Abstract
Physical stimulation of airway surfaces evokes liquid secretion, but the events that mediate this vital protective function are not understood. When cystic fibrosis transmembrane conductance regulator (CFTR) channel activity was used as a functional readout, we found signaling elements compartmentalized at both extracellular and intracellular surfaces of the apical cell membrane that activate apical Cl− conductance in Calu-3 cells. At the outer surface, ATP was released by physical stimuli, locally converted to adenosine, and sensed by A2B adenosine receptors. These receptors couple to G proteins, adenylyl cyclase, and protein kinase A, at the intracellular face of the apical membrane to activate colocalized CFTR. Thus, airways have evolved highly efficient mechanisms to “flush” noxious stimuli from airway surfaces by selective activation of apical membrane signal transduction and effector systems.
Airways continuously remove noxious materials through a mucociliary clearance process that requires liquid secretion (1, 2). cAMP-regulated cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels (3, 4) are expected to participate in liquid secretion in airways, but the two key events in the activation of CFTR by local physical stimuli remain puzzling. First, how do physical stimuli initiate the classic cAMP signaling cascade, a process that is tightly regulated by G-protein-coupled receptors (5)? Second, how does cAMP reach CFTR in the apical membrane? The dogma of G-protein-coupled receptors and adenylyl cyclase (AC) restricted to the basolateral membrane of epithelia does not adequately explain how these events occur (6).
Two ongoing areas of research suggest a potentially more relevant, but as yet not fully tested model for activation of apical CFTR by a local physical stimulus. Airway surface epithelia are poorly innervated, suggesting that mucociliary clearance is subject to autocrine/paracrine control. A leading candidate for mediating mucociliary clearance is the release of cellular nucleotides, because release occurs in response to physical stimuli and luminal nucleotide receptors stimulate apical Cl− conductance, mucus secretion, and ciliary beating (7, 8). The action of ectonucleotidases extends the signaling potential of released ATP on luminal surface by producing adenosine (Ado), a ligand for A2 receptors that couple to AC (9, 10). Recent reports indicate that receptors, intracellular signaling pathways, and scaffolding molecules can form complexes that locally regulate functions in subcellular compartments (11, 12). Thus, a model linking a luminal physical stimulus to activation of CFTR requires specific elements, including ATP release, ectonucleotidases, Ado receptors, G proteins, and AC, to be intimately associated with the apical cell membrane. The goal of the present study was to test this hypothesis in polarized airway epithelial cells.
Methods
Cells.
Human Calu-3 cells were grown as previously described (13) on Costar clear transwells (for HPLC and cAMP assay) or homemade permeable supports (diameter was 1.5 cm for Ussing chambers and 1.5 mm for patch-clamp studies) to confluence with a resistance greater than 100 ohm⋅cm2.
HPLC Analysis of Ado and Its Nucleotides.
Calu-3 epithelia were washed three times with Hanks' balanced salt solution buffer and loaded with 0.5 ml of buffer on the luminal surface. After 3 h at 37°C, 0.3 ml of the surface liquid was carefully collected for HPLC analysis. Adenine-containing species were derivatized with 2-chloroacetaldehyde (14), and the resulting fluorescent 1,N6-ethenopurines were separated by HPLC.
Cl− Secretory Response to Luminal Hypotonic Challenge.
Single-barreled microelectrodes were pulled on a horizontal pipette puller (P5, Narishige, Tokyo) from borosilicate glass (GC 120F, Clarke Electromedical Instruments, Pangbourne, UK) and filled with 3 M KCl. To impale the mucosal surface liquid of Calu-3 epithelia, a macroelectrode (3 M KCl-filled agar bridge/calomel half-cell; World Precision Instruments, Sarasota, FL) was placed in the serosal bath and the microelectrode was positioned by micromanipulator into the mucosal surface liquid so that stable transepithelial potential difference measurements could be recorded with an electrometer (FD 223, World Precision Instruments). Measurements were made over an ≈10-min period after the addition of 20 μl of hypotonic Ringer's solution (200 mOsm) with or without adenosine deaminase (1 unit/ml) to the mucosal surface. Perfluorocarbon (FC-77, 3M Co.) was added to the mucosal surface before and after the addition of Ringer's solution to avoid evaporation of the surface liquid. All experiments were performed at 37°C.
Ussing Chamber Studies.
CFTR-mediated Cl− secretion was measured as described (15). Briefly, cells grown on collagen membrane supports were mounted in conventional Ussing chamber devices. The submucosal bathing solution was Krebs bicarbonate Ringer's solution (KBR) and the mucosal solution was low-Cl− (3 mM) KBR. Bioelectric properties were digitally recorded from the output of voltage clamps (Physiologic Instruments, San Diego, no. VCC600) by using ACQUIRE software (Physiologic Instruments). Voltage was clamped to 0 mV, except for 3-sec pulses to ± 10 mV every 60 sec. Tissue conductance was calculated from the resulting current deflections. For each experiment, basal properties were recorded for 20–40 min. Test compounds (Ado, Ado analogs, A2 antagonists, etc.) were added to the low-Cl− luminal bath. Maximum cAMP-dependent Isc (short circuit current) was recorded after bilateral exposure to 10 μM forskolin. Relative potency was determined by fitting curves to the fractional changes induced by sequential increasing concentrations of test substances, relative to the response to forskolin. These conditions resulted in a basal CFTR-mediated Isc of 30 to 40 μAmp/cm2 and a maximally stimulated CFTR current of ≈150–200 μA/cm2.
Intracellular cAMP Assay.
Calu-3 epithelia were washed three times (160 mM Tris⋅Cl/30 mM sucrose, pH 7.0), and incubated with the same buffer plus 0.3 mM adenosine 5′-[α,β-methylene]diphosphate (AMPCP) for 45 min at room temperature. Subsequently, 1 μM Ado or 1 μM forskolin was added into the luminal bath. After 8 min, cells were lysed and intracellular cAMP was measured by an enzyme immunoassay kit.
Single-Channel Studies.
CFTR Cl− channel activity was recorded at a membrane potential of 60 mV (13).
Cell-attached recording.
Both the pipette and the bath contained 160 mM Tris⋅Cl and 30 mM sucrose, pH 7.0. The resistance of an open pipette was 6–8 MΩ. Compounds tested only in the pipette were added to pipette solution and CFTR channel activity was recorded 300 s after seal formation. Tests of Ado or forskolin outside the pipette were made by cumulative additions to the bath, as indicated.
Outside-out recording.
Pipettes were filled with 40 mM Tris⋅Cl/100 mM Tris⋅gluconate/2 mM MgCl2/5 mM Tes/1 mM EGTA/0.1 mM CaCl2/1 mM MgATP/0.2 mM LiGTP, pH 7.4. The bath buffer was 150 mM Tris⋅Cl/2 mM MgCl/1 mM CaCl2/5 mM Tes/30 mM sucrose/10 mM d-glucose, pH 7.4. After formation of outside-out patches, CFTR channel activity was recorded for 300 s. Ado was then added into the bath and channel activity was recorded for 300–400 s.
Inside-out recording.
Pipette and bath solutions were the same as in the cell-attached recording, and 1 mM MgATP was added in the bath solution. CFTR activity was recorded for 300 s after inside-out patch excision, and then forskolin was added to the bath.
AMPCP was present at 0.3 mM in both pipette and bath solutions in all experiments, except for some experiments in Fig. 2A. The product of CFTR channel number (N) and open probability (po) was calculated as CFTR channel activity. For experiments in Fig. 2 A and C, Npo was calculated from 300 s of recording for each experiment. For all other experiments, control Npo was calculated from the 100 s recorded before test compound exposure. For each test condition, Npo was calculated from the 100-s segment (of 300 s) with the highest Npo.
Figure 2.
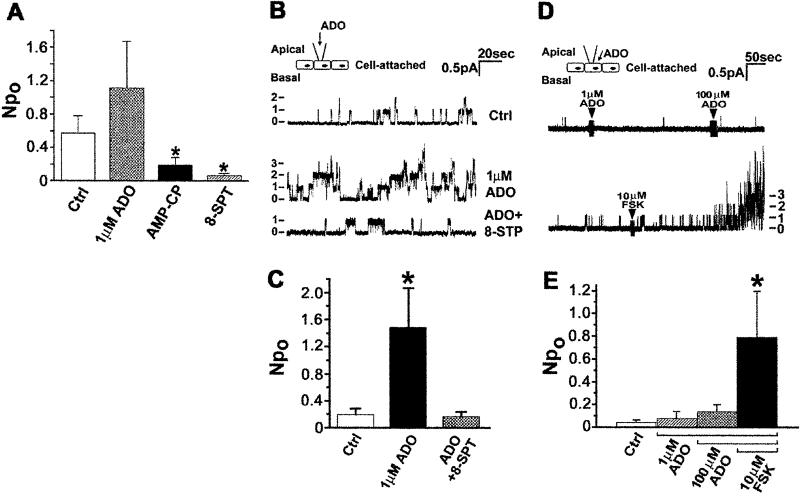
Exogenous and endogenous adenosine stimulates CFTR in cell-attached patches. (A) CFTR cell-attached Npo recorded under control conditions (Ctrl; n = 26) or when the pipette contained 1 μM exogenous Ado (n = 18), 300 μM AMPCP (present in the bath as well; n = 28), or 100 μM 8-SPT (n = 15). *, Different from control (P < 0.05). (B) Effect of exogenous Ado on CFTR channel activity with AMPCP in the pipette and the bath. Numbers and dashes at the left indicate active channel number. (C) Summary data for B. Ctrl (n = 28); 1 μM Ado (n = 28); 1 μM Ado + 100 μM 8-SPT (n = 16). *, Different from Ctrl, P < 0.02. (D) Effect of adenosine added to the bath on CFTR contained in cell-attached patches. Continuous trace showing CFTR activity recorded in the cell-attached mode before and during sequential exposure to 1 and 100 μM Ado, followed by 10 μM forskolin. (E) Summary data for D. (n = 9). *, Different from Ctrl, P < 0.05.
Statistics.
All of the data were expressed as means ± SE and the Student's t test was used for statistical analysis.
Reagents.
ATP, GTP, and the enzymeimmunoassay kit were from Amersham Pharmacia. Guanosine 5′-[β-thio]diphosphate (GDPβS) and guanosine 5′-[γ-thio]triphosphate (GTPγS) were from Boehringer Mannheim. Other reagents were obtained from Sigma.
Results
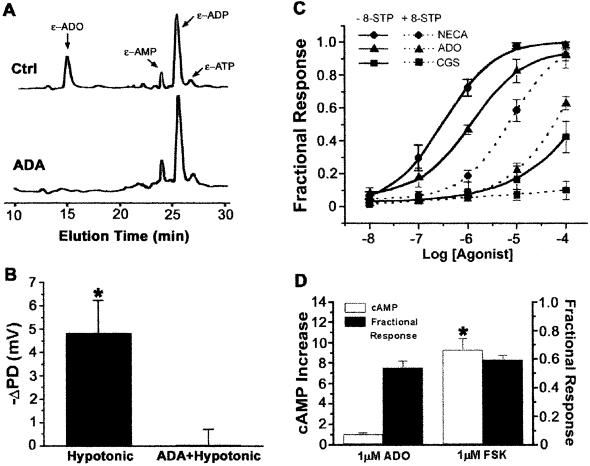
We tested for compartmentalized regulation of CFTR by Ado in Calu-3 cells, a model of serous cells, which are thought to play a critical role in liquid secretion and pathogenesis of cystic fibrosis in human airways (16, 17). We began by asking if Ado was present in airway surface liquid under basal conditions. Liquid sampled from the nondisturbed apical surface of Calu-3 epithelia contained ATP as well as ADP, AMP, and Ado (Fig. 1A), consistent with basal release and metabolism of cellular ATP on the luminal surface (18). Moreover, a hypotonic stimulus stimulated Cl− secretion and this change was prevented by adenosine deaminase (Fig. 1B), which removed Ado from the luminal compartment (Fig. 1 A and B). Because Calu-3 cells do not express P2Y2 receptors (19), this result suggested that the released ATP was metabolized by ectonucleotidases to Ado, a ligand for A2 receptors that couple to AC (9, 10, 20). We tested this possibility by exposing the luminal surface of Calu-3 epithelia to Ado analogs in Ussing chambers. Cl− secretion was stimulated with a rank order potency (5′-N-ethylcarboxamidoadenosine > Ado ≫ CGS-21680) and inhibitor sensitivity (8-SPT), consistent with luminal A2B adenosine receptors (A2BAR) regulating CFTR through a Gs–AC–protein kinase A (PKA) pathway (10, 21) (Fig. 1C). However, when we correlated the effect of luminal Ado on Cl− secretion with cAMP production, we found that 1 μM Ado, the approximate ED50 concentration for stimulation of Cl− secretion (Fig. 1C), barely increased production of cAMP. In contrast, 1 μM luminal forskolin stimulated no more Cl− secretion than 1 μM Ado, but produced 9 times more cAMP (Fig. 1D).
Figure 1.
CFTR regulation by mucosal Ado. (A) HPLC profile of ethenoadenosine (ɛ-Ado) and its nucleotides in the mucosal surface liquid media of Calu-3 cells. The ɛ-adenosine peak was identified by ɛ-adenosine standards and by its sensitivity to 1 unit/ml adenosine deaminase (ADA). y axis, fluorescence in arbitrary units. The amount of ethenopurines in the total 0.5 ml of luminal surface liquid were as follows: ɛ-Ado, 16 pmol; ɛ-AMP, 9 pmol; ɛ-ADP, 28 pmol; and ɛ-ATP, 4 pmol. (B) Cl− secretory response to mucosal hypotonic challenge. (C) Cl− secretion by polarized Calu-3 cells in response to Ado and Ado analogs. Responses to cumulative increases in ligand concentration in the mucosal bath solution are expressed as a percent of the 10 μM forskolin response (solid lines). Dose–effect relationships were also performed in the presence the Ado receptor antagonist 8-(p-sulfophenyl)theophylline (8-SPT; 100 μM) (dashed lines). NECA, 5′-N-ethylcarboxamidoadenosine; CGS, CGS-21680 (n = 3). (D) cAMP accumulation and Cl− secretion. Cl− secretion stimulated by 1 μM Ado or 1 μM forskolin are expressed as a percent of the 10 μM forskolin response (Fractional Response) as in C. The intracellular cAMP concentrations are expressed as fold over control (n = 3 or 4). *, Different from control, P < 0.001.
The efficient stimulation of Cl− secretion by Ado with little change in cAMP level could signify highly localized regulation of CFTR by A2BAR in the apical cell membrane. To better evaluate this possibility we recorded the single-channel activity of CFTR in the apical membranes of polarized Calu-3 cell epithelia under defined patch-clamp conditions. During cell-attached recording with 1 μM exogenous Ado in the pipette, CFTR NPo was nearly 2-fold greater than basal NPo (Fig. 2A). However, the effect only approached statistical significance despite a large sample size Interestingly, CFTR NPo was markedly decreased when the pipette contained an inhibitor of Ado formation, AMPCP (9), or the Ado receptor antagonist 8-SPT (Fig. 2A). Thus, a significant fraction of CFTR activity during basal cell-attached recording conditions is due to endogenous Ado production at the external patched surface. With AMPCP present to limit this production of endogenous Ado, exogenous 1 μM Ado in the pipette caused a dramatic stimulation of CFTR NPo and 8-SPT blocked this action (Fig. 2 B and C). Therefore, A2BAR within an apical cell-attached membrane patch sense Ado and signal to CFTR Cl− channels contained within the same patch. This signaling between Ado at the extracellular surface and CFTR was tightly compartmentalized because 100 μM Ado added to the bath outside the pipette had no effect on CFTR activity within cell-attached patches (Fig. 2 D and E). As a control, CFTR in these same patches was robustly stimulated by the cell-permeant diterpene forskolin (10 μM), which we found to cause a large increase in total intracellular cAMP (Fig. 2 D and E).
Our cell-attached recordings of CFTR in the apical membrane identified A2BAR to CFTR signaling in a near membrane or subapical compartment. To better localize key cAMP-signaling elements, we excised apical membrane patches from polarized Calu-3 cells and used CFTR activity as a functional assay for G proteins, AC, and PKA. In outside-out apical membrane patches with 200 μM LiGTP in the pipette solution to approximate the cytosolic GTP concentration, sequential exposure to 1 μM and 10 μM Ado stimulated CFTR (Fig. 3A). In outside-out patches formed with a PKA inhibitor (PKI) in the pipette, CFTR basal activity was markedly reduced and Ado had no effect (Fig. 3A Inset). PKI sensitivity of Ado stimulation places functional PKA downstream of A2BAR and demonstrates that cAMP-signaling between A2BAR and CFTR is functionally intact in isolated patches of apical cell membrane.
Figure 3.
Regulation of CFTR by extracellular adenosine in excised, outside-out apical membrane patches. (A Upper) Representative trace showing activation of CFTR by extracellular Ado. After 300-s basal recording, Ado addition to the bath is indicated by the arrows, first at 1 μM for 300–400 s, and then at 10 μM for 300 s. (Lower) Summary data for 3A Upper (n = 9). *, Different from control, P < 0.05. (Inset) Effect of PKI (10 μM in pipette) on 1 μM Ado on CFTR activity (n = 6). (B) Effect of 1 μM Ado on CFTR activity when the pipette contained 200 μM GDP (n = 12), 100 μM GDPβS (n = 7), or 10 μM GTPγS (n = 11). *, Different from Before ADO, P < 0.05.
G proteins have been reported to affect CFTR in whole-cell conditions through implied actions on AC (22). When we formed outside-out patches with 200 μM GTP replaced by GDP, Ado no longer stimulated CFTR (Fig. 3B). GDPβS in the pipette caused even lower basal CFTR activity and abrogated the response to extracellular Ado (Fig. 3B). Stimulation of CFTR by Ado was fully supported, however, by just 10 μM GTPγS in the pipette solution (Fig. 3B). These results demonstrate a role for Gs in the regulation of CFTR in excised membrane patches, and reveal physical and functional association of this key element of the cAMP-signaling cascade with the inner apical membrane surface.
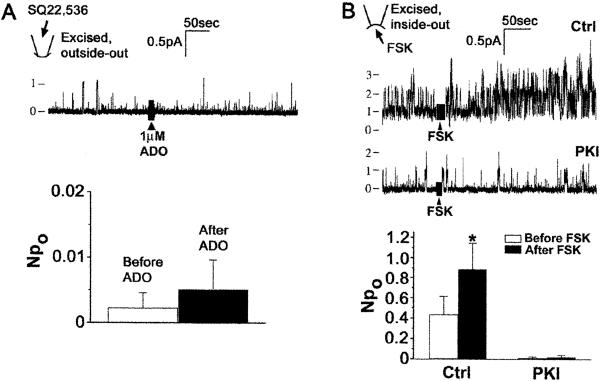
G-protein-dependent, PKA-mediated activation of CFTR by A2BAR in excised patches implies that the integral membrane protein AC is present and functional in the apical membrane of Calu-3 cells. AC expression in the apical membrane of epithelia is controversial (23–27). Therefore, we tested whether conditions that modulate AC affect Ado regulation of CFTR in excised apical membrane patches. When the P-site inhibitor of AC, SQ-22536 (28), was included in the pipette solution used with outside-out patches, we recorded very low basal CFTR activity and detected no increase in NPo in response to extracellular Ado (Fig. 4A, compare with Fig. 3A). As a second approach, patches containing CFTR were excised in the inside-out configuration into a bath that contained 1 mM MgATP. After 5 min, channel activity typically “ran down.” However, the addition of 10 μM forskolin, which directly stimulates AC, to the bath increased CFTR activity; this stimulation was blocked by PKI (Fig. 4B). SQ-22536 sensitivity of Ado regulation of CFTR and forskolin stimulation of CFTR by means of activation of endogenous PKA functionally demonstrate AC activity in the apical membrane.
Figure 4.
CFTR is regulated by apical AC activity. (A Upper) Effect of Ado on CFTR in excised, outside-out patches with the AC inhibitor SQ22,536 in the pipette solution. (Lower) Summary for seven experiments like those in Upper. Note expanded scale for Npo. (B Upper) Single-channel recordings in excised, inside-out patches showing effect of forskolin (FSK) on CFTR in the absence and presence of PKI. (Lower) Summary data for effect of forskolin alone (Ctrl, n = 12) or in the presence of 10 μM PKI (n = 5); *, P < 0.02.
Discussion
The distinctive polarized distribution of ion channels and transporters in epithelia that enables vectoral solute transport has fostered great interest in apical versus basolateral targeting mechanisms. However, descriptions of the signal transduction pathways that are necessary to selectively regulate functions localized in the apical or basolateral cell membranes have been lacking (29). An attractive hypothesis is that polarized transporters are efficiently regulated by signal transduction machinery appropriately colocalized at the apical or basolateral membrane. Testing for such compartmentalization by biochemical methods that disrupt polarity has proved problematic (23, 26, 27). Similarly, immunohistochemical methods often lack the sensitivity to detect a small pool of functionally important proteins localized in a subcellular domain, such as the apical membrane (23–25). Here, we have used patch-clamp technique to physically isolate apical cell membranes, and we have used CFTR single-channel activity as a functional reporter to identify cAMP signaling elements that are present. Our results reveal surprisingly intact A2BAR coupling to CFTR in cell-free apical membrane patches that was sensitive to GTP, to an inhibitor of AC, and to PKI.
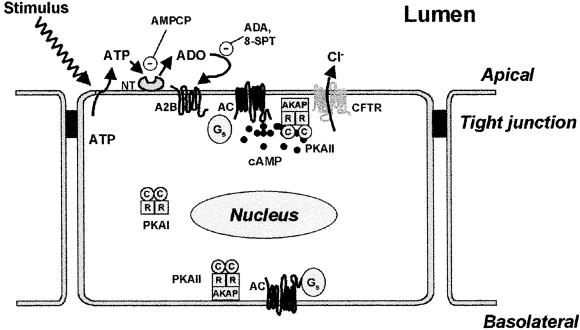
Our results support a model that accounts for activation of CFTR Cl− conductance in the apical membrane by local physical stimuli (Fig. 5). Although we initiated our studies to identify compartmentalized intracellular signaling elements, it became clear that recognition of a local physical stimulus involves organized signaling on the extracellular surface as well. The path begins with release of cellular ATP onto the luminal surface, a process known to occur in response to diverse physical stimuli (18, 30). Although we do not address the identity of the mechanism(s) responsible for ATP release, our HPLC data (Fig. 1A) are consistent with a nonstimulated constitutive release that could be increased by diverse physical stimuli (8, 18). ATP on the luminal surface of Calu-3 cells is converted to Ado. This conversion likely utilizes several ecto-enzymatic activities capable of dephosphorylating nucleotides. Inhibition of basal CFTR NPo by AMPCP and by 8-SPT reveals that Ado generated on the surface of Calu-3 cells activates A2BAR in the apical membrane. Thus, ATP release and metabolism at the extracellular cell surface constitutes a link between a physical stimulus and the intracellular signaling machinery linked to cAMP generation.
Figure 5.
Compartmentalized cAMP-signaling elements on the extracellular and intracellular surfaces of the apical cell membrane. Model depicts how diverse physical stimuli (e.g., hypotonicity and shear stress) regulate CFTR. After release of ATP onto the extracellular surface, Ado is generated by ectonucleotidases (NT). A2B receptors bind Ado and activate AC present in the apical membrane by means of Gs. Sufficient cAMP is generated locally to activate PKA in a diffusionally restricted apical microdomain, but not in other cellular compartments. AKAP, A-kinase anchoring protein.
At the inner apical membrane surface, A2BAR are coupled to CFTR by means of Gs, AC, and PKA. Activation of CFTR by Ado added to the extracellular face of excised, outside-out patches of apical membrane revealed that the intact cAMP-signaling path from receptor to final effector was closely associated with the apical membrane. This interpretation was strengthened by our observations that individually manipulating elements of the pathway affected CFTR activity in excised membrane patches. For example, PKI inhibited signaling, indicating that, as we reported previously (13), PKA exists in excised apical membranes. The pathway was also disrupted by GDP and GDPβS, which are expected for an intact signaling path from A2BAR through Gs (10). Our results also predicted that PKA associated with the excised membrane patch was being activated by locally generated cAMP. In accordance with this prediction, a P-site inhibitor of AC completely blocked activation of CFTR by extracellular Ado. In separate experiments, we showed that CFTR could be activated by exposure of inside-out patches to the AC activator forskolin. Therefore, we were able to observe functional evidence for a path from A2BAR to CFTR that included G proteins, AC, and PKA in apical membrane patches excised from polarized Calu-3 cells.
Our functional evidence of AC in the apical membrane of epithelial cells might be viewed as somewhat surprising, because AC is traditionally viewed as a marker of epithelial basolateral membrane (31, 32). However, our functional approach may be much more sensitive than biochemical or immunocytochemical assays. Importantly, AC function in the apical membrane greatly simplifies models proposed to explain how G-protein-coupled receptors (GPCR) in the apical membrane of epithelia signal to effectors in the same membrane. Specifically, with AC in the apical membrane, it is not necessary to postulate that apical GPCR generate diffusible mediators (Gs, cAMP) for the signal to literally crisscross the cell (33–35). Combined with our previous finding of PKA anchored at the apical membrane by association with A-kinase anchoring proteins (13), the presence of AC in the apical membrane completes a classic GPCR pathway for activated G proteins to regulate apical effectors.
To the best of our knowledge, our finding of membrane- delimited cAMP signaling in the present study is unprecedented. A long-held dogma in the field of ion channel regulation is that signaling from GPCR to ion channels in cell free patches involves no readily diffusible second messenger (36–38). When such “membrane-delimited” signaling is coupled with a lack of change in whole cell second messenger levels, the results are often interpreted as direct modulation of ion channels by G proteins (39). This has been challenged by recent findings that membrane-delimited signaling may involve protein kinase C-mediated phosphorylation (38, 40, 41). However, there is no report showing receptor to ion channel effector regulation in excised membrane patches by an intact classic cAMP pathway, either in epithelia or any other tissues. This is a little unexpected, as there is rich information regarding compartmentalization of PKA with its protein targets, and similarly, of receptors with G proteins. However, we know of no other report of AC close to PKA. The presence of AC in apical membrane patches that contain PKA permits very local cAMP-mediated regulation of CFTR.
In summary, the model presented in this work (Fig. 5) addresses two general problems faced by airway and other barrier epithelial cells. The first is how to regulate effectors in response to physical stimuli for which no specific receptor exists. Diverse physical stimuli are known to release cellular ATP (42, 43). Subsequent conversion of ATP to Ado by ectonucleotidases is, in effect, an extracellular signal transduction pathway through which any stimulus that triggers ATP release can be recognized by an apically localized receptor-driven cAMP-signaling pathway. A second problem for epithelial cells is how to selectively activate the apical functions needed for protective response to luminal stimuli. The dramatically limited spread of cAMP signaling we observed in response to apical A2BAR activation suggests that compartmentalization of cAMP signaling at the inner apical membrane surface allows cells to sensitively regulate apical effectors without global activation of signaling paths that target other cell functions.
Acknowledgments
We thank Mary Lang-Furr and Shuqing Yan for excellent technical assistance. This work is supported by National Institutes of Health Grants HL60280-02 (to M.J.S.) and KO1 DK02777-01 (to P.H.).
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- AC
adenylyl cyclase
- Ado
adenosine
- AMPCP
adenosine 5′-[α,β-methylene]diphosphate
- GDPβS
guanosine 5′-[β-thio]diphosphate
- GTPγS
guanosine 5′-[γ-thio]triphosphate
- 8-SPT
8-(p-sulfophenyl)theophylline
- PKA
protein kinase A
- PKI
PKA inhibitor
- A2BAR
A2B adenosine receptors
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Daviskas E, Anderson S D, Gonda I, Eberl S, Meikle S, Seale J P, Bautovich G. Eur Respir J. 1996;9:725–732. doi: 10.1183/09031936.96.09040725. [DOI] [PubMed] [Google Scholar]
- 2.Winters S L, Yeates D B. J Appl Physiol. 1997;83:1360–1369. doi: 10.1152/jappl.1997.83.4.1360. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M P, Berger H A, Rich D P, Gregory R J, Smith A E, Welsh M J. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 4.Tabcharani J A, Chang X B, Riordan J R, Hanrahan J W. Nature (London) 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- 5.Morris A J, Malbon C C. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 6.Rich T C, Fagan K A, Nakata H, Schaack J, Cooper D M, Karpen J W. J Gen Physiol. 2000;116:147–161. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homolya L, Watt W C, Lazarowski E R, Koller B H, Boucher R C. J Biol Chem. 1999;274:26454–26460. doi: 10.1074/jbc.274.37.26454. [DOI] [PubMed] [Google Scholar]
- 8.Hwang T H, Schwiebert E M, Guggino W B. Am J Physiol. 1996;270:C1611–C1623. doi: 10.1152/ajpcell.1996.270.6.C1611. [DOI] [PubMed] [Google Scholar]
- 9.Moriwaki Y, Yamamoto T, Higashino K. Histol Histopathol. 1999;14:1321–1340. doi: 10.14670/HH-14.1321. [DOI] [PubMed] [Google Scholar]
- 10.Feoktistov I, Biaggioni I. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- 11.Pawson T, Scott J D. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 12.Kuwada S K, Lund K A, Li X F, Cliften P, Amsler K, Opresko L K, Wiley H S. Am J Physiol. 1998;275:C1419–C1428. doi: 10.1152/ajpcell.1998.275.6.C1419. [DOI] [PubMed] [Google Scholar]
- 13.Huang P, Trotter K, Boucher R C, Milgram S L, Stutts M J. Am J Physiol. 2000;278:C417–C422. doi: 10.1152/ajpcell.2000.278.2.C417. [DOI] [PubMed] [Google Scholar]
- 14.Levitt B, Head R J, Westfall D P. Anal Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- 15.Stutts M J, Lazarowski E R, Paradiso A M, Boucher R C. Am J Physiol. 1995;268:C425–C433. doi: 10.1152/ajpcell.1995.268.2.C425. [DOI] [PubMed] [Google Scholar]
- 16.Engelhardt J F, Yankaskas J R, Ernst S A, Yang Y, Marino C R, Boucher R C, Cohn J A, Wilson J M. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 17.Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A, et al. Eur Respir J. 1993;6:169–176. [PubMed] [Google Scholar]
- 18.Watt W C, Lazarowski E R, Boucher R C. J Biol Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- 19.Communi D, Paindavoine P, Place G A, Parmentier M, Boeynaems J M. Br J Pharmacol. 1999;127:562–568. doi: 10.1038/sj.bjp.0702560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musante L, Zegarra-Moran O, Montaldo P G, Ponzoni M, Galietta L J. J Biol Chem. 1999;274:11701–11707. doi: 10.1074/jbc.274.17.11701. [DOI] [PubMed] [Google Scholar]
- 21.Clancy J P, Ruiz F E, Sorscher E J. Am J Physiol. 1999;276:C361–C369. doi: 10.1152/ajpcell.1999.276.2.C361. [DOI] [PubMed] [Google Scholar]
- 22.Hwang T C, Horie M, Nairn A C, Gadsby D C. J Gen Physiol. 1992;99:465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okusa M D, Huang L, Momose-Hotokezaka A, Huynh L P, Mangrum A J. Am J Physiol. 1997;273:F883–F891. doi: 10.1152/ajprenal.1997.273.6.F883. [DOI] [PubMed] [Google Scholar]
- 24.Els W J, Butterworth M B. Microsc Res Tech. 1998;40:455–462. doi: 10.1002/(SICI)1097-0029(19980301)40:6<455::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Richards P D, Els W J. Histochem J. 1994;26:495–503. doi: 10.1007/BF00157895. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann M, Muff R, Stieger B, Biber J, Murer H, Fischer J A. Endocrinology. 1994;134:1173–1178. doi: 10.1210/endo.134.3.8119156. [DOI] [PubMed] [Google Scholar]
- 27.Dixon B S, Sutherland E, Alexander A, Nibel D, Simon F R. Am J Physiol. 1993;265:G686–G698. doi: 10.1152/ajpgi.1993.265.4.G686. [DOI] [PubMed] [Google Scholar]
- 28.Dessauer C W, Tesmer J J, Sprang S R, Gilman A G. Trends Pharmacol Sci. 1999;20:205–210. doi: 10.1016/s0165-6147(99)01310-3. [DOI] [PubMed] [Google Scholar]
- 29.Caplan M J. Am J Physiol. 1997;272:F425–F429. doi: 10.1152/ajprenal.1997.272.4.F425. [DOI] [PubMed] [Google Scholar]
- 30.Grygorczyk R, Hanrahan J W. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 31.Shlatz L J, Schwartz I L, Kinne-Saffran E, Kinne R. J Membr Biol. 1975;24:131–144. doi: 10.1007/BF01868619. [DOI] [PubMed] [Google Scholar]
- 32.Walling M W, Mircheff A K, Van Os C H, Wright E M. Am J Physiol. 1978;235:E539–E545. doi: 10.1152/ajpendo.1978.235.5.E539. [DOI] [PubMed] [Google Scholar]
- 33.Hanson A S, Linas S L. Am J Physiol. 1995;268:F553–F560. doi: 10.1152/ajprenal.1995.268.4.F553. [DOI] [PubMed] [Google Scholar]
- 34.Haraguchi K, Rodbell M. Proc Natl Acad Sci USA. 1990;87:1208–1212. doi: 10.1073/pnas.87.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinne R, Shlatz L J, Kinne-Saffran E, Schwartz I L. J Membr Biol. 1975;24:145–159. doi: 10.1007/BF01868620. [DOI] [PubMed] [Google Scholar]
- 36.Lipscombe D, Kongsamut S, Tsien R W. Nature (London) 1989;340:639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- 37.Thomas S A, Hume R I. J Neurophysiol. 1993;69:1556–1566. doi: 10.1152/jn.1993.69.5.1556. [DOI] [PubMed] [Google Scholar]
- 38.Hu K, Li G R, Nattel S. Am J Physiol. 1999;276:H488–H495. doi: 10.1152/ajpheart.1999.276.2.H488. [DOI] [PubMed] [Google Scholar]
- 39.Robillard L, Ethier N, Lachance M, Hebert T E. Cell Signal. 2000;12:673–682. doi: 10.1016/s0898-6568(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 40.Ribalet B, Eddlestone G T. J Physiol. 1995;485:73–86. doi: 10.1113/jphysiol.1995.sp020713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishizaki T, Ikeuchi Y. FEBS Lett. 1996;378:1–6. doi: 10.1016/0014-5793(95)01399-7. [DOI] [PubMed] [Google Scholar]
- 42.Grygorczyk R, Guyot A. J Physiol. 2001;532:582. doi: 10.1111/j.1469-7793.2001.0582e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson D R, Kennedy I, Burton T J. J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]