Abstract
Objectives
Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Trichomonas vaginalis (TV) are curable, mostly asymptomatic, sexually transmitted infections (STIs) that cause adverse maternal and perinatal outcomes. Most countries do not test for those infections during antenatal care. We implemented a CT, NG, and TV testing and treatment program in an antenatal clinic in Gaborone, Botswana.
Methods
We conducted a prospective study in the antenatal clinic at Princess Marina Hospital in Gaborone, Botswana. We offered pregnant women who were 18 years or older and less than 35 weeks gestation, CT, NG, and TV testing using self-collected vaginal swabs. Testing was conducted using a GeneXpert® CT/NG and TV system. Those who tested positive were given directly observed antibiotic therapy and asked to return for a test of cure. We determined the prevalence of infections, uptake of treatment, and proportion cured. The relationships between a positive STI test and participant characteristics were assessed.
Results
We enrolled 400 pregnant women. Fifty-four (13.5%) tested positive for CT, NG, and/or TV: 31 (8%) for CT, five (1.3%) for NG, and 21 (5%) for TV. Among those who tested positive, 74% (40) received same-day, in person results and treatment. Among those who received delayed results (6), 67% (4) were treated. Statistical comparisons showed that being unmarried and HIV-infected were positively association CT, NG, and/or TV infection. Self-reported STI symptoms were not associated with CT, NG, and/or TV infection.
Conclusion
The prevalence of CT, NG, and/or TV was high, particularly among women with HIV infection. Among women with CT, NG, and/or TV infection, those who received same-day results were more likely to be treated than those who received delayed results. More research is needed on the costs and benefits of integrating highly sensitive and specific STI testing into antenatal care in Southern Africa.
Background
Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Trichomonas vaginalis (TV) infections during pregnancy increase the risk of adverse pregnancy and infant outcomes, including preterm birth.(1) Co-infection with HIV and CT/NG is associated with an increased risk of mother-to-child transmission of human immunodeficiency virus (HIV).(2) Infection with TV is associated with increased risk for HIV acquisition.(3) About 50% of untreated maternal CT and NG infections are transmitted to the neonate during birth.(4, 5) Both infections can cause the eye infection, ophthalmia neonatorum, and untreated gonococcal eye infections can cause blindness.(4) Chlamydial infections are an important cause of neonatal pneumonia as 5–30% of infants born to mothers with CT infection can develop this condition.(4) Infants of mothers infected with TV may contract the infection during delivery, which could result in fever, respiratory problems, and urinary tract infections.(6, 7)
Despite serious risks to maternal and infant health, the prevalence and risk factors for these infections in Southern Africa (including Angola, Botswana, Lesotho, Madagascar, Malawi, Mozambique, Namibia, South Africa, Swaziland, Zambia, and Zimbabwe) are not precisely measured.(8). A recent systematic review on STI prevalence among pregnant women in low- and middle-income countries found only 15 studies based in Southern Africa.(8)
The lack of evidence for the burden of STIs during pregnancy in Southern Africa may be due to the absence of diagnostic testing during antenatal care. A recent study that used both online data and Ministry of Health surveys found that only 14 countries have policies for antenatal screening for CT and/or NG, including: Australia, the Bahamas, Bulgaria, Canada, Estonia, Japan, Germany, Latvia, New Zealand, Democratic People’s Republic of Korea, Romania, Sweden, the UK, and the USA.(9) Forty-three countries did not have antenatal CT/NG testing policies and 139 countries’ policies were unknown.(9) Most countries identify and manage CT, NG, and TV infections using an approach called syndromic management, which was developed for use in countries where laboratory diagnosis was not accessible.(10) The approach uses algorithms to classify symptoms (e.g. abnormal vaginal discharge) into STI syndromes (e.g. vaginal discharge syndrome), which are treated with standardized drug regimens.(10) Diagnosis based on symptoms lacks sensitivity because a large proportion of CT, NG, and TV infections are asymptomatic.(11, 12) For example, a study that assessed the sensitivity and specificity of diagnostic strategies for identifying CT and/or NG among pregnant women in Botswana found that using symptoms alone (including vaginal discharge and/or lower abdominal pain) had a sensitivity of 21% and specific of 78%.(11) Romoren et al. 2004, found the specificity and sensitivity of abnormal vaginal discharge for diagnosing TV was 61% and 74% respectively.(13) A recent South African study of 1,480 women found that more than 50% of CT and other STIs were asymptomatic.(14)
Recent developments in technology have introduced the possibility that antenatal testing for CT, NG, and TV infections will become more accessible with highly sensitive, easy to use, rapid tests. For example, several nucleic acid amplification testing (NAAT)-based platform are in development or newly available for diagnosis of STIs at the point-of-care.(15) These new testing systems have several benefits that may make them more accessible in low and middle income countries including: multi-plex assays that can test for several infections, highly trained laboratory technicians are not required, samples can be self-collected, and samples can be processed on-site thus reducing storage and shipping costs.(15)
Botswana has achieved high levels of antenatal care coverage with over 94% of pregnant women receiving at least one visit, and universal screening for HIV and syphilis are integrated into antenatal care.(16, 17) Nevertheless, Botswana faces high rates of adverse birth outcomes, including preterm birth (15/100 live births) and infant mortality (36/1,000 live births).(18, 19) The absence of diagnostic testing for curable STIs during pregnancy may represent a missed opportunity to reduce the burden of infection and poor maternal outcomes. Our study implemented an antenatal CT, NG, and TV testing and treatment program in a high volume antenatal clinic in Gaborone, Botswana, and assessed the uptake of testing and treatment, the prevalence and correlates of CT, NG, and TV infections, and the proportion of infections cured.
Methods
We conducted a prospective study among 400 consecutively enrolled pregnant women in the antenatal clinic in Princess Marina Hospital in Gaborone, Botswana. Pregnant women attending the antenatal clinic between July 2015 and May 2016, who were over age 18 years, less than 35 weeks of gestation (to ensure the possibility of a test of cure after four weeks prior to birth), and planning to return to the clinic for a follow-up visit were offered testing for CT, NG, and TV infections. With a sample size of 400, a two-sided 95% confidence interval using normal approximation for the assumed prevalence of 13%(11) ranges from 9.9% to 16.7%, which should be narrow enough to give sufficient precision (within half the estimate value on each side) for the estimate of prevalence. Princess Marina Hospital is located in Botswana’s capital city, and is the main referral hospital for southern Botswana. Participants who gave written informed consent provided self-collected vaginal swab specimens after receiving verbal instructions from study staff. Samples were collected in the clinic washroom by participants. Following sample collection, study staff reviewed participants’ obstetric records to collect information related to sociodemographic characteristics (e.g. age, marital status, and education level), obstetric history (e.g. prior pregnancies and births), and HIV and syphilis testing and results. Participants also responded to an interviewer-administered questionnaire conducted in English or Setswana (the language spoken by the majority of people in Botswana), which collected behavioural information (e.g. condom use) and whether participants had been previously diagnosed and/or treated for an STI during the current pregnancy. We also asked if participants were experiencing any symptoms that could be related to having an STI, including abnormal vaginal discharge, painful urination, and/or lower abdominal pain. We chose these symptoms because they are currently used in Botswana’s syndromic management algorithm to identify STIs.(20) Testing and treatment were free of charge and participants were not offered incentives for enrollment in the study.
Testing was conducted by trained study staff using the U.S. Food and Drug Administration-approved Xpert® CT/NG and Xpert® TV nucleic acid amplification assays (Cepheid, Sunnyvale, CA). Xpert allows for 90-minute detection for CT and NG infections and 59 minutes for TV infection.
The goal of the study was to provide participants with results in person on the same day as testing. If a participant left prior to receipt of results, they were to be called and advised to return to the clinic for treatment if necessary. Those who tested positive for CT, NG, and/or TV were offered directly observed treatment. We followed U.S. Centers for Disease Control and Prevention (CDC) treatment guidelines, which included directly observed therapy of 1g oral azithromycin for chlamydial infection, a 250 mg intramuscular injection of ceftriaxone and 1g oral azithromycin for gonococcal infection, and 2g oral metronidazole for trichomonal infection.(21) Participants who were HIV infected were given 400mg of metronidazole twice per day for 7 days.(22) Participants who tested positive were counselled to tell their partner(s) and abstain from sex for seven days, given the option of bringing their partner(s) into the study clinic for treatment, and provided with a contact sheet with instructions for their partners to receive treatment at a clinic of their choosing. Finally, those who tested positive were encouraged to return to the clinic for a follow-up visit, but no sooner than four weeks, for a test of cure. The test of cure is needed to assess persistent infection despite treatment, and the CDC guidelines recommend the test of cure take place 3–4 weeks after treatment completion for pregnant women.(21)
Descriptive statistics were used to characterize sociodemographics and obstetric and medical characteristics of participants. Bivariate comparisons, including chi-square tests of proportions, Fisher’s exact test, Student’s t-tests, and Mann-Whitney U tests were used to examine the relationships between participant characteristics and STI diagnosis. Finally, multivariable logistic regression was used to identify characteristics independently associated with having an STI, adjusting for other factors. Variables were included based on results of stepwise selection for logistic regression analyses, and covariates with a significance level of ≤0.2 were included in the model. Statistical significance of variables in the final model was assessed at the 0.05 level. Stata 13 (StataCorp, College Station, TX) was used for all analyses.
The institutional review boards at the University of Botswana (URB/IRB/1547), the Botswana Ministry of Health, Health Research Development Committee (PPME 13/18/1 IX(434)); and Princess Marina Hospital (PMH 5/79(223-3-2016)) approved the study protocol. The University of California, Los Angeles (15-000692), approved analyses using de-identified data. Cepheid loaned testing equipment and donated the testing reagents used in this study
Results
Between July 2015 and May 2016, we enrolled 400 (86%) of the 466 eligible women recruited (Figure 1). Among those who gave a reason for refusing participation (n=38), the most common reason for refusal was concern about the time it would take to collect the sample (16), 13% (5) said they did not want additional testing, and 11% (4) said they were afraid of the results. The remainder gave no reason or said that they needed to think about it. The ages and gestational ages of women who enrolled and those who declined did not differ (The median age for both those who enrolled and refused was 30 years. The median gestational age for both those who enrolled and refused was 26 weeks). Among those enrolled, we were able to provide results to 99% (399/400) of participants either in person (60%; 240/399) on the same day as testing or by phone (39%; 159/399) within a week.
Figure 1.
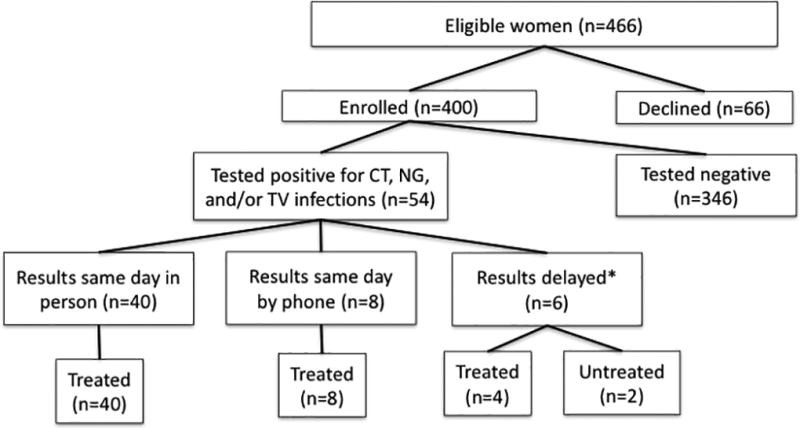
Flow of eligible women and participants in a Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infection testing and treatment study in Gaborone, Botswana.
Note:* Delayed results includes one participant who did not receive results.
As displayed in Table 1, the median age of our sample was 30 years and most women (77%) were unmarried. The HIV prevalence was 23% and among the 319 women with a documented syphilis test, 2 (0.6%) were reactive. Twenty-seven percent of participants reported never using condoms during the three months prior to trying to get pregnant or becoming pregnant; and 47% reported never using condoms during pregnancy. The median number of previous pregnancies was one and 65% had a prior birth. Among those who had given birth, 64 (25%) had experienced at least one prior preterm birth. In terms of current symptoms, 41% reported that they were experiencing at least one CT, NG, and/or TV-related symptom, including vaginal discharge (27%), lower abdominal pain (22%), and/or painful urination (4%). Symptoms related to herpes simplex virus infections such as genital ulcers (1%), and human papillomavirus infections such as warts (4.5%) were rare. Twelve percent had been treated syndromically for an STI (other than HIV) previously during the current pregnancy. Prior treatment did not differ by HIV status.
Table 1.
Characteristics of study participants and associations with Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infection, Gaborone, Botswana. (N=400)
| Total sample (n=400) |
An STI* (n=54) |
CT (n=31) | NG (n=5) | TV (n=21) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| n | % | n | % | ρ | n | % | ρ | n | % | ρ | n | % | ρ | |
| Age, Median [range] | 30 | [19–45] | 29 | [19–43] | 28 | [19–40] | † | 22 | [21–39] | 31 | [21–43] | |||
| 18–25 years | 95 | (23.8) | 17 | (17.9) | 12 | (12.6) | 3 | (3.2) | 4 | (4.2) | ||||
| 26–35 years | 221 | (55.3) | 25 | (11.3) | 14 | (6.3) | 1 | (0.5) | 11 | (5.0) | ||||
| >35 years | 84 | (21.0) | 12 | (14.3) | 5 | (6.0) | 1 | (1.2) | 6 | (7.1) | ||||
|
| ||||||||||||||
| Marital Status (n=396) | ‡ | ‡ | ||||||||||||
| Unmarried | 307 | (76.8) | 49 | (16.0) | 27 | (8.8) | 5 | (1.6) | 20 | (6.5) | ||||
| Married | 89 | (22.3) | 5 | (5.6) | 4 | (4.5) | 0 | (0) | 1 | (1.1) | ||||
|
| ||||||||||||||
| Education (n=392) | † | ‡ | ||||||||||||
| Junior secondary or less | 118 | (29.5) | 22 | (18.6) | 9 | (7.6) | 2 | (1.7) | 12 | (10.2) | ||||
| Senior secondary | 114 | (28.5) | 17 | (14.9) | 12 | (10.5) | 0 | (0) | 5 | (4.4) | ||||
| Tertiary | 160 | (40.0) | 15 | (9.4) | 10 | (6.3) | 3 | (1.2.5) | 4 | (2.5) | ||||
|
| ||||||||||||||
| Gestational Age weeks (LNMP), Median [range] | 26 | [5–36] | 28 | [8–35] | 28 | [8–35] | 22 | [11–32] | 29 | [14–34] | ||||
|
| ||||||||||||||
| Prior pregnancies, Median [range] | 1 | [0–10] | 1 | [0–5] | 1 | [0–5] | 1 | [0–1] | 2 | [0–5] | ||||
| Prior births, Median [range] | 1 | [0–7] | 1 | [0–5] | 1 | [0–5] | 1 | [0–1] | 2 | [0–5] | ||||
| 1 or more births | 261 | (65.3) | 37 | (14.1) | 20 | (7.7) | 3 | (1.1) | 16 | (6.1) | ||||
| Prior preterm birth | 64 | (24.5) | 12 | (18.8) | 10 | (15.6) | ‡ | 1 | (1.6) | 3 | (4.7) | |||
| No prior preterm birth | 192 | (73.5) | 25 | (13.0) | 10 | (5.2) | 2 | (1.0) | 13 | (6.8) | ||||
|
| ||||||||||||||
| Current CT/NG/TV symptoms | 165 | (41.3) | 22 | (13.0) | 10 | (6.0) | 3 | (1.8) | 10 | (6.1) | ||||
| Vaginal discharge | 107 | (26.8) | 15 | (14.0) | 8 | (7.5) | 2 | (1.9) | 6 | (5.6) | ||||
| lower abdominal pain | 88 | (22.0) | 9 | (10.2) | 2 | (2.3) | 1 | (1.1) | 6 | (6.8) | ||||
| Painful urination | 16 | (4.0) | 2 | (12.5) | 2 | (12.5) | 0 | (0) | 0 | (0) | ||||
|
| ||||||||||||||
| Treated syndromicly during pregnancy | 48 | (12.0) | 8 | (16.7) | 5 | (10.4) | 1 | (2.0) | 2 | (4.2) | ||||
|
| ||||||||||||||
| Condom use before pregnancy** | ||||||||||||||
| Never | 110 | (27.5) | 14 | (12.7) | 8 | (7.3) | 1 | (0.9) | 5 | (4.5) | ||||
| Sometimes | 185 | (46.3) | 28 | (15.1) | 17 | (9.2) | 2 | (1.1) | 10 | (5.4) | ||||
| Always | 98 | (24.5) | 12 | (12.6) | 6 | (6.3) | 2 | (2.0) | 6 | (6.1) | ||||
|
| ||||||||||||||
| Condom use during pregnancy | ||||||||||||||
| Never | 188 | (47.0) | 30 | (16.0) | 16 | (8.5) | 4 | (2.1) | 13 | (6.9) | ||||
| Sometimes | 83 | (20.8) | 9 | (10.8) | 6 | (7.2) | 0 | (0) | 3 | (4.6) | ||||
| Always | 75 | (18.8) | 12 | (16.0) | 7 | (9.3) | 1 | (1.3) | 4 | (5.3) | ||||
|
| ||||||||||||||
| HIV infection status | ‡ | ‡ | ||||||||||||
| Infected | 90 | (22.5) | 21 | (23.3) | 10 | (11.1) | 2 | (2.2) | 9 | (10.0) | ||||
| Uninfected | 305 | (76.3) | 32 | 10.5) | 20 | (6.6) | 3 | 1.0) | 12 | (3.9) | ||||
Notes:
An STI means diagnosed with CT, NG, and/or TV infection. LNMP stands for last normal menstrual period.
Women were asked about condom use during the 3 months prior to the pregnancy or trying for the pregnancy. Ranges are in brackets. Percentages are in parentheses, may not add up to 100 due to rounding. The denominator for the total sample column is 400. For the remaining columns, the denominators are derived from the row values in the total sample column.
represent p ≤0.05 and
represents p<0.1, which resulted from Student's T-test, Chi-squared test, Fisher's exact test, or Wilcoxon-Mann-Whitney test. CT, NG, and/or TV symptoms include vaginal discharge, lower abdominal pain and painful urination.
As seen in Table 1, we found the prevalence of CT, NG, and/or TV, hereafter referred to collectively as an STI, to be 13.5% (54/400; 95% CI: 10.3–17.2%). The prevalence of CT was 7.8% (31/400; 95% CI: 4.9–10.2%), NG was 1.3% (5/400; 95% CI: 0.4–2.9%) and TV was 5% (21/400; 95% CI: 3.3–7.9%). Two participants were dually infected with CT and NG, and one was infected CT and TV. As seen in Figure 1, among the women who tested positive for CT, NG, and/or TV, 74% received results and treatment in person prior to leaving the clinic on the same day as testing. Eight women were called and provided results on the same day as testing, and among them five were treated within a week, and three were treated in less than a month. Six women received delayed results (e.g. we were not able to reach them on the same day), and 4 (67%) were treated. In total, 52 (96%) were treated, and 77% were treated on the same day. Further, 41 of the 52 participants treated for CT, NG, and/or TV returned for a test of cure. Among the 41, four (9.8%) retested positive, including three with CT and one with TV. While the reasons for the positive retests were unclear, three women reported that their sex partners were not treated prior to the test of cure.
Bivariate comparisons revealed that marital status (Chi-squared p-value=0.01) and HIV infection status (Chi-squared p-value=0.002) were significantly associated with having CT, NG, and/or NG infection. Unmarried participants were more likely to have an STI (16%) than those who were married (5.6%) and those living with HIV infection were more likely to have an STI (23.3%) than those who were HIV uninfected (10.5%). Among women who had previously given birth, those who experienced a prior preterm birth were more likely to be diagnosed with CT (16%), compared to those with no history of preterm birth (5%). Self-reported STI-related symptoms (abnormal vaginal discharge, lower abdominal pain, and/or painful urination) were not associated with a positive CT, NG, and/or TV diagnosis (Chi-squared p-value=0.89). Among those diagnosed with an STI, 41% (22/54) reported having a symptom related to CT, NG, or TV (28% (15/54) had abnormal vaginal discharge, 17% (9/54) had lower abdominal pain, and 4% (2/54) had painful urination). Among those without an STI, 42% reported having a symptom related to CT, NG, or TV (27% (92/343) had abnormal vaginal discharge, 23% (79/343) had lower abdominal pain, and 4% (14/343) had painful urination).
The stepwise regression analysis was performed using eight predictor variables, including participant age, marital status, HIV infection status, education level, condom use before and after pregnancy, prior births, and STI-related symptoms (abnormal vaginal discharge, lower abdominal pain, and painful urination). Table 2 provides the results from a multivariable logistic regression model with the five independent variables listed in the order in which they were selected by the stepwise procedure. Marital and HIV status were independently and significantly associated with a positive STI diagnosis, after adjustment for age, education, and condom use during pregnancy.
Table 2.
Results of a multivariable logistic regression model assessing participant characteristics associated with Chlamydia trachomatis, Neisseria gonorrhea and Trichomonas vaginalis infection (n=376)
| Characteristics | Positive for CT, NG and/or TV | |
|---|---|---|
|
| ||
| OR (95%CI) | P-value | |
|
|
||
| Age ≤ 25 years | 1.67 (0.84–3.29) | 0.14 |
| Unmarried | 2.89 (1.09–7.67) | 0.03 |
| High school education or less | 1.69 (0.85–3.37 | 0.14 |
| Never used a condom during current pregnancy | 1.59 (0.86–2.96) | 0.14 |
| HIV infected | 2.72 (1.45–5.10) | 0.002 |
Notes: Independent variables (age, marital status, education level, condom use during pregnancy, and HIV infection status.) were included based on a stepwise regression model. Odds ratios reflect increased or decreased likelihood of women with this characteristic being diagnosed with CT, NG, and/or TV.
Discussion
We provided testing and treatment for three curable STIs to 400 pregnant women at one of the largest antenatal clinics in Gaborone, Botswana. The prevalence of one or more STIs was 13.5%. Being unmarried and HIV-infected were significantly associated with testing positive for an STI; and self-reported STI symptoms were not associated with testing positive for an STI. All but one participant received STI test results. Among those who tested positive for an STI, almost three-fourths received results and treatment in person on the same day as testing. However, participants who received delayed results were less likely to be treated.
Our prevalence estimates were high compared to global pooled prevalence estimates among women(23), and similar to pooled prevalences among women in the WHO Africa Region, which were estimated at 3.7% (2.7–5.2) for CT, 1.7% (1.1–2.6) for NG, and 11.5% (9.0–14.6) for TV.(23) Our CT and NG estimates are similar to a 2007 study (using data from 2000/2001) among pregnant women in Botswana, which found the prevalence of CT and NG to be 8% and 3% respectively.(11) However, our estimate of TV prevalence was almost four times lower than the 19% identified by Romoren et al. 2007.(12) These results may be partially explained by differences in our samples. For example, Romoren et al. did not exclude those under 18 years of age, and our participants have a median age that is five years older. Romoren et al. found that age was a predictor of CT, NG, and TV infections, and the prevalence rates of CT and NG infections were highest among teenagers.(11, 12) More research is needed to understand whether the difference in TV prevalence estimates were due to sample differences, variation in diagnostics used, or a change in the prevalence of TV in Gaborone, Botswana over time.
Our study has some limitations. First, women were recruited from a single site, Princess Marina Hospital antenatal clinic, which provides antenatal care, and also serves as a referral clinic for women with high-risk pregnancies. For example, 7% were referred for having a history of miscarriages. However, close to a quarter of our sample (17%) reported that their appointment was a routine check-up, and the remainder was referred for a high-risk condition, including high-blood pressure (22%), rhesus disease (6%), the need for a caesarian section (3%), and diabetes (3%); which are likely not associated with an STI.
It is encouraging to note that the characteristics of our sample did not differ greatly from the population of women in Botswana. The 2007 Botswana Family Health Survey IV Report, which identifies and surveys a representative sample of the population of Botswana on topics related to family planning awareness and basic maternal and child health indicators, also found that very few women were married (18%), 70% reached at least a secondary level of education, and the highest proportion of pregnancies occurred among women between the ages of 30–34.(24) Further, the Botswana AIDS Impact Survey IV Report, which provides HIV incidence and prevalence estimates among the population aged six to 64 years, found that the HIV prevalence among pregnant women was 20.5%. The proportion of preterm births among women who had previously given birth (25%) was expected given that Botswana is one of 11 countries with a national preterm birth rate higher than 15%.(19) Finally, only 80% of our participants had a documented syphilis test, and it is unclear if this is due to a lack of testing or documentation in obstetric records. While our estimate of the prevalence of reactive syphilis tests was low, a 2016 study that used clinic antenatal registers estimated the syphilis prevalence to be 1.15% (95% CI, 0.89–1.41) among pregnant women in Gaborone in 2008.(17)
Additionally, our sample represents women who agreed to participate and may be more likely to include women who believe they are at greater risk for infection. However, this concern is mitigated by the large proportion of women who accepted (86%) out of all those eligible.
This study showed it was possible to integrate CT, NG, and TV diagnostic testing into antenatal care in Botswana. Results highlighted that, even with a 90-minute testing time, it was not possible to provide all participants with results and treatment on the same day as testing. The average wait time at the Princess Marina antenatal clinic was 45 minutes and many women were unwilling or unable to wait for results after their appointment concluded. Thus, to maximize the effectiveness of an STI testing and treatment program, a faster time to result would be useful as would efforts to ensure treatment uptake among those who received delayed results. Since many sub-Saharan African countries have an antenatal care attendance rate of at least 70% and deliver opt-out antenatal HIV testing, antenatal care could provide a framework for CT, NG, and TV testing.(25) As the costs (e.g. capital and supplies) and infrastructure needs (e.g. continuous power and reliable shipping) associated with STI testing may be high for some low and middle-income countries, more research is needed to compare the costs of testing and treatment with the health benefits associated with curing infections and the subsequent savings to the health system. To conserve resources, subgroups, such as women infected with HIV, may be appropriate to target for STI testing.
Our study also found a lack of an association between self-reported STI symptoms, which provides further evidence that syndromic management may not adequately identify STIs in pregnant women. While syndromic management has the benefit of low costs and resource requirements, it is likely that pregnant women are being unnecessarily exposed to antibiotics, which is particularly concerning given the expansion of drug resistant NG.(26) Further, true infections are likely being missed, which may be contributing to the high rates of adverse birth outcomes in Botswana, including preterm birth.
In conclusion, providing testing and treatment for curable STIs to pregnant women in an antenatal clinic in Gaborone, Botswana revealed a high STI prevalence. Participant HIV infection and marital status were significantly positively associated with being diagnosed with CT, NG, and/or TV, and self-reported STI symptoms were not associated with testing positive. Among participants with an STI, those who received same-day results were more likely to be treated than those who received delayed results. The absence of diagnostic tests for STIs during antenatal care likely represents a missed opportunity to improve pregnancy and birth outcomes in Botswana. As highly sensitive and specific, point-of-care testing assays become more widely available, it is important for Health Ministers and other policy makers to assess the short term and long-term cost and benefits of offering STI screening during antenatal care.
Key points.
The prevalence of STIs among pregnant women was high.
HIV infection and being unmarried were associated with being diagnosed with an STI.
Self-reported symptoms (e.g. abnormal vaginal discharge) were not associated with infection.
Among those who tested positive, 74% received in person, same-day results and treatment
Acknowledgments
Funding Statement
This research was supported by (AHRQ; 2T32HS000046), the Center for HIV Identification, Prevention and Treatment Services (CHIPTS; P30MH058107). The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
Footnotes
Contributors
AW managed the study, did the analyses and data collection and wrote the first and final draft. PG was the clinician on the study and approved the final draft. NM, OS, SD, EW, and KD worked on the study, reviewed analyses, wrote sections and edited/approved the final version. DRM helped conceive of the study and data collection protocols, reviewed drafts of the manuscript, and edited/approved of the final version. JDK was the PI on the study, supported analyses, wrote sections and approved the final draft. CM was the PI on the study, supported analyses, wrote sections and approved the final draft.
Competing interests None declared.
References
- 1.Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: Causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis. 2006;25(9):562–9. doi: 10.1007/s10096-006-0190-3. [DOI] [PubMed] [Google Scholar]
- 2.Adachi K, Klausner JD, Bristow CC, Xu J, Ank B, Morgado MG, et al. Chlamydia and Gonorrhea in HIV-Infected Pregnant Women and Infant HIV Transmission. Sexually Transmitted Diseases. 2015;42(10):554–65. doi: 10.1097/OLQ.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClelland RSSL, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. The Journal of Infectious Diseases. 2007;195(5):698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 4.Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clinical infectious diseases: an official publication of the Infectious Diseases Society of American Journal of Obstetrics and Gynecology. 2011;53(Suppl 3):S99–102. doi: 10.1093/cid/cir699. [DOI] [PubMed] [Google Scholar]
- 5.Schachter JGM, Sweet RL, Holt J, Jordan C, Bishop E. Prospective study of perinatal transmission of Chlamydia trachomatis. JAMA: the journal of the American Medical Association. 1986;255(24):3374–7. [PubMed] [Google Scholar]
- 6.Carter JE, Whithaus KC. Neonatal respiratory tract involvement by Trichomonas vaginalis: a case report and review of the literature. Am J Trop Med Hyg. 2008;78(1):17–9. [PubMed] [Google Scholar]
- 7.Hoffman DJ, Brown GD, Wirth FH, Gebert BS, Bailey CL, Anday EK. Urinary tract infection with Trichomonas vaginalis in a premature newborn infant and the development of chronic lung disease. J Perinatol. 2003;23(1):59–61. doi: 10.1038/sj.jp.7210819. [DOI] [PubMed] [Google Scholar]
- 8.Joseph Davey DL, Shull HI, Billings JD, Wang D, Adachi K, Klausner JD. Prevalence of Curable Sexually Transmitted Infections in Pregnant Women in Low- and Middle-Income Countries From 2010 to 2015. Sexually Transmitted Diseases. 2016;43(7):450–8. doi: 10.1097/OLQ.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medline A, Joseph Davey D, Klausner JD. Lost opportunity to save newborn lives: variable national antenatal screening policies for Neisseria gonorrhoeae and Chlamydia trachomatis. International Journal of STD & AIDS. 2016;28(7):660–6. doi: 10.1177/0956462416660483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global Strategy for the Prevention and Control of Sexually Transmitted Infections: 2006–2015. 2007 [Available from: http://www.who.int/hiv/pub/toolkits/stis_strategy[1]en.pdf.
- 11.Romoren M, Sundby J, Velauthapillai M, Rahman M, Klouman E, Hjortdahl P. Chlamydia and gonorrhoea in pregnant Batswana women: time to discard the syndromic approach? BMC Infect Dis. 2007;7:27. doi: 10.1186/1471-2334-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romoren M, Velauthapillai M, Rahman M, Sundby J, Klouman E, Hjortdahl P. Trichomoniasis and bacterial vaginosis in pregnancy: inadequately managed with the syndromic approach. Bull World Health Organ. 2007;85(4):297–304. doi: 10.2471/BLT.06.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romoren MRM, Sundby J, Hjortdahl P. Chlamydia and gonorrhoea in pregnancy: effectiveness of diagnosis and treatment in Botswana. Sex Transm Infect. 2004;80(5):395–400. doi: 10.1136/sti.2003.007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moodley D, Moodley P, Sebitloane M, Soowamber D, McNaughton-Reyes HL, Groves AK, et al. High Prevalence and Incidence of Asymptomatic Sexually Transmitted Infections During Pregnancy and Postdelivery in KwaZulu Natal, South Africa. Sexually Transmitted Diseases. 2015;42(1):43–7. doi: 10.1097/OLQ.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 15.Herbst de Cortina S, Bristow C, Joseph Davey D, Klausner J. A Systematic Review of Point of Care Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infectious Diseases in Obstetrics and Gynecology. 2016 doi: 10.1155/2016/4386127. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UNICEF. Country Statistics: Botswana. 2013 [Available from: https://www.unicef.org/infobycountry/botswana_statistics.html.
- 17.Ganiyu ABML, Mabuz LH. Syphilis sero-positivity among pregnant women attending public antenatal clinics: A five-year analysis from 15 public clinics in Gaborone, Botswana. Southern African Journal of Infectious Diseases. 2016;31(3):91–4. [Google Scholar]
- 18.World Health Organization. Neonatal and Child Health Profile: Botswana. 2013 [Available from: http://www.aho.afro.who.int/profiles_information/index.php/Botswana:Analytical_summary_-_Maternal_and_newborn_health.
- 19.World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. 2012 [Available from: http://www.who.int/pmnch/media/news/2012/preterm_birth_report/en/
- 20.Botswana Ministry of Health. Management of Sexually Transmitted Infections. Reference Manual for Health Workers. 2012 [Google Scholar]
- 21.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. 2015 doi: 10.1093/cid/civ771. [Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm. [DOI] [PubMed]
- 22.Centers for Disease Control and Prevention. 2015 Sexually Transmitted Diseases Treatment Guidelines: Trichomoniasis. 2015 [Available from: https://www.cdc.gov/std/tg2015/trichomoniasis.htm.
- 23.Newman LRJ, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLOS One. 2015;10(12) doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botswana Central Statistical Office and UNICEF. 2007 Botswana Family Health Survey IV Report. Gaborone. 2009 [Google Scholar]
- 25.Kennedy CE, Fonner VA, Sweat MD, Okero FA, Baggaley R, O’Reilly KR. Provider-Initiated HIV Testing and Counseling in Low- and Middle-Income Countries: A Systematic Review. AIDS Behav. 2013;17(5):1571–90. doi: 10.1007/s10461-012-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017;14(7):e1002344. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]


