Short abstract
Background
Pain is a frequent symptom in aquaporin-4-immunoglobulin-G-positive neuromyelitis optica spectrum disorders (AQP4-IgG-pos. NMOSD). Data on pain in myelin-oligodendrocyte-glycoprotein-immunoglobulin-G autoimmunity with a clinical NMOSD phenotype (MOG-IgG-pos. NMOSD) are scarce.
Objective
The objective of this paper is to investigate pain in MOG-IgG-pos. NMOSD, AQP4-IgG-pos. NMOSD and NMOSD without AQP4/MOG-IgG detection (AQP4/MOG-IgG-neg. NMOSD).
Methods
Forty-nine MOG-IgG-pos. (n = 14), AQP4-IgG-pos. (n = 29) and AQP4/MOG-IgG-neg. (n = 6) NMOSD patients were included in this cross-sectional baseline analysis from an ongoing observational study. We identified spinal cord lesions on magnetic resonance imaging, assessed pain by the painDETECT and McGill Pain questionnaires, quality of life by Short Form Health Survey, and depression by Beck Depression Inventory.
Results
Twelve MOG-IgG-pos. NMOSD patients (86%), 24 AQP4-IgG-pos. NMOSD patients (83%), and all AQP4/MOG-IgG-neg. NMOSD patients (100%) suffered from pain. MOG-IgG-pos. NMOSD patients had mostly neuropathic pain and headache; AQP4-IgG-pos. and AQP4/MOG-IgG-neg. NMOSD patients had mostly neuropathic pain. A history of myelitis was less frequent in MOG-IgG-pos. NMOSD than in AQP4-IgG-pos. NMOSD patients. Pain influenced quality of life in all patients. Thirty-six percent of patients with pain received pain medication; none of them were free of pain.
Conclusions
Pain is a frequent symptom of patients with MOG-IgG-pos. NMOSD and is as important as in AQP4-IgG-pos. and AQP4/MOG-IgG-neg. NMOSD. Despite its impact on quality of life, pain is insufficiently alleviated by medication.
Keywords: Aquaporin 4 (AQP4), myelin oligodendrocyte glycoprotein antibody (MOG), neuromyelitis optica (NMO) spectrum disease (NMOSD), pain
Introduction
Pain is one of the most frequent comorbidities of neurologic diseases and severely reduces the quality of life (QoL) of affected patients. In the management of patients with neuroinflammatory diseases, the appropriate treatment of pain is a difficult challenge.
Neuromyelitis optica spectrum disorders (NMOSD) are inflammatory autoimmune diseases of the central nervous system (CNS) associated with immunoglobulin G antibodies (IgG) to the astrocytic water channel aquaporin-4 (AQP4), to myelin oligodendrocyte glycoprotein (MOG) or in some cases without antibody proof.1,2 Currently, it is unclear whether MOG-IgG-positive NMOSD (MOG-IgG-pos. NMOSD) is a disease entity of its own, defined by the presence of anti-MOG-IgG and a clinical phenotype similar to AQP4-IgG-positive NMOSD (AQP4-IgG-pos. NMOSD). Consequently, some authors recognize both diseases as separate disease entities based on different cellular pathologies,3 while others describe them as different forms of a larger disease spectrum, usually under the NMOSD umbrella, based on a similar clinical phenotype characterized by relapsing optic neuritis and/or extensive transverse myelitis leading to blindness, weakness, numbness, cognitive impairment, and pain.4,5
Systematic studies of pain syndromes associated with NMOSD are relatively scarce and have so far been limited to AQP4-IgG-positive or AQP4-IgG-negative NMOSD.6–10 Here, we systematically study pain syndromes of patients suffering from MOG-IgG-pos. NMOSD, contribute to the literature on pain syndromes of patients with AQP4-IgG-pos. and AQP4/MOG-IgG-neg. NMOSD, and provide a comparison of pain syndromes and QoL aspects in MOG-IgG-pos. and AQP4-IgG-pos. NMOSD.
Methods
Ethics, study protocol and patient sample
Data are derived from the baseline visit of an ongoing observational study that is following patients with AQP4-IgG-pos. NMOSD and related disorders. The study was approved by the ethics committee of the Charité–Universitätsmedizin Berlin, Germany (EA1/041/14) and conducted according to the 1964 Declaration of Helsinki in its currently applicable version. All participants provided written informed consent prior to inclusion.
Inclusion criteria for this analysis were diagnosis of NMOSD according to the international consensus diagnostic criteria for NMOSD 20151 or positive proof of anti-MOG-IgG associated with a demyelinating disease of the CNS with a clinical phenotype equivalent to NMOSD diagnosis criteria in patients over 18 years of age.2 Anti-MOG-IgG was detected by a live cell-based assay using HEK293A cells transfected with full-length human MOG and was confirmed by means of a commercial fixed-cell based assay with HEK293 cells transfected with full-length human MOG (Euroimmun, Lübeck, Germany).2,4 We treated MOG-IgG positivity as equivalent to positive AQP4-IgG for fulfilling the diagnostic criteria.
At the time of screening, our database contained 53 baseline visits. Forty-nine patients were included in the analysis. Of these, 14 patients suffered from MOG-IgG-pos. NMOSD and 29 patients from AQP4-IgG-pos. NMOSD.1 Six patients fulfilled the 2015 International Panel for NMO Diagnosis criteria for NMOSD and were negative for anti-MOG-IgG and anti-AQP4-IgG. Three patients were not included in the analysis because of a diagnosis of antibody-negative isolated longitudinally extensive transverse myelitis without fulfilling NMOSD diagnostic criteria or positive MOG-IgG testing. One patient was excluded because of incomplete pain assessment. The resulting female-to-male ratio of 2.5:1 in the MOG-IgG-pos. and of 9:1 in the AQP4-IgG-pos. NMOSD patients corresponds to previous data in the literature.1,4,11,12 Demographic data, Expanded Disability Status Scale (EDSS), and health-related quality of life (hr-QoL) assessment scores were similar between MOG-IgG-pos. and AQP4-IgG-pos. NMOSD patients (Table 1).
Table 1.
Demographics, clinical data and assessment.
| Demography, clinical data, assessment | MOG-IgG-pos. NMOSD patients | AQP4-IgG-pos. NMOSD patients | p valueMOG-IgG-pos./AQP4-IgG-pos. NMOSD patients | AQP4/MOG-IgG-neg. NMOSD patients |
|---|---|---|---|---|
| N of patients (f/m) (ratio) | 14 (10/4) (2.5:1) | 29 (26/3) (9:1) | 0.19 | 6 (5/1) (5:1) |
| Age (years) | 46 (21–79) | 50 (20–72) | 0.47 | 53 (24–71) |
| Disease duration (years) | 5.5 (4.09) (0.3–15.04) | 7.8 (6.83) (0.48–28.08) | 0.147 | 8.24 (6.2) (0.28–22.36) |
| EDSS (0–10) | 2 (1–6) | 3.5 (0-7) | 0.094 | 3.25 (2.5–5) |
| Immunotherapies (n) | 11 | 25 | 5 | |
| Azathioprine | 2 | 5 | 1 | |
| Belimumab | 0 | 1 | 0 | |
| Glatiramer acetate | 0 | 1 | 1 | |
| Mycophenolate mofetil | 0 | 1 | 1 | |
| Prednisolone | 1 | 0 | 0 | |
| Rituximab | 8 | 16 | 1 | |
| Teriflunomide | 0 | 0 | 1 | |
| Tocilizumab | 0 | 1 | 0 | |
| No treatment | 3 | 4 | 1 | |
| VAS General Health (0–100) | 47.50 (51.50) (5–76) | 40.45 (35.00) (0–100) | 0.403 | 52.50 (58) (17–72) |
| VAS Fatigue (0–100) | 46.83 (55.50) (0–76) | 40.17 (40.00) (0–100) | 0.506 | 53.83 (54.00) (33–76) |
| SF-36: PCS (84.15 (90) (0–100)) | 43.47 (41.41) (21.49–57.62) | 40.50 (43.16) (16.58–57.52) | 0.506 | 38.50 (37.16) (19.51–55.38) |
| SF-36: MCS (74.74 (80) (0–100)) | 43.55 (41.18) (27.30–58.99) | 46.38 (49.36) (18.67–60.64) | 0.403 | 46.76 (49.74) (35.97–52.95) |
| BDI-II (0–63) | 8.93 (6.5) (0–25) | 9.31 (8) (0–26) | 0.678 | 15.67 (15) (6–24) |
| Grades (n) | ||||
| No depression (0–9) | 9 | 17 | 1 | |
| Minimal depression (10–19) | 3 | 9 | 3 | |
| Moderate depression (20–29) | 2 | 3 | 2 | |
| Severe depression (30–63) | 0 | 0 | 0 |
AQP4-IgG-pos.: aquaporin-4-immunoglobulin-G-positive; BDI-II: Beck Depression Inventory II; EDSS: Expanded Disability Status Scale; f/m: female/male; MCS: Mental Component Summary; MOG-IgG-pos.: myelin-oligodendrocyte-glycoprotein-immunoglobulin-G-positive; N, n: number; NMOSD: neuromyelitis optica spectrum disorder; PCS: Physical Component Summary; SF-36: Short Form 36 Health Survey; VAS: visual analog scale.
Age, EDSS: median, (range); others: mean, (median), (range); p values: Mann-Whitney U test.
Clinical data
We recorded immunotherapies and pain medication including nonsteroidal anti-inflammatory drugs, anticonvulsants, tricyclic antidepressants, atypical antidepressants, selective serotonin norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors and muscle relaxants. Every patient had a medical interview by a trained neurologist about clinical history and underwent a neurological examination determining the EDSS.
All scales used in this study are established measurements for pain7,13,14 and hr-QoL6,7,9,13,15 both in multiple sclerosis and in NMOSD.
Pain assessment
Pain was recorded whenever a patient reported via the painDETECT questionnaire (PDQ) either current pain or pain within the previous four weeks.
Neuropathic pain is pain caused by a lesion or disease affecting the somatosensory system.16 We classified pain as neuropathic if sensory signs corresponding to the affected nervous structure were examined, and if the magnetic resonance imaging (MRI) confirmed a corresponding CNS lesion.16
We administered the PDQ to ask about pain localization and current and previous pain. The PDQ supports the discrimination of neuropathic and nociceptive pain by seven questions about pain quality. Each quality is rated from 0 to 5 so a maximum subscale score of 35 can be reached. A PDQ-score of 12 or higher is indicative of neuropathic pain,14,17 but was not used as a deciding diagnostic criterion. Similar to previous findings,18 the sensitivity of the PDQ compared to the clinical diagnosis of neuropathic pain was only 70%.
In the McGill Pain Questionnaire (MGQ), patients describe their pain experience with up to 20 words from a list of 78 words, categorized as sensory-discriminative to describe the sense of intensity, location and duration of pain, as motivational-affective to describe unpleasantness, and as cognitive-evaluative to describe cognitions such as appraisal. The chosen words map onto a rating index ranging from 0 to 78 with higher values meaning worse pain.19
hr-QoL
We measured hr-QoL with the Short Form 36 Health Survey (SF-36). A Physical and a Mental Component Summary (PCS/MCS) were calculated using norm-based attaining values from 0 (worst) to 100 (best).20
Self-reported effect of general health and fatigue was acquired by visual analog scales (VAS) asking in German, “How strong is your fatigue/impairment of general health?” labeled with “no fatigue/impairment” as the starting point and “unbearable fatigue/impairment” as the end point. The different positions are related to numbers between 0 (best) and 100 (worst).21
To evaluate depressive symptoms, we administered the Beck Depression Inventory (BDI-II). It contains 21 questions with a score from 0 (best) to 63 (worst) (0–9: nondepressive affect; 10–19: minimal mood disturbance; 20–29:moderate depression; 30 and above: severe depression).22
MRI
MRI was performed with a Siemens 3-Tesla Magnetom Trio scanner (Erlangen, Germany). A neuroradiologist (M.S.) reviewed scans of the brain (three-dimensional (3D) fluid-attenuated inversion recovery, 1 mm isotropic resolution, repetition time (TR)/echo time (TE)/inversion time (TI) = 6000/388/2100 ms) and spinal cord (T2-weighted sagittal, 3 mm slice thickness, TR/TE = 3500/101 ms) to detect residual lesions to confirm or reject the diagnosis of neuropathic pain.
Statistical analysis
We carried out all statistical analyses using IBM SPSS Statistics version 22.0 software (IBM, Armonk, NY, USA). Demographic and clinical characteristics are shown as means plus standard deviation and as range or counts. We performed the Mann-Whitney U test to compare ordinal data, the Fisher exact test to compare binary data between MOG-IgG-pos. and AQP4-IgG-pos. NMOSD patients, and the Pearson correlation test to explore the relationships between the dependent measure of pain intensity and the independent variables of SF-36, VAS and BDI-II. All tests were two tailed and significance was set as p < 0.05. Data derived from AQP4/MOG-IgG-neg. NMOSD patients are descriptively listed because of the low sample size. All statistical analyses have explorative character and should be interpreted as statistical tendency.
Results
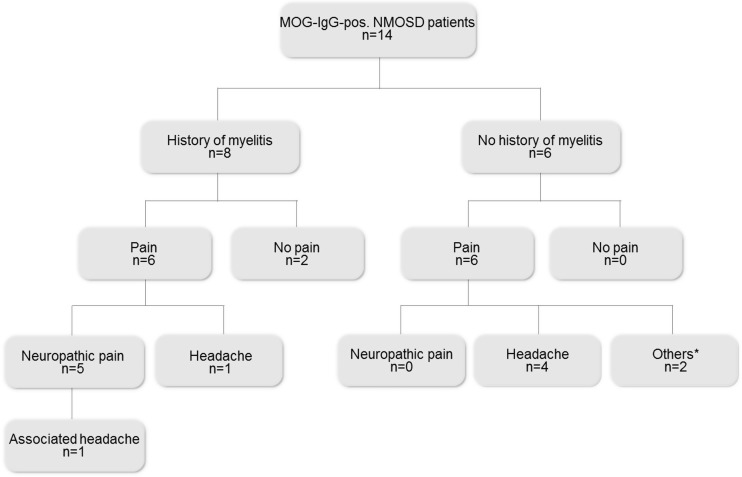
MOG-IgG-pos. NMOSD patients suffered considerably from pain. Pain frequency, intensity, pain type, pain localization and frequency of pain treatment were comparable to AQP4-IgG-pos. NMOSD patients (Tables 2 and 3). Twelve out of 14 MOG-IgG-pos. NMOSD patients (86%) indicated via the PDQ that they suffer from pain. Average pain intensity was mild; one patient (7%) suffered from severe pain. According to medical interview, clinical examination, and MRI, five MOG-IgG-pos. NMOSD patients suffered from neuropathic pain (41.6%), whereas only three patients had a PDQ score >12. Six MOG-IgG-pos. NMOSD patients reported mainly headache/neck pain (50%). Pain types comprised retrobulbar pain (n = 2), tension headache in association with recurrent optic neuritis (n = 1), occipital neuralgia (n = 1) and cervicogenic headache (n = 2). Three patients additionally suffered from migraine, which was aggravated in two of them in the context of MOG-IgG-pos. NMOSD.
Table 2.
Demographics, clinical data, and assessment subject to pain.
| Demographics, clinical data, assessment |
MOG-IgG-pos. NMOSD patients |
|
AQP4-IgG-pos. NMOSD patients |
p value |
AQP4/MOG-IgG-neg. NMOSD patients |
|||
|---|---|---|---|---|---|---|---|---|
| With paina | Without pain | p valuePatients with/without pain | With paina | Without pain | p valuePatients with/without pain | MOG-IgG-pos./AQP4-IgG-pos. NMOSD patients with pain | With paina | |
| N of patients (f/m) | 12 (9/3) | 2 (1/1) | 1 | 24 (21/3) | 5 (5/0) | 0.309 | 6 (5/1) | |
| Age (years) | 46 (21–79) | 41 (34–48) | 0.659 | 50 (20–71) | 40 (31–72) | 0.758 | 0.436 | 53 (24–71) |
| EDSS (0–10) | 2.25 (1–6) | 2 (2) | 0.473 | 3.5 (0–7) | 2.5 (1.5–4) | 0.382 | 0.112 | 3.25 (2.5–5) |
| History of myelitis, n | 8 | 2 | 22 | 5 | 1 | 0.009 b | 6 | |
| Pain type, n (%) | ||||||||
| Neuropathic pain | 5 (41.67%) | 19 (79.17%) | 0.198 | 5 (83.33%) | ||||
| Headache/Neck pain | 5 (41.67%) | 5 (20.83%) | 0.252 | 2 (33.33%) | ||||
| Musculoskeletal | 0 | 0 | NA | 1 (16.67%) | ||||
| Spasticity | 1 (8.33%) | 3 (12.50%) | 1 | 1 (16.67%) | ||||
| Increased tone | 3 (25.0%) | 7 (29.17%) | 0.376 | 2 (33.33%) | ||||
| Othersb | 2 (16.67%) | 4 (16.67%) | 1 | 1 (16.67%) | ||||
| Pain medication, n (%) | 4 (33.33%) | 9 (37.50%) | 0.702 | 3 (50.0%) | ||||
| >1 pain medication | 0 | 4 (16.67%) | 1 | 1 (16.67%) | ||||
| NSAID | 1 | 2 | 0 | |||||
| Opioids | 0 | 3 | 0 | |||||
| Anticonvulsants | 1 | 7 | 3 | |||||
| Antidepressants | 0 | 2 | 1 | |||||
| Muscle relaxants | 1 | 1 | 0 | |||||
| VAS General Health (0–100) | 47.50 (51.50) (5–76) | NA | NA | 43.29 (35) (3–100) | 26.8 (21) (0–51) | 0.270 | 0.679 | 52.50 (58) (17–72) |
| VAS Fatigue (0–100) | 46.83 (55.50) (0–76) | NA | NA | 43.29 (44.50) (1–100) | 25.2 (20) (0–66) | 0.145 | 0.753 | 53.83 (54) (33–76) |
| SF-36 PCS (84.15 (90) (0–100)) |
42.27 (40.98) (21.49–57.62) |
56.64 (56.64) (56.64) |
0.333 | 39.92 (44.27) (16.58–56.58) |
43.23 (43.16) (30.20–57.52) |
0.518 | 0.687 | 38.49 (37.16) (19.51–55.38) |
| SF-36 MCS (74.74 (80) (0–100)) |
42.48 (39.07) (27.30–58.99) |
55.18 (55.18) (55.18) |
0.667 | 45.25 (46.46) (18.67–60.64) |
51.77 (50.77) (45.17–56.86) |
0.201 | 0.430 | 46.76 (49.74) (35.97–52.95) |
| BDI-II (0–63) | 10.33 (7) 1–25 | 0.5 (0.5) (0–1) | 0.022 b | 10.04 (8) 0–26 | 5.8 (4) (1–14) | 0.245 | 1 | 15.67 (15) 6–24 |
AQP4-IgG-pos.: aquaporin-4-immunoglobulin-G-positive; BDI-II: Beck Depression Inventory II; EDSS: Expanded Disability Status Scale; f/m: female/male; MCS: Mental Component Summary; MOG-IgG-pos.: myelin-oligodendrocyte-glycoprotein-immunoglobulin-G-positive; N, n: number; NMOSD: neuromyelitis optica spectrum disorder; NSAID: nonsteroidal anti-inflammatory drug; PCS: Physical Component Summary; SF-36: Short Form 36 Health Survey; VAS: visual analog scale.
aPatients suffering from present pain or pain during the last four weeks.
bConnective tissue disease, arthralgia of unknown origin, vasculitis; Age, EDSS: median, (range); others: mean, (median), (range); p value: Fisher exact test for pain type, pain medication; p value others: Mann-Whitney U test; Significant p values are indicated in bold.
Table 3.
Pain intensity affecting general health and fatigue in relation to pain medication.
| Pain intensity affecting general health and fatigue | MOG-IgG-pos. NMOSD patients with pain |
AQP4-IgG-pos. NMOSD patients with pain |
Pain med |
No pain med |
||||
|---|---|---|---|---|---|---|---|---|
| Pain meda | No pain medb |
p value Pain med/no pain med |
Pain med | No pain med |
p value Pain med/no pain med |
p value MOG-IgG-pos./AQP4-IgG-pos. NMOSD patients with pain |
||
| N of patients | 4 (33.33%) | 8 (66.67%) | 9 (37.5%) | 15 (62.5%) | ||||
| Present pain intensity (0–10) | 3.25 (2.5) (1–7) | 1.222 (0) (0–5) | 0.148 | 4.56 (5) (0–7) | 1.94 (0) (0–7) | 0.025 a | 0.503 | 0.664 |
| Grades: n (%) | ||||||||
| None (0) | 0 | 3 (25%) | 1 (4.17%) | 5 (20.83%) | ||||
| Mild (1–4) | 3 (25%) | 3 (25%) | 3 (12.5%) | 6 (25%) | ||||
| Moderate (5–6) | 0 | 1 (8.33%) | 3 (12.5%) | 1 (4.17%) | ||||
| Severe (7–10) | 1 (8.33%) | 0 (one missing) | 2 (8.33%) | 2 (8.33%) (one missing) | ||||
| Most intense pain: last four weeks (0–10) | 5.25 (5.5) (2–8) | 3.67 (4) (0–8) | 0.414 | 6.56 (7) (3–8) | 3.72 (3.5) (0–9) | 0.041b | 0.604 | 1 |
| Average pain: last four weeks (0–10) | 3.5 (3.5) (1–6) | 1.89 (1) (0–5) | 0.199 | 4.56 (5) (0–9) | 2.56 (2.5) (0–7) | 0.118 | 0.710 | 0.561 |
| VAS General Health (0–100) | 65.25 (69.5) (46–76) | 38.63 (30.5) (5–72) | 0.048 a | 47.0 (35) (3–100) | 37.5 (33.5) (0–75) | 0.472 | 0.330 | 1 |
| VAS Fatigue (0–100) | 67.25 (68.5) (58–74) | 36.63 (37.5) (0–76) | 0.154 | 53.67 (59) (6–100) | 34.1 (24.0) (0–78) | 0.069 | 0.503 | 0.823 |
AQP4-IgG-pos.: aquaporin-4-immunoglobulin-G-positive; MOG-IgG-pos.: myelin-oligodendrocyte-glycoprotein-immunoglobulin-G-positive; N, n: number; NMOSD: neuromyelitis optica spectrum disorder; VAS: visual analog scale.
Mean (median) (range), p values: Mann-Whitney U test.
aPain med: patients under pain with pain medication. bNo pain med: patients under pain without pain medication. Significant p values are indicated in bold.
All MOG-IgG-pos. NMOSD patients who received pain medication reported pain and tended to have stronger pain than those without pain-relieving treatment.
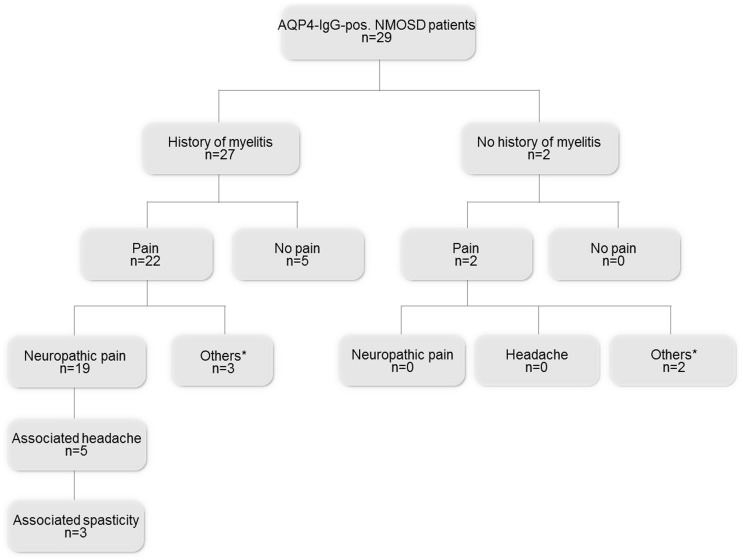
Similar to the pain frequency among MOG-IgG-pos. NMOSD patients, 24 out of 29 AQP4-IgG-pos. NMOSD patients (83%) suffered from pain. The average pain intensity was mild. However, four AQP4-IgG-pos. NMOSD patients (14%) suffered from severe pain. Nineteen AQP4-IgG-pos. NMOSD patients were diagnosed with neuropathic pain (82.6%). Among these, 17 patients had a PDQ-score indicating probable neuropathic pain. Five AQP4-IgG-pos. NMOSD patients suffered mainly from headache/neck pain (21.7%). Headache types comprised tension headache (n = 3) that occurred in two patients as a primary symptom of the NMOSD, occipital neuralgia (n = 1) and cervicogenic headache (n = 1). Eight AQP4-IgG-pos. NMOSD patients with pain who received pain medication still reported pain. Present pain intensity (p = 0.025) and maximal pain intensity in the previous four weeks (p = 0.041) were higher in patients with pain medication than in those without. Detailed results of the PDQ are shown in Supplementary Table 1. Table 3 provides details on pain intensity and hr-QoL subject to pain medication.
Pain intensity had a substantial impact on patients’ well-being. Present pain intensity was correlated with higher depression scores in MOG-IgG-pos. NMOSD patients (p = 0.001) and with emotional affect (measured with MCS) both in MOG-IgG-pos. NMOSD (p = 0.047) and in AQP4-IgG-pos. NMOSD patients (p = 0.031). With growing pain intensity, the latter showed moreover a stronger effect on general health (p < 0.001), PCS (p = 0.008) and fatigue (p < 0.001) (Supplementary Table 1). Thus, MOG-IgG-pos. and AQP4-IgG-pos. NMOSD patients were similar with respect to different quantitative and qualitative aspects of pain as well as to related aspects of their hr-QoL.
To describe their pain experience, MOG-IgG-pos. and AQP4-IgG-pos. NMOSD patients chose different words within the MGQ, but the number of chosen words, and the pain intensity in all categories, were similar. The cognitive-evaluative component was the most affected. MOG-IgG-pos. NMOSD patients described their pain experience in the MGQ mainly by the sensory-discriminative word “throbbing” (50%), by the motivational-affective word “punishing” (62.5%) and by the cognitive-evaluative word “annoying” (50%). AQP4-IgG-pos. NMOSD patients described their pain quality mainly by the sensory-discriminative words “flashing” (55.6%), “pricking” (61.11%) and “tingling” (62.5%). A detailed evaluation of MGQ is provided in Supplementary Table 2.
MOG-IgG-pos. and AQP4-IgG-pos. NMOSD patients showed a similar effect on their hr-QoL. As expected, all patient groups showed impaired mental and physical function in the SF-36 survey (Table 1). There was no difference between MOG-IgG-pos. and AQP4-IgG-pos. NMOSD patients (PCS: p = 0.687; MCS: p = 0.430). Both groups indicated a clear effect on general health and fatigue (Tables 1 and 2). MOG-IgG-pos. NMOSD patients with pain-relieving treatment reported a higher impairment of general health than those without pain medication (p = 0.048; Table 3). Both MOG-IgG-pos. NMOSD patients without pain did not participate in the VAS, while one of them also did not participate in the SF-36. MOG-IgG-pos. NMOSD patients compared to AQP4-IgG-pos. NMOSD patients with pain had similar depression scores (p > 0.999). Five MOG-IgG-pos. NMOSD patients (36%) and 12 AQP4-IgG-pos. NMOSD patients (42%) suffered from mild or moderate depression according to the BDI-II (Table 1). MOG-IgG-pos. NMOSD patients with pain had higher depression scores than those without pain (p = 0.022). Table 2 displays the BDI-II results in patients with and without pain.
During assessment, all patients were stable; none of them had an acute relapse. The different clinical phenotypes were the following: Both of the MOG-IgG-pos. NMOSD patients without pain and two MOG-IgG-pos. NMOSD patients with pain had a history of recurrent myelitis and optic neuritis. Six MOG-IgG-pos. NMOSD patients with pain had a history of recurrent optic neuritis without history of myelitis, three MOG-IgG-pos. NMOSD patients with pain had a history of one-time (n = 2) or recurrent (n = 1) myelitis without history of optic neuritis. Of the latter one patient additionally had a history of encephalitis and one patient a history of brainstem syndrome. One MOG-IgG-pos. NMOSD patient with pain had a clinical presentation of one episode of atypical facial pain and one episode of cranial allodynia.
Twelve AQP4-IgG-pos. NMOSD patients with pain had a clinical history of recurrent myelitis and optic neuritis. Three of them additionally had a history of brainstem syndrome. Two AQP4-IgG-pos. NMOSD patients with pain had a history of recurrent (n = 2) optic neuritis without history of myelitis, 10 AQP4-IgG-pos. NMOSD patients with pain had a history of one-time (n = 1) or recurrent (n = 9) myelitis without history of optic neuritis. One of them had an episode of area postrema syndrome and one of them had a history of brainstem syndrome.
Five AQP4-IgG-pos. NMOSD patients without pain had a clinical history of recurrent myelitis and optic neuritis (n = 3) or myelitis without history of optic neuritis (n = 2).
Three AQP4/MOG-IgG-neg. NMOSD patients had a history of recurrent myelitis and optic neuritis. Two AQP4/MOG-IgG-neg. NMOSD patients had a history of one-time optic neuritis without history of myelitis and one AQP4/MOG-IgG-neg. NMOSD patient had a history of myelitis and area postrema syndrome.
A clinical history of myelitis occurred less frequently in MOG-IgG-pos. NMOSD patients than in AQP4-IgG-pos. NMOSD patients (p = 0.009). All patients with neuropathic pain showed residual spinal cord lesions, whereas other pain types occurred independently from former myelitis attacks. The respective pain types and their relationships to myelitis attacks are illustrated in Figures 1 and 2.
Figure 1.
Flowchart illustrating current pain conditions in MOG-IgG-pos. NMOSD patients with and without a history of myelitis. n: number of patients; MOG-IgG-pos.: myelin-oligodendrocyte-glycoprotein-immunoglobulin-G-positive; NMOSD: neuromyelitis optica spectrum disorder. *Others: connective tissue disease, arthralgia of unknown origin, vasculitis.
Figure 2.
Flowchart of current pain conditions in AQP4-IgG-pos. NMOSD patients with and without a history of myelitis. AQP4-IgG-pos.: aquaporin-4-immunoglobulin-G-positive; n: number of patients; NMOSD: neuromyelitis optica spectrum disorder. *Others: connective tissue disease, arthralgia of unknown origin, vasculitis.
Current immunomodulatory treatment is listed in Table 1. Four MOG-IgG-pos. NMOSD patients (33%) and nine AQP4-IgG-pos. NMOSD patients (37.5%) had a pain treatment, whereas the majority of the patients with pain did not have any pain medication. All patients undergoing pain treatment still suffered from pain. Table 2 provides a list of the different pain medication classes.
Discussion
MOG-IgG-pos. NMOSD patients suffer from pain at a frequency comparable to AQP4-IgG-pos. NMOSD patients. The high prevalence of pain in more than 80% of AQP4-IgG-pos. NMOSD is consistent with previous findings.6,7,9
Frequent pain syndromes in NMOSD include neuropathic pain, spasticity-associated pain, musculoskeletal pain and headache.10,23,24 These pain syndromes were equally present in our cohort of MOG-IgG-pos. NMOSD patients. Moreover, we included patients with pain due to coexisting autoimmune-modulated diseases such as connective tissue disease and vasculitis as they may jointly emerge with and may be causally linked to NMOSD.1,4
Different dominant pain qualities between the groups might be generated by different underlying pathophysiological mechanisms in MOG-IgG-pos. and AQP4-IgG-pos. NMOSD.
Under healthy conditions the neuropeptide nerve growth factor (NGF) has a high affinity to tropomyosin receptor kinase A (TrkA). TrkA is expressed on unmyelinated nociceptive axons of the spinal cord and regulates synaptic strength and plasticity of sensory neurons.25 NGF has a high affinity to bind MOG, thus loss of MOG by antibody-mediated destruction in MOG-IgG-pos. NMOSD may cause abundant NGF-concentrations in the CNS. This might lead to aberrant sprouting of unmyelinated nociceptive fibers in the posterolateral tract of the spinal cord,26 causing chronic neuropathic pain. Depending on the level of the lesion, this process could lead to occipital neuralgia or to more distal neuropathic pain syndromes.
Furthermore, the brainstem is involved in up to one-third of MOG-IgG-pos. NMOSD patients.27 The brainstem is a critical region in the pathophysiology of migraine and trigeminal neuralgia.28,29 Cervicogenic headache can result from inflammatory lesions in the superior cervical spine leading to musculoskeletal dysfunction.24
In AQP4-IgG-pos. NMOSD the high frequency of spinal cord lesions might be a risk factor for neuropathic pain.8,10 In line with recent data by Jarius et al.,2 we show that APQ4-IgG-pos. NMOSD patients had a higher incidence of clinical myelitis compared to MOG-IgG-pos. NMOSD patients.
Under healthy conditions AQP4 is coexpressed with excitatory amino acid transporter 2. Loss of AQP4 in NMOSD may lead to an excessive accumulation of glutamate in the extracellular space, interrupting the glutamine-glutamate-GABA axis. This may disrupt the balance between excitation and inhibition in nociceptive pathways23 and lead to neuropathic pain in AQP4-IgG-pos. NMOSD.
Whereas MOG-IgG-pos. NMOSD patients characterized their pain experience mainly as throbbing, punishing and annoying, AQP4-IgG-pos. NMOSD patients described it as flashing, pricking and tingling. The more affective words used by MOG-IgG-pos. NMOSD patients might reflect the important influence of pain on their state of mind. MOG-IgG-pos. NMOSD patients with pain also showed a stronger impairment by depression than those without pain, as well as a positive correlation between pain intensity and depression grade, indicating a possible interaction of pain and mood disorders. Both symptoms are known to make the emergence and exacerbation of the other symptom more likely.30 Furthermore, differences in descriptive words could be related to the more common diagnosis of headaches in the MOG-IgG-pos. NMOSD patients. To our knowledge, however, a predominance of certain words to describe headache compared to other pain conditions is not provided in the literature. All patients showed a clear impairment of general health and strong restrictions caused by fatigue.31 MOG-IgG-pos. NMOSD patients with pain reported a higher impairment by fatigue than those without pain. This may be a neuropsychological effect whereby chronic pain could exhaust the patient and lead to fatigue. It could also be caused by a biochemical process, whereby increased levels of cytokines present in neuroimmunological diseases32 may contribute to the development of fatigue33,34 and pain.23 In addition, deep gray matter pathology, cortical atrophy and axonal damage in the CNS are presumably involved in the development of fatigue and chronic pain conditions in various diseases.35 Side effects of medication could also contribute to fatigue in pain patients.
We show that pain intensity in all patient groups correlated with physical and mental impairment of the patient’s hr-QoL. In contrast, the EDSS did not differ between patients with and without pain. Pain is not represented in the EDSS and therefore needs to be carefully examined using patient medical history and specific evaluation tools.
In line with previous NMOSD studies,7 we show that MOG-IgG-pos. NMOSD patients with pain are currently not effectively treated. Less than 40% of patients with pain in both diagnostic categories receive pain treatment, and even those were not pain free. Pain intensity, impairment of general health, and fatigue were even more severe in patients with pain treatment than in patients without. These findings might indicate that patients who complain about severe pain are more likely to receive pain medication than those who suffer from light pain; however, it seems that pain treatment is not effective in improving the patients’ QoL.
These alarming results show the necessity of exploring the efficacy of a multimodal and multidisciplinary approach to pain management in MOG-IgG-pos. NMOSD as well as in AQP4-IgG-pos. and AQP4/MOG-IgG-neg. NMOSD since pharmacologic interventions are currently insufficient.
Limitations
Results from single-center studies are not generalizable to a broader population, especially since sample sizes are small. Nonetheless, the overlap of our results in NMOSD patients with previously published data supports validity in a broader context. Patients who tested AQP4-IgG and MOG-IgG negative might show antibody positivity if tests were more sensitive, but fulfill the current criteria for antibody-negative NMOSD. Because of the small number of AQP4/MOG-IgG-neg. NMOSD patients, we used descriptive data only and did not include them in statistical comparisons. Finally, our results do not allow any certain pathophysiological conclusion.
Conclusions
Pain is a frequent and severe symptom of patients suffering from MOG-IgG-pos., AQP4-IgG-pos. and AQP4/MOG-IgG-neg. NMOSD. The vast majority of MOG-IgG-pos. and AQP4-IgG-pos. NMOSD patients suffer from pain. MOG-IgG-pos. NMOSD patients suffer from headache or neuropathic pain, whereas AQP4-IgG-pos. NMOSD patients mostly suffer from neuropathic pain. These findings might be linked to different underlying pathophysiological mechanisms in AQP4-IgG-pos. NMOSD patients, causative for neuropathic pain. Both patient groups describe their pain experience differently, while its impact on the patients’ QoL is similar. Pain is currently insufficiently controlled by medication. Only between one-third and one-half of patients receive pain medication and among those, not one is permanently free of pain. Therefore, it is important to systematically record pain, to carefully distinguish among different pain types, and to concentrate in future research on effective pain management in patients suffering from NMOSD.
Supplemental Material
Supplemental material, Supplemental Table1 for Pain in AQP4-IgG-positive and MOG-IgG-positive neuromyelitis optica spectrum disorders by Susanna Asseyer, Felix Schmidt, Claudia Chien, Michael Scheel, Klemens Ruprecht, Judith Bellmann-Strobl, Alexander U Brandt and Friedemann Paul in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Supplemental Material
Supplemental material, Supplemental Table2 for Pain in AQP4-IgG-positive and MOG-IgG-positive neuromyelitis optica spectrum disorders by Susanna Asseyer, Felix Schmidt, Claudia Chien, Michael Scheel, Klemens Ruprecht, Judith Bellmann-Strobl, Alexander U Brandt and Friedemann Paul in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Acknowledgements
We are grateful to Ivonne Hinz for excellent organizational assistance and to Susan Pikol and Cynthia Kraut for MRI assistance.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S. Asseyer has nothing to declare. F. Schmidt has received speaker honoraria from Genzyme. C. Chien has nothing to declare. M. Scheel holds a patent for manufacturing of phantoms for computed tomography imaging with 3D printing technology and has received research support from the Federal Ministry of Economics and Technology. K. Ruprecht has served on scientific advisory boards for Sanofi-Aventis/Genzyme, Novartis, and Roche; has received travel funding and/or speaker honoraria from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi-Aventis/Genzyme, Teva Pharmaceuticals, Novartis, and the Guthy Jackson Charitable Foundation; is an academic editor for PLoS One; receives publishing royalties from Elsevier; and has received research support from Novartis, Merck Serono, and the German Ministry of Education and Research. J. Bellmann-Strobl has received travel funding and speaking fees from Bayer Healthcare, Sanofi-Aventis/Genzyme, Merck, and Teva Pharmaceuticals. A.U. Brandt has served on the scientific advisory board for Biogen; has received travel funding and/or speaker honoraria from Novartis and Biogen; has patents pending for methods and systems for foveal and optic nerve head morphometry, perceptive visual computing-based postural control analysis, MS serum biomarkers, and perceptive sleep motion analysis; has consulted for Nexus and Motognosis; and has received research support from Novartis Pharma, Biogen Idec, BMWi, BMBF, and the Guthy Jackson Charitable Foundation. F. Paul serves on the scientific advisory board for Novartis; has received speaker honoraria and travel funding from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; is an academic editor for PLoS One; is an associate editor for Neurology® Neuroimmunology & Neuroinflammation; has consulted for Sanofi Genzyme, Biogen Idec, MedImmune, Shire, and Alexion; and has received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, the German Research Council, Werth Stiftung of the City of Cologne, the German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, the EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, the Guthy Jackson Charitable Foundation, and National Multiple Sclerosis of the USA.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a research grant from the German Research Foundation (DFG Exc. 257 to F.P.).
Supplemental material
Supplemental material is available for this article online.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation 2016; 13: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm 2015; 2: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarius S, Ruprecht K, Kleiter I.et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016; 13: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: A UK study. Brain 2017; 140: 3128–3138. [DOI] [PubMed] [Google Scholar]
- 6.Kanamori Y, Nakashima I, Takai Y, et al. Pain in neuromyelitis optica and its effect on quality of life: A cross-sectional study. Neurology 2011; 77: 652–658. [DOI] [PubMed] [Google Scholar]
- 7.Qian P, Lancia S, Alvarez E, et al. Association of neuromyelitis optica with severe and intractable pain. Arch Neurol 2012; 69: 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tackley G, Vecchio D, Hamid S, et al. Chronic neuropathic pain severity is determined by lesion level in aquaporin 4-antibody-positive myelitis. J Neurol Neurosurg Psychiatry 2017; 88: 165–169. [DOI] [PubMed] [Google Scholar]
- 9.Pellkofer HL, Havla J, Hauer D, et al. The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica. PLoS One 2013; 8: e71500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S, Mutch K, Elsone L, et al. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult Scler 2014; 20: 1658–1661. [DOI] [PubMed] [Google Scholar]
- 11.Uzawa A, Mori M, Kuwabara S. MOG antibody disorders and AQP4 antibody NMO spectrum disorders share a common immunopathogenesis. J Neurol Neurosurg Psychiatry Epub ahead of print 6 June 2018. DOI: 10.1136/jnnp-2018-318231. [DOI] [PubMed]
- 12.dos Passos GR, Oliveira LM, da Costa BK, et al. MOG-IgG-associated optic neuritis, encephalitis, and myelitis: Lessons learned from neuromyelitis optica spectrum disorder. Front Neurol 2018; 9: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitmann H, Biberacher V, Tiemann L, et al. Prevalence of neuropathic pain in early multiple sclerosis. Mult Scler 2016; 22: 1224–1230. [DOI] [PubMed] [Google Scholar]
- 14.Chavarro VS, Mealy MA, Simpson A, et al. Insufficient treatment of severe depression in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflammation 2016; 3: e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veauthier C, Gaede G, Radbruch H, et al. Poor sleep in multiple sclerosis correlates with Beck Depression Inventory values, but not with polysomnographic data. Sleep Disord 2016; 2016: 8378423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008; 70: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 17.Freynhagen R, Baron R, Gockel U, et al. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006; 22: 1911–1920. [DOI] [PubMed] [Google Scholar]
- 18.Epping R, Verhagen AP, Hoebink EA, et al. The diagnostic accuracy and test-retest reliability of the Dutch painDETECT and the DN4 screening tools for neuropathic pain in patients with suspected cervical or lumbar radiculopathy. Musculoskelet Sci Pract 2017; 30: 72–79. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R, Torgerson WS. On the language of pain. Anesthesiology 1971; 34: 50–59. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: Manual and interpretation guide. Lincoln, RI: QualityMetric Inc, 1993. [Google Scholar]
- 21.Kos D, Nagels G, D’Hooghe MB, et al. A rapid screening tool for fatigue impact in multiple sclerosis. BMC Neurol 2006; 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 23.Bradl M, Kanamori Y, Nakashima I, et al. Pain in neuromyelitis optica—prevalence, pathogenesis and therapy. Nat Rev Neurol 2014; 10: 529–536. [DOI] [PubMed] [Google Scholar]
- 24.Masters-Israilov A, Robbins MS. Headache in neuromyelitis optica. Curr Pain Headache Rep 2017; 21: 20. [DOI] [PubMed] [Google Scholar]
- 25.Bennett D, Shelton D, McMahon SB. Sequestration of endogenous nerve growth factor in the adult rat using a trkA-IgG fusion molecule blocks hyperalgesia associated with carrageenan inflammation. J Physiol 1995; 487P: 216P. [Google Scholar]
- 26.von Büdingen HC, Mei F, Greenfield A, et al. The myelin oligodendrocyte glycoprotein directly binds nerve growth factor to modulate central axon circuitry. J Cell Biol 2015; 210: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarius S, Kleiter I, Ruprecht K, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 3: Brainstem involvement—frequency, presentation and outcome. J Neuroimmunol 2016; 13: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kister I, Caminero AB, Herbert J, et al. Tension-type headache and migraine in multiple sclerosis. Curr Pain Headache Rep 2010; 14: 441–448. [DOI] [PubMed] [Google Scholar]
- 29.Russo A, Silvestro M, Tedeschi G, et al. Physiopathology of migraine: What have we learned from functional imaging? Curr Neurol Neurosci Rep 2017; 17: 95. [DOI] [PubMed] [Google Scholar]
- 30.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Med Rev 2014; 17: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.König HH, Bernert S, Angermeyer MC. Gesundheitszustand der deutschen Bevölkerung: Ergebnisse einer repräsentativen Befragung mit dem EuroQol-Instrument [Health status of the German population: Results of a representative survey using the EuroQol questionnaire] [in German]. Gesundheitswesen 2005; 67: 173–182. [DOI] [PubMed] [Google Scholar]
- 32.Uzawa A, Masahiro M, Kuwabara S. Cytokines and chemokines in neuromyelitis optica: Pathogenetic and therapeutic implications. Brain Pathol 2014; 24: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanson JB, Zéphir H, Collongues N, et al. Evaluation of health-related quality of life, fatigue and depression in neuromyelitis optica. Eur J Neurol 2011; 18: 836–841. [DOI] [PubMed] [Google Scholar]
- 34.Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 2017; 13: 662–675. [DOI] [PubMed] [Google Scholar]
- 35.Ruscheweyh R, Deppe M, Lohmann H, et al. Pain is associated with regional grey matter reduction in the general population. Pain 2011; 152: 904–911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Table1 for Pain in AQP4-IgG-positive and MOG-IgG-positive neuromyelitis optica spectrum disorders by Susanna Asseyer, Felix Schmidt, Claudia Chien, Michael Scheel, Klemens Ruprecht, Judith Bellmann-Strobl, Alexander U Brandt and Friedemann Paul in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Supplemental material, Supplemental Table2 for Pain in AQP4-IgG-positive and MOG-IgG-positive neuromyelitis optica spectrum disorders by Susanna Asseyer, Felix Schmidt, Claudia Chien, Michael Scheel, Klemens Ruprecht, Judith Bellmann-Strobl, Alexander U Brandt and Friedemann Paul in Multiple Sclerosis Journal—Experimental, Translational and Clinical