Abstract
The infective, microscopic Strongyloides stercoralis larvae in contaminated soil can penetrate human skin with the help of excretory/secretory proteases. These proteases play a critical role in infection and transmigration of the parasite to the intestines. Strongylastacin is similar to astacin (from the digestive gland of the crayfish Astacus astacus), a multi‐domain protein with a signal peptide, a pro‐enzyme, a catalytic domain containing the zinc binding consensus astacin family signature sequence HEXXHXXGFXHEXXRXDR, and a second conserved zinc binding motif SIMHY at N‐ terminal region. An EGF‐1 like domain and a CUB domain are located at the COOH‐ terminal. In this study, the excretory/secretory Strongylastacin gene from S. stercoralis infective larval stage was cloned and expressed as a 45 kDa in Escherichia coli. Immunoblot analysis showed the presence of natural IgG antibodies against strongylastacin in six infected and six non‐endemic normal sera. These findings were confirmed in an ELISA of 32 S. stercoralis infected and 32 presumed normal human sera; all contained natural anti‐strongylastacin IgG antibodies. By contrast, IgE antibodies specific to strongylastacin were present in sera from individuals infected with S. stercoralis but not in uninfected control sera. Moreover, recombinant strongylastacin did not cross‐react with IgE antibodies either from patients infected with filaria or patients with tropical pulmonary eosinophilic (TPE) who had increased IgE antibodies. The present authors conclude that strongylastacin, an excretory/secretory antigen, elicits specific IgE antibodies in S. stercoralis infected humans. Non‐specific IgG antibodies to strongylastacin are present in both infected and normal humans. Further investigation is needed to understand the role of the host protective response against strongylastacin.
INTRODUCTION
Nematode parasites produce a variety of proteases that play several important functional roles in the development of larval stages, digestion, assimilation and ovulation (1), organogenesis, differentiation and molting (2). Proteases of parasites are presumed to mediate a variety of functions that include tissue penetration and migration by degrading the extra‐cellular matrix (3–9), immune evasion (10, 11) and disruption of host blood coagulation cascades (12, 13). Metalloendopeptidases (meprins) cleave a variety of peptides and proteins and have a preference for peptides with more than six amino acids, indicating a wide range of substrate binding sites (14, 15). Meprins cleave bioactive peptides such as gastrin, cholecystokinin and parathyroid hormone; cytokines such as osteopontin and monocyte chemotactic peptide‐1; as well as proteins such as gelatin, collagen IV, fibronectin and casein (16). Metalloproteases have been identified as immunodominant antigens with the potential to elicit protective immune responses (17, 18). They are potential targets for chemotherapy (19, 20).
Strongyloides stercoralis is an obligate skin penetrating parasite. The infective third‐stage larva of S. stercoralis is quite efficient at hydrolyzing elements of the skin matrix such as fibronectin, laminin and bovine elastin (9, 21). It has been shown that tissue penetration of S. stercoralis L3 stage larvae can be blocked in vitro by a metalloprotease inhibitor, 2 mM o‐ phenanthroline (4). Three metalloproteases in the somatic extracts of S. stercoralis have been identified (4), these include a 40 kDa metalloproteinase known as strongylastacin which belongs to the astacin family of zinc metalloproteases (22). Although biochemical characterization of the astacin protein family is well advanced (23), little is known about the immune response of these proteases. We undertook the present study to explore humoral immune responses to strongylastacin in S. stercoralis infected patient sera. Further, we compared these responses with sera from patients infected with the lymphatic filarial parasite W. bancrofti, since there is often serological cross‐reactivity between S. stercoralis and W. bancrofti.
MATERIALS AND METHODS
Cloning, expression and purification of strongylastacin
A S. stercoralis cDNA library constructed in lambda Zap XR phage has been described previously (24). The phage encoding strongylastacin was in vivo excised into pBluescript. Plasmid DNA was isolated and the region corresponding to the open reading frames of the strongylastacin was amplified by PCR using specific primers (forward 5′‐ AATAGAAATAATTTAGAATCTATTG‐3′; reverse primer 5′‐TTATTTAAAACTTTTGAACTTAATTG‐3′) designed to the sequence containing a pro‐astacin domain without its signal sequence. The PCR‐amplified gene was cloned into a pET directional TOPO vector (Invitrogen, San Diego, CA, USA) as described by the manufacturer. In order to obtain active metalloprotease, E. coli BL21 (Origami) was transformed with the TOPO construct to express active protein. The strongylastacin recombinant protein was produced and purified using standard techniques. Briefly, LB broth (6 L, with 100 μg ampicillin per mL of LB in six bacterial culture flasks; Beckman, Fullerton, CA, USA) was inoculated with an overnight culture of E. coli BL21 (DE3, Origami) containing the plasmid strongylastacin cDNA gene in pET. The flasks were shaken at 37°C and 150 rpm until the optical density of the culture at 600 nm reached 0.6. IPTG was added to a concentration of 0. 8 mM, and the cultures shaken overnight at 28°C. The cells were harvested by centrifugation, washed with 20 mM Tris HCl (pH 7.5), and homogenized. After centrifugation at 100,000 ×g for 1 hr at 4°C, the supernatant was purified using the His Bind kit (Novagen, Madison, WI, USA). Four mL of 50% saturated resin was added to the 20‐mL of sample supernatant containing 20 mM imidazole and shaken for 15 min. The mixture was packed under gravity flow on a polypropylene column and washed with 40 column volumes of wash buffer (20 mM Tris HCl [pH 7.5], 300 mM NaCl, 20 mM imidazole). The protein was eluted with 10 column volumes of elution buffer (20 mM Tris/HCl [pH 7.5], 300 mM NaCl, 250 mM imidazole), and extensively dialyzed against PBS. The recombinant protein sample was concentrated to 10 mL by ultra filtration through Amicon filters (Amicon, Beverly, MA, USA) with 10‐kDa cutoffs. The concentration of the protein was estimated using the micro‐bicinchoninic acid method (Pierce, Rockford, IL, USA).
Activation of zymogen strongylastacin
In order to activate strongylastacin (by removal of the propeptide), purified astacin was subjected to limited digestion with trypsin immobilized on activated cyanogen bromide 4B (CNBr 4B, Amersham Biosciences, Piscataway, NJ, USA). In brief, 1 mL of 3 mg/mL trypsin (Sigma, St Louis, MO, USA) in phosphate buffer (pH 5.5) was mixed with 1 mL of 50% activated cyanogen bromide Sepharose 4B saturated in PBS (pH 5.5) and coupling was allowed for 3 hr on a rocker at 4°C. The matrix was washed with 5 column volume of chilled PBS (pH 5.5), unbound active sites were blocked with chilled 100 mM Tris HCl (pH 8.0) for 1 hr and the same step was repeated once more. The resin was washed three times alternately with the following buffers: 0.1M acetate buffer (pH 4.0) containing 0.5M NaCl and 0.1M Tris HCl (pH 8.0) containing 0.5M NaCl. The coupled matrix was stored in PBS (pH 5.0) at 4°C until used. An aliquot of 100 μL CNBr Sepharose coupled trypsin slurry was mixed with 100 μg of purified zymogen strongylastacin in 200 μL of buffer containing 20 mM Tris HCl (pH 8.0) and 1 mM CaCl2 and incubated at 37°C rocker for 15 min. The sample was removed and immediately centrifuged at 4000 rpm at 4°C in a refrigerated microcentrifuge for 2 min. The supernatant, containing active strongylastacin, was then transferred to a fresh Eppendorf tube. The sample was mixed with 6 × sample solubilizing Laemelli buffer without reducing agent and without boiling and stored at 4°C until use.
Zymogram
The gelatinase activity of astacin was assessed on gelatin incorporated SDS‐PAGE gels (Invitrogen). The astacin was loaded onto the gel without heating. The electrophoretic procedure was carried out at 4°C and at a constant current of 25 mA. After termination of electrophoretic separation the gel was washed extensively five times in renaturation buffer containing 50 mM Tris HCl (pH 7.5), 2 mM ZnSO4, and 2.5% Triton X 100 at 37°C to remove excess SDS. The gel was then incubated overnight at 37°C in renaturation buffer containing 1% (v/v) Triton X‐100 to facilitate degradation of gelatin by astacin. Subsequently, the gels were stained with Coomassie Brilliant Blue and destained in a solution of 7% (v/v) acetic acid and 20% (v/v) methanol. Hydrolysis of the gelatin is demonstrated by the presence of clear bands against a blue background where gelatin has remained intact.
Immunoblot
Purified recombinant strongylastacin was separated by 10% NuPAGE Bis Tris polyacrylamide gels (Invitrogen) at 70 milliamps for 40 min (200 volts constant) and transferred to nitrocellulose membrane under 25 volts (constant) for 90 min. Blots or strips were blocked overnight with 5% non‐fat milk powder in TBST (0.1% Tween 20) at 4°C. Blots or strips were washed three times in TBST, each wash for 5 min. For the IgG immunoblot, the test sera were diluted to 1:200 times in TBST containing 3% skim milk powder. Pooled S. stercoralis infected sera at 1:500 dilution was used as positive control and pooled normal sera at 1:200 as negative control (1: 200). After washing, strips were incubated separately with 1:5000 times diluted goat anti human (Fc specific, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) conjugated to alkaline phosphatase. Blots were developed with an alkaline phosphatase substrate (Promega, Madison WI, USA). For IgE detection, IgG was depleted to enrich IgE antibody by incubating 1:4 times diluted sera with protein G coupled Sepharose overnight at 4°C as described by the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Each sample of this IgG depleted serum was diluted one fold (1:8) in TBST (0.1% Tween 20) containing 3% skim milk powder and incubated with strips for one hour at room temperature. After washing, blots were incubated with 1:4000 times diluted goat anti human IgE (Kirkegaard & Perry, Gaithersburg, MD, USA) conjugated to alkaline phosphatase at room temperature for two hours. Blots were developed with alkaline phosphatase substrate (Promega) after washing with TBST.
Enzyme linked immunosorbent assay
An ELISA was used to measure the specific IgG antibodies to strongylastacin antigen in pooled sera from different groups of individuals. Thirty‐two normal sera were from the NIH blood bank, and 32 S. stercoralis infected sera were from stool‐positive individuals who were originally from South‐East Asia. Briefly, Immulon 2HB plates (Dynex Technologies, Chantilly, VA, USA) were coated overnight with purified recombinant strongylastacin at a concentration of 2.5 μg/mL in 0.05 M carbonate buffer (pH 9.6). Wells were blocked for 1 hr with 5% non‐fat milk in phosphate‐buffered saline containing 0.1% Tween 20. The wells were washed in PBST and a goat anti‐human IgG (Fc fragment‐specific, Jackson ImmunoResearch Laboratories) alkaline phosphatase conjugate was added at 1:5000 dilutions in PBST and incubated for 1 hr at 37°C. Plates were washed and measured at 405 nm (Dynatech MR 5000, Dynatech, Chantilly, VA, USA) after being developed with paranitrophenol phosphate (pNPP, Kirkegaard and Perry, Gaithersburg, MD, USA). The standard positive serum pool containing nine high titered antibodies to S.stercoralis somatic antigen was always tested in duplicate at twofold dilutions ranging from 1:16 to 1:1024 to establish a curve of reactivity. A standard antibody‐negative pool of serum was always tested at dilutions of 1:16, 1:32 and 1:64. Also the test sera were serially diluted to 1:16, 1:32 and 1:64 in TBST containing 5% skim milk (in duplicate) and incubated for 1 hr at 37°C. The starting dilution of 1:16 of the positive serum pool in standard curve construction was assigned an arbitrary unit value of 1000 U/mL. Arbitrary unit values for each of the test sera were obtained by interpolation of the standard curve. For IgE ELISA, IgG antibodies were depleted to enrich IgE antibody by incubating 1:4 times diluted sera with protein G coupled Sepharose overnight at 4°C as described by the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Recombinant strongylastacin antigen‐coated 96 well plates were used as described for IgG ELISA. Each sample of of this IgG depleted serum was diluted 1:10 times in TBST (0.1% Tween 20) containing 3% skim milk powder and incubated at 37°C for an hour. After washing, blots were incubated with 1:8000 times diluted goat anti human IgE (Kirkegaard & Perry) conjugated to alkaline phosphatase at 37°C for 1 hr. Plates were washed and measured at 405 nm (Dynatech MR 5000) after developing with pNPP.
Source of patient sera
Parasite‐positive sera were obtained from patients in whom S. stercoralis larvae had been demonstrated in fecal specimens within the month prior to being tested for the presence or absence of antibody. Most of these positive test samples were collected during 1991 from South‐East Asian immigrants who had settled in the Lowell area of Massachusetts, USA. A pool of nine high titered sera was used as a positive control in ELISA. A negative control serum pool was made from eight patients seen at the NIH Clinical Center for other illnesses such as cutaneous leishmaniasis and unexplained eosinophilia. The strongyloides‐negative sera used to screen the strongylastacin for reactivity came from normal blood donors at the NIH blood bank. Sera were collected from TPE patients, a disease caused by the filarial parasite W. bancrofti. These patients have an immunologically hyper‐responsive syndrome associated with high concentrations of antifilarial IgE antibodies and eosinophilia.
Statistical analysis
The significance of variance between control and experimental groups were determined by a Mann–Whitney U test using a StatView program (SAS Institute, Cary, NC, USA).
RESULTS
Strongylastacin protein expression, purification and Western blot
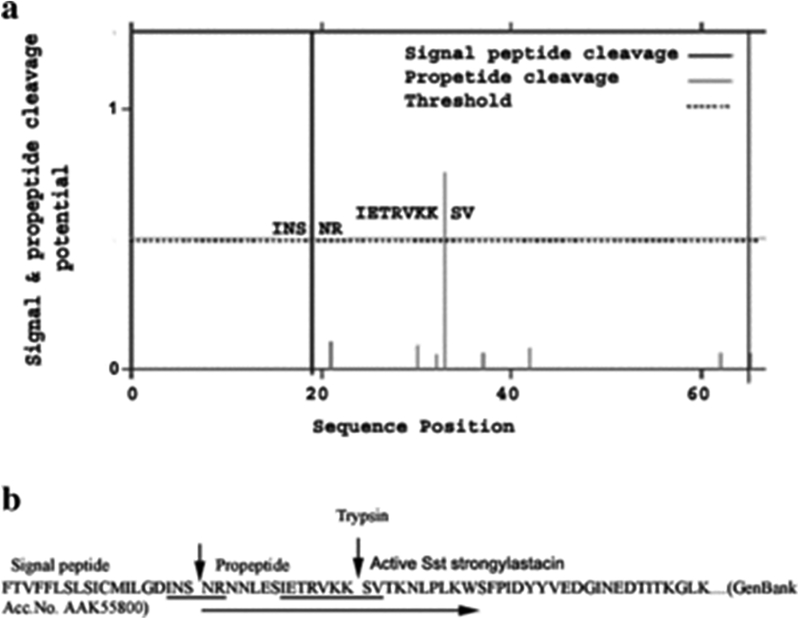
Panels A and B of Figure 1 show that the amino acid residues of the signal peptide (INS‐NR) and propeptide (IETRVKK‐SV) cleavage sites were predicted well above the threshold line by proP server (25, 26). Based on the predicted result, a forward PCR primer was designed to express strongylastacin protein with the propeptide sequence lacking its signal sequence in E. coli (Fig. 1 panel B). The strongylastacin cDNA (AF118022) of 1074 bp encoding 358 amino acids was cloned into the pET expression system (Invitrogen). Recombinant metalloproteinase strongylastacin with N‐terminal 6‐Histidine tag was expressed in E. coli after inducing with IPTG. Subsequently the 45 kDa strongylastacin was purified using Ni‐NTA column (Fig. 2 panel A). The recombinant strongylastacin protein was reactive with monoclonal anti His tag (Clontech, Palo Alto, CA, USA) antibody and with pooled S. stercoralis infected sera (data not shown). To obtain active strongylastacin protein, we used E. coli Origami (DE3) pLys S as the host bacteria to facilitate disulfide bond formation (27).
Figure 1.
ProP server program predicted the locations of signal peptide and ProPeptide cleavage sites in amino acid sequence at N‐terminal portion of strongylastacin of S. stercoralis. Panel (a) The plot shows that the signal peptide specific cleavage site, indicated by a strong vertical line, passes across amino acid residues S (serine) and N (asparagine). Amino acid residues of propeptide specific cleavage site are shown by a grey vertical line which passes between amino acids K (lysine) and S (serine) residues well above the dotted horizontal threshold line. Panel (b) Underlined amino acid sequence of strongylastacin (GenBank accession AAK55800) shows the protease recognition sites specific to signal and propeptide residues (indicated by vertical arrows). Recombinant strongylastacin 45 kDa protein expressed in E. coli with a propeptide without signal sequence is shown by a long arrow under the amino acid sequence.
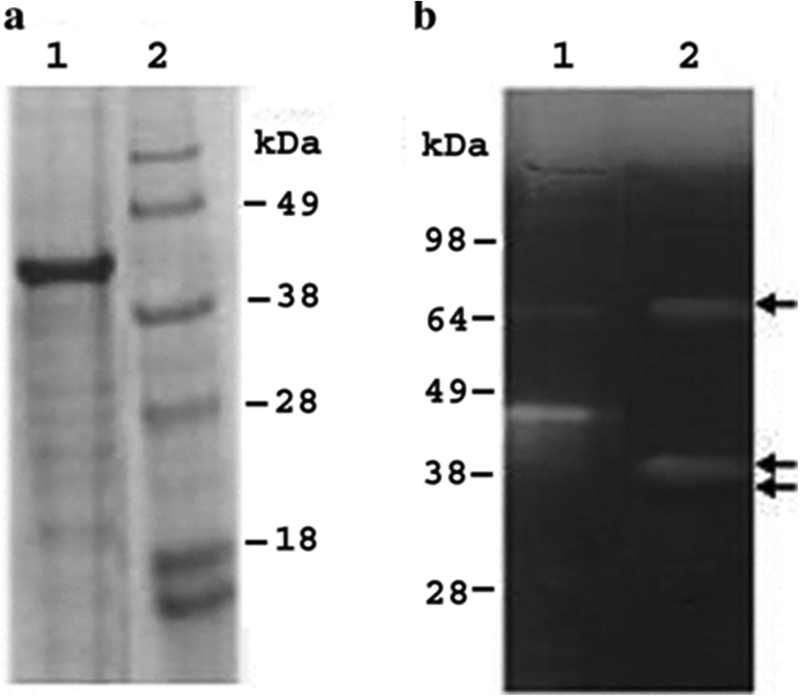
Figure 2.
SDS‐PAGE and zymogram analysis of recombinant strongylastacin. Panel (a) Recombinant bacteria expressed strongylastacin with His tag at the N‐ terminal was purified using Ni2+ charged agarose. The sample was separated by SDS‐PAGE and stained with Coomassie brilliant blue. Lane 1, purified strongylastacin at 45 kDa, Lane 2, molecular weight marker. Panel (b) Zymogram showing gelatinolytic activity of recombinant strongylastacin subjected to limited trypsin digestion. Lane 1, SeeBlue pre‐stained protein molecular weight marker (Invitrogen) showing gelatinolytic activity due to protease contamination. Lane 2, two bands of gelatinolytic activity at 39 kDa and 68 kDa. The 39 kDa is a monomer and 68 kDa might be a dimer. An inactive third band at approximately 37 kDa is not clearly visible. Arrows indicate bands of gelatinolytic activity.
Zymogram
In order to determine the activity of purified strongylastacin, we tested the enzymatic activity in a zymogram gel incorporated with gelatin. Limited trypsin digestion of the recombinant strongylastacin precursor of 45 kDa resulted in both activation of the enzyme and removal of its prosequence. Panel B of Figure 2 shows strong gelatinolytic activity at ~39 kDa and its dimer at ~68 kDa. The recombinant strongylastacin has both an active, and a smaller inactive, band at ~37 kDa (indicated by an arrow since it is not clearly visible in the figure). This experiment suggests that purified strongylastacin exists in three forms; an active dimer formed due to intermolecular disulfide bonds, and an active and an inactive monomer.
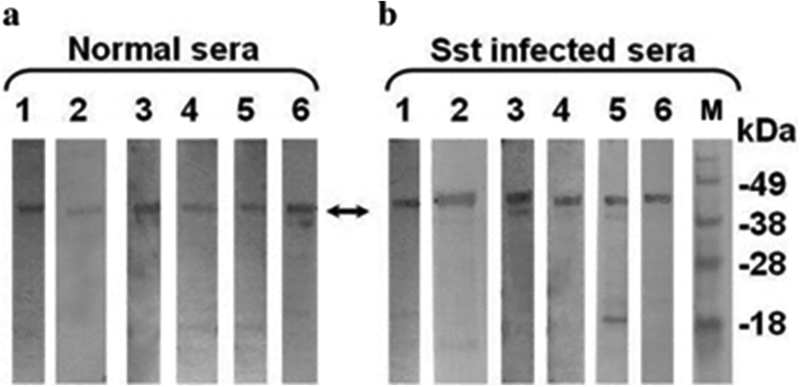
Immunoblot screening of human IgG sera with recombinant strongylastacin
IgG antibodies of six S. stercoralis infected and six normal North American sera from the NIH blood bank were reactive with the 45 kDa protein (Fig. 3, panels A and B). To raise rabbit antibodies against recombinant strongylastacin, we collected five pre‐immunized rabbit sera in order to choose a suitable rabbit for strongylastacin immunization. However, all five rabbit sera were reactive with recombinant antigen at various serum dilutions (not shown). This indicates that anti‐strongylastacin antibodies are naturally present in control rabbits. This finding corroborates our observations that anti‐strongylastacin IgG antibodies are present in strongyloides uninfected North American individuals.
Figure 3.
Detection of strongylastacin specific IgG antibodies in sera of normal donors and S. stercoralis infected patients. Affinity purified strongylastacin antigen was resolved by using NuPAGE gel electrophoresis; strips were prepared after transfer of protein from gel to nitrocellulose. Panel (a) strips of nitrocellulose membrane containing the strongylastacin were probed with 1:100 times diluted human sera from each of six normal individuals or, Panel (b) each of six S. stercoralis infection sera. Molecular mass standards (lane M) are shown at the right hand side of the immunoblots. Sst, S. stercoralis.
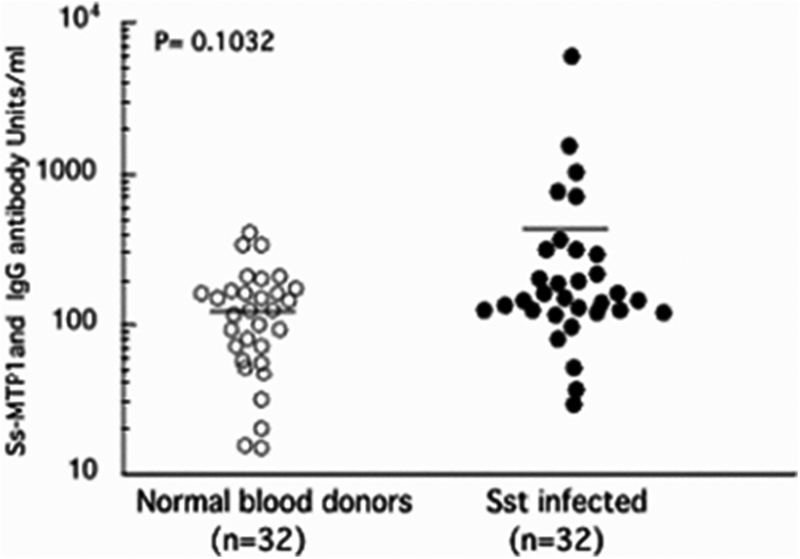
IgG ELISA of human sera with recombinant strongylastacin
Because strongylastacin antigen was recognized by IgG antibodies from both S. stercoralis infected and normal North American human sera, we further investigated the titer of anti‐strongylastacin antibodies in S. stercoralis infected and uninfected control sera. Samples from 32 individuals with parasitologically confirmed S. stercoralis infections were tested against the recombinant strongylastacin by ELISA. All strongyloidiasis sera had previously been found to have significantly increased IgG concentrations to S. stercoralis L3 larval stage somatic extract and were positive to the NIE antigen from S. stercoralis by ELISA (28). Similarly, 32 normal sera representing the Strongyloides negative population were obtained from the NIH blood bank. The negative status of infection for all individual sera in this population was confirmed by serological assays targeting S. stercoralis antigens (28). Intriguingly, both Strongyloides infected and uninfected sera showed the presence of anti‐strongylastacin antibodies. There was no statistically significant difference between S. stercoralis infected and normal uninfected human population in anti‐strongylastacin IgG antibody concentrations (Fig. 4). Thus, both immunoblot and ELISA results confirmed that IgG antibodies specific for strongylastacin occur naturally in humans and rabbits, even in the absence of infection with S. stercoralis.
Figure 4.
Distribution of IgG antibody levels to purified strongylastacin antigen as determined by ELISA in sera from 32 (uninfected) normal and 32 S. stercoralis infected individuals. The mean values of two groups are not significantly different (P, 0.1032). Sst, S. stercoralis.
Strongylastacin specific IgE antibody in human sera by Western blot and ELISA assay
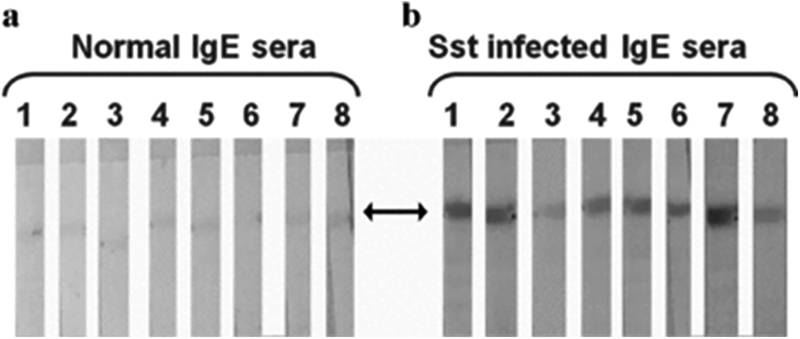
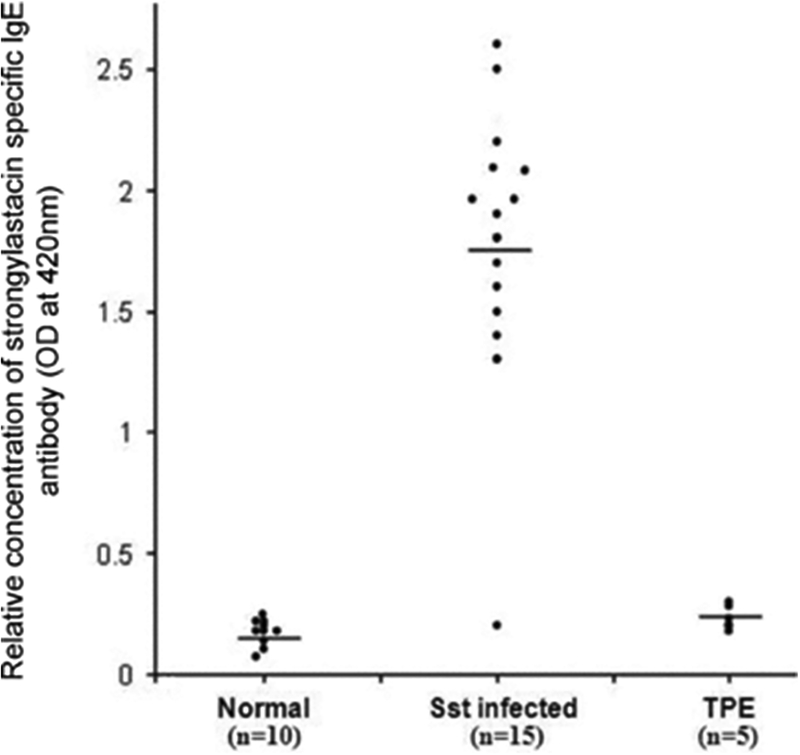
To determine whether the IgE isotype conferred specificity to strongylastacin, we performed IgE immunoblots with S. stercoralis infected sera. Each of these showed distinct reactivity compared to normal sera (Fig. 5). For further confirmation, we performed IgE ELISA against strongylastacin (Fig. 6). Fourteen of 15 (93%) S. stercoralis infected human sera, but none of the normal (n= 10) and TPE (n= 5) sera, reacted with strongylastacin. Moreover the concentrations of IgE to strongylastacin in the S. stercoralis infected group was significantly increased compared to both the normal (P < 0.0001, Mann–Whitney U‐test) and the TPE (P < 0.0001, Mann–Whitney U‐test) groups.
Figure 5.
Western blot analysis of IgE antibodies in sera from control and S. stercoralis infected patients probed with purified strongylastacin antigen. Purified strongylastacin antigen was separated on NuPAGE gel and strips were made after transferring protein from the gel to the nitrocellulose membrane. Both normal and S. stercoralis infected human sera were pre‐absorbed with protein G sepharose to enrich IgE antibodies. Strips were separately incubated with 1:8 times diluted sera for 1 hr. Blots were stained with a second antibody labeled with goat anti‐ human IgE antibody conjugated to alkaline phosphatase. Panel (a) none of the eight normal sera reacted. Panel (b) IgE antibodies in seven of eight strongly reacted with strongylastacin and one showed weak reactivity. Sst, S. stercoralis.
Figure 6.
Distribution of IgE antibody levels to strongylastacin as determined by ELISA. The sera analyzed were from 10 normal individuals, 15 S. stercoralis infected individuals and five W. bancrofti infected patients with symptoms of tropical TPE. Strongylastacin specific IgE antibodies were significantly greater in S. stercoralis infected patients compared to normal blood donors and TPE patients (P < 0.0001; Mann–Whitney U‐test). There was no statistically significant difference between normal donors and TPE patients.
DISCUSSION
Our previous studies showed that metalloproteases are present in excretory/secretory products of infective S. stercoralis larvae and that these metalloproteases play an important role in larval penetration of the host body (4, 22, 29). Recently it was shown that astacin from the S. stercoralis parasite contains multi‐domains such as a signal peptide, propeptide, protease, EGF‐1 like and CUB domains (22). These domains are found in the majority of the astacin protein family with a Zn2+ binding motif, whereas the number of EGF‐1 like and CUB domains varies among species, or orthologs of the same species (22). The CUB and EGF motifs are involved in substrate binding and required for proper folding and secretion of the enzyme to the extracellular compartment (30, 31). In the current study, the immunoblots revealed the presence of IgG antibodies to strongylastacin in all individuals, irrespective of S. stercoralis infection status. Similar results obtained in normal rabbits housed in a well‐maintained laboratory (data not shown) suggest that the strongylastacin IgG antibody is ubiquitous. Indeed IgG specific ELISA with recombinant strongylastacin confirmed the presence of antibodies in all 32 normal and 32 S. stercoralis infected human sera (Fig. 4). Titers of IgG antibody were not statistically significant between these two groups. These antibodies may result from zinc metalloproteases, including astacin‐like enzymes in food, for example shellfish or mushrooms ingested by humans (and indeed laboratory rabbits) and/or in the normal gut biota. In any event, these naturally occurring anti‐strongylastacin antibodies may neutralize the strongylastacin of invading L3 S. stercoralis parasites, thus protecting host proteins from proteolysis. Previously, we showed the presence of antibodies to recombinant heat shock protein 70 (HSP70) of W. bancrofti in sera of patients infected with this organism (i.e. microfilaremic patients) as well as in endemic normal individuals and in control North American volunteers who had never been exposed to this pathogen (32). Our studies established that strongylastacin‐specific IgE antibodies are present in S. stercoralis infected individuals but not in control (normal) individuals, suggesting that active infection elicits an IgE response to strongylastacin (Figs 4 and 5). It is possible that protection by IgE antibodies (33) is disabled by naturally found IgG antibodies that compete for the same epitope (34). Dogs vaccinated with the hookworm enzyme Ac‐MTP‐1, an ortholog of astacin from A. caninum, were significantly protected against subsequent challenge with hookworm larvae compared to unvaccinated dogs. It is pertinent to note that this report also demonstrated the presence of Ac‐MTP‐specific IgE antibodies in the vaccinated dogs (17). In like fashion, vaccination of hamsters with a cocktail of two antigens, the hookworm aspartic protease Ay‐ASP‐2 and the A. ceylanicum hookworm ortholog of strongylastacin, Ay‐MTP‐1, reduced worm burden and egg counts to 43% and 59% respectively, and significantly improved hemoglobin values and body weights compared to either each antigen singly or adjuvant alone (18). In contrast, recombinant astacin expressed in insect cells from the strongylid nematode Ostertagia ostertagi failed to protect vaccinated calves against parasite challenge (35). Nonetheless, based on the results presented here and these published reports, we propose that naturally occurring antibodies have a dual role that confers protection by neutralizing parasite proteases to protect host proteins from degradation, and these cross reactive IgG antibodies may block the protection otherwise provided by S. stercoralis specific IgE antibodies.
Contributor Information
Ravi Varatharajalu, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892.
Vijayalakshmi Parandaman, Department of Bioinformatics, Johns Hopkins University, Shady Grove Campus, Maryland 20878; USA.
Momar Ndao, National Reference Laboratory for Parasitology, McGill University, Montreal Quebec, Canada.
John F. Andersen, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892
Franklin A. Neva, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892
REFERENCES
- 1.Hishida R, Ishihara T, Kondo K, Katsura I (1996) Hch‐1, a gene required for normal hatching and normal migration of a neuroblast in C. elegans, encodes a protein related to TOLLOID and BMP‐1. EMBO J 15: 4111–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki M, Sagoh N, Iwasaki H, Inoue H, Takahashi K (2004) Metalloproteases with EGF, CUB, and thrombospondin‐1 domains function in molting of Caenorhabditis elegans. Biol Chem 385: 565–8. [DOI] [PubMed] [Google Scholar]
- 3.McKerrow JH, Keene WE, Jeong K, Werb Z (1983) Degradation of extracellular matrix by larvae of Schistosoma mansoni. I. Degradation by cercariae as a model for initial parasite invasion of host. Lab Invest 49: 195–200. [PubMed] [Google Scholar]
- 4.McKerrow JH, Brindley P, Brown M, Gam AA, Staunton C, Neva FA (1990) Strongyloides stercoralis: identification of a protease that facilitates penetration of skin by the infective larvae. Exp Parasitol 70: 134–43. [DOI] [PubMed] [Google Scholar]
- 5.Fetterer RH, Rhoads ML (1997) Extracellular matrix: A tool for defining the extracorporeal function of parasite proteases. Parasitol Today 13: 119–22. [DOI] [PubMed] [Google Scholar]
- 6.Haffner A, Guilavogui AZ, Tischendorf FW, Brattig NW (1998) Onchocerca volvulus: microfilariae secrete elastinolytic and males nonelastinolytic matrix‐degrading serine and metalloproteases. Exp Parasitol 90: 26–33. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji N, Miyoshi T, Islam MK, Isobe T, Yoshihara S, Arakawa T, Matsumoto Y, Yokomizo Y (2004) Recombinant Ascaris 16‐Kilodalton protein‐induced protection against Ascaris suum larval migration after intranasal vaccination in pigs. J Infect Dis 190: 1812–20. [DOI] [PubMed] [Google Scholar]
- 8.Semerene AR, Lino Rde S Jr., Oliveira JA, Magalhaes AV, Stefani MM, Barbosa AP, Campos DM (2004) Experimental lagochilascariosis: histopathological study of inflammatory response to larval migration in the murine model. Mem Inst Oswaldo Cruz 99: 393–8. [DOI] [PubMed] [Google Scholar]
- 9.Williamson AL, Lustigman S, Oksov Y, Deumic V, Plieskatt J, Mendez S, Zhan B, Bottazzi EL, Hotez PJ, Loukas A (2006) Ancylostoma caninum MTP‐1, an astacin‐ like metalloprotease secreted by infective hookworm larvae, is involved in tissue migration. Infect Immun 74: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semnani RT, Law M, Kubofcik J, Nutman TB (2004) Filaria‐induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host‐parasite interface. J Immunol 172: 6229–38. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, Sher A (2002) Evasion of innate immunity by parasitic protozoa. Nat Immunol 3: 1041–7. [DOI] [PubMed] [Google Scholar]
- 12.Suchitra S, Joshi P (2005) Characterization of Haemonchus contortus calreticulin suggests its role in feeding and immune evasion by the parasite. Biochim Biophys Acta 1722: 293–303. [DOI] [PubMed] [Google Scholar]
- 13.Morgan ER, Shaw SE, Brennan SF, De Waal TD, Jones BR, Mulcahy G (2005) Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol 21: 49–51. [DOI] [PubMed] [Google Scholar]
- 14.Bertenshaw GP, Turk BE, Hubbard SJ, Matters GL, Bylander JE, Crisman JM, Cantley LC, Bond JS (2001) Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J Biol Chem 276: 13,248–55. [DOI] [PubMed] [Google Scholar]
- 15.Butler PE, McKay MJ, Bond JS (1987) Characterization of meprin, a membrane‐ bound metalloendopeptidase from mouse kidney. Biochem J 241: 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villa JP, Bertenshaw GP, Bond JS (2003) Critical amino acids in the active site of meprin metalloproteinases for substrate and peptide bond specificity. J Biol Chem 278: 42,545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotez PJ, Ashcom J, Zhan B, Bethony J, Loukas A, Hawdon J, Wang Y, Jin Q, Jones KC, Dobardzic A, Dobardzic R, Bolden J, Essiet I, Brandt W, Russell PK, Zook BC, Howard B, Chacon M (2003) Effect of vaccination with a recombinant fusion protein encoding an astacin like metalloprotease (MTP‐1) secreted by host‐ stimulated Ancylostoma caninum third‐stage infective larvae. J Parasitol 89: 853–5. [DOI] [PubMed] [Google Scholar]
- 18.Mendez S, Zhan B, Goud G, Ghosh K, Dobardzic A, Wu W, Liu S, Deumic V, Dobardzic R, Liu Y, Bethony J, Hotez PJ (2005) Effect of combining the larval antigens Ancylostoma secreted protein 2 (ASP‐2) and metalloprotease 1 (MTP‐1) in protecting hamsters against hookworm infection and disease caused by Ancylostoma ceylanicum. Vaccine 23: 3123–30. [DOI] [PubMed] [Google Scholar]
- 19.Coombs GH, Mottram JC (1997) Parasite proteinases and amino acid metabolism: possibilities for chemotherapeutic exploitation. Parasitology 114 Suppl: S61–80. [PubMed] [Google Scholar]
- 20.Todorova VK (2000) Proteolytic enzymes secreted by larval stage of the parasitic nematode Trichinella spiralis. Folia Parasitol (Praha) 47: 141–5. [DOI] [PubMed] [Google Scholar]
- 21.Hotez P, Haggerty J, Hawdon J, Milstone L, Gamble HR, Schad G, Richards F (1990) Metalloproteases of infective Ancylostoma hookworm larvae and their possible functions in tissue invasion and ecdysis. Infect Immun 58: 3883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallego SG, Loukas A, Slade RW, Neva FA, Varatharajalu R, Nutman TB, Brindley PJ (2005) Identification of an astacin‐like metallo‐proteinase transcript from the infective larvae of Strongyloides stercoralis. Parasitol Internat 54: 123–133. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GD, Bond JS (1997) Activation mechanism of meprins, members of the astacin metalloendopeptidase family. J Biol Chem 272: 28126–32. [DOI] [PubMed] [Google Scholar]
- 24.Moore TA, Ramachandran S, Gam AA, Neva FA, Lu W, Saunders L, Williams SA, Nutman TB (1996) Identification of novel sequences and codon usage in Strongyloides stercoralis. Mol Biochem Parasitol 79: 243–8. [DOI] [PubMed] [Google Scholar]
- 25.Duckert P, Brunak S, Blom N (2004) Prediction of proprotein convertase cleavage sites. Protein Eng Des and Select 17: 107–112. [DOI] [PubMed] [Google Scholar]
- 26.Bendtsen JD, Nielsen H, Gunnar von Heijne, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795. [DOI] [PubMed] [Google Scholar]
- 27.Di Lorenzo M, Hidalgo A, Haas M, Bornscheuer UT (2005) Heterologous production of functional forms of Rhizopus oryzae lipase in Escherichia coli. Appl Environ Microbiol 71: 8974–8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA (2002) Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3‐ stage larvae. Mol Biochem Parasitol 125: 73–81. [DOI] [PubMed] [Google Scholar]
- 29.Brindley PJ, Gam AA, McKerrow JH, Neva FA(1995) Ss40: the zinc endopeptidase secreted by infective larvae of Strongyloides stercoralis. Exp Parasitol 80: 1–7. [DOI] [PubMed] [Google Scholar]
- 30.Sieron AL, Tretiakova A, Jameson BA, Segall ML, Lund‐Katz S, Khan MT, Li S, Stöcker W (2000) Structure and function of procollagen C‐proteinase (mTolloid) domains determined by protease digestion, circular dichroism, binding to procollagen type I, and computer modeling. Biochemistry 39: 3231–9. [DOI] [PubMed] [Google Scholar]
- 31.Garrigue‐Antar L, François V, Kadler KE. (2004) Deletion of epidermal growth factor‐ like domains converts mammalian tolloid into a chordinase and effective procollagen C‐ proteinase. J Biol Chem 279: 49835–41. [DOI] [PubMed] [Google Scholar]
- 32.Ravi V, Kubofcik J, Bandopathyaya S, Geetha M, Narayanan RB, Nutman TB, Kaliraj P (2004) Wuchereria bancrofti: cloning and characterization of heat shock protein 70 from the human lymphatic filarial parasite. Exp Parasitol 106: 1–10. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad A, Wang CH, Bell RG (1991) A role for IgE in intestinal immunity. Expression of rapid expulsion of Trichinella spiralis in rats transfused with IgE and thoracic duct lymphocytes. J Immunol 146: 3563–3570. [PubMed] [Google Scholar]
- 34.Bell RG (1996) IgE, allergies and helminth parasites: A new perspective on an old conundrum. Immunol Cell Biol 74: 337–345. [DOI] [PubMed] [Google Scholar]
- 35.De Maere V, Vercauteren I, Geldhof P, Gevaert K, Vercrysse J, Claerebout E (2005) Molecular analysis of astacin‐like metalloproteases of Ostertagia ostertagi. Parasitology 130: 89–98. [DOI] [PubMed] [Google Scholar]