Abstract
Background/Aim: Prognostic impact of p16expression in patients with oropharyngeal squamous cellcarcinoma (OSCC) undergoing surgery is not fullyexamined. The aim of this study was to clarify these issues.Patients and Methods: Sixty-four OSCC subjects wereanalyzed. Immuno-histochemical staining of p16, asurrogate marker for human papillomavirus (HPV), wasperformed histopathologically. Data were retrospectivelyanalyzed according to p16 positivity and factors linked toprognosis were also analyzed. Results: No significantdifference was observed in the prognosis between the p16-positive group (n=28) and the p16-negative group (n=36).In patients undergoing post-operative radiation, the p16-positive group (n=18) had a significantly better prognosisthan the p16-negative group (n=6). On multivariateanalysis, transoral surgery was a significant predictor ofoverall survival (p=0.0173). Conclusion: Prognostic impactof p16 can be emphasized in a subgroup of OSCC patientsundergoing surgery. Surgery with sufficient surgical marginmay be chosen as the first treatment for HPV-negativeOSCC in some cases.
Keywords: Oropharyngeal cancer, surgery, Human papillomavirus, p16, clinical outcome
Among head and neck squamous cell carcinomas (HNSCCs),the incidence of oropharyngeal SCC (OSCC) is increasingcompared with carcinomas of other origins, therebyattracting public attention (1,2). Like other malignancies inthe upper gastrointestinal tract, OSCC is attributed to thecarcinogenic effects of tobacco and alcohol (3). Prediagnosiscigarette smoking can be a prognostic factor foroverall survival (OS) in OSCC patients (4). Therapeuticoptions for early stage OSCC include both surgery andradiotherapy as single treatment modality, while those foradvanced stage include surgery, chemoradiation andchemotherapy (5,6).On the other hand, in recent years, human papillomavirus(HPV) is known to be involved, in addition to drinking andsmoking, in oropharyngeal carcinogenesis (1,7-10). HPVis the most common sexually transmitted disease. However,the overwhelming majority of patients with HPV clear theinfection. A small percentage of patients with HPV developoncogenic HPV types, especially HPV-16, andconsequently, SCCs can develop in such patients. HPVcauses more than 5% of malignancies worldwide (1,7-10).Over 70% of OSCCs are currently thought to be linked tooncogenic HPV infection. Immunohistochemistry for p16protein is often used as a surrogate marker for oncogenicHPV in the oropharyngeal tissues (11,12). The clinicalprofile of patients with HPV-related OSCC differs quitenotably from that of non-HPV-related OSCC, and theclinical outcome for HPV-related OSCC is reported to besignificantly better due to high sensitivity for radiationtherapy (2,7,8).Numerous clinical studies have examined the role oftransoral surgical resection (SR) for the treatment oforopharyngeal malignancies and have shown similar clinicaloutcomes and improved functional outcomes compared withchemoradiation therapies (5,13). However, the relationshipbetween p16 expression and prognosis in OSCC patientsundergoing surgery has not been fully examined. Thus, thereis urgent need for elucidating these issues. The aims of thisstudy are therefore to clarify the relationship between p16expression and prognosis in patients with OSCC undergoing surgery.
Patients and Methods
Between January 2000 and December 2009, 94 consecutive patientsdiagnosed as OSCC were admitted to the Department of Head andNeck Surgery, Osaka International Cancer Institute, Osaka, Japan(Former Osaka Medical Center for Cancer and CardiovascularDiseases). All patients were treatment naive for OSCC. Of these, 64patients for which immuno-histochemical staining for p16 insurgical specimens was available, were analyzed in this study. Therewere 51 male and 13 female patients with a median age of 62 years(range=41-80 years). For all cases, indication for surgery wascarefully reviewed through discussion with surgeons, oncologist andradiologists.Clinical stage for OSCC was determined based on Union forInternational Cancer Control (UICC) classification system (ver. 7)(14). In terms of T classification, T1 was found in 16 cases, T2 in 22,T3 in 10 and T4a in 16. In terms of N classification, N0 was foundin 32 cases, N1 in 6, N2a in 1, N2b in 11, N2c in 11 and N3 in 3.In terms of patients with p16 positivity, a positive cell rate of25% or more in tumor cells in the immuno-histochemical stain ofp16 was defined as positive using surgical specimen, according toprevious report (15). Positivity for p16 was determined byexperienced pathologists.Indications for post-operative radiation therapy were: (1)insufficient surgical margin of primary lesion; or (2) 2 or morelymph node metastases; or (3) the presence of lymph nodemetastases with extra-nodal invasion.Baseline characteristics, details of uncontrollable lesions andclinical outcomes were retrospectively compared. Factors linked toOS and disease specific survival were analyzed using univariate andmultivariate analyses.The ethical committee in our hospital acknowledged our currentstudy protocol (approval number, 1503315272) and this studystrictly followed all regulations of the Declaration of Helsinki.Statistical analysis. The categorical parameters in the p16-positiveand p16-negative groups were analyzed by the Fisher’s exact test,while the numerical parameters were analyzed either with unpairedStudent t-test or with Mann-Whitney U-test, as appropriate. OS anddisease-specific survival curves were created by using the Kaplan-Meier method and compared by using the log-rank test. Variableswith p-values<0.05 in univariate analysis were entered into themultivariate analysis with the Cox proportional hazards model. Forthe purpose of analyzing the significance of predictors inmultivariate analyses, analyzed variables were divided by themedian values for all cases and treated as nominal covariates. OSwas defined as the time interval from SR until death (due to anycause) or the last follow-up visit. Disease specific survival wasdefined as the time interval from SR until death due to the disease(i.e., OSCC) or the last follow-up visit and all deaths with othercauses were treated as censored population. Data were expressed asmedian values (range) unless otherwise stated. The significancethreshold in the current analysis was set at p<0.05. All statisticalanalyses were performed using the JMP 13 software (SAS InstituteInc., Cary, NC).
Results
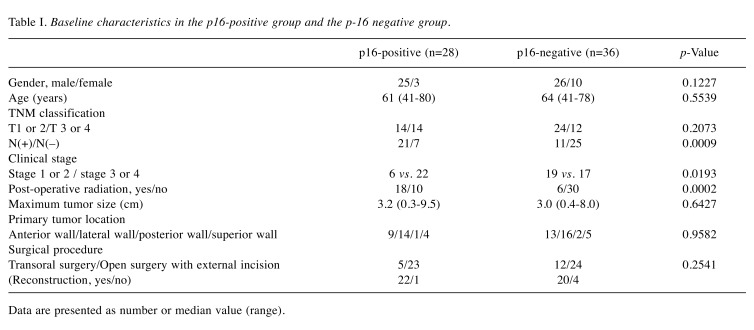
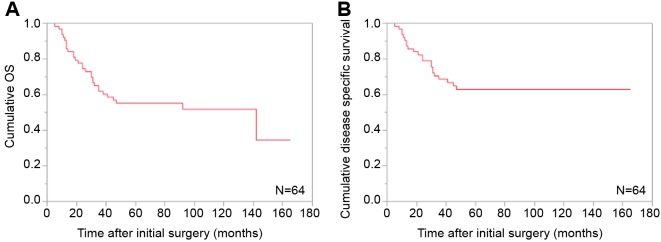
Baseline characteristics. Baseline characteristics in thep16-positive and p16-negative groups are demonstrated inTable I. There were 28 patients (43.75%) in the p16-positive group and 36 patients (56.25%) in the p16-negativegroup. As for gender and age, no significant difference wasfound in the two groups (p=0.1227 for gender andp=0.5539 for age). As for N factor in TNM classification,the proportion of patients with positivity for N factor in thep16-positive group was significantly higher than that in thep16-negative group (21/28 vs. 11/36, p=0.0009). As fortumor location, both groups had the largest number oftumors located on the lateral side walls (14/28 (50.0%) inthe p16-postive group and 16/36 (44.4%) in the p16-negative group). Post-operative radiation therapy wasperformed in 18 patients in the p16-positive group (totalradiation dose: 50 gray (Gy) in one patient, 60 Gy in 15, 66 Gy in 2 and 70 Gy in none) and 6 patients in the p16-negative group (total radiation dose: 50 Gy in one patient,60 Gy in 4, 66 Gy in none and 70 Gy in one).Cumulative OS rates for all cases and comparison of OS anddisease specific survival rates between the p16-positivegroup and the p16-negative group. The median follow-upperiod following SR for all cases was 55 months (range=1-165 months). For all cases, the 1-, 3- and 5-year cumulativeOS rates were 90.5, 61.9 and 55.3%, respectively, while the1-, 3- and 5-year cumulative disease specific survival rateswere 90.5, 68.7 and 63.0%, respectively (Figure 1A and B).The median follow-up period following SR was 55 months(range=1-165 months) in the p16-positive group and 50.5months (range=5-137 months) in the p16-negative group.The 1-, 3- and 5-year cumulative OS rates were 96.3, 63.0and 55.6%, respectively, in the p16-positive group, and 86.1,61.1 and 55.1%, respectively, in the p16-negative group(p=0.633; Figure 2A). The 1-, 3- and 5-year cumulativedisease specific survival rates were 96.3, 66.7 and 58.8%,respectively, in the p16-positive group, and 86.1, 70.9 and67.2%, respectively, in the p16-negative group (p=0.7305;Figure 2B).Causes of mortality. During the observation period, 30patients (46.9%) succumbed to disease. In the p16-positivegroup, 13 (46.4%) patients succumbed during the observationperiod. Causes for death were tumor progression in 11 andmiscellaneous causes in 2. In the p16-negative group, 17(47.2%) patients succumbed during the observation period.Causes for death were tumor progression in 11 andmiscellaneous causes in 6.
Table I. Baseline characteristics in the p16-positive group and the p-16 negative group.
Data are presented as number or median value (range).
Figure 1. Cumulative overall survival (A) and disease-specific survival (B) for all cases (n=64).
Figure 2. Cumulative overall survival (A) and disease-specific survival (B) for all cases stratified by p16 positivity.
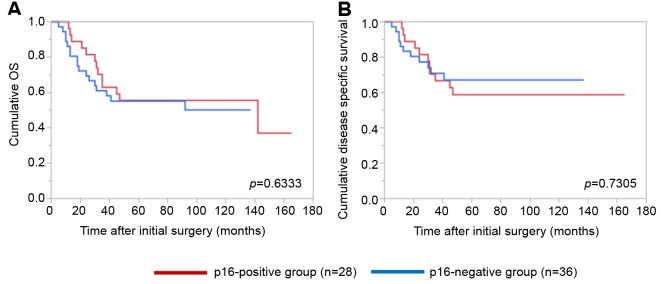
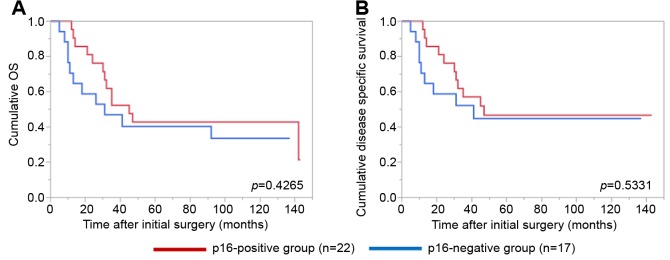
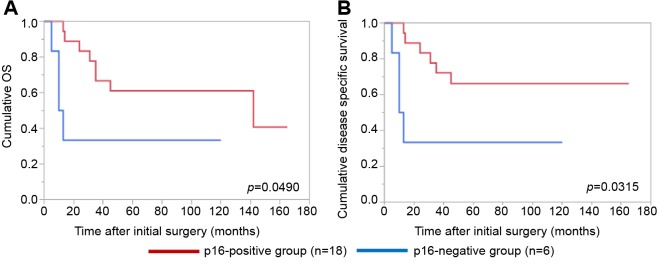
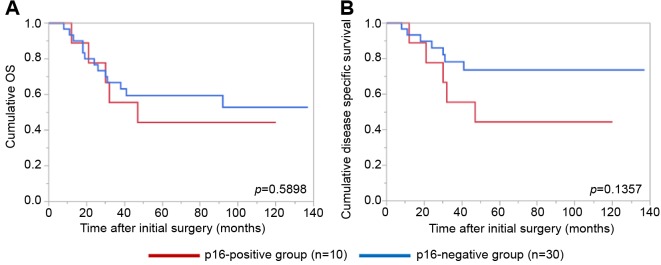
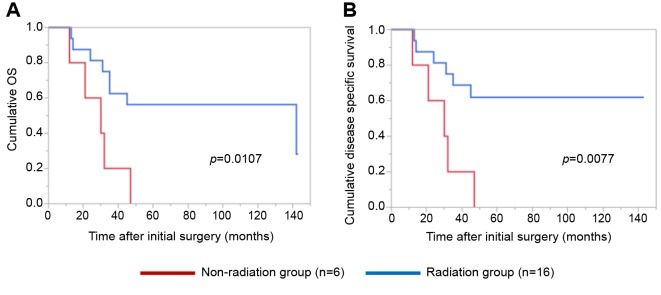
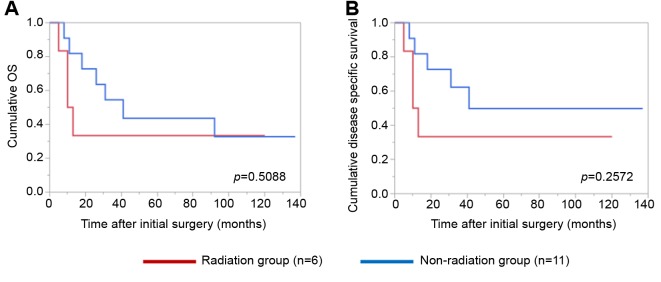
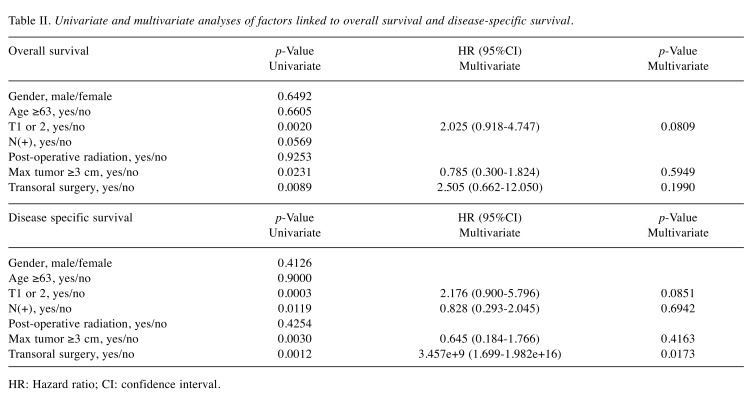
Details of uncontrollable lesions as assessed by T, N and Mfactors in the p16-positive group and the p16-negative groupduring the follow-up period. Details of uncontrollable lesionsin the p16-positive group and the p16-negative group were:T in 3 cases, N in 2, M in 5, T and M in 1 and N and M in1 in the p16-positive group and T in 3 cases, N in 3, M in 4and N and M in 1 in the p16-negative group (p=0.8664).Comparison of OS and disease specific survival ratesbetween the p16-positive group and the p16-negative groupaccording to the tumor stage. In patients with stage I or II(n=25), the 1-, 3- and 5-year cumulative OS rates were 100,100 and 100%, respectively, in the p16-positive group (n=6),and 100, 73.7 and 68.0%, respectively, in the p16-negativegroup (n=19) (p=0.1348; Figure 3A). The 1-, 3- and 5-yearcumulative disease specific survival rates were 100, 100 and100%, respectively, in the p16-positive group, and 100, 87.5and 87.5%, respectively, in the p16-negative group(p=0.3787; Figure 3B).In patients with stage III or IV, the 1-, 3- and 5-yearcumulative OS rates were 95.2, 52.4 and 42.9%, respectively,in the p16-positive group (n=22), and 70.6, 47.1 and 40.3%,respectively, in the p16-negative group (n=17) (p=0.4265;Figure 4A). The 1-, 3- and 5-year cumulative disease specificsurvival rates were 95.2, 57.1 and 46.8%, respectively, in thep16-positive group, and 70.6, 58.8 and 44.8%, respectively,in the p16-negative group (p=0.5331; Figure 4B).Comparison of OS and disease specific survival ratesbetween the p16-positive group and the p16-negative groupin vivo 32: 927-935 (2018)930 stratified by the post-operative radiation therapy. In patientsthat underwent post-operative radiation therapy (n=24), the1-, 3- and 5-year cumulative OS rates were 100, 66.7 and61.1%, respectively, in the p16-positive group (n=18), and50, 33.3 and 33.3%, respectively, in the p16-negative group(n=6) (p=0.0490; Figure 5A). The 1-, 3- and 5-yearcumulative disease specific survival rates were 100, 72.2 and66.2%, respectively, in the p16-positive group, and 50.0,33.3 and 33.3%, respectively, in the p16-negative group(p=0.0315; Figure 5B).Patients that did not have post-operative radiation therapy(n=40), the 1-, 3- and 5-year cumulative OS rates were 88.9,55.6 and 44.4%, respectively, in the p16-positive group(n=10), and 93.3, 66.7 and 59.4%, respectively, in the p16-negative group (n=30) (p=0.5898; Figure 6A). The 1-, 3- and5-year cumulative disease-specific survival rates were 88.9,55.6 and 44.4%, respectively, in the p16-positive group, and93.3, 78.3 and 73.3%, respectively, in the p16-negative group(p=0.1357; Figure 6B).Comparison of OS and disease-specific survival ratesbetween stage III or IV patients with and without postoperativeradiation therapy stratified by p16 positivity. Instage III or IV patients with p16 positivity (n=22), the 1-, 3-and 5-year cumulative OS rates were 100, 62.5 and 56.3%,respectively, in the postoperative radiation group (n=16), and80.0, 40.0 and 20.0%, respectively, in the non-postoperativeradiation group (n=6) (p=0.0107; Figure 7A) and the 1-, 3- and 5-year disease-specific survival rates were 100, 68.8 and61.9%, respectively, in the postoperative radiation group, and80.0, 40.0 and 20.0%, respectively, in the non-postoperativeradiation group (p=0.0077; Figure 7B).In stage III or IV patients without p16 positivity (n=17),the 1-, 3- and 5-year cumulative OS rates were 50.0, 33.3and 33.3%, respectively, in the postoperative radiationgroup (n=6), and 81.8, 54.6 and 43.6%, respectively, in thenon-postoperative radiation group (n=11) (p=0.5088;Figure 8A) and the 1-, 3- and 5-year disease specificsurvival rates were 50.0, 33.3 and 33.3%, respectively, inthe postoperative radiation group, and 81.8, 62.3 and49.9%, respectively, in the non-postoperative radiationgroup (p=0.2572; Figure 8B).Univariate and multivariate analyses of parameterscontributing to overall survival and disease specific survival.The univariate analysis identified that the following factorssignificantly contributed to OS for all cases (n=64): T1 or 2,yes/no (p=0.0020); maximum tumor size ≥3 cm, yes/no(p=0.0231); transoral surgery, yes/no (p=0.0089) (Table II).The hazard ratios (HRs) and 95% confidence intervals (CIs)determined by multivariate analysis for the three significantvariables (selected based on a p<0.05 in univariate analysis)are detailed in Table II. On multivariate analysis, T factor 1or 2 was identified as marginally significant predictorassociated with OS (p=0.0809).Likewise, the univariate analysis identified that thefollowing factors significantly contributed to disease-specific survival for all cases: T1 or 2, yes/no (p=0.0003); N(+),yes/no (p=0.0119); maximum tumor size ≥3 cm, yes/no(p=0.0030); transoral surgery, yes/no (p=0.0012) (Table II).The HRs and 95% CIs determined by multivariate analysisfor the four significant variables are detailed in Table II. Onmultivariate analysis, transoral surgery was identified as asignificant predictor associated with OS (p=0.0173).
Figure 3. Cumulative overall survival (A) and disease-specific survival (B) in stage I or II patients (n=25) stratified by p16 positivity.
Figure 4. Cumulative overall survival (A) and disease-specific survival (B) in stage III or IV patients (n=39) stratified by p16 positivity .
Figure 5. Cumulative overall survival (A) and disease-specific survival (B) in patients with postoperative radiation therapy (n=24) stratified by p16 positivity.
Figure 6. Cumulative overall survival (A) and disease-specific survival (B) in patients without postoperative radiation therapy (n=40) stratified by p16 positivity.
Figure 7. Cumulative overall survival (A) and disease-specific survival (B) in the p16-positive stage III or IV patients with and without post-operative radiation therapy.
Figure 8. Cumulative overall survival (A) and disease-specific survival (B) in the non-p16-positive stage III or IV patients with and without postoperative radiation therapy .
Table II. Univariate and multivariate analyses of factors linked to overall survival and disease-specific survival.
HR: Hazard ratio; CI: confidence interval.
Discussion
The frequency of HPV-related HNSCCs is reported to be 35-80% in Western countries and 30-50% in Asian countries (9,16-20). Since HPV-positive and negative HNSCCs presentquite different clinical characteristics, personalized therapyaccording to HPV infection is currently being extensivelystudied.Protein p16 is an important protein controlling the cellcycle, and its altered expression has been noticed in manytypes of cancers (21). In UICC/AJCC ver.8 published in2017, OSCCs were classified into the p16-positive group andthe p16-negative group (11,12). Although p16 immunohistochemicalstaining cannot directly detect HPV, it wasadopted as a surrogate marker to detect HPV infectionindirectly because p16 overexpression is observed in manyof HPV-related OSCCs. In terms of criteria regardingpatients with p16-positivity, wide range of p16 immunopositivecut-off from 5-80% was reported in previous studies(15-19). Difference of clinical prognoses could not berecognized in these reports. Thus, in this study, experiencedpathologists in our hospital defined patients with theimmune-positive cell ratio of 25% or more in tumor cells ashaving p16 positivity, according to previous report (15). Inthis study, the p16-positive rate was 43.75%, which is in linewith previous data (9,20,22-25).In Western countries, regarding tumor stage of OSCCs,there are more reports in which the number of T1 or T2 islarger in the HPV-positive group than in the negative group,while regarding N factor, it is reported that the prevalence ofpatients with advanced N factor is higher in the HPV positivegroup than in the negative group, even in patients with lessadvanced T factor (16,26,27). In our data, the proportion ofN factor-positive patients was significantly higher in the p16-positive group than in the p16-negative group. The higherproportion of N factor-positive patients in the p16-positivegroup can explain the higher proportion of stage III or IVpatients in the p16-positive group in this study.Previous reports have shown that HPV-positive OSCCshave favorable sensitivity to chemotherapy and radiotherapyand good prognosis (7,8). However, in surgically treatedOSCC cases, there are only few large studies regarding theprognostic impact of p16 expression. Quon, et al. reportedthat p16 was not a prognostic factor in resectable OSCC(n=48) when treated with an initial transoral robotic surgeryapproach, which was similar to our current results (28).While in our subgroup analyses of patients with postoperativeradiation therapy, patients in the p16-positivegroup had significantly better prognosis. Additionally, in thep16-postive stage III or IV patients, those that had postoperative radiation therapy had better prognosis thanthose without, however, in the non-p16-positive stage III orIV patients, those that had postoperative radiation therapydid not have better prognosis than those without. Theseresults indicated that in the p16-positive group, OS rate canbe expected to increase by postoperative radiation therapy,but not in the p16-negative group. Both in stage I or II andstage III or IV, p16-positive group had better prognosis inour results. These results may be linked to the effect ofradiation therapy in the p16-positive group.In a subgroup analysis of patients without post-operativeradiation therapy, the p16-positive group did not survivelonger than the p16-negative group. Rather, the p16-negativegroup had better prognosis than the p16-positive group. In asense, for patients with non-HPV-related OSCC, the surgicalstrategy should be considered due to the poor response toradiation therapy. However, it is important to ensuresufficient surgical margin in such patients.Previous studies reported that sensitivity to radiationtherapy can differ according to tumor location or macroscopictumor type (29,30). In particular, OSCCs with invasion intothe anterior pillar, soft palate and tongue base, and intrusiveinvasion type and ulcer formation type are reported to beassociated with poor sensitivity to radiation therapy. In p16-negative OSCCs with such poor response to radiation,surgical indication can be especially considered. However,extensive SR requires for reconstructive surgery, which cancause postoperative dysphagia and dysarthria. For surgicalindications, careful consideration will be thus needed.In our multivariate analysis of factors linked to OS ordisease-specific survival, only transoral surgery was asignificant factor. Transoral surgery is a minimally-invasiveapproach (31). Indication for transoral surgery is limited tosmall volume OSCCs and this can be associated with ourcurrent results.
Conclusion
Although our study was retrospective in its nature, ourresults demonstrated that the prognostic impact of p16 canbe emphasized in OSCC patients undergoing post-operativeradiation. In addition, in OSCC patients without HPV,indication for surgery should be strongly considered.Clinicians should be aware of these in daily clinical practice.
Conflicts of Interest
All Authors declare that they have no conflicts of interest.
Conflicts of Interest
All Authors declare that they have no conflicts of interest. Institute, for the radiotherapy, and the help they provided to our technical assistants, Mrs. Yui Okajima, Ms. Yumi Kida and Mrs. Midori Tanide.
References
- 1.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association betweenhuman papillomavirus and a subset of head and neck cancers. JNatl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Okami K. Clinical features and treatment strategy for HPV-relatedoropharyngeal cancer. Int J Clin Oncol. 2016;21:827–835. doi: 10.1007/s10147-016-1009-6. [DOI] [PubMed] [Google Scholar]
- 3.Granados-García M. Oropharyngeal cancer: an emergentdisease. Salud Publica Mex. 2016;58:285–290. doi: 10.21149/spm.v58i2.7798. [DOI] [PubMed] [Google Scholar]
- 4.Giraldi L, Leoncini E, Pastorino R, Wünsch-Filho V, de CarvalhoM, Lopez R, Cadoni G, Arzani D, Petrelli L, Matsuo K, Bosetti C, La Vecchia C, Garavello W, Polesel J, Serraino D, Simonato L, Canova C, Richiardi L, Boffetta P, Hashibe M, Lee YCA andBoccia S. Alcohol and cigarette consumption predict mortality inpatients with head and neck cancer: a pooled analysis within theInternational Head and Neck Cancer Epidemiology (INHANCE)Consortium. Ann Oncol. 2017;28:2843–2851. doi: 10.1093/annonc/mdx486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner MT, Byrd JK, Ferris RL. Current Role of Surgery inthe Management of Oropharyngeal Cancer. J Oncol Pract. 2016;12:1176–1183. doi: 10.1200/JOP.2016.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelstein D, Gillison ML, Pfister DG, Spencer S, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Eisele DW, Fenton M, Foote RL, Gilbert J, HaddadRI, Hicks WL Jr, Hitchcock YJ, Jimeno A, Leizman D, Lydiatt WM, Maghami E, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rocco J, Rodriguez CP, Shah JP, Weber RS, Witek M, Worden F, Yom SS, Zhen W, Burns JL, Darlow SD. NCCNGuidelines Insights: Head and Neck Cancers, Version 2.2017. JNatl Compr Canc Netw. 2017;15:761–770. doi: 10.6004/jnccn.2017.0101. [DOI] [PubMed] [Google Scholar]
- 7.Lassen P, Eriksen JG, Krogdahl A, Therkildsen MH, Ulhøi BP, Overgaard M, Specht L, Andersen E, Johansen J, Andersen LJ, Grau C, Overgaard J; Danish Head and Neck Cancer Group(DAHANCA) The influence of HPV-associated p16-expressionon accelerated fractionated radiotherapy in head and neckcancer: Evaluation of the randomized DAHANC6 and 7 trial. Radiother Oncol. 2011;100:49–55. doi: 10.1016/j.radonc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, Tornillo L, Wolfensberger M, Terracciano LM. Is theimproved prognosis of p16 positive oropharyngeal squamouscell carcinoma dependent of the treatment of the treatmentmodality. Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 9.Marur S, D’Souza G, Westra WH, Forastiere AA. HPVassociatedhead and neck cancer: a virus-related cancerepidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman TA, Schiller JT. Human papillomavirus in cervicalcancer and oropharyngeal cancer: One cause, two diseases. Cancer. 2017;123:2219–2229. doi: 10.1002/cncr.30588. [DOI] [PubMed] [Google Scholar]
- 11.Mizumachi T, Homma A, Sakashita T, Kano S, Hatakeyama H. Confirmation of the eighth edition of the AJCC/UICCTNM staging system for HPV-mediated oropharyngeal cancer inJapan. Int J Clin Oncol. 2017;22:682–689. doi: 10.1007/s10147-017-1107-0. [DOI] [PubMed] [Google Scholar]
- 12.Doescher J, Veit JA, Hoffmann TK. The 8th edition of the AJCC Cancer Staging Manual: Updates in otorhinolaryngology, head and neck surgery. HNO. 2017;65:956–961. doi: 10.1007/s00106-017-0391-3. [DOI] [PubMed] [Google Scholar]
- 13.Monnier Y, Simon C. Surgery versus radiotherapy for earlyoropharyngeal tumors: a never-ending debate. Curr TreatOptions Oncol. 2015;16:42. doi: 10.1007/s11864-015-0362-4. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee onCancer: the 7th edition of the AJCC cancer staging manual andthe future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, von Knebel DoeberitzM. Overexpression of p16 (INK4A) as a specific marker fordysplastic and neoplastic epithelial cells of the cervix uteri. IntJ Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 16.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients withoropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai RK, Erickson J, Pourmand N, Kong CS. p16INK4Aimmunohistochemical staining may be helpful in distinguishingbranchial cleft cysts from cystic squamous cell carcinomasoriginating in the oropharynx. Cancer Cytopathol. 2009;117:108–111. doi: 10.1002/cncy.20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen PW, Man M, Lam KY, Kwong YL. Clinicopathologicalsignificance of p16 gene expression in the surgical treatment ofhead and neck squamous cell carcinomas. J Clin Pathol. 2002;55:58–60. doi: 10.1136/jcp.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymphnodes: a highly effective strategy for localizing site of tumororigin. Clin Cancer Res. 2003;9:6469–6475. [PubMed] [Google Scholar]
- 20.Luginbuhl A, Sanders M, Spiro JD. Prevalence, morphology,and prognosis of human papillomavirus in tonsillar cancer. AnnOtol Rhinol Laryngol. 2009;118:742–749. doi: 10.1177/000348940911801010. [DOI] [PubMed] [Google Scholar]
- 21.Urszula C, Tomasz Z, Katarzyna N, Katarzyna RW, Jedrzej G, Aleksandra P, Mateusz O, Bartosz P, Marzenna PO, Piotr D. Expression of cell cycle-related proteins p16, p27 and Ki-67proliferating marker in laryngeal squamous cell carcinomas andin laryngeal papillomas. Anticancer Research. 2017;37(5):2407–2415. doi: 10.21873/anticanres.11580. [DOI] [PubMed] [Google Scholar]
- 22.Egawa N, Egawa K, Griffin H, Doorbar J. Humanpapillomaviruses; Epithelial tropisms, and the development ofneoplasia. Viruses. 2015;7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruyama H, Yasui T, Ishikawa-Fujiwara T, Morii E, Yamamoto Y, Yoshii T, Takenaka Y, Nakahara S, Todo T, Hongyo H. Human papillomavirus and p53 mutations in headand neck squamous cell carcinoma among Japanese population. Cancer Sci. 2014;105:409–417. doi: 10.1111/cas.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Deng Z, Chen Y, Suzuki M, Xie M. Is there ahigher prevalence of human papillomavirus infection in Chineselaryngeal cancer patients? A systematic review and metaanalysis. Eur Arch Otorhinolaryngol. 2016;273:295–303. doi: 10.1007/s00405-014-3345-3. [DOI] [PubMed] [Google Scholar]
- 25.Hama T, Tokumaru Y, Fujii M, Yane K, Okami K, Kato K, Masuda M, Mineta H, Nakashima T, Sugasawa M, Sakihama N, Yoshizaki T, Hanazawa T, Kato H, Hirano S, Imanishi Y, Kuratomi Y, Otsuki N, Ota I, Sugimoto T, Suzuki S. Prevalence of human papillomavirus in oropharyngeal cancer: amulticenter study in Japan. Oncology. 2014;87:173–182. doi: 10.1159/000360991. [DOI] [PubMed] [Google Scholar]
- 26.Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ. Human papillomavirus inoropharynx cancer in TAX 324-A subset analysis from aninternational phase Ⅲ trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, Solomon B, Choi J, O'Sullivan B, Kenny LM, McArthur GA. Prognostic significance of p16ink-4a and humanpapillomavirus in patients with oropharyngeal cancer treated onTROG 02. 02Phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quon H, Cohen MA, Montone KT, Ziober AF, Wang LP, Weinstein GS, O’Malley BW. Transoral robotic surgery andadjuvant therapy for oropharyngeal carcinomas and the influenceof p16 INK4a on treatment outcomes. Laryngoscope. 2013;123:635–640. doi: 10.1002/lary.22172. [DOI] [PubMed] [Google Scholar]
- 29.Kraus AP, Huvos AG, Spiro RH. Surgicalmanagement of squamous cell carcinoma of the base of thetongue. Am J Surg. 1993;166:384–388. doi: 10.1016/s0002-9610(05)80338-1. [DOI] [PubMed] [Google Scholar]
- 30.Lee WR, Mendenhall WM, Parsons JT, Million RR, Cassisi SP. Carcinoma of the tonsillar resion: Amultivariate analysis of 243 patients treated with radicalradiotherapy. Head Neck. 1993;15:283–288. doi: 10.1002/hed.2880150402. [DOI] [PubMed] [Google Scholar]
- 31.Howard J, Masterson L, Dwivedi RC, Riffat F, Benson R, Jefferies S, Jani P, Tysome JR, Nutting C. Minimallyinvasive surgery versus radiotherapy/chemoradiotherapy forsmall-volume primary oropharyngeal carcinoma. CochraneDatabase Syst Rev. 2016;12 doi: 10.1002/14651858.CD010963.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]