Abstract
Pixantrone (PIX) is an anticancer drug approved for the treatment of multiple relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. It is an aza-anthracenedione synthesized to have the same anticancer activity as its predecessors, anthracyclines (e.g. doxorubicin) and anthracenediones (e.g. mitoxantrone), with lower cardiotoxicity. However, published data regarding its possible cardiotoxicity are scarce. Therefore, this work aimed to assess the potential cytotoxicity of PIX, at clinically relevant concentrations (0.1; 1; and 10 μM) in both non-differentiated and 7-day differentiated H9c2 cells. Cells were exposed to PIX for 48 h and cytotoxicity was evaluated through phase contrast microscopy, Hoescht staining and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction and neutral red (NR) uptake assays. Cytotoxicity was observed in differentiated and non-differentiated H9c2 cells, with detached cells and round cells evidenced by phase contrast microscopy, mainly at the highest concentration tested (10 μM). In the Hoechst staining, PIX 10 μM showed a marked decrease in the number of cells when compared to control but with no signs of nuclear condensation. Furthermore, significant concentration-dependent mitochondrial dysfunction was observed through the MTT reduction assay. The NR assay showed similar results to those obtained in the MTT reduction assay in both differentiated and non-differentiated H9c2 cells. The differentiation state of the cells was not crucial to PIX effects, although PIX toxicity was slightly higher in differentiated H9c2 cells. To the best of our knowledge, this was the first in vitro study performed with PIX in H9c2 cells and it discloses worrying cytotoxicity at clinically relevant concentrations.
Keywords: Pixantrone, H9c2 cells, cardiotoxicity
ABBREVIATIONS
- DMEM
Dulbecco´s Modified Eagle medium
- DOX
Doxorubicin
- EDTA
Ethylenediamine tetraacetic acid
- EMA
European Medicines Agency
- FBS
Fetal bovine serum
- FDA
Food and Drug Administration
- HBSS
Hanks’ balanced salt solution
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- MTX
Mitoxantrone
- NHL
Non-Hodgkin’s lymphoma
- NR
Neutral Red
- PBS
Phosphate buffered saline
- PIX
Pixantrone
- RA
Retinoic acid
- ROS
Reactive oxygen species
Introduction
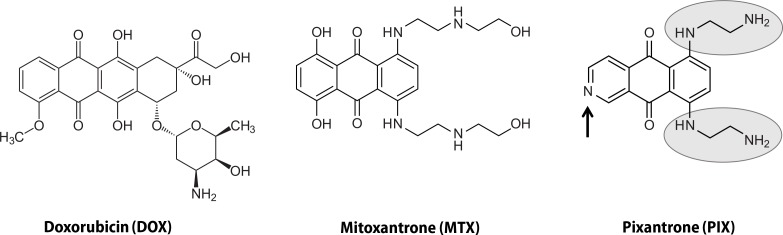
Chemotherapy is still one of the most frequent strategies used against cancer. Anticancer drugs, however, lack total selectivity, which results in serious side effects (Hrynchak et al., 2017; Reis-Mendes et al., 2015). Cardiotoxicity is one of the most worrying side effect found in patients treated with anticancer drugs of several pharmacological groups, like anthracyclines [e.g. doxorubicin (DOX)] and anthracenediones [e.g. mitoxantrone (MTX)] (Hrynchak et al., 2017; Reis-Mendes et al., 2015). DOX has been used for almost 50 years, but cardiotoxicity has been a critical and limiting side effect in its clinical use. MTX was synthesized with the purpose to maintain an effective anticancer potential yet without the cardiotoxicity, which characterized DOX (Conner, 1984). However, early data of MTX demonstrated that cardiac toxicity was still a matter of concern (Stuart-Harris et al., 1984). It was thus necessary to study the cardiotoxicity mechanisms of these compounds in order to find new ones that would be, in fact, less cardiotoxic. The mechanisms of cardiotoxicity of MTX are still poorly known, however several hypotheses have been suggested. The 5,8-dihydroxyphenyl ring of MTX was implicated in its cardiotoxicity through the formation of free radicals (Corbett et al., 1981; El-Helw & Hancock, 2007). Additionally, MTX can form complexes with Fe3+ and lead to oxidative stress, although the Fe3+-MTX complex is eight times less potent than Fe3+-DOX in the production of the hydroxyl radical (Herman et al., 1997; Malisza & Hasinoff, 1995). Actually, the oxidative stress hypothesis was the first considered as the culprit for MTX-induced cardiotoxicity, possibly because it has been linked to DOX-induced cardiotoxicity (Costa et al., 2013). Therefore, the 5,8-dihydroxyphenyl ring of MTX was replaced by a pyridine ring to create an aza derivative. Moreover, the (ethylamino)-diethylamino side chains were substituted for (hydroxyethylamino)-ethylamino side chains, forming a powerful cytotoxic agent against in vitro and murine models of cancer, BBR 2778. This compound was later named pixantrone (PIX) (6,9-bis [(2-aminoethyl) amino] benzo[g]isoquinoline-5, 10-dione) (Krapcho et al., 1994) (Figure 1).
Figure 1.
Chemical structure of DOX, MTX and PIX. DOX is composed by a tetracyclic quinone-hydroquinone chromophore, a carbonyl-containing side chain, and an aminosugar (daunosamine). MTX is a three-ring quinone-hydroquinone anthracenedione; its side chains lack carbonyl groups and MTX also lacks daunosamine. PIX differs from MTX in its lack of the hydroquinone, insertion of a nitrogen heteroatom in the same ring (black arrow), and substitution of (ethylamino)-diethylamino for (hydroxyethylamino)-ethylamino side chains (grey circles).
PIX (Pixuvri®) was approved by the European Medicines Agency (EMA) in monotherapy in adult patients with relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma (NHL) (EMA 2017; Péan et al., 2013). PIX seems to have similar anticancer mechanisms when compared with MTX and anthracyclines, namely DOX (Volpetti et al., 2014). Anthracyclines or anthracenediones kill tumor cells by intercalating in the DNA, inhibiting topoisomerase IIα, and forming double-strand breaks (Beggiolin et al., 2001; Hasinoff et al., 2016). These mechanisms are essentially due to the presence of the planar ring system that is composed by the four rings in anthracyclines and an amino-sugar, daunosamine, and the three rings in anthracenediones without any amino-sugar (Beggiolin et al., 2001; Hasinoff et al., 2016). Moreover, the nitrogen atom in PIX was added in the anthracenedione chromophore to create an additional hydrogen bond, and in turn, modulate the DNA affinity and the interaction with topoisomerase II (El-Helw & Hancock, 2007). In fact, PIX works as a topoisomerase II inhibitor, as PIX induces formation of linear DNA in a cleavage assay with topoisomerase IIα and topoisomerase IIβ. In K562 cells, PIX also showed to cause DNA double-strand breaks (Hasinoff et al., 2016).
While some efficacy has been demonstrated in relapsed aggressive NHL (Borchmann et al., 2003; Pettengell et al., 2012), the Food and Drug Administration USA (FDA) has not approved PIX use and it has only been conditionally approved in the EU for aggressive NHL (EMA, 2017). In fact, data regarding its cardiotoxicity are scarce, and lack of FDA approval is, in part, due to the inconsistencies shown by some of the studies carried out so far (FDA, 2010). In a Phase I clinical trial performed by Faivre et al. six patients with advanced solid tumors received a cumulative dose of 500 mg/m2 (range, 560–1 460 mg/m2) of PIX without any evidence of cardiotoxicity (Faivre et al., 2001). Although not so widely used as its analogues, from the cardiac point of view, most authors consider PIX a safer drug than DOX or MTX. However, in a study by Dawson and collaborators, a patient treated with total dose of 1 120 mg/m2, four cycles, showed signs of decreased left ventricular ejection (Dawson et al., 2000) and a Phase III clinical trial using lower cumulative doses showed decreased ejection fraction in 19% of the patients treated with PIX (Pettengell et al., 2012). As reported by the FDA, Phase II and Phase III clinical studies showed several inconsistences regarding PIX cardiotoxicity (FDA, 2010). There are also few in vitro studies aimed to evaluate the effects of PIX on cardiac cells (Hasinoff et al., 2016), thus a better clarification concerning its possible cardiotoxicity is essential. Therefore, the main purpose of this work was to study PIX cardiotoxicity in vitro in non-differentiated and differentiated H9c2 cells as to elucidate its possible cardiotoxicity.
Materials and methods
Materials
Trypsin-EDTA solution, trypan blue solution 0.4% (w/v) and Dulbecco´s Modified Eagle Medium (DMEM) high glucose, sodium dodecyl sulphate, hydrochloric acid, sodium bicarbonate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), neutral red (NR) solution, Hoescht 33258 solution, dimethyl sulfoxide, retinoic acid (RA) and paraformaldehyde were obtained from Sigma-Aldrich (Germany). PIX was acquired from Abcam (United Kingdom). All sterile plastic material was obtained from Corning Costar (USA). Penicillin/ streptomycin (10 000 units/mL/10 000 μg/mL) and the phosphate buffer solution (PBS) without calcium and magnesium were obtained from Biochrom (Germany). Fetal bovine serum (FBS), PBS with calcium and magnesium and Hank’s saline solution (HBSS or “Hanks’ balanced salt solution”) were obtained from Gibco (United Kingdom).
Cell culture
Experiments were performed using H9c2 cells, a subclone of the original cell line derived from embryonic BD1X rat heart tissue (Kimes & Brandt, 1976). The rat cardiomyocyte derived H9c2 cell line was obtained from the European Collection of Cell Cultures [H9c2 cell line from rat (BDIX heart myoblast), Sigma-Aldrich (Germany)]. H9c2 cells have morphological similarities with immature embryonic cardiomyocytes, while preserving characteristics of adult cardiac cells (Hescheler et al., 1991) and their metabolic characteristics are similar to those of the rat heart (Zordoky & El-Kadi, 2007). The cells are not fully differentiated in cardiomyocytes and remain in the proliferative state when 10% FBS is present in the culture medium (Rossato et al., 2013). They are one of the most widely used in vitro model to study cardiotoxicity (Rossato et al., 2013; Sardão et al., 2009; Zordoky & El-Kadi, 2007). To obtain differentiated H9c2 cells, the cells are maintained in a low FBS (1%) medium enriched with 10 nM RA for 7 days (medium change every other day). This procedure enhances the cells’ cardiac adult phenotype and decreases myogenic differentiation and cellular division (Menard et al., 1999). Since PIX acts on cellular division, this later model can be seen as a good model to avoid the bias of high proliferation rates.
Experimental procedures in cell culture
Cell maintenance
While proliferating, cells were placed at 37 °C with 5% CO2 in DMEM high glucose enriched by 10% of FBS, 100 units/ml penicillin and 100 μg/ml streptomycin. To prevent loss of myoblast cells, cell cultures were subcultured through trypsinization before reaching 70–80% confluence and were used before passage 29 (Reis-Mendes et al., 2017; Ruiz et al., 2012).
H9c2 were used in two differentiation states: non-differentiated and differentiated. The trypsinization step was performed prior to each seeding and to maintain cells in culture. Non-differentiated H9c2 cells were seeded at a density of 8 750 cells/cm2 (Sardão et al., 2009). Twenty-four hours after seeding, the cells were incubated with different PIX concentrations (0.1, 1 and 10 μM) for 48 h. The concentrations were chosen based on the plasma values of PIX found in a Phase I clinical trial (Faivre et al., 2001). Maximum concentration (Cmax) of PIX 1 h after intravenous doses (37.5 to 150 mg/m2) ranged between 1–7 μM (Faivre et al., 2001).
H9c2 cells were also differentiated with DMEM supplemented with 1% FBS, RA 10 nM and antibiotics, with medium change every other day for 7 days, after being seeded in a density of 6 000 cells/cm2 (Reis-Mendes et al., 2017). After the 7-day differentiation, cells were incubated with PIX (0.1, 1 or 10 μM) for 48 h.
Stock solutions of PIX were prepared in sterile PBS and PBS was used as control. PIX solutions were made monthly and frozen at –20 °C to guarantee stability.
Phase contrast microscopy
Phase-contrast microscopy morphological evaluation was performed to determine the effects of PIX after a 48-h incubation in both non-differentiated and differentiated cells. A Nikon Eclipse TS100 equipped with a Nikon DS-Fi1 camera (Japan) was used.
Hoescht staining
To evaluate the effects of PIX on the nuclear morphology of both non-differentiated and differentiated H9c2 cells, Hoechst staining was performed, as previously described (Reis-Mendes et al., 2017). Cells were examined in a Nikon Eclipse TS100 equipped with a Nikon DS-Fi1 camera (Japan) using a fluorescent filter (λexcitation = 346 nm and λemission = 460 nm).
Cytotoxicity evaluation
For the evaluation of PIX putative cytotoxicity, two assays were used: the NR lysosomal uptake and the MTT reduction assays. Both methods were performed 48 h after the cells’ exposure to PIX and the assays are described as follows.
MTT reduction assay
The MTT colorimetric assay is based on the reduction of the tetrazolium salt with formazan formation and it was performed as previously described (Reis-Mendes et al., 2017; Rossato et al., 2013). The spectrophotometric measurement of the formazans was done at 550 nm in a multi-well plate reader (PowerWaveXS, BioTek Instruments Inc., USA).
Neutral red lysosomal uptake assay
The NR dye enters the viable cells and concentrates in lysosomes. The NR uptake assay was performed as previously described (Reis-Mendes et al., 2017). The absorbance was read at 540 and 690 nm (reference) on a multi-well plate reader (Biotech Synergy HT, USA).
Statistical analysis
The results are expressed as mean ± standard deviation. Outliers were evaluated by the ROUT test. The D’Agostino & Pearson normality test was used to evaluate data distribution. A parametric analysis of variance (ANOVA) was performed when data distribution was normal, followed by the Tukey’s post-hoc test. When data did not follow a normal distribution, statistical analysis was performed using the Kruskal-Wallis test, followed by the Dunn’s post-hoc test when a significant p-value was reached. Statistical significance was reached when p<0.05. All statistical analyses were performed in GraphPad Prism 7 software (USA). All details of the statistical analyses can be found in the figure legends.
Results
Morphological evaluation of pixantrone-exposed non-differentiated H9c2 cells showed cytotoxicity at the highest concentration tested with no signs of condensed nuclei
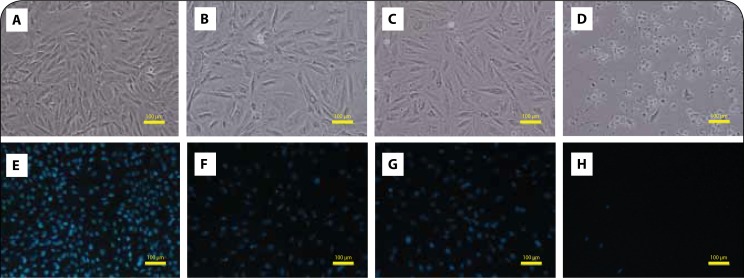
Cells were morphologically assessed through phase-contrast microscopy after a 48-h exposure to PIX. H9c2 cells were incubated with PIX (0.1, 1 and 10 μM), as described in the Methods section. Control cells had mostly cells with polygonal form attached to the bottom of the wells with very few detached cells (Figure 2A). At 48 h, PIX 0.1 and 1 μM had no signs of cellular damage (Figures 2B and 2C), while PIX 10 μM caused a clear cytoplasmic injury in non-differentiated H9c2 cells and most cells were round and detached (Figure 2D). Hoescht staining was used to evaluate the nuclear morphology after the 48-h incubation. A slight decrease in the number of cells in PIX 0.1 μM (Figure 2F) and PIX 1 μM (Figure 2G) exposed cells was observed when compared to the control wells (Figure 2E), with some nuclear fragmentation in non-differentiated H9c2 cells incubated with PIX 0.1 μM (Figure 2F). PIX 10 μM caused a marked decrease in the number of cells, with very few fluorescent nuclei in the field (Figure 2H).
Figure 2.
PIX causes cytotoxicity in non-differentiated H9c2 without signs of condensed nuclei. Phase contrast microscopy (A, B, C, D) and fluorescence microscopy (Hoechst 33258 staining) (E, F, G, H) images of non-differentiated H9c2 cells after a 48-h exposure to PBS (A and E), 0.1 μM PIX (B and F), 1 μM PIX (C and G) or 10 μM PIX (D and H). Images are representative of two independent experiments (scale bar 100 μm).
Pixantrone caused significant cytotoxicity in non-differentiated H9c2 cells according to the neutral red and MTT assays
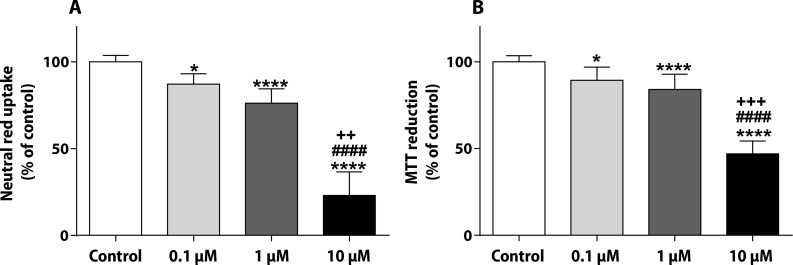
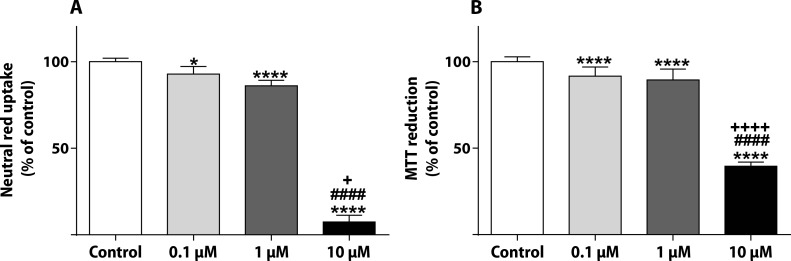
The NR uptake and the MTT reduction assays were done after the 48-h incubation in non-differentiated H9c2 cells (Figure 3). In the NR assay, PIX caused a concentration dependent decrease in the uptake of the vital dye: 0.1 μM presented values 87.07±6.07%, 1 μM of 76.25±8.21% and 10 μM of 23.08±13.53% (Figure 3A). In the MTT assay, PIX produced a significant mitochondrial dysfunction at 0.1 μM (89.32±7.49%), 1 μM (84.06±8.82%) and 10 μM (47.00±7.39%) concentrations when compared to control cells (100.0±3.51%) (Figure 3B).
Figure 3.
PIX leads to lysosomal and mitochondrial damage in non-differentiated H9c2. NR uptake (A) and MTT reduction (B) assays in non-differentiated H9c2 cells exposed to 0.1, 1 or 10 μM PIX for 48 h. Values are expressed as percentage of control and are shown as mean ± standard deviation. The results were obtained from 4–5 independent experiments using 20–24 wells. The statistical analyses were made using the Kruskal-Wallis test, followed by the Dunn’s post-hoc test (*p<0.05 and ****p<0.0001 versus control; ####p<0.0001 versus PIX 0.1 μM; ++p<0.01, +++p<0.001 versus PIX 1 μM).
Pixantrone caused higher cellular damage in differentiated than in non-differentiated H9c2 cells
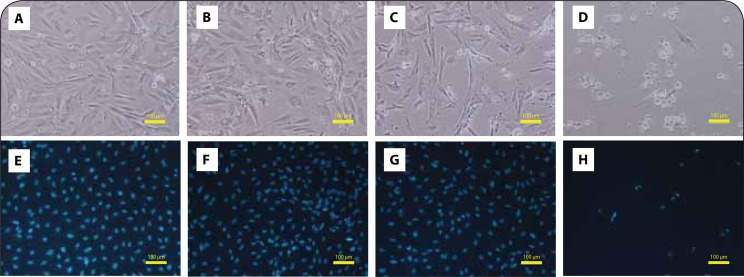
The differentiation with 10 nM RA and 1% FBS for 7 days led to morphological changes, making H9c2 cells more resembled to the cardiac phenotype. The differentiation protocol dramatically reduced cell division, and the cell bodies became smaller and fusiform. Overall, differentiated control cells showed a more organized network, bearing a cardiac-like morphology, as shown by phase-contrast morphology (Figure 4A). In differentiated H9c2 cells at 48 h, PIX incubation caused cell injury with cytoplasmic injury observed at PIX 1 μM and PIX 10 μM. In the latter concentration, the cellular damage was substantial (Figure 4D). In the Hoescht staining, a slight decrease in the number of cells for PIX 0.1 μM (Figure 4F) and PIX 1 μM (Figure 4G) was observed when compared to the control wells (Figure 4E). PIX 10 μM was able to cause a marked decrease in the number of cells, with very few fluorescent nuclei observed in the field (Figure 4H).
Figure 4.
PIX evokes cellular damage in a concentration dependent manner in differentiated H9c2 cells. Phase contrast microscopy (A, B, C, D) and fluorescence microscopy (Hoechst 33258 staining) (E, F, G, H) images of 7-day differentiated H9c2 cells after a 48-h exposure to PBS (A and E), 0.1 μM PIX (B and F), 1 μM PIX (C and G) or 10 μM PIX (D and H). Images are representative of two independent experiments (scale bar 100 μm).
Pixantrone caused a concentration-dependent mitochondrial and lysosome dysfunction in differentiated H9c2 cells
In the NR assay performed in differentiated H9c2 cells, the highest concentration of PIX (10 μM) caused a substantial impairment of lysosomal uptake of the vital dye (7.44±3.83%) when compared to control cells (100.0±2.03%). NR uptake in PIX 0.1 μM was 93.51±3.22% and in PIX 1 μM, it was 86.15±3.06% (Figure 5A). In the MTT reduction assay, PIX 10 μM (39.54±2.40%) caused a significant mitochondrial dysfunction when compared to control cells (100.0±2.90%) (Figure 5B). The highest concentration of PIX (10 μM) caused a significant higher cytotoxicity when compared to PIX 1 μM or PIX 0.1 μM (89.42±6.29% and 91.59±5.27%, respectively).
Figure 5.
PIX induces lysosomal and mitochondrial disruption in differentiated H9c2 cells. NR uptake (A) and MTT reduction (B) assays in differentiated H9c2 cells exposed to 0.1, 1 or 10 μM PIX for 48 h. Values are expressed as percentage of control and are shown as mean ± standard deviation. The results were obtained from 4 independent experiments using 20 wells. The statistical analyses were made using the Kruskal-Wallis test, followed by Dunn’s post-hoc test (A) and the ANOVA test, followed by the Tukey post hoc test (B) (*p<0.05 and ****p<0.0001 versus control; ####p<0.0001 versus PIX 0.1 μM; +p<0.05, ++++p<0.0001 versus PIX 1 μM).
Discussion
The present study is the first report on the toxicity of pharmacologically relevant concentrations of PIX (0.1; 1 and 10 μM) in H9c2 cells. PIX was cytotoxic at all concentrations tested, as evaluated by phase contrast microscopy, MTT reduction and NR uptake assays, in both differentiated and non-differentiated H9c2 cells.
A Phase III, multicentre, open-label, randomized trial was used to test the efficacy and safety of PIX in heavily pre-treated patients with relapsed or refractory aggressive NHL. The frequency of cardiac adverse events was higher in the PIX group than in the comparator group, where patients were treated with vinorelbine, oxaliplatin, ifosfamide, etoposide, MTX, or gemcitabine in a single agent therapy. The authors justified the higher frequency of cardiac adverse events in the PIX group by the presence of five patients within this group (compared with none in the comparator group) with previous histories of congestive heart failure or cardiomyopathy at the time of study enrolment (Pettengell et al., 2012). However, two major issues, in our point of view, must be discussed that make PIX eligible as culprit of those cardiac events. More cardiac adverse events occurred in the PIX treatment group [24 of 68 (35.3%)] than in the comparator [14 of 67 (20.9%)], even when the comparator group had a higher median previous DOX dose equivalent of 315.5 mg/m2 (range 15–681 mg/m2), when compared to 292.9 mg/m2 (range 51–472 mg/m2) in the PIX group (Pettengell et al., 2012). Moreover, patients in the comparator arm were treated with acknowledged cardiotoxic drugs (Pettengell et al., 2012) but presented lower incidence of cardiotoxicity. In fact, FDA raised several issues regarding PIX cardiotoxicity, suggesting that PIX is indeed cardiotoxic, but gave no conclusions regarding the comparison of its toxicity to anthracyclines/anthracenediones (FDA, 2010). Our results support the idea that PIX is, in fact, cardiotoxic. In the morphological evaluation done through phase contrast microscopy, cellular damage was not evident following a 48-h exposure to 0.1 or 1 μM PIX, in non-differentiated H9c2 cells. Only at 10 μM PIX a significant damage was observed. Despite the clear reduction in the number of nuclei, especially seen in the highest PIX concentration tested (10 μM), in both differentiated and non-differentiated cells, no significant changes on nuclear morphology were evident when compared to control cells. These data indicate that PIX cytotoxicity may not elicit apoptosis in H9c2 cells in the time-point analyzed. Moreover, in the present work, two methods were used to evaluate the cytotoxicity of PIX, the MTT reduction and the NR uptake assays, which are extremely sensitive in assessing cellular homeostasis impairment. The MTT colorimetric assay is based on mitochondrial activities of NADH dehydrogenase (complex I) and/or succinate dehydrogenase (complex II) on the tetrazolium salt with formazan formation (Costa et al., 2009), whereas the NR uptake assay is based on the ability of viable cells to uptake the supravital dye that penetrates cell membranes and concentrates in lysosomes (Repetto et al., 2008). Significant cytotoxicity was reported in both assays at all concentrations tested, being more pronounced for 10 μM PIX at 48 h, both in differentiated and non-differentiated H9c2 cells. The concentrations used here are clinically relevant, since 1 h after intravenous administration of doses ranging between 37.5 to 150 mg/m2, the Cmax of PIX on the plasma of patients in a Phase I clinical trial was between 1–7 μM (Faivre et al., 2001). The recommended dose of PIX is 50 mg/m2 administered on days 1, 8, and 15 of each 28-day cycle for up to 6 cycles for the treatment of adult patients with multiple relapsed or refractory aggressive NHL (Péan et al., 2013).
To the best of our knowledge, there is only one study with PIX in cardiac cellular models (Hasinoff et al., 2016). For that reason, our results will be compared with the published data of other acknowledged cardiotoxic anticancer drugs, DOX and MTX, in in vitro models. Non-differentiated H9c2 cells exposed to 1 μM DOX at several time points (between 0 and 48 h) evidenced an increase in cell death with increasing time of exposure, evaluated through phase contrast microscopy (Zhang et al., 2015). Regarding MTX, our group assessed the cytotoxicity of 5 μM and 2 μM MTX in differentiated H9c2 cells. After a 48-h exposure, an increase in cell death with increasing MTX concentrations was found (Reis-Mendes et al., 2017). Based on these morphological data, PIX seems to cause lower damage when compared to DOX or MTX in the same cellular model, since only at the highest concentration tested (10 μM), a significant damage was seen. Moreover, in our study with MTX, no signs of nuclear morphological alterations were observed after exposure to 2 or 5 μM MTX for 48 h in differentiated H9c2 cells (Reis-Mendes et al., 2017), as evidenced here with PIX. However, in non-differentiated H9c2 cells, a 24-h exposure to 1.60 μM MTX or 0.9 μM DOX caused morphological signals of apoptosis (Kluza et al., 2004) and caspase 3 activation occurred in non-differentiated H9c2 cells exposed for 24 h to 100 nM and 1 μM MTX (Rossato et al., 2013).
In non-differentiated H9c2 cells, DOX elicited mitochondrial dysfunction as seen through the MTT reduction assay, following a 24-h exposure to 0.2, 1 and 5 μM (Zhang et al., 2015). Regarding MTX, also in non-differentiated H9c2 cells, it led to a time- and concentration-dependent cytotoxicity (Rossato et al., 2013). At 48 h, 10 μM MTX had values of around 20% when compared to control (100.0%) in the MTT assay, whereas 1 and 0.1 μM MTX showed values of approximately 80 and 90%, respectively, when compared to control cells (Rossato et al., 2013). In the study carried out by our group using differentiated H9c2 cells exposed to various concentrations of MTX (0.01, 0.1, 1, 2 and 5 μM) for 48 h, 5 μM MTX showed values of around 75% in the MTT reduction assay, whereas 1 and 0.1 μM of 80% and 90%, respectively, when compared to control cells (Reis-Mendes et al., 2017). The cytotoxicity caused by MTX is, in fact, very similar to what was observed in the present work with similar concentrations of PIX in non-differentiated and differentiated cells. Moreover, PIX, as did DOX, decreased the mitochondrial membrane potential of cardiac rat neonatal cardiomyocytes, thus demonstrating the ability of PIX to alter mitochondrial homeostasis (Hasinoff et al., 2016) and its cardiotoxic potential.
Moreover, in the present work, 10 μM PIX caused a higher cytotoxicity in the NR assay in differentiated H9c2 cells when compared to non-differentiated cells. This assay also seems more sensitive to detect PIX cytotoxicity. On the other hand, using the same assay, differentiated H9c2 cells exposed for 48 h to 1 μM MTX incorporated 60% of NR when compared to control cells (set to 100%) (Reis-Mendes et al., 2017), whereas with PIX, at the same incubation time and using the same concentration, the NR uptake was 76.25±8.21% in non-differentiated cells and 86.15±3.06% in differentiated cells. Additionally, 0.1 μM MTX did not cause any significant toxicity in the NR assay at 48 h in differentiated H9c2 cells (Reis-Mendes et al., 2017), while 0.1 μM PIX decreased the dye’s cellular incorporation to 87.07±6.09% in non-differentiated cells and to 93.51±3.22% in differentiated cells at the same incubation time point, further demonstrating the cardiotoxic potential of PIX. Most authors agree that using several cytotoxicity tests with different inherent mechanisms can help elucidate the underlying cytotoxicity of the drugs tested (Lopes et al., 2008; Reis-Mendes et al., 2017; Soares et al., 2013).
The mechanisms involved in PIX putative cardiotoxicity are still undisclosed. In human leukemia K562 cells and contrasting with DOX, PIX produced no detectable semi-quinones or reactive oxygen species (ROS), possibly due to a lower PIX accumulation in those cells. The intracellular accumulation of PIX (1.5 nmol) was shown to be low when compared to MTX (8.1 nmol) or DOX (6.5 nmol) under the same experimental conditions (1 h incubation at 37 °C in a final concentration of 10 nmol) (Hasinoff et al., 2016). Also, the spectrophotometric titrations of PIX suggested that, unlike what happens with DOX or MTX, PIX does not bind to Fe3+, probably due to its lack of hydroquinone functionality (Herman et al., 1997; Malisza and Hasinoff 1995). Fe3+ causes oxidative stress via the Fenton reaction and through formation of the highly toxic hydroxyl radical (Costa et al., 2011). It is widely known that, in the heart, DOX forms a semiquinone free radical and Fe3+-DOX complexes, which are responsible for a futile redox cycle that creates oxidative stress (Costa et al., 2013). MTX does not form ROS easily, but it affects the heart mitochondria (Reis-Mendes et al., 2017; Rossato et al., 2014; Rossato et al., 2013), with the heart being largely susceptible to energetic dysfunction. According to Rossato et al., the endpoint of toxicity induced by MTX is the electron transport chain and its dysfunction can create a mild oxidative stress that results in fatal cardiotoxicity (Rossato et al., 2014; Rossato et al., 2013). As stated before, PIX is able to decrease the mitochondrial membrane potential of cardiac rat neonatal cardiomyocytes (Hasinoff et al., 2016) and mitochondria can be targets for PIX.
Additionally, compared to MTX and anthracyclines, PIX shows better selectivity for topoisomerase IIα (the form present in cancer cells) instead of IIβ (the form present in cardiomyocytes) at the 1–10 μM concentration range (Hasinoff et al., 2016). The higher selectivity of PIX towards topoisomerase IIα led to the belief that PIX elicits low cardiotoxicity, since topoisomerase IIβ is the isoform present in cardiomyocytes and its inhibition can be responsible, at least partially, for the cardiotoxicity of DOX and MTX (Capranico et al., 1992; Hasinoff et al., 2016; Zhang et al., 2012). However, the cardiac concentration of PIX after therapy is not known, and it may be sufficient to inhibit the physiological cardiac form of topoisomerase and thus cause cardiac dysfunction. In fact, cardiac dysfunction has been observed after PIX therapy, even if its use is not largely disseminated (Pettengell et al., 2012; Volpetti et al., 2014).
Overall, the two cytotoxicity assays performed in the present work show to be more sensitive to detect PIX-induced toxicity than Hoescht staining or phase contrast microscopy. In addition, differentiated H9c2 cells are slightly more prone to PIX induced cytotoxicity than non-differentiated H9c2 cells. Thus PIX toxicity is not dependent on its effects on cellular division, as the former model has a reduced proliferative state (Menard et al., 1999; Reis-Mendes et al., 2017). Despite the structural changes performed in the synthesis of PIX and its lower potential to form ROS and toxic drug metal complexes in human myocardial strip (Salvatorelli et al., 2013), our work clearly shows its potential cardiac toxicity. This contradicts some of the data obtained in the scarce clinical and preclinical studies done with PIX, where PIX is claimed as a cardiosafe drug when compared with its analogues (Beggiolin et al., 2001; Borchmann et al., 2003; Cavalletti et al., 2007; Hasinoff et al., 2016; Pettengell et al., 2012). Actually, compared to the reports made for its predecessors, mainly with the works done in our group with MTX in the same cellular models (Reis-Mendes et al., 2017; Rossato et al., 2013), the data obtained here show that PIX has similar cardiac toxicity as MTX and should be regarded as a potential cardiotoxic drug.
Acknowledgments
ARM and VMC acknowledge Fundação da Ciência e Tecnologia (FCT) for their grants (SFRH/BD/129359/2017 and SFRH/BPD/110001/2015, respectively). This work was supported by FEDER funds through the Operational Programme for Competitiveness Factors – COMPETE and by national funds by the FCT within the project “PTDC/ DTP-FTO/1489/2014 – POCI-01-0145-FEDER-016537”.
REFERENCES
- Beggiolin G, Crippa L, Menta E, Manzotti C, Cavalletti E, Pezzoni G, Torriani D, Randisi E, Cavagnoli R, Sala F, Giuliani FC, Spinelli S. Bbr 2778, an aza-anthracenedione endowed with preclinical anticancer activity and lack of delayed cardiotoxicity. Tumori. 2001;87:407–416. doi: 10.1177/030089160108700611. [DOI] [PubMed] [Google Scholar]
- Borchmann P, Morschhauser F, Parry A, Schnell R, Harousseau JL, Gisselbrecht C, Rudolph C, Wilhelm M, Gunther H, Pfreundschuh DM, Camboni G, Engert A. Phase-II study of the new aza-anthracenedione, BBR 2778, in patients with relapsed aggressive non-Hodgkin’s lymphomas. Haematologica. 2003;88:888–894. [PubMed] [Google Scholar]
- Capranico G, Tinelli S, Austin CA, Fisher ML, Zunino F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta. 1992;1132:43–48. doi: 10.1016/0167-4781(92)90050-a. [DOI] [PubMed] [Google Scholar]
- Cavalletti E, Crippa L, Mainardi P, Oggioni N, Cavagnoli R, Bellini O, Sala F. Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: comparative studies against doxorubicin and mitoxantrone. Invest New Drugs. 2007;25:187–195. doi: 10.1007/s10637-007-9037-8. [DOI] [PubMed] [Google Scholar]
- Conner CS. Mitoxantrone: a replacement for doxorubicin? Drug Intell Clin Pharm. 1984;18:479–480. doi: 10.1177/106002808401800604. [DOI] [PubMed] [Google Scholar]
- Corbett TH, Griswold DP, Jr., Roberts BJ, Schabel FM., Jr Absence of delayed lethality in mice treated with aclacinomycin A. Cancer Chemother Pharmacol. 1981;6:161–168. doi: 10.1007/BF00262337. [DOI] [PubMed] [Google Scholar]
- Costa VM, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F. Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr Med Chem. 2011;18:2272–2314. doi: 10.2174/092986711795656081. [DOI] [PubMed] [Google Scholar]
- Costa VM, Carvalho F, Duarte JA, Bastos ML, Remiao F. The heart as a target for xenobiotic toxicity: the cardiac susceptibility to oxidative stress. Chem Res Toxicol. 2013;26:1285–1311. doi: 10.1021/tx400130v. [DOI] [PubMed] [Google Scholar]
- Costa VM, Silva R, Tavares LC, Vitorino R, Amado F, Carvalho F, Bastos ML, Carvalho M, Carvalho RA, Remiao F. Adrenaline and reactive oxygen species elicit proteome and energetic metabolism modifications in freshly isolated rat cardiomyocytes. Toxicology. 2009;260:84–96. doi: 10.1016/j.tox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Dawson LK, Jodrell DI, Bowman A, Rye R, Byrne B, Bernareggi A, Camboni G, Smyth JF. A clinical phase I and pharmacokinetic study of BBR 2778, a novel anthracenedione analogue, administered intravenously, 3 weekly. Eur J Cancer. 2000;36:2353–2359. doi: 10.1016/s0959-8049(00)00342-7. [DOI] [PubMed] [Google Scholar]
- El-Helw LM, Hancock BW. Pixantrone: a novel aza-anthracenedione in the treatment of non-Hodgkin’s lymphomas. Expert Opin Investig Drugs. 2007;16:1683–1691. doi: 10.1517/13543784.16.10.1683. [DOI] [PubMed] [Google Scholar]
- EMA . Pixuvri (pixantrone): EU summary of product characteristics. London, UK: European Medicines Agency; 2017. [Google Scholar]
- Faivre S, Raymond E, Boige V, Gatineau M, Buthaut X, Rixe O, Bernareggi A, Camboni G, Armand JP. A phase I and pharmacokinetic study of the novel aza-anthracenedione compound BBR 2778 in patients with advanced solid malignancies. Clin Cancer Res. 2001;7:43–50. [PubMed] [Google Scholar]
- FDA . In: FDA ODAC Briefing Document: Pixuvri (pixantrone) U.S.D.o.H.a.H. Services, editor. Maryland, USA: 2010. [Google Scholar]
- Hasinoff BB, Wu X, Patel D, Kanagasabai R, Karmahapatra S, Yalowich JC. Mechanisms of action and reduced cardiotoxicity of pixantrone; a topoisomerase II targeting agent with cellular selectivity for the topoisomerase II alpha isoform. J Pharmacol Exp Ther. 2016;356:397–409. doi: 10.1124/jpet.115.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman EH, Zhang J, Hasinoff BB, Clark JR, Jr, Ferrans VJ. Comparison of the structural changes induced by doxorubicin and mitoxantrone in the heart, kidney and intestine and characterization of the Fe(III)-mitoxantrone complex. J Mol Cell Cardiol. 1997;29:2415–2430. doi: 10.1006/jmcc.1997.0477. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- Hrynchak I, Sousa E, Pinto M, Costa VM. The importance of drug metabolites synthesis: the case-study of cardiotoxic anticancer drugs. Drug Metab Rev. 2017;49:158–196. doi: 10.1080/03602532.2017.1316285. [DOI] [PubMed] [Google Scholar]
- Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- Kluza J, Marchetti P, Gallego MA, Lancel S, Fournier C, Loyens A, Beauvillain JC, Bailly C. Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene. 2004;23:7018–7030. doi: 10.1038/sj.onc.1207936. [DOI] [PubMed] [Google Scholar]
- Krapcho AP, Petry ME, Getahun Z, Landi JJ, Jr, Stallman J, Polsenberg JF, Gallagher CE, Maresch MJ, Hacker MP, Giuliani FC, Beggiolin G, Pezzoni G, Menta E, Manzotti C, Oliva A, Spinelli S, Tognella S. 6,9-Bis[(aminoalkyl) amino]benzo[g]isoquinoline-5,10-diones. A novel class of chromophoremodified antitumor anthracene-9,10-diones: synthesis and antitumor evaluations. J Med Chem. 1994;37:828–837. doi: 10.1021/jm00032a018. [DOI] [PubMed] [Google Scholar]
- Lopes MA, Meisel A, Dirnagl U, Carvalho FD, Bastos ML. Doxorubicin induces biphasic neurotoxicity to rat cortical neurons. Neurotoxicology. 2008;29:286–293. doi: 10.1016/j.neuro.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Hasinoff BB. Production of hydroxyl radical by iron(III)-anthraquinone complexes through self-reduction and through reductive activation by the xanthine oxidase/hypoxanthine system. Arch Biochem Biophys. 1995;321:51–60. doi: 10.1006/abbi.1995.1367. [DOI] [PubMed] [Google Scholar]
- Menard C, Pupier S, Mornet D, Kitzmann M, Nargeot J, Lory P. Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9c2 cardiac cells. J Biol Chem. 1999;274:29063–29070. doi: 10.1074/jbc.274.41.29063. [DOI] [PubMed] [Google Scholar]
- Péan E, Flores B, Hudson I, Sjöberg J, Dunder K, Salmonson T, Gisselbrecht C, Laane E, Pignatti F. The European Medicines Agency review of pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non-hodgkin’s b-cell lymphomas: Summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2013;18:625–633. doi: 10.1634/theoncologist.2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettengell R, Coiffier B, Narayanan G, de Mendoza FH, Digumarti R, Gomez H, Zinzani PL, Schiller G, Rizzieri D, Boland G, Cernohous P, Wang L, Kuepfer C, Gorbatchevsky I, Singer JW. Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: a phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2012;13:696–706. doi: 10.1016/S1470-2045(12)70212-7. [DOI] [PubMed] [Google Scholar]
- Reis-Mendes A, Gomes AS, Carvalho RA, Carvalho F, Remiao F, Pinto M, Bastos ML, Sousa E, Costa VM. Naphthoquinoxaline metabolite of mitoxantrone is less cardiotoxic than the parent compound and it can be a more cardiosafe drug in anticancer therapy. Arch Toxicol. 2017;91:1871–1890. doi: 10.1007/s00204-016-1839-z. [DOI] [PubMed] [Google Scholar]
- Reis-Mendes AF, Sousa E, de Lourdes Bastos M, Costa VM. The role of the metabolism of anticancer drugs in their induced-cardiotoxicity. Curr Drug Metab. 2015;17:75–90. doi: 10.2174/1389200216666151103114926. [DOI] [PubMed] [Google Scholar]
- Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Rossato LG, Costa VM, Dallegrave E, Arbo M, Silva R, Ferreira R, Amado F, Dinis-Oliveira RJ, Duarte JA, de Lourdes Bastos M, Palmeira C, Remiao F. Mitochondrial cumulative damage induced by mitoxantrone: late onset cardiac energetic impairment. Cardiovasc Toxicol. 2014;14:30–40. doi: 10.1007/s12012-013-9230-2. [DOI] [PubMed] [Google Scholar]
- Rossato LG, Costa VM, Vilas-Boas V, de Lourdes Bastos M, Rolo A, Palmeira C, Remiao F. Therapeutic concentrations of mitoxantrone elicit energetic imbalance in H9c2 cells as an earlier event. Cardiovasc Toxicol. 2013;13:413–425. doi: 10.1007/s12012-013-9224-0. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Courilleau D, Jullian JC, Fortin D, Ventura-Clapier R, Blondeau JP, Garnier A. A cardiac-specific robotized cellular assay identified families of human ligands as inducers of PGC-1alpha expression and mitochondrial biogenesis. PLoS One. 2012;7:e46753. doi: 10.1371/journal.pone.0046753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatorelli E, Menna P, Paz OG, Chello M, Covino E, Singer JW, Minotti G. The novel anthracenedione, pixantrone, lacks redox activity and inhibits doxorubicinol formation in human myocardium: insight to explain the cardiac safety of pixantrone in doxorubicin-treated patients. J Pharmacol Exp Ther. 2013;344:467–478. doi: 10.1124/jpet.112.200568. [DOI] [PubMed] [Google Scholar]
- Sardão VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB. Doxorubicin-induced mitochondrial dysfunction is secondary to nuclear p53 activation in H9c2 cardiomyoblasts. Cancer Chemother Pharmacol. 2009;64:811–827. doi: 10.1007/s00280-009-0932-x. [DOI] [PubMed] [Google Scholar]
- Soares AS, Costa VM, Diniz C, Fresco P. Potentiation of cytotoxicity of paclitaxel in combination with Cl-IB-MECA in human C32 metastatic melanoma cells: A new possible therapeutic strategy for melanoma. Biomed Pharmacother. 2013;67:777–789. doi: 10.1016/j.biopha.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Stuart-Harris RC, Bozek T, Pavlidis NA, Smith IE. Mitoxantrone: an active new agent in the treatment of advanced breast cancer. Cancer Chemother Pharmacol. 1984;12:1–4. doi: 10.1007/BF00255899. [DOI] [PubMed] [Google Scholar]
- Volpetti S, Zaja F, Fanin R. Pixantrone for the treatment of adult patients with relapsed or refractory aggressive non-Hodgkin B-cell lymphomas. Onco Targets Ther. 2014;7:865–872. doi: 10.2147/OTT.S34055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Meng C, Zhang XM, Yuan CH, Wen MD, Chen Z, Dong DC, Gao YH, Liu C, Zhang Z. Ophiopogonin D attenuates doxorubicin-induced autophagic cell death by relieving mitochondrial damage in vitro and in vivo. J Pharmacol Exp Ther. 2015;352:166–174. doi: 10.1124/jpet.114.219261. [DOI] [PubMed] [Google Scholar]
- Zordoky BN, El-Kadi AO. H9c2 cell line is a valuable in vitro model to study the drug metabolizing enzymes in the heart. J Pharmacol Toxicol Methods. 2007;56:317–322. doi: 10.1016/j.vascn.2007.06.001. [DOI] [PubMed] [Google Scholar]