standard diet) for 10 weeks. All experimental procedures were approved by the Institution for Nutritional Sciences Institutional Animal Care and Use Committee, Shanghai Institutes for Biological Sciences, Shanghai University of Traditional Chinese Medicine. At the end of HFD feeding, mice were fasted overnight and blood was collected for the assay of blood glucose, non-esterified free fatty acids (NEFAs), triglyceride (TG) and total cholesterol (TC) contents with commercial kits (Jiancheng Bioengineering Institute, Nanjing, China). Liver were isolated and assayed immediately or stored at minus 80 degrees Celsius until analysis. Liver index were calculated as the ratio of liver weight to the body weight. TG contents in isolated liver tissue were assayed by triglycerides assay Kit (Jiancheng Bioengineering Institute, Nanjing, China).
For fasting and refeeding experiments, normal chow diet-fed mice (22–25 g) were fasted overnight with or without oral administration of berberine (100 mg·kg−1) or metformin (200 mg·kg−1) twice (before fasting and 2 h prior to the treatment), while the refed mice were refed with normal chow diet for another 6 h. Blood was collected and the levels of non-esterified free fatty acids (NEFAs) were measured using Non-Esterified Free fatty acids Assay Kit. TG contents in the liver were determined with triglycerides assay Kit, while diacylglycerol (DAG) contents in the skeletal muscle were assayed with DAG ELISA Kit (Shuojia, Shanghai, China). For the determination of protein kinase B (Akt) phosphorylation (Thr308) in skeletal muscle, mice were orally administrated with glucose 2 g/kg to induce insulin secretion and skeletal muscle was collected 1 h later.
2.3. Glucose, Pyruvate and Glucagon Tolerance Tests
For glucose tolerance test, overnight fasted mice were administered with glucose (2 g·kg−1) by gavage. For pyruvate or glucagon tolerance test, fasted mice intraperitoneally injected with pyruvate (2 g·kg−1) or glucagon (2 mg·kg−1). Blood glucose concentration at regular intervals was assayed using a commercial Kit (Jiancheng Bioengineering Institute, Nanjing, China). Blood glucose area under carve (AUC) was calculated as the follows: 0.5 × [Bg0 + Bg0.5]/2 + 0.5 × [Bg0.5 + Bg1.0]/2 + [Bg1 + Bg2]/2 (Bg0, Bg0.5, Bg1.0 and Bg2.0 referred to the blood glucose content at 0, 0.5, 1.0 and 2.0 h).
2.4. Preparation of Rimary Mouse Hepatocytes
For the preparation of primary mouse hepatocytes, fasted mice were anesthetized and liver was perfused in situ with Hank's Balanced Salt Solution (HBSS) without supplement of Ca2+ & Mg2+ through the hepatic inferior vena cava to remove the blood, followed by digesting with collagenase type IV. The whole liver was then isolated and centrifuged to obtain the hepatocytes. After filtering, the hepatocytes were resuspended and cultured for further treatment in DMEM supplemented with 10% FBS and incubated at 37 °C in an atmosphere of 5% CO2. The purity of hepatocytes was ensured by cytokeratin 18 (CK-18) staining.
2.5. Measurements of Acetyl CoA and Oxaloacetate Concentration
The freshly isolated liver was rapidly homogenized in ice-cold RIPA lysis buffer. The homogenates were then centrifuged and the supernatants were collected for the detection of acetyl CoA and oxaloacetate concentrations using commercial kits (Shuojia, Shanghai, China) according to the manufacturer's instructions. For intracellular acetyl CoA measurement in hepatocytes, primary hepatocytes were incubated with PA (100 μM) for 24 h.
2.6. Detection of Mitochondrial Complex I, II Activity and Fatty Acids β-oxidation Rate
The primary mouse hepatocytes were pretreated with berberine (10 μM) or metformin (1 mM) for 30 min and then exposed to PA for 2 h. The mitochondria were isolated by Cell Mitochondria Isolation Kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's instructions. Colorimetric method was used to quantitatively analyze mitochondrial respiratory chain complex I, II activity and fatty acids β-oxidation rate with commercial kits (GenMed Scientific Inc., USA), and the results were normalized by corresponding mitochondrial protein contents according to the manufacturer's instructions. Data were expressed as relative activity normalized to the control group.
2.7. Measurement of Hepatic Lactate/Pyruvate and NAD+/NADH Ratio
The lactate and pyruvate levels in the liver were examined according to the manufacturer's instructions for the lactate and pyruvate assay kit (Jiancheng, Nanjing, China). The NAD+/NADH ratio in the liver was assayed using an NAD/NADH Quantitation Kit (Sigma-Aldrich) according to the manufacturer's instructions. For the measurement of lactate production in hepatocytes, cells were treated with indicative agents in the presence of 10 mM pyruvate (Sigma) for 8 h. The cell culture medium was collected and assayed immediately for lactate. To measure the ratio of NAD+/NADH in hepatocytes, cells were treated with indicated agents in the presence of 10 mM pyruvate, lactate, alanine and glutamine for 8 h or 100 μM PA for 24 h. The cells were collected and assayed for NAD+/NADH ratio.
2.8. Assay of Hepatic Glucose Production
The primary hepatocytes isolated from mice (fasting overnight) or pcDNA3.1-MPC1 transfected HepG2 cells were cultured in DMEM medium supplemented with 10% FBS and then pretreated with indicated agents followed by 100 μM PA insult for 24 h. After washing, the medium was replaced by glucose-free medium supplemented with 10 mM pyruvate or 10 mM methyl pyruvate for 6 h. Glucose contents in the medium were assayed. For the assay of hepatic glucose production in the presence of different substrates, the primary hepatocytes were cultured with glucose-free medium supplemented with 10 mM pyruvate, lactate, alanine and glutamine for 6 h, Glucose contents in the medium were detected.
2.9. Oxygen Consumption Rate (OCR) Assay
Pyruvate-supported respiration was measured using a XF96 Extracellular Flux analyzer (Seahorse Bioscience, North Billerica, MA). HepG2 cells (Cell Bank of Chinese Academy of Sciences, Shanghai, China) were seeded at 10,000 cells/well in XF96 microplates (Seahorse Bioscience) and incubated overnight in DMEM supplemented with 10% FBS. Cells were treated with berberine (10 μM), metformin (1 mM) or UK-5099 (10 μM) for 2 h prior to the analysis in no-glucose DMEM containing 10 mM sodium pyruvate as the sole respiratory substrate. The oxygen consumption rate (OCR) was measured by injections of oligomycin (0.5 μM), FCCP (2.5 μM), and rotenone (0.5 μM) + antimycin A (0.5 μM) at indicated time points (XF Cell Mito Stress Test Kit; Seahorse Bioscience). Experimental treatments were performed on 6 wells of each plate as biological replicates.
2.10. HepG2 Cell Transfection
Three SIRT3 small interfering RNAs (siRNA), pcDNA3.1-SIRT3 and pcDNA3.1-MPC1 synthesized and provided by Genomeditech Co., Ltd. (Shanghai, China) were transfected into HepG2 cells using Lipofectamine™ 2000 Transfection Reagent (Invitrogen) according to the manufacturer's instructions. A control siRNA and pcDNA3.1 were used as a control. After transfection for 48 h, the cells were treated with berberine (10 μM) in the presence of pyruvate (10 mM) for 8 h. Lactate production, MPC1 protein expression and MPC1 acetylation were examined.
2.11. Western Blotting Analysis
Hepatocytes or liver tissue were lysed with ice-cold RIPA buffer and the protein extraction were immunoblotted to analysis the protein expression. The protein lysates were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore Co, Ltd.). Immunoblotting was performed according to standard procedures with the following primary antibodies: G-6-Pase (sc-15840, 1:500) and SIRT3 (sc-365175, 1:500) antibodies were from Santa Cruz Biotechnology, Inc. (Santa, CA, USA), antibodies for PEPCK (ab70358, 1:1000), PC (ab128952, 1:1000), p-PDH (S293) (ab177461, 1:1000) were from Abcam (Cambridge, MA, USA), antibodies of MPC1 (14462, 1:1000), PDH (2784, 1:1000) and Acetylated-Lysine (9441, 1:1000) were obtained from Cell Signaling Technology (Beverly, MA, USA), VDAC1 (CY5416, 1:1000) antibody was from Abways Technology, Inc. and antibodies of GAPDH (AP0063, 1:3000), Goat Anti-Rabbit IgG (H + L) HRP (BS13278, 1:10000) and Goat Anti-Mouse IgG (H + L) HRP (BS12478, 1:10000) were from Bioworld Technology (St. Paul, MN, USA). To determine the mitochondrial acetylated-lysine in the liver, mitochondrial protein was prepared with Tissue Mitochondria Isolation Kit (Beyotime Institute of Biotechnology, Shanghai, China) as manufacturer's instructions.
2.12. Immunoprecipitation
Primary mouse hepatocytes or transfected HepG2 cells were pretreated with indicated agents and then incubated with 100 μM PA for 24 h or 10 mM pyruvate for 8 h. After treatment, cells were washed with ice-cold PBS and lysed with RIPA lysis buffer. Cell lysates were collected and control IgG antibody (Beyotime Institute of Biotechnology, Shanghai, China) or anti-MPC1 antibody was immunoprecipitated. Standard immunoprecipitation procedures were performed according to the protocols detailed by Beyotime Institute of Biotechnology (Shanghai, China). Acetylated-Lysine, SIRT3 and MPC1 expressions were analysis by western blotting.
2.13. Immunofluorescence
To observe the combination of SIRT3 and MPC1 in mitochondria, the hepatocytes were pretreated with berberine for 30 min and then incubated with 10 mM pyruvate for 8 h. After treatment, the cells were incubated with 200 nM Mito Tracker Red CMXRos (Invitrogen) for 40 min at 37 °C. After fixing in the 4% paraformaldehyde for 20 min, the cells were permeabilized with 0.3% Triton X100 for 15 min and then incubated with 3% BSA for 2 h, followed by incubating with primary antibodies (anti-MPC1 and SIRT3) overnight at 4 °C in a humidified chamber. After washings, the cells were incubated with Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) and Goat Anti-Mouse IgG H&L (Alexa Fluor® 647) for 1 h at 37 °C and the images were visualized using confocal microscopy (TCS SP8, Leica, Germany).
2.14. Detection of 2-NBDG Uptake in C2C12 Cells
C2C12 mouse myoblasts (Cell Bank of Chinese Academy of Sciences, Shanghai, China) were cultured in DMEM with 10% FBS and differentiated into myotubes by replacing the growth medium with differentiation medium (DMEM supplemented with 2% horse serum) for 7 days. For glucose uptake in skeletal muscle cells, differentiated C2C12 cells were pretreated with berberine (10 μM) and metformin (1 mM) for 30 min and then cultured with PA (100 μM) in non-glucose DMEM for 2 h. After washing, the medium was replaced by 2-NBDG probe (500 μM, Invitrogen, Oregon, USA) with insulin (0.1 μM) for another 40 min. Glucose uptake was visualized using Olympus BX43 microscope (Olympus Corporation, Tokyo, Japan).
2.15. Real-time Quantitative RT-PCR
Real-time quantitative RT-PCR was used to determine the relative expression levels of mRNAs. Total RNA was extracted from liver tissue or primary hepatocytes using Total RNA Extraction Reagent (Yeasen, Shanghai, China) and cDNA was synthesized using the Hieff™ First Strand cDNA Synthesis Kit (Yeasen, Shanghai, China) according to the manufacturers' instructions. Real-time quantitative PCR was performed using Hieff™ SYBR Green Master Mix (Yeasen, Shanghai, China) and the CFX96™ Real-Time PCR Detection System (BIO-RAD, USA). The primer sequences were obtained from Generay Biotechnology (Shanghai, China) and listed in supplementary Table 1. Gene expressions was normalized with β-actin or 18S as housekeeping genes.
2.16. Hematoxylin and Eosin (HE) Staining
Isolated liver of HFD mice was fixed in 4% paraformaldehyde and embedded in paraffin. After deparaffinization and dehydration with xylene and ethanol, liver sections were stained with hematoxylin and eosin, and then the histopathological changes were visualized using Olympus BX43 microscope (Olympus Corporation, Tokyo, Japan).
2.17. Statistical Analyses
The results were expressed as the mean ± SD. The significance of differences was analyzed using one-way ANOVA followed by Tukey test. GraphPad Prism 6.0 was used for statistical analysis. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. Berberine Reduced Endogenous Glucose Production
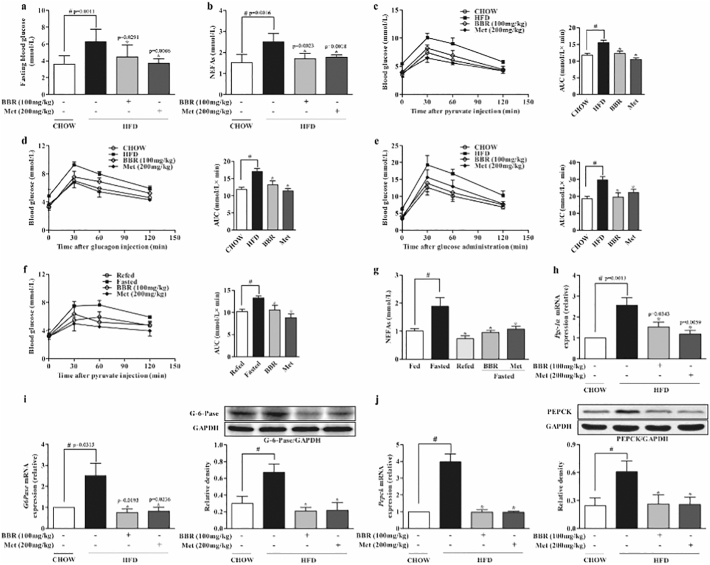
Hepatic glucose output in the fasting state is accompanied with free fatty acid mobilization. In HFD-fed mice berberine administration reduced elevated levels of fast blood glucose (6.25 ± 1.52 mmol/L) and non-esterified fatty acids (NEFAs) (2.50 ± 0.40 mmol/L) by 28.5% and 31.3%, respectively (Fig. 1a, b). Pyruvate tolerance test is an indicator for endogenous glucose production. In response to pyruvate load, the total blood glucose (AUC) in HFD-fed mice was significantly higher (15.51 ± 0.72 mmol/L*min) than that in the chow-fed mice (11.77 ± 0.57 mmol/L*min), indicative of pyruvate intolerance, but this alteration was reversed by berberine (Fig. 1c). Moreover, berberine attenuated hyperglycemic response to glucagon challenge in HFD-fed mice by 22.4% when compared with mice in the control (Fig. 1d). These results well demonstrated the suppressive effect of berberine on endogenous glucose production. HFD feeding also impaired glucose tolerance test, but the increased total blood glucose was reduced by 34.2% in berberine-treated mice (Fig. 1e). Similarly, berberine attenuated the hyperglycemic response to pyruvate load and reduced blood NEFAs in fasted chow-fed mice (Fig. 1f, g). Berberine suppressed Pgc-1α gene expression with downregulation of gene and protein expressions of PEPCK and G-6-Pase in the liver of HFD-fed mice (Fig. 1h–j), indicating that berberine reduced endogenous glucose production via suppression of hepatic gluconeogenesis. Anti-diabetic agent metformin demonstrated a similar regulation as berberine.
Fig. 1.
Berberine reduced endogenous glucose production. (a–b): Fasting blood glucose and NEFAs in HFD-fed mice. (c–e): Pyruvate tolerance, glucagon tolerance and glucose tolerance test in HFD-fed mice. (f): Pyruvate tolerance test in fasted mice. (n = 8). (g): The levels of NEFAs in fasted mice. (h): Hepatic gene expression of Pgc-1α in HFD-fed mice. (i–j): Hepatic gene and protein expressions of PEPCK and G-6-Pase in HFD-fed mice. (BBR, berberine; Met, metformin). Date were showed as mean ± SD (n = 5–6). ⁎p < 0.05 vs. HFD or fasted mice; #p < 0.05 vs. chow-fed diet mice.
Besides, oral administration of berberine reduced blood levels of triacylglycerol and total cholesterol in HFD mice. The increases in hepatic index and body weight gain were also reduced by berberine without influence on food intake (Supplementary Fig. 1a–e).
3.2. Berberine Regulated Hepatic Lipid Metabolism
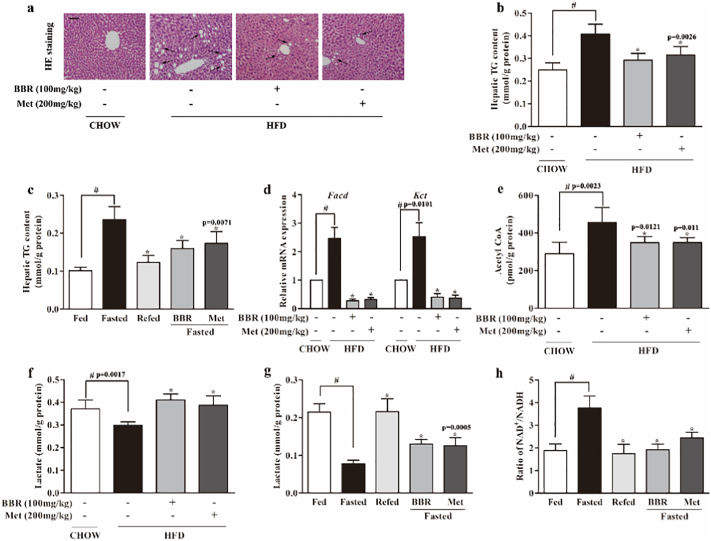
Consistent with elevated levels of circulating NEFAs, HFD increased hepatic lipid deposition, indicated by hepatocellular vacuolation, but this alteration was reversed by berberine (Fig. 2a). Consistently, berberine reduced the increased triacylglycerol contents in the liver from 0.41 ± 0.04 mmole/g protein to 0.29 ± 0.03 mmole/g protein in HFD-fed mice (Fig. 2b). Similarly, berberine reduced hepatic triacylglycerol accumulation by 32.6% in fasted chow-fed mice (Fig. 2c). Fatty acyl CoA dehydrogenase (FACD) and β-ketoacyl CoA thiolase (KCT) are the key enzymes in β-oxidation, and their transcriptional expression increased in the liver of HFD-fed mice, indicating the enhanced β-oxidation upon abundant NEFAs. Oral administration of berberine and metformine attenuated fatty acid oxidation by normalizing gene expressions of Facd and Kct (Fig. 2d). HFD feeding increased acetyl CoA from 290.79 ± 60.15 pmol/g protein to 456.38 ± 79.68 pmol/g protein, but the increased acetyl CoA was reduced by 23.47% in berberine-treated mice (Fig. 2e). Although the concentration of berberine in the blood is low after oral administration, it can accumulate in the liver at the concentration about 2 μM in rats (200 mg kg−1) [28]. Therefore, we observed the effects of berberine in hepatocytes at concentrations ranging from 0.1 to 10 μM. Consistently, in PA-treated primary hepatocytes, berberine concentration-dependently reduced Facd and Kct gene expressions (Supplementary Fig. 2 a, b), reduced mitochondrial fatty acids β-oxidation rate and the activities of mitochondrial complex I and II (Supplementary Fig. 2c–e), and thereby limited acetyl CoA generation by 59.8% at the concentration of 10 μM (Supplementary Fig. 2f). Fatty acid oxidation inhibitor trimetazidine and l-carnitine transport inhibitor mildronate also showed similar effects in PA-treated primary hepatocytes. Since acetyl CoA is an end product of β-oxidation, these results suggested that suppression of fatty acid β-oxidation could prevent acetyl CoA accumulation.
Fig. 2.
Berberine regulated hepatic lipid metabolism. (a): HE stained liver tissue of HFD-fed mice (Bar, 50 μm). The view is one from five independent experiments. (b–c): Triacylglycerol (TG) contents in the liver of HFD-fed mice and fasted mice. (d): Gene expressions of Facd and Kct in the liver of HFD-fed mice. (e): Acetyl CoA contents in the liver of HFD-fed mice. (f): Lactate contents in the liver of HFD-fed mice. (g–h): Lactate contents and NAD+/NADH ratio in the liver of fasted mice. (BBR, berberine, Met, metformin). Data were expressed as the mean ± SD (n = 5–6). ⁎p < 0.05 vs. HFD or fasted mice; #p < 0.05 vs. chow-fed diet mice.
To increase blood glucose during fasting state, gluconeogenesis is increased with decreased glycolysis. In line with this, HFD feeding decreased hepatic lactate generation, but the reduced lactate levels (0.30 ± 0.01 mmol/g protein) were restored to 0.41 ± 0.03 mmol/g protein by berberine treatment (Fig. 2f). In fasted chow-fed mice, berberine also restored lactate accumulation from 0.08 ± 0.01 mmol/g protein to 0.13 ± 0.01 mmol/g protein in the liver (Fig. 2g). Meanwhile, we also observed that NAD+/NADN ratio increased in the liver of fasted chow-fed mice, whereas the change was reversed by berberine and metformin treatment (Fig.2h).
3.3. Berberine Reduced Hepatic MPC1 Induction With Regulation of PDH and PC
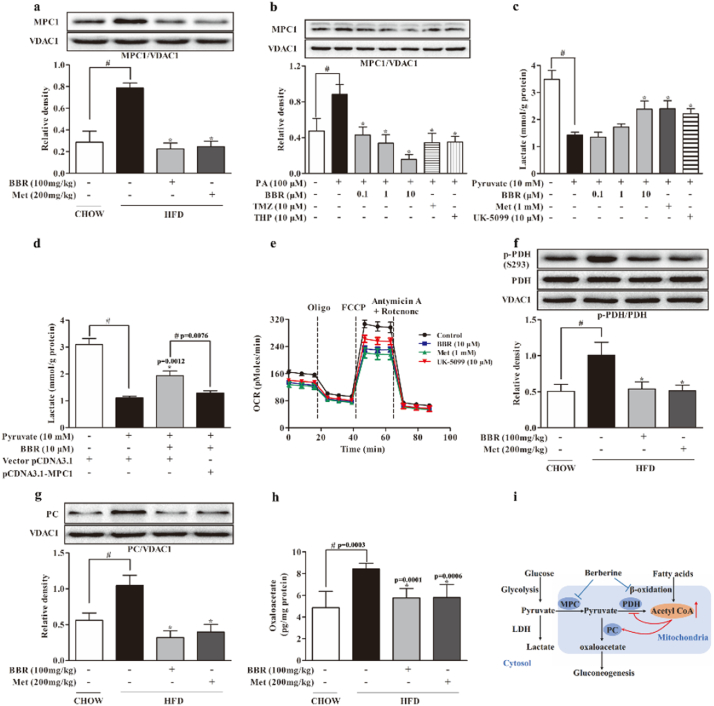
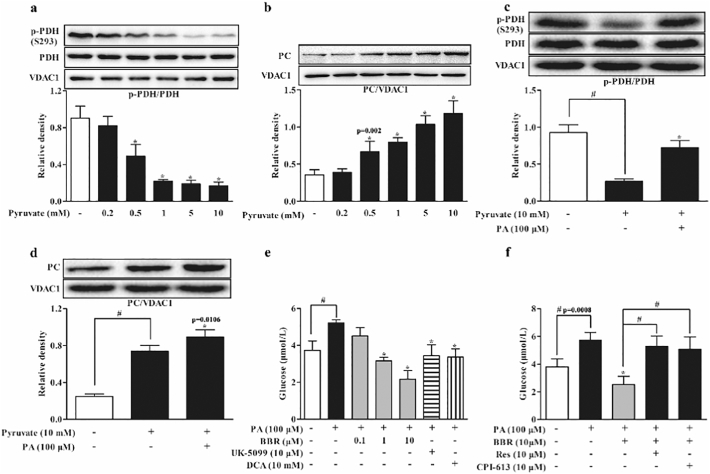
MPC1 is an indispensable subunit for MPC activity. Mitochondrial pyruvate is converted either to acetyl CoA by PDH for oxidation, or to oxaloacetate by PC for carboxylation and subsequent gluconeogenesis. HFD significantly increased MPC1 protein expression by 1.7 folds, but this alteration was normalized by berberine as well as metformin in the liver of HFD-fed mice (Fig. 3a). A similar regulation was also found in hepatocytes when exposed to PA (Fig. 3b). When pyruvate was used as the major fuel for mitochondrial metabolism in hepatocytes, hepatic lactate accumulation was decreased by 59.05%. (Fig. 3c), but the reduced lactate was restored by berberine and metformin treatment. The increased lactate accumulation by berberine should be a result from inhibition of MPC1 function, because the action was blocked by MPC1 overexpression in HepG2 cells (Fig. 3d). Berberine and metformin also reduced mitochondrial oxygen consumption rate (OCR) when pyruvate was added as the substrate (Fig. 3e), indicative of reduced pyruvate oxidation in mitochondria. As an MPC inhibitor, UK-5099 inhibited pyruvate oxidation rate in hepatocytes, indicative of the positive correlation between MPC function and mitochondrial pyruvate oxidation. Berberine and metformin treatment inhibited Pdk4 gene expression (Supplementary Fig. 3a), and then normalized PDH activity by dephosphorylation at Ser 293 in the liver of HFD-fed mice (Fig. 3f). Meanwhile, berberine inhibited PC protein expression, and thus reduced oxaloacetate production by 31.66% in the liver of HFD-fed mice (Fig. 3g, h). In line with the regulation in vivo, berberine inhibited Pdk4 gene expression, preserved PDH activity by dephosphorylation and inhibited PC induction in PA-treated hepatocytes (Supplementary Fig. 3b–d). Fatty acid oxidation inhibitors trimetazidine and mildronate demonstrated similar regulations as berberine. Because the metabolism of mitochondrial pyruvate is competitively regulated by PDH for oxidation or by PC for gluconeogenesis [14, 15], these results raised the possibility that the shift of mitochondrial pyruvate in metabolism could be influenced by fatty acid oxidation (Fig. 3i).
Fig. 3.
Berberine reduced hepatic MPC1 induction with regulation of PDH and PC. (a–b): MPC1 protein expression in the liver of HFD-fed mice and primary mouse hepatocytes incubated with PA for 24 h. (c–d): Lactate production in primary mouse hepatocytes or HepG2 cells transfected with pCDNA3.1-MPC1 in the presence of 10 mM pyruvate for 8 h. (e): Oxygen consumption rate in HepG2 cells pretreated with BBR, Met and UK-5099 for 2 h. (f–h): PDH phosphorylation, PC protein expression and oxaloacetate contents in the liver of HFD-fed mice. (i): The mechanic pathway for berberine to restrain pyruvate-driven hepatic glucose production in the setting of lipid disorder. (BBR, berberine; Met, metformin; TMZ, trimetazidine; THP, mildronate; PA, palmitate). Data were expressed as the mean ± SD (n = 5–6). ⁎p < 0.05 vs. HFD mice, PA or pyruvate-treated cells; #p < 0.05 vs. chow-fed diet mice or untreated cells.
3.4. Berberine Reduced SIRT3 Induction and Preserved MPC1 Acetylation in the Liver
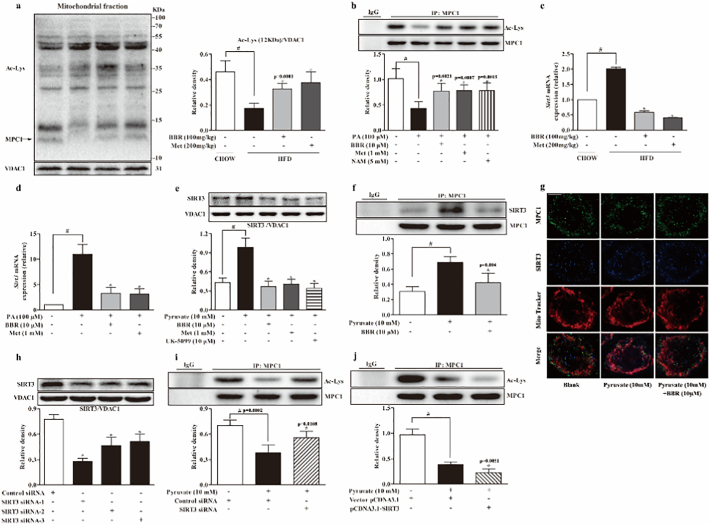
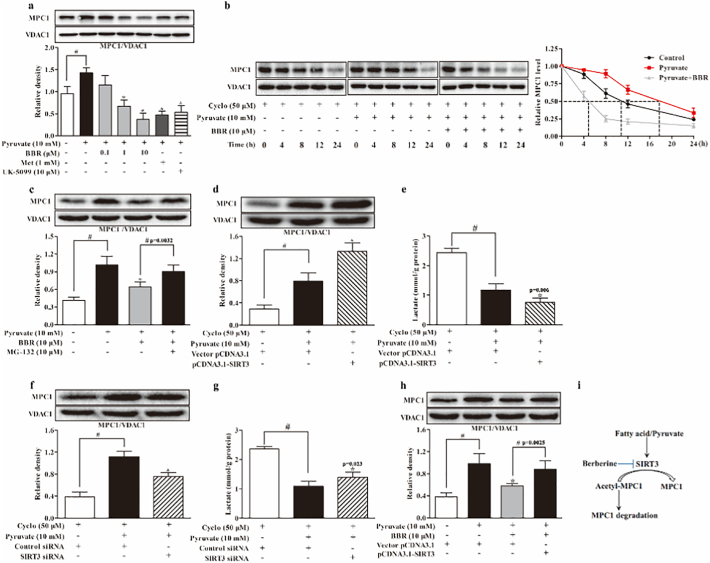
Theoretically, acetyl CoA accumulation upon hepatic fatty acid load should increase the acetylation of mitochondrial proteins. We investigated the acetylation of MPC1 in the liver. Intriguingly, HFD feeding resulted in decreased MPC1 acetylation in the liver, however, the loss of acetylation of MPC1 was restored by berberine and metformin treatment (Fig. 4a). In PA-treated hepatocytes, the result of immunoprecipitation showed that berberine as well as metformin increased acetyl-lysine expression in MPC1 (Fig. 4b). These findings suggested the possibility that berberine and metformin reduced MPC1 activity by preserving acetylation. As a sirtuins inhibitor, nicotinamide preserved acetyl-lysine in MPC1, indicative of the regulation of acetylation of MPC1 by SIRT. Deacetylation of protein in mitochondria is predominantly mediated by SIRT3, and we observed increased hepatic SIRT3 gene expression in the liver of HFD-fed mice and in PA-treated primary hepatocytes (Fig. 4c, d). Berberine and metformin reduced SIRT3 gene expression, explanting their actions to protect MPC1 acetylation. In primary hepatocytes, pyruvate-induced SIRT3 protein induction was also attenuated by berberine as well as metformin (Fig. 4e). Interestingly, MPC inhibitor UK-5099 downregulated SIRT3 expression, a regulation likely due to the limitation of mitochondrial pyruvate metabolism. Co-immunoprecipitation and confocal microscopy examinations showed that berberine prevented SIRT3 binding to MPC1 (Fig. 4f, g). Transfection of HepG2 cells with three SIRT3 siRNA duplexes and the result showed that SIRT3 siRNA-1 had the highest knockdown efficiency (Fig. 4h). Therefore, SIRT3 siRNA-1 was used for subsequent experiments. As shown in Fig. 4i, knockdown of SIRT3 increased the acetylation of MPC1, while overexpression of SIRT3 reduced MPC1 acetylation. These results demonstrated that hepatic MPC1 acetylation is mediated by SIRT3 (Fig. 4i, j).
Fig. 4.
Berberine reduced SIRT3 induction and preserved MPC1 acetylation in the liver. (a): Acetylated-Lysine protein expression in the liver mitochondria. (b): Acetylation in precipitated MPC1 in primary mouse hepatocytes incubated with PA for 24 h. (c–d): SIRT3 gene expression in the liver of HFD-fed mice and PA-treated hepatocytes. (e): SIRT3 protein expression in primary hepatocytes in the presence of 10 mM pyruvate for 8 h. (f): Western blot examination of SIRT3 in precipitated MPC1 protein in hepatocytes incubated with pyruvate for 8 h. (g): Confocal image of mitochondrial MPC1 and SIRT3 colocalization in hepatocytes (Blue: MPC1; Green: SIRT3; Red: MitoTracker Red cMXRos. Scale bars: 5 μm), The view is one of five independent experiments. (h): Knockdown efficiency of three SIRT3 siRNAs transfected in HepG2 cells. (i): MPC1 protein expression in HepG2 with SIRT3 knockdown when cultured with pyruvate (10 mM) for 8 h. (j): Acetylation of MPC1 expression in HepG2 cells overexpressed with SIRT3 when cultured with pyruvate (10 mM) for 8 h. (BBR, berberine; Met, metformin; NAM, nicotinamide; PA, palmitate). Data were expressed as the mean ± SD (n = 5–6). ⁎p < 0.05 vs. HFD mice, PA or pyruvate-treated cells; #p < 0.05 vs. chow-fed diet mice or untreated cells.
3.5. Berberine Reduced MPC1 Expression Via Degradation
Berberine concentration-dependently reduced pyruvate-induced MPC1 protein expression in hepatocytes, (Fig. 5a). When protein synthesis was inhibited by cycloheximide, MPC1 protein expression decreased after 8 h. Adding pyruvate improved MPC1 protein stabilization, but this action was abrogated by berberine (Fig. 5b), indicating that berberine attenuated MPC1 protein expression via degradation, at least in part. Proteasome inhibitor MG-132 blocked the action of berberine to reduce MPC1 protein expression (Fig. 5c), further confirming the regulation. In the presence of cycloheximide, overexpression of SIRT3 further enhanced MPC1 expression in response to pyruvate with decreased lactate accumulation (Fig. 5d, e). In contrast, SIRT3 knockdown attenuated MPC1 expression with increased lactate accumulation (Fig. 5f, g). These results raised the possibility that deacetylation modification by SIRT3 influences MPC1 expression and activity via post-translational regulation, likely due to the inhibition of protein degradation. SIRT3 overexpression blocked the inhibitory effect of berberine on MPC1 expression (Fig. 5h), suggesting that berberine impaired MPC1 stability via preserving acetylation (Fig. 5i).
Fig. 5.
Berberine reduced MPC1 expression via degradation. (a): MPC1 protein expression in primary mouse hepatocytes incubated with pyruvate (10 mM) for 8 h. (b): MPC1 protein degradation in primary mouse hepatocytes. (c): MPC1 protein expression in primary mouse hepatocytes incubated with pyruvate for 8 h in the presence of MG-132. (d–e): MPC1 protein expression and lactate accumulation in HepG2 cells overexpressed with SIRT3. (f–g): MPC1 protein expression and lactate generation in HepG2 cells when SIRT3 was knockdown. (h): MPC1 protein expression in SIRT3 overexpressed HepG2 cells. (i) The possible mechanism for berberine to reduce MPC1 activity. (BBR, berberine; Met, metformin; Cyclo, cycloheximide). Data were expressed as the mean ± SD (n = 5–6). ⁎p < 0.05 vs. pyruvate-treated cells; #p < 0.05 vs. untreated cells or indicated groups.
3.6. Berberine Restrained Mitochondrial Pyruvate Carboxylation in Hepatocytes
In hepatocytes, pyruvate load activated PDH activity by dephosphorylation and induced PC protein expression in a concentration-dependent manner (Fig. 6a, b), and this regulation should promote mitochondrial pyruvate metabolism through both oxidation and carboxylation pathways. MPC inhibitor UK-5099 further augmented PDH activity by dephosphorylation with a reduction in PC expression (Supplementary Fig. 4a, b), suggesting the possibility that MPC inhibition facilitates mitochondrial pyruvate oxidation. In contrast, PA treatment impaired PDH activity by increasing phosphorylation and further enhanced PC induction in response to pyruvate (Fig. 6c, d), indicating that fatty acid load shifts mitochondrial pyruvate from oxidation to carboxylation for gluconeogenesis. In deed, when PDH activity was inhibited by CPI-613, pyruvate further increased PC induction (Supplementary Fig. 4c), providing evidence that inhibition of pyruvate oxidation can enhance pyruvate carboxylation. When PA potentiated glucose production form 3.73 ± 0.50 μM to 5.22 ± 0.16 μM, berberine concentration-dependently reduced pyruvate-driven hepatic glucose production, and the glucose contents in berberine treatment at 10 μM was even lower than the basal level (Fig. 6e). MPC inhibitor UK-5099 and PDH activator DCA also effectively restrained hepatic glucose production (Fig. 6e). SIRT activator resveratrol and PDH inhibitor CPI-613 attenuated the inhibitory effect of berberine on hepatic glucose production (Fig. 6f), further conforming that SIRT inhibition and PDH activity protection by berberine contributed to restraining pyruvate-driven hepatic glucose production.
Fig. 6.
Berberine restrained mitochondrial pyruvate carboxylation in hepatocytes. (a–b): PDH phosphorylation and PC expression in primary mouse hepatocytes incubated with pyruvate at indicative concentrations for 8 h. (c–d): PDH phosphorylation and PC protein expression in primary mouse hepatocytes incubated with pyruvate with or without PA for 8 h. (e–f): Glucose production in primary mouse hepatocytes pretreated with indicated agents and then incubated with PA for 24 h. (BBR, berberine; Res, resveratrol; DCA, dichloroacetate; PA, palmitate). Data were expressed as the mean ± SD (n = 5–6). ⁎p < 0.05 vs. PA or pyruvate-treated cells; #p < 0.05 vs. untreated cells or indicated treatments.
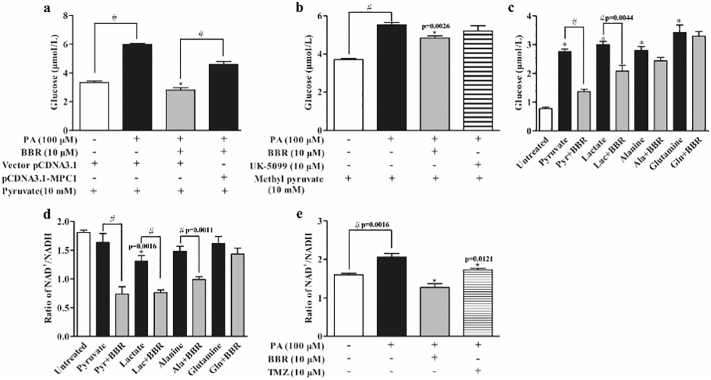
3.7. Berberine Reduced Pyruvate-driven HGP Dependent on MPC1 Inhibition
Although mitochondrial pyruvate import via the MPC is a central step in hepatic gluconeogenesis, pyruvate transformations and other alternative substrates enable MPC-independent gluconeogenesis [9, 10]. In hepatocytes, pyruvate was added as the major substrate, berberine reduced the increased glucose production by 53.13%, but the inhibitory effect was diminished by MPC1 overexpression (Fig. 7a). Different from pyruvate, methyl pyruvate is inner membrane-permeable, entering into mitochondria independent of MPC. Berberine mildly reduced methyl pyruvate-mediated glucose production by 12.51%, while MPC inhibitor UK5009 failed to limit methyl pyruvate-driven HGP (Fig. 7b), providing evidence that berberine restrained pyruvate-driven glucose production dependent on MPC1 inhibition. Meanwhile, we also observed the effects of berberine on HGP in response to different substrates and found that berberine reduced pyruvate and lactate-driven hepatic glucose output, but no significant effects were observed when alanine and glutamine were used as altered substrates (Fig. 7c). Because alanine and glutamine induce hepatic glucose production independent of MPC [9, 10], this result further confirmed that berberine reduced HGP mainly by restraining pyruvate/lactate-derived glucose production dependent on MPC1 inhibition. Although pyruvate, alanine and glutamine failed to influence NAD+/NADH ratio, berberine reduced NAD+/NADH ratio in the presence of pyruvate and alanine, despite no effect in the presence of glutamine (Fig. 7d). Different from other substrates, lactate mildly reduced NAD+/NADH ratio, presumably due to the conversion of lactate to pyruvate by LDH (Fig. 7d). These results suggested that the regulation of NAD+/NADH ratio by berberine was independent of gluconeogenic substrates. PA stimulation resulted in an increase in NAD+/NADH ratio, but this alteration was reversed by berberine and fatty acid β-oxidation inhibitor trimetazidine (Fig. 7e), indicating that berberine reduced the increased NAD+/NADH ratio by limiting fatty acid oxidation.
Fig. 7.
Berberine reduced pyruvate-driven HGP dependent on MPC1 inhibition. (a): Glucose production in HepG2 cells overexpressed with MPC1 and then exposed to PA for 24 h. (b): Methyl pyruvate-driven glucose output in primary mouse hepatocytes incubated with PA for 24 h. (c–d): Glucose production and NAD+/NADH ratio in primary mouse hepatocytes in response to 10 mM different substrates. ⁎p < 0.05 vs. untreated cells; #p < 0.05 vs. indicated treatments. (e): NAD+/NADH ratio in primary mouse hepatocytes incubated with PA for 24 h. (BBR, berberine; TMZ, trimetazidine; Pyr, Pyruvate; Lac, Lactate; Ala, Alanine; Gln, glutamine). Data were expressed as the mean ± SD (n = 5–6). ⁎p < 0.05 vs. PA-treated cells; #p < 0.05 vs. untreated cells or indicated treatments.
Because lipid disorders are associated with insulin resistance, we observed the effect of berberine on insulin sensitivity. In the muscle of fasted mice, berberine and metformin reduced diacylglycerol (DAG) accumulation (Supplementary Fig. 5a), which is demonstrated to impair insulin sensitivity [12, 29]. Consistently, berberine and metformin improved insulin signaling by restoration of Akt phosphorylation in the muscle of fasted chow-fed mice and promoted glucose uptake in muscle cells (Supplementary Fig. 5b, c). Insulin action in the liver is to inhibit gluconeogenesis. In hepatocytes, PA attenuated the inhibitory effect of insulin on glucose production, but this change was reversed by berberine treatment (Supplementary Fig. 5d). Together, these results demonstrated that berberine ameliorated systemic insulin resistance, contributing to reducing excessive hepatic glucose output in metabolic disorders.
4. Discussion
To reduce fasting hyperglycemia in type 2 diabetes, more therapeutic strategies are designed to restrain hepatic glucagon response, mainly mediating gluconeogenic signaling [24, [30], [31], [32]]. Herein, we showed that berberine reduced fasting hyperglycemia by reducing mitochondrial pyruvate import via inhibiting MPC1 function in the setting of lipid overload. Different from upstream regulation of gluconeogenic signaling, such as regulations of cAMP/PKA and CREB/TORC2 activation [24, 31, 32], this finding provides a distinct regulation, likely independent of the hepatic glucagon response, to reduce hepatic glucose overproduction by mitochondrial pyruvate supply.
Derangements in lipid metabolism is closely associated with accelerated hepatic gluconeogenesis, while its potential mechanism remains unclear [33, 34]. In the present study, we found that HFD feeding increased hepatic lipid deposition due to the increased fatty acid trafficking through circulation. In fasting state, adipose lipolysis increases circulating NEFAs, and oxidation of hepatic fatty acids supplies energy for glucose production [12]. Metformin mildly suppresses mitochondrial respiration, and this action is proposed to be related to the reduced cellular energy charge [24]. Berberine is also shown to inhibit mitochondrial respiratory chain complex I in muscle cells [35]. Therefore, it is proposed that modest inhibition of respiratory chain could restrain hepatic gluconeogenesis while increase glucose utilization in peripheral tissues [30]. Therefore, we reasoned that limiting fatty acid oxidation by berberine should contribute to reducing hepatic gluconeogenesis, especially in the setting of increased fatty acid load. We found that berberine and metformin inhibited mitochondrial complex I and II activity in hepatocytes, contributing to reducing acetyl CoA production via limiting fatty acid oxidation. Theoretically, limiting fatty acid oxidation by berberine raised the possibility to induce hepatic lipid accumulation; however. we found that hepatic lipid accumulation was reduced by berberine. The decreased levels of circulation NEFAs by berberine may partially explain the reduced hepatic lipid deposition. We should note that mitochondrial complex inhibition could activate AMPK, and published studies show that berberine reduces hepatic gluconeogenesis via AMPK activation [21, 22]. Reduced energy charge by complex I inhibition is an upstream event of AMPK activation, and in this context, we believe that regulation of hepatic glucose and lipid metabolism by berberine is independent of AMPK activation.
More than a metabolic intermediate, acetyl CoA is now recognized as a second messenger in cellular energy metabolism [36]. Acetyl CoA allosterically inhibits PDH by activating PDK [14]. Consistent with this, we observed that berberine preserved PDH activity by dephosphorylation with reduced PDK gene expression in the liver of HFD-fed mice. Theoretically, pyruvate generated by cytosolic glycolysis flows into mitochondria to be metabolized through competing metabolic pathways, oxidation by PDH or carboxylation by PC for anaplerotic reactions or gluconeogenesis [37]. When PDH is impaired, it is logical to speculate that more mitochondrial pyruvate would be shifted from oxidation to oxaloacetate by PC, enhancing gluconeogenesis. Consistent with the published study which shows that acetyl CoA allosterically activates PC [15], increased PC expression and oxaloacetate production in the liver of HFD-fed mice supported our hypothesis. Berberine and metformin reduced acetyl CoA accumulation and improved PDH activity, partially explaining its action to reduce PC expression and oxaloacetate generation in the liver of HFD-fed mice. Moreover, this regulation should contribute to restraining pyruvate-driven hepatic glucose production by facilitating pyruvate oxidation. Berberine is documented to reduce hepatic gluconeogenesis with improved lipid metabolism through different mechanisms [[20], [21], [22]], and herein, we provided evidence that limitation of acetyl CoA accumulation may be a functional event linking improved lipid metabolism to reduced HGP.
As an end product of glycolysis, pyruvate locates at a crucial metabolic branch. Cytosolic pyruvate is either reduced in the cytosol by lactate dehydrogenase (LDH) or imported into the mitochondrial matrix by MPC. Interestingly, we found that lactate accumulation was reduced during fasting state, a regulation likely due to reduced glycolysis, because increased gluconeogenesis is accompanied with reduced glycolysis. Given that the suppression of MPC1 by berberine, more cytosolic pyruvate was converted to lactate by LDH, thereby increasing lactate accumulation. Consistent with this, metformin is shown to elicit an increase in the cytosolic redox state with an increased ratio of lactate to pyruvate in both plasma and liver [38]. Recent studies demonstrated that MPC1 is required for efficient regulation of hepatic gluconeogenesis and diet-induced obesity increases hepatic MPC expression and function [9]. In the present study, HFD feeding or PA incubation increased hepatic MPC1 expression, and this change should facilitate mitochondrial import of pyruvate for gluconeogenesis when PDH activity is impaired. In this context, suppression of MPC1 expression by berberine should contribute to reducing pyruvate-driven hepatic glucose production, especially when PDH was impaired by acetyl CoA accumulation.
Recently, protein lysine acetylation and deacetylation emerge as a key post-translational modification in the regulation of cellular metabolism. MPC activity could be regulated by acetylation, a regulation found to inactivate MPC activity in diabetic mice heart and cancer cells [19, 39]. As acetyl CoA is the major donor of the acetyl groups for acetylation [16], accumulated acetyl CoA should increase protein acetylation. Intriguingly, we observed that HFD feeding in mice increased acetylated protein in liver mitochondria, but the acetylation of MPC1 was reduced (Fig. 4a). This discrepancy suggested another regulation likely to modulate MPC1 deacetylation in mitochondria. As a NAD+-dependent protein deacetylase, SIRT3 is localized in the mitochondrial matrix where it reduces acetylation levels of metabolic enzymes by deacetylation [19].
In fasting state, increased hepatic gluconeogenesis is associated with reduced glycolysis. Consistently, we found reduced lactate accumulation and increased NAD+/NADH ratio in the liver of fasted mice (Fig. 2g, h). In addition, fasting also increases mitochondrial NAD+ generation in the liver due to the enhanced fatty acid oxidation. Although NADH or NAD+ is unable to pass through the inner membrane of mitochondria, the balance of NAD+/NADH pools in the cytoplasm and mitochondria can be maintained by malate-aspartate shuttle. In this context, the decreased cellular redox state during fasting, indicated by the increased NAD+/NADN ratio, might be an acceptable explanation for SIRT3 induction. We should note that this explanation is non-exclusive, because other metabolic pathways are also involved in the altered redox-state, especially in the setting of metabolic disorders. As a result, SIRT3 interacted with MPC1 to protect MPC1 function via deacetylation modification, ensuring mitochondrial import of pyruvate for gluconeogenesis. As mentioned above, berberine limited fatty acid oxidation and the reduced NAD+ contents may partially explain its action to prevent SIRT3 induction. In line with our finding, berberine is reported to inhibit sirtuins at transcriptional level as well as at translational level in human hepatoma cells, mediating mitochondria-dependent apoptosis through FoxO1/3a and p53 acetylation [40]. Berberine attenuated MPC1 protein expression via degradation regulation, and the acetylation modification should be a reasonable cause for its action (Fig. 5i). In addition, hepatic SIRT3 is induced during fasting to promote fatty acid oxidation via reversible enzyme deacetylation in the liver [41], indicative of the special role of SIRT3 in hepatic gluconeogenesis. Therefore, we reasoned that berberine inhibited SIRT activity to impair MPC1 stability via acetylation regulation in a nutrition-sensing way.
MPC1 mediates mitochondrial pyruvate import and we confirmed that berberine reduced pyruvate-driven glucose production in a manner dependent on MPC1 inhibition. We observed that berberine failed to suppress alanine and glutamine-derived glucose production, because alanine and glutamine enter into tricarboxylic acid cycle (TAC) to drive gluconeogenesis through malate/oxaloacetic acid pathway, independent of MPC [9, 10]. These results further indicated that although other alternative substrates also mediate hepatic glucose output, berberine mainly restrained gluconeogenesis by blocking the supply of mitochondrial pyruvate. Moreover, we showed that the altered redox state was mediated by increased fatty acid oxidation, but not gluconeogenetic substrates. Berberine inhibited mitochondrial complex activity, well explaining its action to restore redox homeostasis by limiting fatty acid oxidation during gluconeogenesis.
Interestingly, berberine and metformin exhibited most similar effects in this study. In fact, accumulated evidence indicates that both berberine and metformin are versatile drugs, sharing similar effects on the treatment of metabolic diseases [42]. Berberine and metformin activate AMPK and improve insulin sensitivity, contributing to attenuating hyperglycemia in diabetes [42]. Metformin improves lipid metabolism by lowering plasma triglycerides and reduces hepatic steatosis, presumably mediated by activation of fatty acid oxidation and inhibition of lipogenesis [43, 44]. Similary, berberine is also shown to improve lipid metabolism [25, 26]. Although metformin and berberine improve glucose and lipid metabolism though multiple mechanisms, inhibition of mitochondrial respiratory chain complex I may be a common mechanism. Both berberine and metformin exert the ability to reduce cellular energy charge by mild inhibition of complex I [35, 45]. Recently, a study reports that mitochondrial complex I inhibition by rotenone ameliorates hyperglycemia and insulin resistance in obese mice [46]. Rotenone also enhanced glycolysis and repressed gluconeogenesis correlated with a reduction in cellular NAD+/NADH ratio, providing evidence to support our conclusion [46]. Therefore, it is of interesting for further study to elucidate the implication of mitochondrial complex I inhibition in the management of metabolic disorders.
In conclusion, our work showed that in response to pyruvate load, SIRT3 improved MPC1 stabilization via deacetylation, facilitating the transport of pyruvate into mitochondria for gluconeogenesis when PDH activity was impaired by accumulated acetyl CoA. Berberine reduced hepatic acetyl CoA accumulation by limiting fatty acid oxidation and preserved MPC1 acetylation by SIRT3 inhibition, eventually blocking mitochondrial pyruvate supply for gluconeogenesis due to MPC1 protein degradation. These results demonstrated the impact of fatty acid oxidation on hepatic pyruvate response and elucidated a previously unrecognized role for berberine to restrain hepatic glucose overproduction.
The following are the supplementary data related to this article.
Oligonucleotide primer sequences used for RT-PCR.
Supplementary figures
Funding Sources
This work is partially supported by the National Natural Science Foundation of China (No.81603353) and Natural Science Foundation of Jiangsu Province (BK20160762). The funders have no role in study design, data collection, data analysis, interpretation and writing of the report.
Declaration of Interests
All authors declare that no conflict of interest exists regarding data reported in the present study.
Author Contributions
AYL, NZ, YY and BLL designed the research. AYL performed, analyzed, interpretated the data and drafted the manuscript. QLiu and QLi collected data and reviewed the manuscript. BLL, NZ, YY contributed to the discussion and critical revision of the manuscript. All authors approved the final version of the paper.
Acknowledgments
We are grateful to Phenotype Analysis Center of Nanjing Medical University, China, for providing technical assistance during oxygen consumption rate assay. Furthermore, we gratefully acknowledge Shanghai Education Committee, China (No. A1-Z1730251040404), for their financial support.
Contributor Information
Yang Yang, Email: yangyang@shutcm.edu.cn.
Ning Zhang, Email: ningzhang@shutcm.edu.cn.
References
- 1.Cahill G.F. Starvation in man. N Engl J Med. 1970;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 2.Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz J., Tayek J.A. Recycling of glucose and determination of the Cori cycle and gluconeogenesis. Am J Physiol. 1999;277(3 Pt 1):E401–E407. doi: 10.1152/ajpendo.1999.277.3.E401. [DOI] [PubMed] [Google Scholar]
- 4.Satapati S., Sunny N.E., Kucejova B., Fu X., He T.T., Méndez-Lucas A. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53(6):1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 6.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.C. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigueira P.A., McCommis K.S., Schweitzer G.G., Remedi M.S., Chambers K.T., Fu X. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 2014;7(6):2042–2053. doi: 10.1016/j.celrep.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Divakaruni A.S., Wiley S.E., Rogers G.W., Andreyev A.Y., Petrosyan S., Loviscach M. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110(14):5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray L.R., Sultana M.R., Rauckhorst A.J., Oonthonpan L., Tompkins S.C., Sharma A. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab. 2015;22(4):669–681. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCommis K.S., Chen Z., Fu X., McDonald W.G., Colca J.R., Kletzien R.F. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 2015;22(4):682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postic C., Dentin R., Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30(5):398–408. doi: 10.1016/s1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- 12.Perry R.J., Camporez J.P., Kursawe R., Titchenell P.M., Zhang D., Perry C.J. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Zhang B., Huang F., Liu B., Xie Y. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J Lipid Res. 2016;57(7):1243–1255. doi: 10.1194/jlr.M067397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugden M.C., Holness M.J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284(5):E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 15.Adina-Zada A., Zeczycki T.N., St Maurice M., Jitrapakdee S., Cleland W.W., Attwood P.V. Allosteric regulation of the biotin-dependent enzyme pyruvate carboxylase by acetyl CoA. Biochem Soc Trans. 2012;40(3):567–572. doi: 10.1042/BST20120041. [DOI] [PubMed] [Google Scholar]
- 16.Choudhary C., Weinert B.T., Nishida Y., Verdin E., Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15(8):536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- 17.Jing E., Emanuelli B., Hirschey M.D., Boucher J., Lee K.Y., Lombard D. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108(35):14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschey M.D., Shimazu T., Jing E., Grueter C.A., Collins A.M., Aouizerat B. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44(2):177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang L., Li Q., Huang L., Li D., Li X. SIRT3 binds to and deacetylates mitochondrial pyruvate carrier 1 to enhance its activity. Biochem Biophys Res Commun. 2015;468(4):807–812. doi: 10.1016/j.bbrc.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Xia X., Yan J., Shen Y., Tang K., Yin J., Zhang Y. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M., Lv X., Li J., Meng Z., Wang Q., Chang W. Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis. Mol Cell Endocrinol. 2012;363(1–2):122–130. doi: 10.1016/j.mce.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang S.J., Dong H., Li J.B., Xu L.J., Zou X., Wang K.F. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J Gastroenterol. 2015;21(25):7777–7785. doi: 10.3748/wjg.v21.i25.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller R.A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang B., Zhao L.H., Zhou Q., Zhao T.Y., Wang H., Gu C.J. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:905749. doi: 10.1155/2015/905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S., Zhang M., Yu Y., Lan X., Yao F., Yan X. Berberine attenuates development of the hepatic gluconeogenesis and lipid metabolism disorder in type 2 diabetic mice and in palmitate-incubated HepG2 cells through suppression of the HNF-4α miR122 pathway. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0152097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumashiro N., Beddow S.A., Vatner D.F., Majumdar S.K., Cantley J.L., Guebre-Egziabher F. Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes. 2013;62(7):2183–2194. doi: 10.2337/db12-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan X.S., Ma J.Y., Feng R., Ma C., Chen W.J., Sun Y.P. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szendroedi J., Yoshimura T., Phielix E., Koliaki C., Marcucci M., Zhang D. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111(26):9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 31.Altarejos J.Y., Montminy M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozcan L., Wong C.C., Li G., Xu T., Pajvani U., Park S.K. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 2012;15(5):739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma R., Sinha S., Danishad K.A., Vikram N.K., Gupta A., Ahuja V. Investigation of hepatic gluconeogenesis pathway in non-diabetic Asian Indians with non-alcoholic fatty liver disease using in vivo ((31)P) phosphorus magnetic resonance spectroscopy. Atherosclerosis. 2009;203(1):291–297. doi: 10.1016/j.atherosclerosis.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Sunny N.E., Parks E.J., Browning J.D., Burgess S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14(6):804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu M., Xiao Y., Yin J., Hou W., Yu X., Shen L. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pietrocola F., Galluzzi L., Bravo-San Pedro J.M., Madeo F., Kroemer G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015;21(6):805–821. doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Jeoung N.H., Harris C.R., Harris R.A. Regulation of pyruvate metabolism in metabolic-related diseases. Rev Endocr Metab Disord. 2014;15(1):99–110. doi: 10.1007/s11154-013-9284-2. [DOI] [PubMed] [Google Scholar]
- 38.Madiraju A.K., Erion D.M., Rahimi Y., Zhang X.M., Braddock D.T., Albright R.A. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vadvalkar S.S., Matsuzaki S., Eyster C.A., Giorgione J.R., Bockus L.B., Kinter C.S. Decreased mitochondrial pyruvate transport activity in the diabetic heart: Role of mitochondrial pyruvate carrier 2 (MPC2) acetylation. J Biol Chem. 2017;292(11):4423–4433. doi: 10.1074/jbc.M116.753509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shukla S., Sharma A., Pandey V.K., Raisuddin S., Kakkar P. Concurrent acetylation of FoxO1/3a and p53 due to sirtuins inhibition elicit Bim/PUMA mediated mitochondrial dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol Appl Pharmacol. 2016;291:70–83. doi: 10.1016/j.taap.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Hirschey M.D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D.B. SIRT3 regulates fatty acid oxidation via reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., Zhu C., Ying Y., Luo L., Huang D., Luo Z. Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget. 2017;9(11):10135–10146. doi: 10.18632/oncotarget.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geerling J.J., Boon M.R., van der Zon G.C., van den Berg S.A., van den Hoek A.M., Lombès M. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes. 2014;63(3):880–891. doi: 10.2337/db13-0194. [DOI] [PubMed] [Google Scholar]
- 44.Lin H.Z., Yang S.Q., Chuckaree C., Kuhajda F., Ronnet G., Diehl A.M. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6(9):998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 45.El-Mir M.Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 46.Hou W.L., Yin J., Alimujiang M., Yu X.Y., Ai L.G., Bao Y.Q. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J Cell Mol Med. 2018;22(2):1316–1328. doi: 10.1111/jcmm.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oligonucleotide primer sequences used for RT-PCR.
Supplementary figures