Abstract
Background/Aim: The combination of oxaliplatin, leucovorin and fluorouracil (FOLFOX) has been established as postoperative adjuvant chemotherapy for stage III colon cancer. However, the safety and efficacy of neoadjuvant FOLFOX in patients with rectal cancer are still controversial. This prospective pilot study aimed to evaluate the feasibility of neoadjuvant FOLFOX therapy without radiation for baseline resectable rectal cancer (RC). Patients and Methods: The study included 30 patients with clinical stage II/III RC between February 2012 and December 2015. The patients were treated with six cycles of FOLFOX followed by elective surgery. The primary endpoint was the R0 resection rate. The secondary endpoints were the scheduled treatment completion rate, adverse events, pathological response and the disease-free survival (DFS) rate. Results: All the patients underwent elective R0 resection after neoadjuvant FOLFOX therapy. The completion rate of the 6-cycle regimen was 93.3% (28/30 patients). Grade 3-4 adverse events occurred in seven patients (23.3%). Pathological complete response was noted in two patients (6.7%). The 3-year DFS rate was 77.5% (95% confidence interval, 61.4%-93.7%). Conclusion: Neoadjuvant FOLFOX therapy without radiation is a feasible therapeutic strategy for baseline resectable RC.
Keywords: Rectal cancer, neoadjuvant chemotherapy, FOLFOX, feasibility
Surgery is the standard treatment for rectal cancer (RC). Total mesorectal excision (TME) is a widely accepted surgical procedure for RC, and it can reduce the local recurrence rate dramatically (1). Research over the past two decades has shown perioperative radiotherapy with or without chemotherapy followed by TME can reduce the incidence of local recurrence in patients with locally advanced RC further. However, perioperative radiotherapy was not found to improve overall survival (OS) (2-5). To improve oncological outcomes, distant metastasis should be prevented, since distant metastasis is the most important prognostic factor for colorectal cancer.
Postoperative adjuvant chemotherapy can improve the prognosis of patients with stage II/III colon cancer undergoing curative surgery. This improvement is associated with the inhibitive action of these adjuvant chemotherapeutic agents on distant micrometastases that are present at the time of elective surgery. Some cytotoxic agents are used in adjuvant chemotherapy regimens. Among them, oxaliplatin-based regimens are considered the most effective for improving the rates of disease-free survival (DFS) and OS (6-8). Therefore, neoadjuvant chemotherapy (NAC) using oxaliplatin-based regimens for RC is a promising therapeutic strategy for preventing local recurrence through shrinkage of the primary tumour, but also systemic relapse through the action of diminishing latent micrometastases.
However, the efficacy and safety of NAC for the treatment of RC have not been adequately established. Therefore, this single-institution, pilot study aimed to determine the efficacy and safety of NAC involving FOLFOX therapy (5-fluorouracil + L-leucovorin + oxaliplatin).
Patients and Methods
Patient selection. The protocol of this single-arm, single-institution, phase II study was approved by the institutional review board of Nippon Medical School (Tokyo, Japan) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all the participants prior to their inclusion in this study.
Baseline clinical staging was assessed by colonoscopy, chest and abdominopelvic computed tomography (CT) and pelvic magnetic resonance imaging. Tumours were staged according to the American Joint Committee on Cancer International Union Against Cancer (seventh edition). The presence of a lymph node with a short axis ≥5 mm was considered as confirmation of metastasis (9).
The inclusion criteria were as follows: (i) histologically proven rectal adenocarcinoma, (ii) location of the centre of the primary tumour under the lower edge of the second sacrum, (iii) RC confirmed as cT3-4,anyN,M0, (iv) no distant metastasis on chest, abdominal and pelvic CT, (v) good physical fitness for elective surgery, (vi) Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, (vii) age 20-80 years and (viii) adequate hematologic, hepatic and renal functions (i.e. neutrophil count >1500/ml; platelet count >100,000/μl; estimated glomerular filtration rate >40 ml/min/1.73 m2; total bilirubin concentration less than twice the upper limit of normal and liver transaminase or alkaline phosphatase levels less than thrice the upper limit of normal).
The exclusion criteria were as follows: (i) intestinal obstruction despite ileostomy or colostomy construction; (ii) T4 tumour invasion of an adjacent organ or the pelvic wall, which was deemed unresectable before administration of any preoperative therapy on the basis of imaging examination; (iii) history of prior chemotherapy and/or radiotherapy of the pelvis; (iv) presence of any other active malignant disease or severe co-morbidity and (v) pregnancy.
Neoadjuvant chemotherapy. Six courses of FOLFOX (day 1, intravenous bolus of oxaliplatin at 85 mg/m2, L-leucovorin at 400 mg/m2 and fluorouracil at 400 mg/m2, followed by continuous infusion at 2,400 mg/m2 for 46 h) were administered before surgery. Adverse events of chemotherapy were evaluated according to the National Cancer Institute Common Terminology for Adverse Events, version 4.0.
Surgical procedure. Surgery was performed 2-6 weeks after completion of neoadjuvant FOLFOX therapy. Total or tumour-specific mesorectal excision was performed, with preservation of the bilateral autonomic nerves. Lateral pelvic lymphadenectomy was performed when the tumour was located below the peritoneal reflection and the lateral lymph node was judged as metastatic before neoadjuvant FOLFOX therapy, regardless of the response to therapy.
Pathological evaluation. The extent of the tumour response to neoadjuvant FOLFOX therapy was categorised according to the number of viable carcinoma cells within the tumour as described by the Japanese Classification of Colorectal Carcinoma Version 2 (10) as follows: grade 0, no regression; grade 1a, minimal effect (necrosis of less than one-third of the lesion); grade 1b, mild effect (necrosis of less than two-thirds but one-third or more of the lesion); grade 2, moderate effect (necrosis of more than two-thirds of the lesion) and grade 3, no tumour cells (pathological complete response [pCR]). Patients with a tumour response of grades 2 to 1b were considered to have a partial response.
Statistical analysis. The primary endpoint of this study was the R0 resection rate. The secondary endpoints were the scheduled treatment completion rate, adverse events, pathological response, downstaging rate and 3-year DFS rate. The Kaplan–Meier method was used to calculate the event rates. SPSS v21 software (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses.
Results
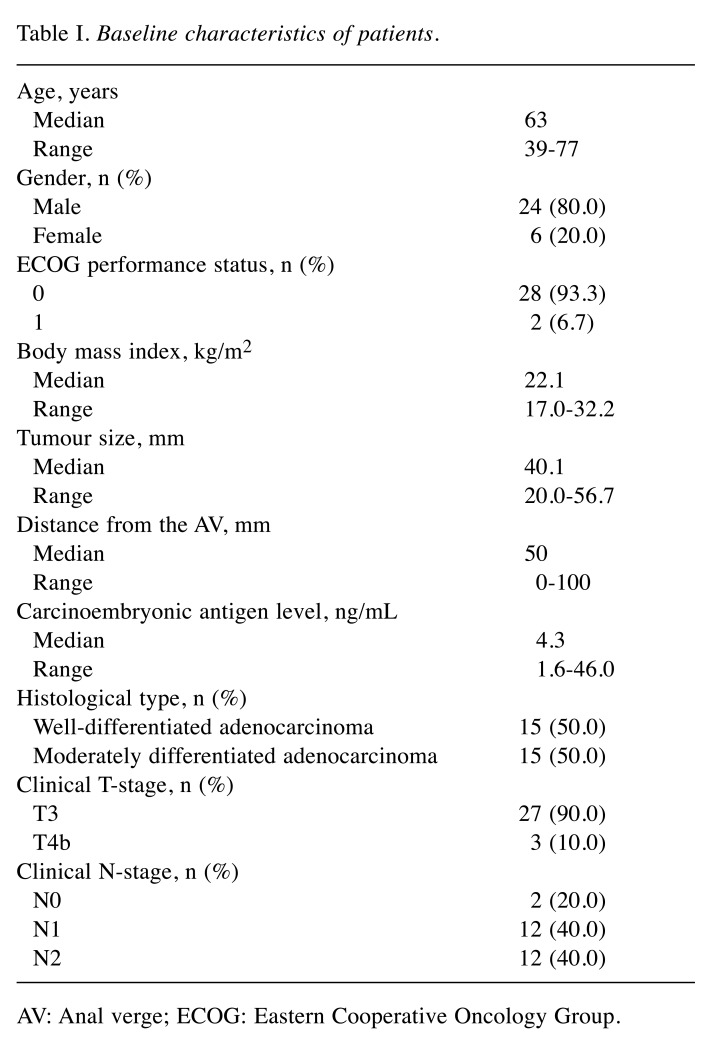
Patient characteristics. This study enrolled 30 patients between February 2012 and December 2015. Table I shows the patient characteristics. The majority of patients (90%) had cT3 tumours. Three patients had cT4b tumours, and of these patients, two showed invasion of the levator ani muscle and one showed invasion of the seminal vesicles. All cT4b tumours were considered resectable at baseline assessment.
Table I. Baseline characteristics of patients.
AV: Anal verge; ECOG: Eastern Cooperative Oncology Group.
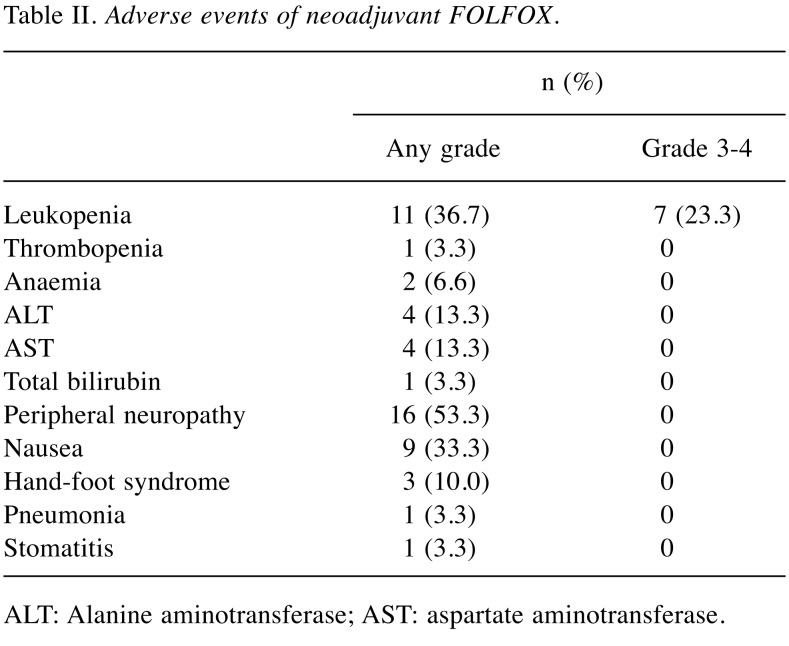
Treatment exposure and adverse events. The completion rate of the scheduled 6-cycle treatment regimen was 93.3% (28/30). Two patients received five cycles. One patient with an adverse event of peripheral neuropathy and another with nausea refused the last cycle. The relative dose intensities of oxaliplatin, bolus 5-FU and infused 5-FU were 95.8%, 98.9% and 98.9%, respectively.
Table II shows the adverse events. The overall rate of any grade of adverse events during the scheduled treatment regimen was 80.0% (24/30). The most common adverse event was peripheral neuropathy (53.3%, 16/30). Grade 3-4 adverse events developed in 23.3% (7/30) of patients, and all patients had neutropenia. No patients had febrile neutropenia.
Table II. Adverse events of neoadjuvant FOLFOX.
ALT: Alanine aminotransferase; AST: aspartate aminotransferase
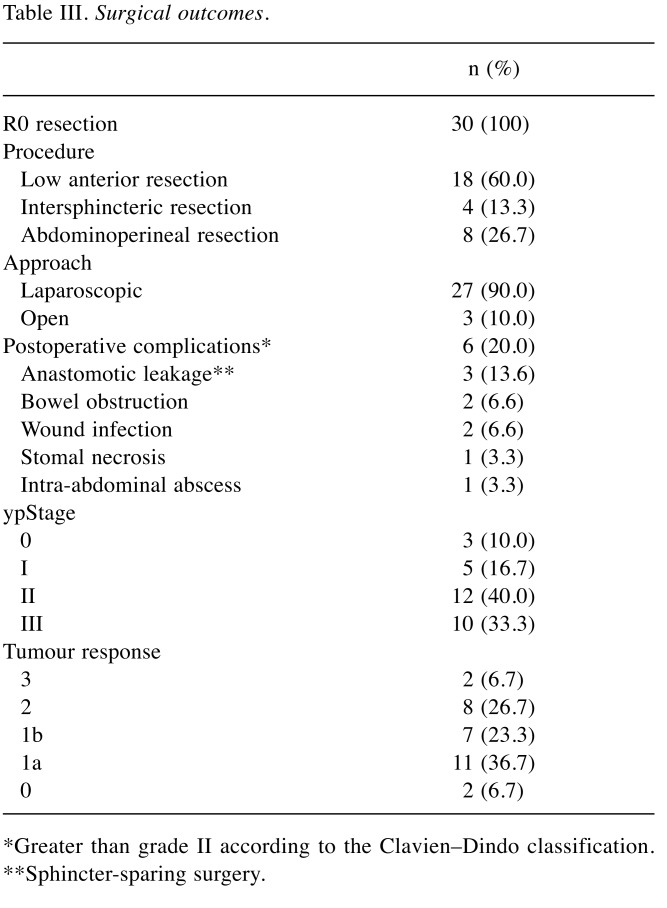
Surgery. All the 30 patients scheduled for elective surgery underwent R0 resection. The median duration from the last cycle of neoadjuvant FOLFOX therapy to tumour resection was 32 days (range=16-46 days). One patient requested to undergo surgery beyond the planned interval at day 46. Table III shows the surgical outcomes. Three patients with cT4 tumours underwent radical resection combined with total mesorectal excision involving adjacent organs (the levator ani in two patients and the seminal vesicle in one patient). Sphincter-sparing resection was performed in 73.3% (22/30) of the patients. Postoperative complications (grade≥2) were noted in 20% (6/30) of the patients. Reoperation was needed in patients with stomal necrosis. Three patients with anastomotic leakage were treated with tubal drainage, and no further surgery was performed.
Table III. Surgical outcomes.
*Greater than grade II according to the Clavien–Dindo classification. **Sphincter-sparing surgery.
Pathological outcomes. Table III shows the pathological results. pCR was observed in two patients (6.7%), and partial response was observed in 15 patients (50.0%). Although 13 patients (43.3%) had a poor response, including two patients (6.7%) with no response, planned surgery was performed and all patients underwent R0 resection.
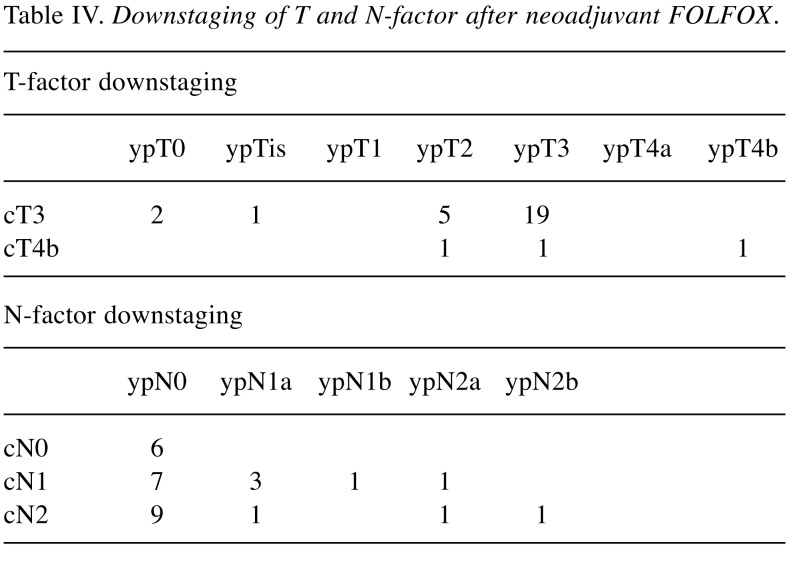
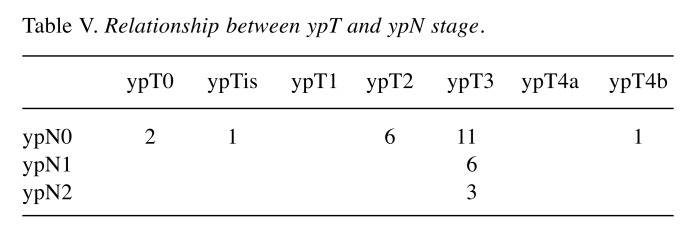
Among the 30 patients, 18 (60%) showed clinical downstaging (cStage II to ypStage 0-I and cStage III to ypStage 0-III) after NAC when compared with the clinical stage before NAC. Table IV shows T and N-factor downstaging. T-factor downstaging was observed in 10 (33.3%) patients. Among 27 patients with cT3 tumours, ypT0 was noted in two patients, ypTis in 1, ypT2 in 5 and ypT3 in 19. Among 3 patients with cT4b tumours, ypT2, ypT3 and ypT4b (seminal vesicle) were each noted in one patient. N-factor downstaging (cN1 to ypN0 and cN2 to ypN0-1) was observed in 17 (70.8%) of 24 patients. All six patients with cN0 were judged as ypN0. Of the 24 patients, 16 did not have lymph node metastasis histopathologically. Table V shows the relationship between ypT and ypN. Nine patients showed T-factor downstaging to less than T2, and they did not have lymph node metastasis, although 6 patients were judged as cN+. On the other hand, among 20 patients with ypT3, 9 (40.0%) had lymph node metastasis.
Table IV. Downstaging of T and N-factor after neoadjuvant FOLFOX.
Table V. Relationship between ypT and ypN stage.
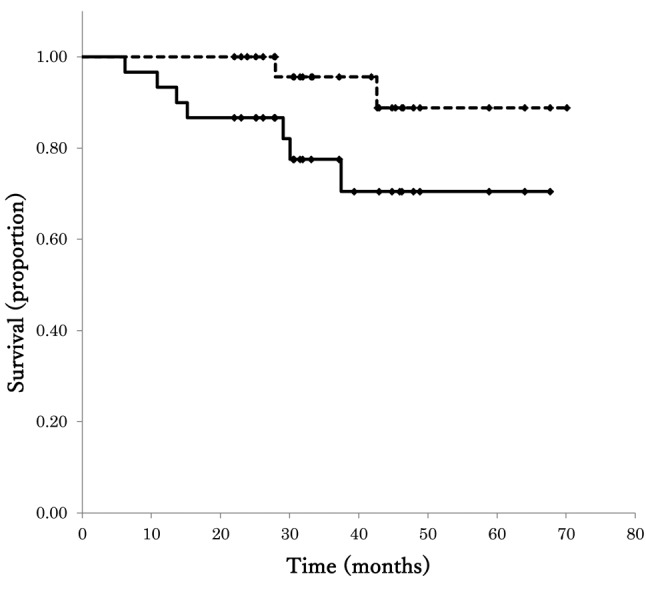
Survival. The median follow-up period was 31.1 months (range=9-54 months). The 3-year OS and DFS rates in all patients were 95.7% (95% confidence interval [CI]=87.3%-100%) and 77.5% (95%CI=61.4%-93.7%), respectively (Figure 1). Two patients had anastomotic recurrence, and both patients underwent a second surgery to excise the recurrence. They have had no relapse after the second surgery. Five patients developed distant metastases (lung 2 and liver 3), and two patients died from metastatic disease (lung 1 and liver 1).
Figure 1. Kaplan–Meier plots of disease-free survival (DFS) and overall survival (OS) for the 30 study patients.
Discussion
In this study, NAC involving six cycles of neoadjuvant FOLFOX followed by elective surgery was associated with a R0 resection rate of 100% and a pCR rate of 6.7%. Additionally, the scheduled treatment completion rate was 93.3%, and grade 3-4 adverse events were observed in 23.3% of patients.
The advantage of NAC is that effective chemotherapeutic agents are administered at an early stage of treatment when the tumour is still intact. Tumour shrinkage can improve the local control of surgery, and exposure of undetected distant micrometastases may reduce the risk of recurrence after surgery. NAC could be considered a promising therapeutic strategy for improving the oncological outcomes of RC. Also, the R0 resection rate was used as the primary endpoint. R0 resection rate after NAC was 100% among patients who were judged to be capable of undergoing radical excision at baseline. Previous reports also showed the R0 resection rates of NAC for cT3-4 (except T4b) were high, ranging from 98.3% to 100% (11-14). An important problem inherent in preoperative treatment is that surgical curability may be impaired by tumour growth during the treatment. In this study, although 13 patients showed no response histologically, the tumours did not develop clinically during NAC and the therapy did not hinder surgical curability. This is an important finding to support future research on whether NAC will improve prognosis.
Our strategy had a therapy completion rate of 93.3% with high relative dose intensity. A neoadjuvant setting has the advantage of administering planned systemic chemotherapy, as patients are in a good performance status. In order to increase the effects of adjuvant chemotherapy, it is necessary not only to administer a powerful cytotoxic agent but also to maintain a high dose intensity. Although the grade 3-4 neutropenia rate was 23.3%, which was higher than that reported in previous oxaliplatin-based NAC studies (0%-4.0%) (14-18), adverse events were controlled by appropriate drug reduction or withdrawal. Previous studies reported that 32.6%-42% of patients had grade 3-4 neutropenia after 6 months of postoperative adjuvant chemotherapy (FOLFOX/CapeOX [capecitabine + L-leucovorin + oxaliplatin]) (19-21). Additionally, the TOSCA trial investigated the optimal therapy period (3 vs. 6 months) and reported that the rate of grade 3-4 neutropenia in the 3-month arm was 19.3% (22). Based on these results, our results are considered reasonable.
In locally advanced RC, NACRT combined with TME can result in a low local recurrence rate. However, NACRT did not improve OS in previous studies (2-5). Several factors might be associated with this result. First, the effect of the chemotherapeutic agent 5-FU used as a radiosensitiser might be insufficient to decrease distant metastasis. Second, the induction rate of adjuvant chemotherapy after NACRT might be low, and the rate in EORTC22921 was 43% (5) and in CHRONICLE was 48% (23). The final goal of NAC is prognostic improvement. In the neoadjuvant setting it is reasonable to administer chemotherapeutic agents for decreasing distant metastasis and improving OS. To reveal the prognostic outcomes of the NAC approach, the PROSPECT study (US, NCT01515787) comparing neoadjuvant FOLFOX followed by selective use of NACRT to standard NACRT is ongoing.
In this study, the response rate was 56.7% and the pCR rate was 6.7%. Previous studies on oxaliplatin-based regimens without a molecular-target drug revealed a pCR rate of 6.6%-12.2% (14,17,18,24). The pCR rate might increase with the addition of a molecular-target drug. The pCR rate was 13.3%-25% for regimens that involved bevacizumab (11-13,15) and was 18% for regimens that involved cetuximab (13). However, the relationship between histological response and prognosis in NAC has not been clarified, though many reports on NACRT showed pCR and good histological response were associated with a good prognosis (25-28). AlGizawy et al. (14) reported on NAC involving XELOX plus bevacizumab and mentioned that this strategy had a high pCR of 20% and a 2-year DFS rate of 70%. On the other hand, our strategy had a low pCR of 6.7% and a 3-year DFS rate of 77.5%. Thus, any positive response may be the goal of NAC for improving prognosis. To judge the validity of adding a molecular-target drug, it is necessary to clarify the significance of increasing the pCR rate in NAC.
The additive effect of a molecular-target drug on prognostic improvement in postoperative adjuvant chemotherapy of colon cancer has not been shown. Presently, it is questionable to add a molecular-target drug to NAC for resectable RC to improve prognostic outcomes. With regard to T4 and bulky T3 tumours that cannot easily undergo R0 resection, a phase two study on XELOX and a bevacizumab regimen for such locally advanced RC reported R0 resection rates of 90-100% (16,29). The addition of a molecular-target drug to NAC may help in the treatment of borderline and unresectable RC.
For locally advanced RC, the standard approach is TME (LAR or APR) (low anterior resection or abdominoperineal resection). However, a paradigm shift is occurring recently. Selected patients with complete (ycT0N0) and good (ycT1-2N0) responses to NACRT are considered as candidates for organ preservation strategies, including local excision (LE) (30,31) or strict follow-up without operation (watch-and-wait approach) (32-34). Organ preservation is a promising strategy to decrease postoperative complications and impairments in anorectal function and quality of life, which are associated with TME. Although the indication of organ preservation is debatable still, a meta-analysis by Shaikh et al. reported that OS and DFS in cT3anyN patients, who underwent NACRT, showed no difference between LE and TME (35). In our study, nine patients (30.0%) with downstaging to less than ypT2 had ycN0 despite including six patients with cN+. Shrinkage of the primary tumour by NAC can reflect the disappearance of latent lymph node metastasis, and the absence of lymph node metastasis is essential for LE. Our results suggested that NAC may be an option for organ preservation.
The present study had certain limitations. Only RC cases judged as resectable before treatment were included. Therefore, further studies are needed to evaluate the R0 and local recurrence rates of neoadjuvant FOLFOX in cases of borderline or unresectable RC. In addition, this was a single-institution, pilot study and the number of samples was relatively small. Therefore, to clarify the effects of FOLFOX on the prognosis of RC patients after surgery, a larger randomised control trial is needed.
In conclusion, NAC involving FOLFOX is a safe and feasible treatment for baseline resectable RC. NAC is a promising therapeutic strategy that avoids the adverse events of radiation, which are problems in NACRT, and it can improve prognosis.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
References
- 1.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, L. Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 5.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, Zapas JL, Goodwin JW, Fehrenbacher L, Ramanathan RK, Conley BA, Flynn PJ, Soori G, Colman LK, Levine EA, Lanier KS, Wolmark N. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 7.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 8.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa S, Hida J, Ike H, Kinugasa T, Ota M, Shinto E, Itabashi M, Kameoka S, Sugihara K. Selection of lymph node-positive cases based on perirectal and lateral pelvic lymph nodes using magnetic resonance imaging: study of the Japanese Society for Cancer of the Colon and Rectum. Ann Surg Oncol. 2016;23:1187–1194. doi: 10.1245/s10434-015-5021-2. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Society for Cancer of the Colon and Rectum: Japanese classification of colorectal carcinoma. Tokyo, Kanehara & Co. 2009 [Google Scholar]
- 11.Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub KJ, Shia J, Guillem JG, Temple LK, Paty PB, Saltz LB. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Martos C, Brown G, Estevan R, Salud A, Montagut C, Maurel J, Safont MJ, Aparicio J, Feliu J, Vera R, Alonso V, Gallego J, Martin M, Pera M, Sierra E, Serra J, Delgado S, Roig JV, Santos J, Pericay C. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19:1042–1043. doi: 10.1634/theoncologist.2014-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa S, Goto S, Matsumoto T, Hida K, Kawada K, Matsusue R, Yamaguchi T, Nishitai R, Manaka D, Kato S, Kadokawa Y, Yamanokuchi S, Kawamura J, Zaima M, Kyogoku T, Kanazawa A, Mori Y, Kanai M, Matsumoto S, Sakai Y. A multicenter phase 2 study on the feasibility and efficacy of neoadjuvant Chemotherapy without radiotherapy for locally advanced rectal cancer. Ann Surg Oncol. 2017;24:3587–3595. doi: 10.1245/s10434-017-5967-3. [DOI] [PubMed] [Google Scholar]
- 14.AlGizawy SM, Essa HH, Ahmed BM. Chemotherapy alone for patients with stage II/III rectal cancer undergoing radical surgery. Oncologist. 2015;20:752–757. doi: 10.1634/theoncologist.2015-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya H, Ohmiya N, Goto H, Nagino M. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II trial. Jpn J Clin Oncol. 2013;43:964–971. doi: 10.1093/jjco/hyt115. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa J, Nishimura J, Mizushima T, Miyake Y, Kim HM, Takemoto H, Tamagawa H, Noura S, Fujii M, Fujie Y, Kato T, Miwa H, Takemasa I, Ikeda M, Yamamoto H, Sekimoto M, Nezu R, Doki Y, Mori M. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol. 2014;73:1079–1087. doi: 10.1007/s00280-014-2417-9. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya T, Uehara K, Nakayama G, Ishigure K, Kobayashi S, Hiramatsu K, Nakayama H, Yamashita K, Sakamoto E, Tojima Y, Kawai S, Kodera Y, Nagino M. Early results of multicenter phase II trial of perioperative oxaliplatin and capecitabine without radiotherapy for high-risk rectal cancer: CORONA I study. Eur J Surg Oncol. 2016;42:829–835. doi: 10.1016/j.ejso.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Ueki T, Manabe T, Inoue S, Ienaga J, Yamanaka N, Egami T, Ishikawa M, Konomi H, Ikubo A, Nagayoshi K, Nakamura M, Tanaka M. A feasibility study of neoadjuvant XELOX without radiotherapy for locally advanced lower rectal cancer. Anticancer Res. 2016;36:741–747. [PubMed] [Google Scholar]
- 19.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins JN, Seay TE, Fehrenbacher L, O’Reilly S, Chu L, Azar CA, Wolmark N. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385–3390. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andre T, Boni C, Monudeji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A, Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 21.de Gramont A, van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im SA, Bodoky G, Salazar R, Maindrault-Goebel F, Shacham-Shmueli E, Bajetta E, Makrutzki M, Shang A, André T, Hoff PM. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase III randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 22.Lonardi S, Sobrero A, Rosati G, Di Bartolomeo M, Ronzoni M, Aprile G, Scartozzi M, Banzi M, Zampino MZ, Pasini F, Marchetti P, Cantore M, Zaniboni A, Rimassa L, Ciuffreda L, Ferrari D, Barni S, Zagonel V, Maiello E, Rulli E, Labianca R. Phase III trial comparing 3-6 months of adjuvant FOLFOX4/XELOX in stage II-III colon cancer: Safety and compliance in the TOSCA trial. Ann Oncol. 2016;27:2074–2081. doi: 10.1093/annonc/mdw404. [DOI] [PubMed] [Google Scholar]
- 23.Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, Ledermann J, Sebag-Montefiore D. Chronicle: Results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25:1356–1362. doi: 10.1093/annonc/mdu147. [DOI] [PubMed] [Google Scholar]
- 24.Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: Initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34:3300–3307. doi: 10.1200/JCO.2016.66.6198. [DOI] [PubMed] [Google Scholar]
- 25.Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr., Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 26.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, Geisler D, Dietz DW, Lavery IC, Fazio VW, Kalady MF. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18:1590–1598. doi: 10.1245/s10434-010-1506-1. [DOI] [PubMed] [Google Scholar]
- 27.Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 28.Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S, Mattia FO, Friso KL, Genovesi D, Vidali C, Gambacorta MA, Buffoli A, Lupattelli M, Favretto MS, La Torre G. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Uehara K, Ishiguro S, Sakamoto E, Maeda A, Inoue M, Tojima Y, Kobayashi S, Omiya N, Ishizuka N, Nakao A, Goto H, Nagino M. Phase II trial of neoadjuvant chemotherapy with XELOX plus bevacizumab for locally advanced rectal cancer. Jpn J Clin Oncol. 2011;41:1041–1044. doi: 10.1093/jjco/hyr084. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): Results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16:1537–1546. doi: 10.1016/S1470-2045(15)00215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, Faucheron JL, Jafari M, Portier G, Meunier B, Sileznieff I, Prudhomme M, Marchal F, Pocard M, Pezet D, Rullier A, Vendrely V, Denost Q, Asselineau J, Doussau A. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390:469–479. doi: 10.1016/S0140-6736(17)31056-5. [DOI] [PubMed] [Google Scholar]
- 32.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr., Silva e Sousa AH Jr., Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–718. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JC, Lindebjerg J, Rafaelsen SR, Jakobsen A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:2015–2016. doi: 10.1016/S1470-2045(15)00120-5. [DOI] [PubMed] [Google Scholar]
- 34.Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP, Wilson MS, Scott N, Dwyer O’. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174–183. doi: 10.1016/S1470-2045(15)00467-2. [DOI] [PubMed] [Google Scholar]
- 35.Shaikh I, Askari A, Ourû S, Warusavitarne J, Athanasiou T, Faiz O. Oncological outcomes of local excision compared with radical surgery after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2014;30:19–29. doi: 10.1007/s00384-014-2045-1. [DOI] [PubMed] [Google Scholar]