Abstract
Epilepsy is one of the most common chronic neurologic disorders that affects individuals of all ages. It is primarily managed with antiepileptic drugs (AEDs), with the goal of maintaining complete seizure control combined with minimal or no adverse effects. Oral administration is the mainstay of AED delivery for patients with chronic epilepsy and consists essentially of immediate-release (IR) and modified-release (delayed-release and extended-release [ER]) dosage formulations. Extended-release formulations (hydrophilic or hydrophobic matrix systems, reservoir systems, and osmotic-release systems) release a drug in a controlled manner during an extended period of time following administration. Extended-release formulations have many advantages compared with IR formulations, including simplification of dosing regimens, reduction in pill burden, and reduction in the peak-to-trough fluctuations in serum drug concentration that may be associated with a decreased risk of adverse effects and of seizures. These advantages have the potential to increase adherence to antiepileptic therapy, improve the quality of life of patients, and reduce health care costs. This article, which is intended as a practical guide for clinicians, reviews the properties of the different ER AED formulations currently available and discusses the advantages of ER over IR formulations. Subsequently, an explanation of the technologic basis of the different oral ER formulations, the critical attributes that differentiate ER products, and their individual strengths and weaknesses is provided. Specific recommendations to practitioners on treating patients with ER formulations are included.
Keywords: antiepileptic drugs, dosage formulations, epilepsy, extended-release, immediate-release, modified-release formulations, seizure, treatment
Introduction
Oral administration is the mainstay of antiepileptic drug (AED) delivery for patients with chronic epilepsy.1 Efforts to enhance the ease of administration are important because lack of adherence or partial adherence to medication can have immediate detrimental consequences, such as uncontrolled or recurrent seizures,2–4 or can lead to adverse effects (AEs) associated with restarting a previous dose without titration.
There are 2 main categories of oral drug delivery systems (DDSs): immediate-release (IR) and modified-release dosage formulations. The latter can be further subdivided into delayed-release (DR) and extended-release (ER) formulations. Extended-release formulations offer many advantages for most patients with epilepsy. However, not all ER products have the same pharmaceutical properties. For the clinician to use them to their fullest advantage, it is important to understand the nature of the different DDSs, and the relative strengths and weaknesses of each.
This review will focus on the ER formulations of AEDs currently available to treat epilepsy. The objectives of this review are to discuss their advantages compared with IR formulations, differentiate between the various types of oral ER formulations, and review and explain the technologic basis of the different oral ER formulations. It aims to be an informative and practical guide for clinicians who provide care for patients with epilepsy.
Terminology
Many AEDs are approved for use in multiple formulations (e.g., IR, DR, and ER). The release and subsequent absorption of a drug are influenced by its physical and chemical properties (e.g., aqueous solubility, salt form, particle size, acid dissociation constant, and DDS, and the physiologic properties of the site of absorption). Different formulations may contain the same drug at identical strengths; however, they may not be bioequivalent. Each formulation has its own specific dosage instructions and titration instructions and is differently suited to a specific patient's need. Consequently, the different formulations of an AED are not directly interchangeable.
IR Formulations. Immediate-release dosage formulations are developed to dissolve without delaying or prolonging dissolution or absorption of the drug. Generally, an IR tablet or capsule is swallowed whole and instantaneously disintegrates to make the drug available for absorption and subsequent pharmacologic action.1,5 Immediate-release products are used when the rapid onset of action of a drug is advantageous.1 They usually include “superdisintegrants” (e.g., croscarmellose sodium) as excipients.6 These components rapidly disintegrate, de-aggregate, and/or dissolve, when they come into contact with water or with the gastrointestinal track.1,5
In general, 70% to 80% of a drug should be released, preferably within an hour, and certainly within 4 hours, after ingestion.1,6 When in vitro dissolution tests are performed for IR formulations, no less than 85% of the drug is expected to have dissolved within 60 minutes or less in an aqueous medium.7,8 For poorly soluble drugs, a longer dissolution time may be considered normal.8
Modified-Release Formulations. A goal of pharmacotherapy for epilepsy is to maintain consistent serum drug concentration (SDC) while minimizing AEs. Modified-release dosage formulations have been developed to achieve this goal by delaying or extending the rate of drug absorption, or by altering its site of release.9 In doing so, these products may enhance therapeutic outcomes or improve convenience for the patient.
DR Formulations. The US Pharmacopeia (USP) defines DR as a dosage form that does not release the medication promptly after administration.10 In other words, once a certain amount of time has lapsed, the drug is immediately and completely released. The delayed release may be time sensitive or dependent on the environment (e.g., gastrointestinal pH). For example, an enteric coating can be used to intentionally delay drug release until after the tablet has passed through the stomach. This may reduce drug irritation of the gastric mucosa or prevent gastric juices from inactivating an acid-labile drug.10 An example of a DR formulation of an AED is Depakote DR or Depakote Sprinkles (divalproex sodium, Abbott Laboratories, North Chicago, IL).
ER Formulations. Since the introduction of the first modified-release formulations about 70 years ago, many terms have been used to describe the properties of ER formulations, including sustained release, sustained action, prolonged action, controlled release, time-released, and long-acting. Although these terms are frequently used interchangeably, individual products may differ in design and performance.
The USP defines ER as a dosage form designed to release the medication in a controlled manner during an extended period of time, at a predetermined rate, duration, and location following administration.10,11 At steady state, the rate of absorption is approximately equivalent to the rate of elimination due to metabolism and excretion.12 Figure 1A shows a schematic representation of change in SDC over time following ingestion of IR, DR, and ER formulations. Table 1 lists the currently available ER formulations of AEDs.
Figure 1.
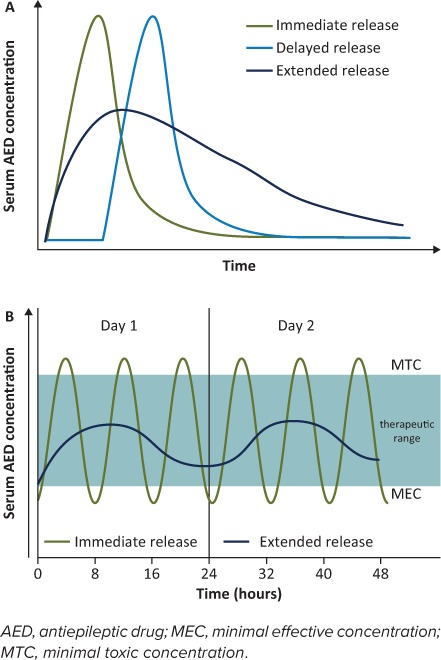
Schematic diagrams of changes in blood serum drug concentration (SDC) over time for different drug delivery systems. (A) For the immediate-release (IR) formulation, almost immediately following ingestion, the SDC rises and quickly peaks before rapidly falling off. For the delayed-release formulation, the SDC profile is similar to the IR formulation, but there is a lag phase following ingestion before the drug is released. For the extended-release (ER) formulation, the SDC rises more slowly from the time of ingestion, forming a much broader concentration curve over time. (B) Simulated SDC–time curve at steady state for IR (administered 3 times a day) and ER (administered once daily) AED formulations during a 2-day period. Repeated doses of the IR formulation are required to maintain SDC within the therapeutic zone for optimal seizure control; this can result in wide peak-to-trough fluctuations in concentration. For some patients, seizure control with the IR formulation may require drug doses that result in a peak concentration that is above the therapeutic zone shortly after administration. This increases the likelihood of concentration-related toxicity. The SDC troughs shortly before the next IR dose and may be below the therapeutic zone, increasing the risk of seizures. By comparison, the once-daily dosing of the ER formulation minimizes peak-to-trough fluctuations, maintaining the SDC within the therapeutic zone, and thus avoiding an increase in the likelihood of either toxicity or seizures.
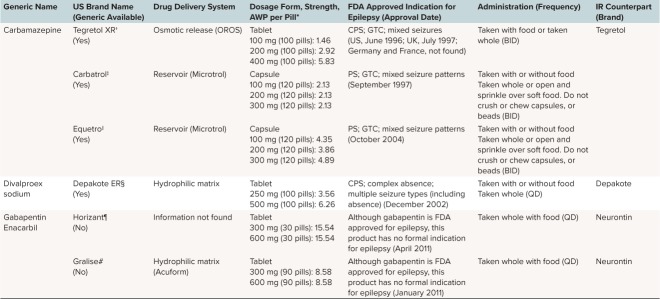
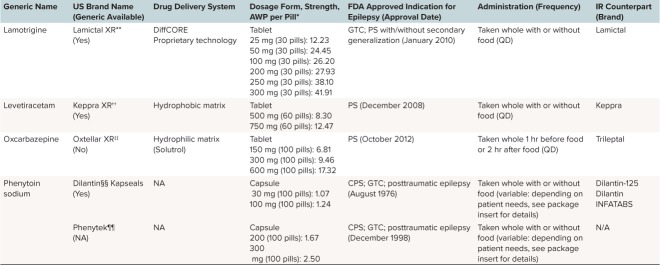
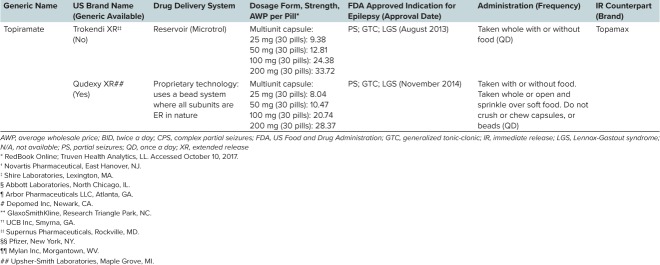
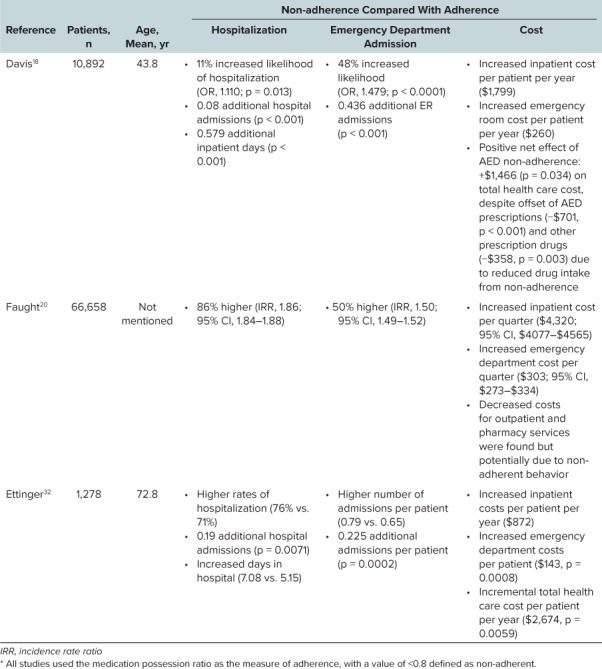
Table 1.
Overview of Currently Available Extended-Release (ER) Formulations of Antiepileptic Drugs
Table 1.
Overview of Currently Available Extended-Release (ER) Formulations of Antiepileptic Drugs (cont.)
Table 1.
Overview of Currently Available Extended-Release (ER) Formulations of Antiepileptic Drugs (cont.)
Extended-release formulations can allow for reduction in dose frequency, which may enhance convenience and thereby improve adherence.9 The aim of ER formulations is to minimize fluctuations in SDC between doses.13 Although a “flat” serum concentration may not necessarily improve efficacy, it can simplify AED therapy and can minimize concentration-related AEs.14,15
Phenytoin was approved by the US Food and Drug Administration (FDA) for the treatment of epilepsy in 1953, but it was not until 1976 that Dilantin Kapseals (active drug phenytoin, Pfizer, New York, NY) became the first oral ER formulation of an AED available in the United States.16 Two decades later, the next ER formulations with carbamazepine as the active drug arrived: Tegretol XR (1996; Novartis, East Hanover, NJ) and Carbatrol (1997; Shire Inc, Lexington, MA). Today there are more than 10 ER products for treating epilepsy (Table 1).
Although ER formulations of AEDs are widely available, IR products are most frequently prescribed worldwide. This popularity may be largely driven by lower medication costs and order of introduction to the market, because IR formulations are typically introduced first. Age is an important consideration in the pediatric populations because younger infants and some children are unable to swallow a solid dosage formulation, and few ER formulations are available in a sprinkle formulation (e.g., Carbatrol, Trokendia XR, and Qudexy XR). Use of these dosage formulations is also limited by the inability to titrate by small increments, which may limit their use in growing children. Independent of cost considerations, for most patients who are able to swallow, there are many advantages to using ER over IR formulations.
Advantages of ER Formulations
There are many inherent advantages to using ER formulations. They may enhance adherence to AED therapy, minimize fluctuations in SDC, improve seizure control, and reduce toxicities associated with peak concentrations compared with IR formulations.
Enhanced Medication Adherence and Improved Quality of Life. Non-adherence to AEDs is a problem in the management of epilepsy, and it can have serious or even fatal consequences if patients experience inadequate seizure control (Table 2).17–19 In a US survey of 661 patients with epilepsy, 71% noted dose omissions and 45% reported a seizure following a missed dose.19 A large retrospective database analysis in a US managed-care adult population showed that during a mean follow-up of 27 months, 39% of patients with epilepsy were non-adherent.18 In another retrospective open-cohort study of claims data for 33,658 US Medic-aid patients with epilepsy, non-adherence to AEDs was associated with a more than 3-fold increase in mortality compared with adherence, and during periods of non-adherence patients had significantly higher incidences of emergency department visits, hospital admissions, motor vehicle injuries, and fractures.20
Table 2.
Summary of Retrospective Studies on Claims Data That Compared Costs Associated With Antiepileptic Drug (AED) Adherence and Non-adherence *
Evidence shows that patients receiving an ER formulation are more likely to continue with therapy than patients receiving IR formulations, and that there are no significant differences in AEs between the 3 formulations (Tegretol [ER carbamazepine], Carbatrol [ER carbamazepine], and a generic IR carbamazepine).21 In a review of studies that compared ER, conventional, and IR formulations of AEDs, several studies noted increases in adherence following a change from an IR to an ER formulation.17 In general, ER formulations were associated with reduced AEs, greater tolerability, improved dosing convenience, increased efficacy, and improvements in quality of life.17 Finally, because the likelihood of missing a dose increases with dosing frequency, and with the number of tablets/capsules taken,19 the simplification of dosing regimens with ER formulations is an important approach to improving adherence.13,15,17,22
Decreased Fluctuation in Peak-and-Trough SDC. Many AEDs have short half-lives. For IR formulations, this necessitates frequent dosing to maintain SDCs within the targeted range for optimal seizure control. The resulting wide peak-to-trough fluctuations in concentrations (Figure 1B) may increase the likelihood of both seizures and AEs.13,15,22,23
Extended-release formulations enable the dosing interval to be increased, which maintains concentration within the targeted range while decreasing fluctuations in peak-to-trough concentrations (Figure 1B). The decreased fluctuations may result in reduced toxicity and fewer concentration-related AEs compared with IR formulations.14,15,24 This may make ER formulations more forgiving to occasional irregular dosing compared with IR formulations. A pharmacokinetic simulation of Trokendi XR (topiramate; Supernus Pharmaceuticals, Rockville, MD) showed that following a delayed or omitted dose, SDC could be restored by giving the ER dose at any point within the next dosing interval, or by giving an additional ER dose together with the next scheduled dose.25
Less Frequent Local and Systemic AEs. Extended-release DDSs typically have slower release rates, which can result in fewer concentration-related AEs and can prevent side effects that may occur during the absorption phase with IR formulations.26 Lower peak concentrations may allow some patients to have their total daily dose increased without experiencing AEs, resulting in improved seizure control for the same chemical moiety when an ER formulation is used.
Uthman17 reviewed more than 15 studies comparing ER formulations of AEDs to IR, DR, or placebo products. A total of 4 of the 7 studies that directly compared ER and IR formulations showed significantly fewer AEs, better tolerability, and enhanced compliance for ER formulations of carbamazepine,27,28 levetiracetam,29 and valproate.30
Similarly to DR formulations, ER products have the potential to improve adherence by reducing local AEs, such as the gastrointestinal intolerance that can be associated with IR formulations. Additionally, ER formulations that are enteric coated may improve gastrointestinal tolerability in some patients. For example, the gastrointestinal AEs associated with valproic acid have been reduced by the introduction of enteric-coated formulations.31
Overall Decrease in Health Care Costs. The use of AED ER formulations is generally associated with better adherence and seizure control than IR formulations.17 Several studies in adults have shown that an increase in adherence was associated with a decrease in the costs of care and hospitalization (Table 2)18,32,33; hence, ER formulations have the potential to reduce overall health care costs compared with IR formulations. Helmers et al34 studied the economic burden associated with generic versus branded AEDs in the United States. They found that the periods of generic use were associated with higher total medical service cost (i.e., $3186) when compared to period of brand product use. Likewise, Labiner et al35 reported that generic AED use was associated with significantly greater use of medical services and increased risk of epilepsy-related medical events compared with brand use. Within an institutional setting, ER products require less pharmacy preparation and less nursing time related to administration (e.g., QD versus TID).
Disadvantages
The ER formulations of AEDs also have some inherent disadvantages compared with IR formulations. The pharmaceutical formulation is generally more expensive to the manufacturer and may suffer from a limited number of available strengths.
Manufacturing Costs. Certain ER formulations are easier to manufacture (e.g., matrix system) than others (e.g., reservoir or osmotic systems).9 However, in general, compared with IR formulations, the manufacturing process and development of ER formulations are more technologically complex and time-consuming, consequently translating into higher production costs.9
Dose Limitations. The currently available ER formulations are not suited for all patients with epilepsy, especially those who require very large doses to maintain seizure control. These doses may not be available as ER formulations, or may not have been tested (e.g., for Equetro [carbamazepine; Validus Pharmaceutical, Parsippany, NJ], doses larger than 1600 mg have not been studied). Given the limitations on the amount of medication that can be placed within an ER DDS, patients who require large total daily doses may still need to take multiple ER pills. For instance, a patient requiring 3000 mg/day Keppra XR (levetiracetam; USB Inc, Smyrna, GA) may require four 750-mg ER tablets at bedtime. Although the pill burden is high, the frequency of administration is more convenient because it is reduced to once daily.
Additionally, the limited number of available strengths for some ER formulations may make direct conversion from an IR to an ER dose difficult. For example, the prescription information for Depakote ER suggests that, “For patients whose DEPAKOTE total daily dose cannot be directly converted to DEPAKOTE ER, consideration may be given at the clinician's discretion to increase the patient's DEPAKOTE total daily dose to the next larger dosage before converting to the appropriate total daily dose of DEPAKOTE ER.” For more information on conversion of IR doses to ER doses, see the section “Conversion to ER DDS From Current AED Therapy.”
Many children with seizure have comorbidities that may require the use of feeding tubes for medication administration. An inability to crush ER tablets and administer the medication via an enteral feeding tube is a distinct disadvantage to the use of these products.
Forgiveness Period. Although once-daily dosing with ER formulations may improve adherence, some concerns have been raised about the so-called forgiveness period of ER formulations. The forgiveness period is the time that a patient is unlikely to experience adverse consequences following a missed dose of a drug. Some suggest that the effective SDC of AEDs following administration of once-daily ER formulations may not extend beyond 24 hours; therefore, the risk of a seizure is greater following a missed dose of an ER formulation compared with an IR formulation that is part of a multiple–daily-dose regimen.13,14 In other words, the forgiveness period for ER formulations may be shorter than for IR formulations.
A discussion of a “forgiveness period” is often complicated by a lack of understanding of the traditional versus the effective half-life of a drug. For IR drugs with linear pharmacokinetics and rapid absorption, a traditional calculation of half-life may be an accurate measure of serum concentration decline. However, for ER formulations that have slower absorption, the calculation of a traditional half-life may be a poor predictor of drug accumulation and fluctuation in serum concentrations, and therefore not clinically useful.
Although valproic acid has a traditionally calculated half-life of 12 to 16 hours, its prolonged drug release leads to an effective half-life of divalproex-ER of 40 hours, which supports its once-per-day administration.36 Similarly, Topamax XR displayed a 1.5-fold longer effective half-life (55.7 hours) than the IR formulation (37.1 hours).37
It has also been proposed that if a patient forgets to take a once-daily dose in the morning, he or she has the whole day to remember to take it,17 whereas for an IR product, trying to make up a missed dose is often not tolerated. Additionally, computer simulation studies have assessed the possible impact of dosing irregularities with ER versus IR formulations of AEDs. Brittain and Wheless25 used a population pharmacokinetic model to predict topiramate SDC following dosing irregularities with a once-daily ER formulation (Trokendi XR) compared with a twice-daily IR formulation (Topamax). They found that the trough SDC following an omitted ER dose was only slightly lower than that following an omitted IR dose. In a second study, Pellock and Brittain38 demonstrated that the forgiveness period for once-daily Trokendi XR can equal or exceed that of twice-daily IR topiramate. Both studies concluded that dosing irregularities with the ER formulation of topiramate posed no greater risk than with the IR formulation.25,38
In general, when treating any chronic disease, physicians and pharmacists should instruct the patient on what to do when he or she is late with or misses a dose of medication. Upon realizing that an ER dose has been forgotten, even if this realization occurs 24 hours later (e.g., when the next dose is due), the patient should make up for the missed dose as soon as possible. This is recommended because the larger forgiveness period with ER formulations compared with IR formulations allows the patient to return to his or her daily schedule more quickly.
Oral ER AED Delivery Systems
The AEDs that are currently available as ER formulations are detailed in Tables 1 and 3. There are currently 3 common types of oral ER DDSs: matrix DDS, osmotic-release DDS, and reservoir DDS (or membrane-controlled DDS).9 Drug release from these systems employs one or more of the following processes: dissolution and diffusion of the drug, system swelling, erosion of a matrix containing the drug, and osmotic pressure–induced release of the drug.9 Properties of the drug, such as solubility, can determine which DDS is most appropriate to deliver it.9
Table 3.
Extended-Release (ER) Formulations According to Drug Delivery System (DDS)
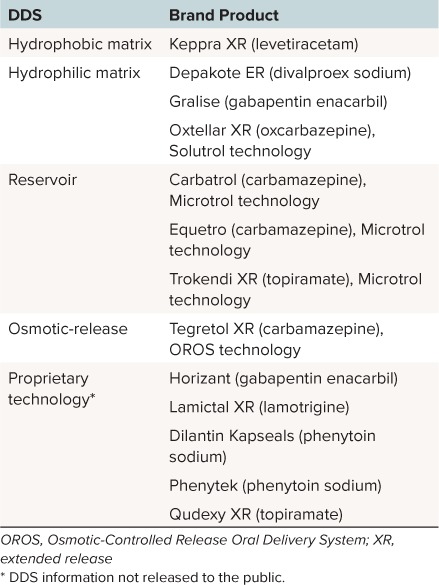
Matrix DDS. In matrix DDS, the drug is homogenously mixed within a matrix of one or more rate-controlling substances, and other inert substances.9 The drug is released either by diffusion out of the matrix and/or by the erosion of the matrix itself (Figure 2).9,39,40 Depending on the properties of the matrix material, matrix systems are defined as either hydrophilic or hydrophobic.
Figure 2.
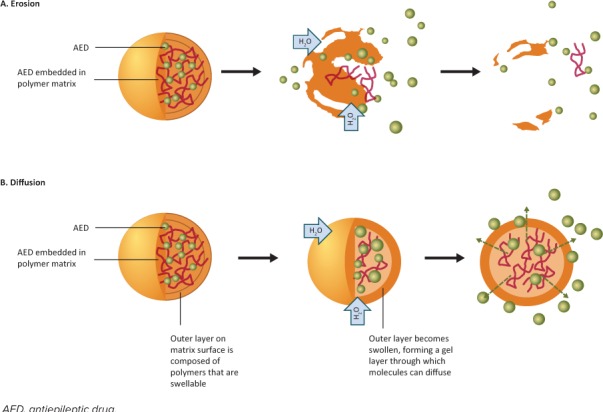
Matrix drug delivery system. The drug embedded in a polymer matrix is released from the matrix by the processes of (A) erosion and/or (B) diffusion. In hydrophilic matrix systems, water enters the matrix, which begins to swell. The dissolved drug is released by diffusion out of the matrix and by erosion of the matrix itself. In hydrophobic matrix systems, there is minimal swelling of the matrix, but a swollen gel layer forms on the matrix surface. As water enters the matrix, the drug dissolves and is predominantly released by diffusion out of the matrix, through the surface gel layer.
Hydrophilic Matrix DDS. In hydrophilic matrix systems, the drug is dispersed throughout a polymer matrix of hydrophilic material.9,41 The rate of drug release is controlled by both diffusion and erosion.9 When water is absorbed by the matrix, the matrix swells and the polymer on the surface of the pill hydrates. This polymer changes from a crystalline state to a gel state, forming a gel layer on the surface that controls the rate of release of the drug.39 As the gel layer increases, the polymer chains closest to the surface begin to relax and lose consistency, which is followed by a gradual erosion of the matrix.39 Water-soluble drugs dissolve and are released by a combination of diffusion out of the matrix, through the gel layer, and as a result of the erosion of the matrix itself.9,39
Many factors affect the rate of drug release from hydrophilic matrix systems, including the concentration of polymer in the matrix, particle size of the polymer, the viscosity of the polymer in solution, and solubility of the drug itself.41,42 The AED formulations that use a hydrophilic matrix system (Table 3) include Oxtellar XR (oxcarbazepine; Supernus Pharmaceuticals, Rockville, MD) and Depakote ER.
Hydrophobic Matrix DDS. In hydrophobic matrix systems, the drug is dispersed throughout a polymer matrix of inert hydrophobic material.9 The hydrophobic matrix undergoes no or minimal swelling on contact with water. When water enters the matrix, the drug dissolves and is predominately released by diffusion out of the matrix (Figure 2).
In such diffusion-based matrix systems, the drug is not uniformly released over time, because the diffusion front of the drug gradually moves further into the matrix. Also, because drug solubility is usually dependent on pH, the rate of release of the drug varies with the pH of the environment. Consequently, the rate of drug release from hydrophobic matrix systems can be altered by factors such as non-uniform loading of the drug within the matrix, and the incorporation of pH modifiers.9 Currently, only 1 AED formulation, Keppra XR (levetiracetam, UCB Inc), uses a hydrophobic matrix system (Table 3).
Bimodal matrix DDSs combine an IR component with an ER dosage form.9 For example, a bimodal formulation of lamotrigine is under development, combining an IR segment to deliver a loading dose of the drug with an ER segment to deliver a controlled maintenance dose.43
Matrix DDSs are widely used, not only because they are relatively simple to formulate and easy and inexpensive to manufacture, but also for their ability to contain drugs of various concentrations and moieties with different physiochemical properties, as well as their ability to deliver high–molecular weight drugs.9,42
Reservoir DDS. In reservoir systems, a core containing a water-soluble drug is surrounded by an insoluble membrane (Figure 3A).9 A reservoir system typically consists of many subunits, such as beads, pellets, or mini-tablets, containing the drug. A key feature of a system containing multiple subunits is that it is possible to incorporate subunits with different release characteristics allowing for a multimodal IR plus ER DDS.9
Figure 3.
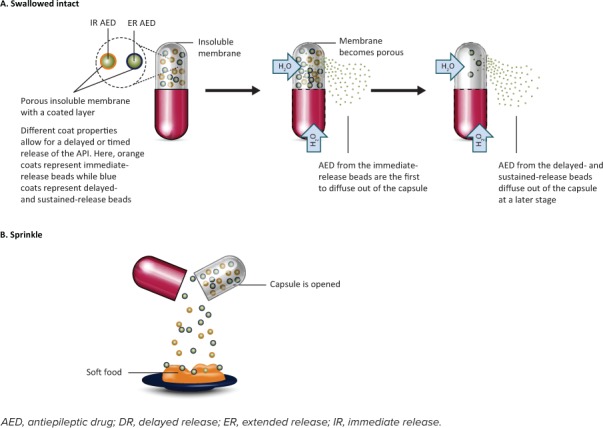
Reservoir drug delivery system. These systems typically contain many subunits (beads or pellets) containing the drug. Upon contact with water, the insoluble membrane becomes porous. (A) Swallowed intact: As water enters the system, water-soluble drug is released from the subunits and diffuses out through the pores in the membrane. The timing and rate of release of the drug can be modified by including a mixture of subunits with different drug-releasing characteristics (IR, DR, ER) in a single capsule, together with changing the thickness of the insoluble membrane and the size of the membrane pores. (B) Sprinkled: As an alternative to swallowing the capsule, it can be opened by the user, and the subunits can then be sprinkled onto a spoon of soft food. This mixture of food and drug is swallowed without chewing or crushing.
On contact with water, the insoluble membrane surrounding the core becomes porous due to the incorporation of water-soluble or leachable additives into its structure (Figure 3A). When water enters the system via the membrane pores, the water-soluble drug is released from the subunits and diffuses through the pores. The rate of release of the drug in a reservoir system can be controlled by factors such as the pore size, coating materials, coating thickness, and membrane thickness. As with matrix systems, if the solubility of the drug is pH dependent, drug release can also be modified by the addition of buffering agents.
Some reservoir systems can be manually opened and their contents sprinkled onto soft food (Figure 3B), which is then swallowed without chewing. This mode of delivery can be advantageous for pediatric patients who have difficulty swallowing pills. For adult and adolescent patients, it is possible for these sprinkles to be delivered whole (not crushed) via an enteral feeding tube. However, in practice, most patients are not given their medication via feeding tubes due to concerns about clogging.43,44 This is especially true for children who require feeding tubes of a smaller diameter. It should also be noted that non-reservoir IR formulations, such as Depakote and Topamax, can be sprinkled. The AED formulations that use a reservoir system (Table 3) include Carbatrol (carbamazepine) and Trokendi XR (topiramate).
Osmotic-Release DDS. Similar to reservoir systems, osmotic-release DDSs (also called osmotic-pump DDS) consist of a drug-containing core surrounded by an insoluble but semipermeable membrane capsule or coating. This membrane contains an orifice through which the soluble drug is forced by osmotic pressure that builds up inside the capsule on contact with water.9,41,45
There are single- and double-chamber capsules (Figure 4). In single-chamber capsules, the drug and an osmotic agent are contained in a single compartment. As water is drawn through the semipermeable membrane by the osmotic agent, the drug dissolves, and the osmotic agent becomes hydrated and swells, forcing the drug out through the orifice in the capsule. Single-chamber capsules are only suitable for more soluble drugs.9,41,45 In double-chamber capsules, the drug is contained in one compartment and the osmotic agent is contained in a separate compartment known as the “push compartment.” As the osmotic agent draws water through the semipermeable membrane, the drug dissolves or a suspension is formed, and the osmotic agent becomes hydrated. Additionally, the polymer contained in the push compartment begins to swell, pressurizing the drug-containing compartment, which forces the drug through the membrane orifice. Double-chamber capsules are more suited for less soluble drugs or for when a larger drug loading is required.9,41,45
Figure 4.
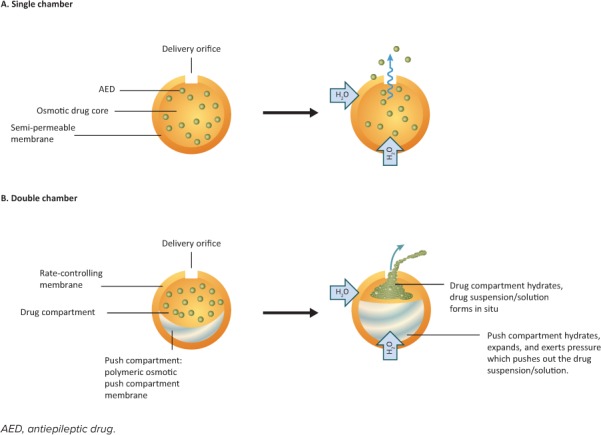
Osmotic-release drug delivery systems. These systems consist of the drug and an osmotic agent surrounded by a semipermeable membrane capsule containing a small orifice. (A) In single chamber capsules, water is drawn into the capsule by the osmotic agent, which begins to swell, forcing the drug through the orifice. (B) Double-chamber capsules have an additional push chamber containing a highly swellable polymer. As this polymer swells, the push chamber expands, exerting pressure on the drug-containing chamber, forcing the drug through the orifice.
The release of the drug by osmotic-release DDS is not easily affected by environmental influences, such as agitation and pH.9,41,45 However, the manufacture of osmotic-release DDS, and particularly that of 2-chamber systems, is complex and expensive.9 The AED formulations that use a single-chamber osmotic-release DDS include Tegretol XR (carbamazepine; Table 3). The matrix, reservoir, and osmotic DDS can be found in the form of a single-layered or multilayered tablet or a capsule.9
Clinical Application and Practical Considerations
Administration. Different ER formulations have specific instructions for administration (Table 1). The main factors to consider are: 1) whether the tablet should be administered with or without food; and 2) whether the tablet should be swallowed whole and not crushed, or whether it can be opened and the contents sprinkled onto soft food.
Ghost Tablets. A feature of some DDS products is the phenomenon of “ghost tablets” (e.g., Tegretol XR). This occurs when the shell that houses the drug does not disintegrate or become digested, but passes out intact in the stool. This can be a source of anxiety for patients and caretakers if they are not preinformed that this is part of the normal functioning of the DDS and does not affect efficacy or indicate any absorption problem.46 It should be noted that all osmotic-release DDSs leave a ghost tablet. However, the same is not true of matrix or reservoir DDSs. Because the drug is released via diffusion, some leave a ghost tablet, whereas others do not.46
Storage. According to the individual labeling requirements, most formulations should be: 1) protected from moisture—unlike most IR formulations, moisture can destroy the releasing characteristics of ER formulations; hence, storage in bathroom cabinets is strongly discouraged; 2) stored at 25°C (77°F), with excursions permitted to 15°C to 30°C (59°F –86°F); and 3) protected from light. Storage conditions should be in accordance with USP guidance on controlled room temperature.47 For further details on the storage of individual formulations, the reader should consult the prescribing information.
Conversion to ER DDS From Current AED Therapy
The IR and ER formulations of an AED are not directly interchangeable because of possible differences in the rate or extent of absorption; therefore, switching from IR to ER formulations requires the careful monitoring of clinical response and the consideration of possible dose adjustments.13 For many of the ER formulations, specific guidance on the practical aspects of switching formulations is not provided in the package insert. We have provided suggestions, based on an understanding of the pharmacology and pharmacokinetics of the IR and ER products and the desire to maintain seizure control during this transition.
IR to an ER Formulation. When transitioning from an IR formulation to an ER formulation, for most of the ER AEDs, a milligram-to-milligram conversion is advised. At times a milligram-to-milligram conversion is not possible because of the limited range of strengths available (Depakote ER), or when transitioning from a phenytoin sodium salt formulation to a phenytoin free acid formulation. Specific recommendations for these situations can be found in the prescribing information for the AED.
An example of this transition, based upon the authors' experiences, is noted below for a patient on oxcarbazepine who has a required total daily dose of 1200 mg. On day 1, the patient should receive the IR formulation as usual: 600 mg in the morning and 600 mg at night. On day 2, the patient is switched, taking 600 mg of the IR formulation in the morning and taking the full daily dose of 1200 mg of the ER formulation in the evening. Although this means that on the conversion day the patient receives a total dose of 1800 mg, he or she will not receive an overdose, because the IR formulation is absorbed quickly, whereas the ER dose is slowly absorbed overnight. On day 3, the patient takes the full daily dose (1200 mg) of the ER formulation in the evening.
Intravenous to ER Transition. In general, when starting an oral ER medication for a patient who has been maintained on an intravenous (IV) formulation of the same medication, the ER dose should be administered simultaneously with the last IV dose to account for the different pharmacokinetic properties of the formulations, and to ensure continued serum concentrations in the therapeutic range. If simultaneous administration is not possible, the ER formulation should be administered within 2 hours (before or after) the last IV dose. Boggs and Preis48 found that prior to discharging a patient from the hospital, a protocol involving an IV dose followed within 1 hour by a similar dose of an ER formulation was well tolerated and provided good seizure control.
Considerations in Product Selection
AED Costs. An overview of the average wholesale prices of the currently available AEDs in the US is given in Table 1. This standard reference for costs is included to give a relative idea of the costs of the ER formulations. Readers should be aware that the final price paid by the patient is dependent on multiple factors. Prices in the United States are primarily determined by insurance plans and copay schedules for medications. Prices in Europe are determined by a combination of country approval, cost negotiations, and the level of government support.
The ER formulations are generally more expensive than the IR formulations. However, as previously discussed, there are several benefits associated with ER formulations that will likely reduce the overall health care costs. The main benefit is that use of ER formulations improves adherence, which may reduce downstream direct and indirect costs associated with care and hospitalization. However, formal studies on the financial impact of ER formulations are lacking and are needed.
Brand or Generic Formulations. Generic substitution has the potential to reduce the cost of AED therapy, but some drugs with a narrow therapeutic range may exhibit differences that could be clinically important. Generic formulations are produced by several manufacturers and are distributed by numerous intermediate suppliers. Although this may facilitate lower costs through open market competition, recent studies34,35,49 suggest a critical examination of this cost savings, in light of increased uses of medical resource and emergency services occurring after a change from a brand to generic formulation. One study noted the propensity for patients receiving lamotrigine to switch back to brand medications was higher than that reported for antihypertensive or antihyperlipidemic agents.49 At times, it is difficult to determine which generic product a patient has been receiving because chain pharmacies often align with the strongest and best-positioned suppliers, which may result in inventory disruption and product switches.
Brand ER formulations of AEDs that are available on the market have usually undergone evaluation in randomized controlled trials to demonstrate their efficacy and tolerability. Most of these brand ER formulations also have one or more generic formulations available. For a generic to be marketed, bioequivalence to the brand formulation must have been shown.
According to the FDA 2 drugs are bioequivalent if 90% of the confidence interval of the pharmacokinetics parameters falls within 80% to 125% of the brand-name drug (Figure 5). Most generic formulations differ by =10% in AUC0-t compared with the reference brand drug. This means that the 2 products exhibit no substantial difference in the rate and extent of drug absorption.50 Although these parameters may be acceptable for most drugs, these tolerance limits may be too broad for AEDs that have a narrow therapeutic range or for some patients with difficult-to-control epilepsy. Important to the discussion is the lack of any requirement for the generic drug to use the same DDS as the brand formulation.
Figure 5.
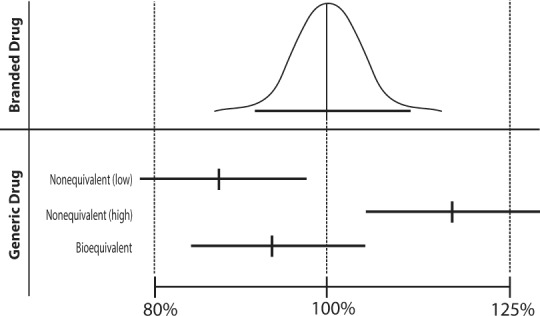
Testing for bioequivalence.
Ting et al51 tested the FDA bioequivalence standard of generic IR lamotrigine and the brand version, Lamictal (lamotrigine; GlaxoSmithKline, Research Triangle Park, NC) and confirmed the soundness of the FDA bioequivalence standard, with few patients having seizure exacerbations or tolerability issues that could be attributed to switching. In a different study, switching between 2 generic versions of IR lamotrigine was also shown to result in no detectable differences in clinical effects.52 However, in an analysis of bioequivalence data from 258 studies on 141 generic AEDs, the results suggested that switching between generic AEDs may cause greater changes in SDC than switching between brand reference products and generic drugs.53 A study on the bioequivalence of brand and generic ER AEDs found that although most formulations had comparable bioequivalence ratios, for some products, the pharmacokinetic ratios were close to acceptable limits.54
Switching between generic versions of ER AEDs is not recommended because there can be a greater variation between generic ER AEDs than between generic IR AEDs.49,53 Staying with the same generic also provides comfort to the patient in terms of identifying medication and knowing what doses are being administered and refilled, and makes dosage adjustments easier for physicians.
The American Epilepsy Society supports the use of generic AEDs and has published a position statement on this issue.50 In particular, it is recommended that health care professionals should “ensure that a bioequivalent FDA-approved generic product is substituted for the brand or another generic AED,” and that they should counsel patients and caregivers on the equivalence between brands and generics, informing them of changes in tablet or capsule appearance (e.g., color or shape, when switching between medications).50 They also recommend counseling the patient to continue with the same generic supplier for a specific AED, something especially important for an ER product.
The Future of Oral ER DDSs in Epilepsy
Although the cost of an ER product should be considered in product selection, in general, ER formulations can provide several benefits over IR formulations of the same drug for most patients. However, the current types of ER DDSs in epilepsy may not suit all patients, and certain drugs may not be easily incorporated into an ER DDS. For example, there are rare occurrences of patients who benefit from timed higher peak concentrations, for whom an IR formulation may be the better option.
Developments in other areas of medicine, where there are multiple additional types of ER delivery, may be applicable to the treatment of epilepsy. For instance, to provide additional treatment options not based on pills/capsules, especially for the pediatric population and those with difficulty swallowing, a number of ER DDSs within the broader field of neurology have been developed for the treatment of attention-deficit/hyper-activity disorder, which include a transdermal patch (Daytrana [methylphenidate; Noven Pharmaceuticals, Miami, FL]) and liquid suspensions (Dyanavel XR [amphetamine; Tris Pharma Inc, Monmouth, NJ] and Quillivant XR [methylphenidate; Pfizer, New York, NY]). So far, none of these ER DDS have been developed for the treatment of epilepsy, but with further advances in technology, they may appear in the future.
Conclusions
Oral administration will remain the dominant route of AED delivery to treat epilepsy, and oral ER systems are a valuable tool for achieving improved adherence and seizure control, and reduced toxicity. With this review, the authors hope to convince clinicians of the importance of differentiating between the different ER DDSs currently used in epilepsy therapy, and of the benefits of using ER formulations to treat patients with epilepsy. We believe that there are clear benefits for the use of ER formulations compared with IR formulations, and that ER formulations should be used for most patients with epilepsy, when appropriate for the patient, and when available for their prescribed medication. The tables included in this review should provide a useful and practical reference guide for clinicians.
Acknowledgments
We wish to thank Nietzsche Lam, PhD, for his review of this manuscript and valuable comments regarding content.
ABBREVIATIONS
- AE
adverse effects
- AED
antiepileptic drugs
- DDS
drug delivery systems
- DR
delayed-release
- ER
extended-release
- FDA
US Food and Drug Administration
- IR
immediate-release
- IV
intravenous
- SDC
serum drug concentration
- USP
US Pharmacopeia
Footnotes
Disclosures Dr Wheless receives grants from Acordia, GW Pharmaceuticals, INSYS Inc, LivaNova, Lundbeck, Mallinckrodt, Neuralis, NeuroPace, the National Institutes of Health, Shainberg Foundation, Upsher-Smith, and Zogenix, is on the speaker's bureau for Eisai, LivaNova, Lundbeck, Mallinckrodt, Supernus, and Upsher-Smith, and also serves as a Consultant for CombiMatrix, Eisai, GW Pharmaceuticals, Lundbeck, NeuroPace, Sun Pharmaceuticals, Supernus, and Upsher-Smith. Dr Phelps has no conflicts of interest.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org
REFERENCES
- 1.Bhandari N., Kumar A., Choudhary A. et al. A review on immediate release drug delivery system. Int Res J Pharm App Sci. 2014;49(1):78–87. [Google Scholar]
- 2.Eatock J., Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatr Dis Treat. 2007;3(1):117–131. doi: 10.2147/nedt.2007.3.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manjunath R., Davis KL, Candrilli SD et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372–378. doi: 10.1016/j.yebeh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Samsonsen C., Reimers A., Bråthen G. et al. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125–e128. doi: 10.1111/epi.12801. [DOI] [PubMed] [Google Scholar]
- 5.Mangal M., Thakral S., Goswami M. et al. Superdisintegrants: an updated review. Int Pharmacy Pharmaceut Sci Res. 2012;2(2):26–35. [Google Scholar]
- 6.Jaimini M., Ranga S., Kumar A. et al. A review on immediate release drug delivery system by using design of experiment. J Drug Discov Therap. 2013;1(12):21–27. [Google Scholar]
- 7.US Food and Drug Administration Centre for Drug Evaluation and Research Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms. Silver Spring; MD: 1997. [Google Scholar]
- 8.Long M., Chen Y. Dissolution testing of solid products. In: Qiu Y., Chen Y., Zhang GGZ, editors. Developing Solid Oral Dosage Forms. San Diego, CA: Academic Press; 2009. pp. 319–340. ., eds. [Google Scholar]
- 9.Qiu Y., Zhou D. Understanding design and development of modified release solid oral dosage forms. J Validation Technol. 2011;17(2):23–32. [Google Scholar]
- 10.United States Pharmacopeia. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1151s87.html Accessed May 24, 2017.
- 11.Holquist C., Fava W. A look at delayed- vs. extended-release Rxs. Drug Topics. 2007 Jul 23; http://drugtopics.modernmedicine.com/drug-topics/news/clinical/pharmacy/fda-safety-page-delayed-release-vs-extended-release-rxs?page=full Accessed April 10, 2017.
- 12.Sakai JB. Practical Pharmacology for the Pharmacy Technician. Philadelphia, PA: Lippincott Williams & Wilkins; Pharmacokinetics: The absorption, distribution, and excretion of drugs; pp. 27–40. [Google Scholar]
- 13.Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9(6):153–157. doi: 10.1111/j.1535-7511.2009.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21(9):765–774. doi: 10.2165/00023210-200721090-00005. [DOI] [PubMed] [Google Scholar]
- 15.Pellock JM, Smith MC, Cloyd JC et al. Extended-release formulations: simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5(3):301–307. doi: 10.1016/j.yebeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 16.French JA, Gazzola DM. New generation antiepileptic drugs: what do they offer in terms of improved tolerability and safety? Ther Adv Drug Saf. 2011;2(4):141–158. doi: 10.1177/2042098611411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uthman BM. Extended-release antiepilepsy drugs – review of the effects of once-daily dosing on tolerability, effectiveness, adherence, quality of life, and patient preference. US Neurol. 2014;10(1):30–37. [Google Scholar]
- 18.Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49(3):446–454. doi: 10.1111/j.1528-1167.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Glassman M., Rienzi V. The relationship between poor medication compliance and seizures. Epilepsy Behav. 2002;3(4):338–342. doi: 10.1016/s1525-5050(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 20.Faught E., Duh MS, Weiner JR et al. Nonadherence to antiepileptic drugs and increased mortality. Findings from the RANDOM study. Neurology. 2008;71(20):1572–1578. doi: 10.1212/01.wnl.0000319693.10338.b9. [DOI] [PubMed] [Google Scholar]
- 21.Garnett WR, Gilbert TD, O'Connor P. Patterns of care, outcomes, and direct health plan costs of antiepileptic therapy: a pharmacoeconomic analysis of the available carbamazepine formulations. Clin Ther. 2005;27(7):1092–1103. doi: 10.1016/j.clinthera.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Leppik IE, Hovinga CA. Extended-release antiepileptic drugs: a comparison of pharmacokinetic parameters relative to original immediate-release formulations. Epilepsia. 2013;54(1):28–35. doi: 10.1111/epi.12043. [DOI] [PubMed] [Google Scholar]
- 23.Reed CR, Dutta S., Liu W. Once-daily dosing is appropriate for extended-release divalproex over a wide dose range, but not for enteric-coated, delayed-release divalproex: evidence via computer simulations and implications for epilepsy therapy. Epilepsy Res. 2009;87(2–3):260–267. doi: 10.1016/j.eplepsyres.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Somerville KW. Bioequivalence in development of antiepileptic drugs. Epilepsy Res. 2006;68(1):82–84. doi: 10.1016/j.eplepsyres.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Brittain ST, Wheless JW. Pharmacokinetic simulations of topiramate plasma concentrations following dosing irregularities with extended-release vs. immediate-release formulations. Epilepsy Behav. 2015;52(pt A):31–36. doi: 10.1016/j.yebeh.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Steinhoff BJ, Wendling AS. Short-term impact of the switch from immediate-release to extended-release oxcarbazepine in epilepsy patients on high dosages. Epilepsy Res. 2009;87(2–3):256–259. doi: 10.1016/j.eplepsyres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Ficker DM, Privitera M., Krauss G. et al. Improved tolerability and efficacy in epilepsy patients with extended-release carbamazepine. Neurology. 2005;65(4):593–595. doi: 10.1212/01.wnl.0000172932.95985.51. [DOI] [PubMed] [Google Scholar]
- 28.Miller AD, Krauss GL, Hamzeh FM. Improved CNS tolerability following conversion from immediate-to extended-release carbamazepine. Acta Neurol Scand. 2004;109(6):374–377. doi: 10.1111/j.1600-0404.2004.00291.x. [DOI] [PubMed] [Google Scholar]
- 29.Richy FF, Banerjee S., Brabant Y. et al. Levetiracetam extended release and levetiracetam immediate release as adjunctive treatment for partial-onset seizures: an indirect comparison of treatment-emergent adverse events using meta-analytic techniques. Epilepsy Behav. 2009;16(2):240–245. doi: 10.1016/j.yebeh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Doughty J., Baker GA, Jacoby A. et al. Compliance and satisfaction with switching from an immediate-release to sustained-release formulation of valproate in people with epilepsy. Epilepsy Behav. 2003;4(6):710–716. doi: 10.1016/j.yebeh.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Davis R., Peters DH, McTavish D. Valproic acid: a reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1994;47(2):332–372. [PubMed] [Google Scholar]
- 32.Ettinger AB, Manjunath R., Candrilli SD et al. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14(2):324–329. doi: 10.1016/j.yebeh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Faught RE, Weiner JR, Guérin A. et al. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501–509. doi: 10.1111/j.1528-1167.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 34.Helmers SL, Paradis PE, Manjunath R. et al. Economic burden associated with the use of generic antiepileptic drugs in the United States. Epilepsy Behav. 2010;18(4):437–444. doi: 10.1016/j.yebeh.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Labiner DM, Paradis PE, Manjunath R. et al. Generic antiepileptic drugs and associated medical resource utilization in the United States. Neurology. 2010;74(20):1566–1574. doi: 10.1212/WNL.0b013e3181df091b. [DOI] [PubMed] [Google Scholar]
- 36.Dutta S., Reed RC. Functional half-life is a meaningful descriptor of steady-state pharmacokinetics of an extended-release formulation of a rapidly cleared drug: as shown by once-daily divalproex-ER. Clin Drug Investig. 2006;26(12):681–690. doi: 10.2165/00044011-200626120-00002. [DOI] [PubMed] [Google Scholar]
- 37.Gidal BE, Clark AM, Anders B., Gilliam F. The application of half-life in clinical decision making: comparison of the pharmacokinetics of extended-release topiramate (USL255) and immediate-release topiramate. Epilepsy Res. 2017;129:26–32. doi: 10.1016/j.eplepsyres.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Pellock JM, Brittain ST. Use of computer simulations to test the concept of dose forgiveness in the era of extended-release (XR) drugs. Epilepsy Behav. 2016;55:21–23. doi: 10.1016/j.yebeh.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Maderuelo C., Zarzuelo A., Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J Control Release. 2011;154(1):2–19. doi: 10.1016/j.jconrel.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Nokhodchi A., Raja S., Patel P. et al. The role of oral controlled release matrix tablets in drug delivery systems. Bioimpacts. 2012;2(4):175–187. doi: 10.5681/bi.2012.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabor F., Fillafer C., Neutsch L. et al. Improving oral delivery. Handb Exp Pharmacol. 2010;197:345–398. doi: 10.1007/978-3-642-00477-3_12. [DOI] [PubMed] [Google Scholar]
- 42.Ghori MU, Conway BR. Hydrophilic matrices for oral control drug delivery. Am J Pharmacol Sci. 2015;3:103–109. [Google Scholar]
- 43.Poonuru RR, Gonugunta CS. Bimodal gastroretentive drug delivery systems of lamotrigine: formulation and evaluation. Indian J Pharm Sci. 2014;76(6):476–482. [PMC free article] [PubMed] [Google Scholar]
- 44.Clark AM, Pellock JM, Holmay M. et al. Clinical utility of topiramate extended-release capsules (USL255): bio-equivalence of USL255 sprinkled and intact capsule in healthy adults and an in vitro evaluation of sprinkle delivery via enteral feeding tubes. Epilepsy Behav. 2016;57(pt A):105–110. doi: 10.1016/j.yebeh.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Conley R., Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin. 2006;22(10):1879–1892. doi: 10.1185/030079906x132613. [DOI] [PubMed] [Google Scholar]
- 46.Tungaraza TE, Talapan-Manikoth P., Jenkins R. Curse of the ghost pills: the role of oral controlled-release formulations in the passage of empty intact shells in faeces: two case reports and a literature review relevant to psychiatry. Ther Adv Drug Saf. 2013;4(2):63–71. doi: 10.1177/2042098612474681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Pharmacopeia USP 33–NF 28 reissue: general notices and requirements; applying to standards, tests, assays, and other specifications of the United States Pharmacopeia. http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/USP33-NF28-ReissueGeneralNotices.pdf Accessed May 3, 2017.
- 48.Boggs JG, Preis K. Successful initiation of combined therapy with valproate sodium injection and divalproex sodium extended-release tablets in the epilepsy monitoring unit. Epilepsia. 2005;46(6):949–951. doi: 10.1111/j.1528-1167.2005.69703.x. [DOI] [PubMed] [Google Scholar]
- 49.LeLorier J., Duh MS, Paradis PE et al. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy. Neurology. 2008;70(22, pt 2):2179–2186. doi: 10.1212/01.wnl.0000313154.55518.25. [DOI] [PubMed] [Google Scholar]
- 50.Vossler DG, Anderson GD, Bainbridge J. AES position statement on generic substitution of antiepileptic drugs. Epilepsy Curr. 2016;16(3):209–211. doi: 10.5698/1535-7511-16.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ting TY, Jiang WL, Lionberger R. et al. Generic lamotrigine versus brand-name Lamictal bioequivalence in patients with epilepsy: a field test of the FDA bioequivalence standard. Epilepsia. 2007;56(9):1415–1424. doi: 10.1111/epi.13095. [DOI] [PubMed] [Google Scholar]
- 52.Privitera MD, Welty TE, Gidal BE et al. Generic-to-generic lamotrigine switches in people with epilepsy: the randomised controlled EQUIGEN trial. Lancet Neurol. 2016;15(4):365–372. doi: 10.1016/S1474-4422(16)00014-4. [DOI] [PubMed] [Google Scholar]
- 53.Krauss GL, Caffo B., Chang YT et al. Assessing bio-equivalence of generic antiepilepsy drugs. Ann Neurol. 2011;70(2):221–228. doi: 10.1002/ana.22452. [DOI] [PubMed] [Google Scholar]
- 54.Johnson EL, Chang YT, Davit B. et al. Assessing bio-equivalence of generic modified-release antiepileptic drugs. Neurology. 2016;86(17):1597–1604. doi: 10.1212/WNL.0000000000002607. [DOI] [PMC free article] [PubMed] [Google Scholar]