Abstract
Background:
Polycystic kidney disease (PKD) leads to progressive chronic kidney disease (CKD) with a subsequent risk of adverse events such as cardiac disease, infections, end-stage kidney disease (ESKD), and mortality.
Objectives:
To determine the risks of CKD-related adverse outcomes in patients with PKD compared with patients without PKD.
Setting:
Canadian study of prediction of death, dialysis and interim cardiovascular events (CanPREDDICT) was a prospective pan-Canadian cohort study from 2008-2013 involving 28 facilities with adjudicated outcomes.
Patients:
Adult CKD patients (estimated glomerular filtration rate [eGFR] = 15-45 mL/min/1.73 m2) under the care of a nephrologist.
Measurements:
Polycystic kidney disease as identified by the treating physician.
Methods:
Patients with PKD (PKD) and non-PKD were propensity score (PS) matched (1:4) using demographics, comorbidities, and laboratory values. We used conditional Cox proportional hazards models to examine the risk of cardiac disease (defined as coronary artery disease or congestive heart failure), infection, ESKD, or all-cause mortality in patients with PKD compared with no PKD.
Results:
Among a total of 2370 patients, 105 with PKD were matched with 416 without PKD with a baseline mean age and eGFR of 62.6 years and 27.8 mL/min, respectively. During 1680 person-years of follow time (median follow-up: 3.8 years), there were a total of 43 cardiac, 83 ESKD, 117 infectious, and 39 all-cause mortality events. PKD was associated with a higher risk of cardiac events (9.5% vs 7.9%, hazard ratio [HR] = 1.46, 95% confidence interval [CI] = 1.04-2.04) and ESKD (25.7% vs 13.5%, HR = 2.00, 95% CI = 1.33-3.01), and with similar risks for infection (21.9% vs 22.6%, HR = 1.16, 95% CI = 0.75-1.82) or all-cause mortality (6.7% vs 7.7%, HR = 0.87, 95% CI = 0.40-1.91) compared with non-PKD. There were no differences in the types of infections (urinary, respiratory, hematologic, or other) between the 2 groups (P = .585).
Conclusions:
Patients with PKD with advanced CKD are at a potentially higher risk of ESKD and cardiac events compared with patients without PKD. These findings, if confirmed in larger cohorts, suggest that monitoring and treatment for adverse outcomes in patients with PKD, especially related to cardiac disease, may be beneficial.
Keywords: polycystic kidney disease, chronic kidney disease, cardiac events, mortality, end-stage kidney disease, infection
Abrégé
Contexte:
La maladie polykystique des reins (MPR) mène à l’insuffisance rénale chronique (IRC) progressive, laquelle augmente à son tour la probabilité d’apparition de pathologies subséquentes, notamment cardiopathie, infections et insuffisance rénale terminale (IRT), ainsi que le risque de décès du patient.
Objectifs de l’étude:
L’étude visait à évaluer le risque de survenue d’une pathologie associée à l’IRC chez les patients atteints d’une MPR en comparaison avec les patients non atteints d’une MPR.
Cadre et type d’étude:
Il s’agit d’une étude de cohorte prospective pancanadienne, la CanPREDDICT (Canadian Study of Prediction of Death, Dialysis and Interim Cardiovascular Events). L’étude s’est tenue entre 2008 et 2013 au sein de 28 établissements, et les issues étaient rigoureusement définies et jugées par un comité.
Participants:
Des patients adultes atteints d’IRC (DFGe = 15-45 mL/min/1,73 m2) suivis par un néphrologue.
Mesure:
La maladie polykystique des reins (MPR) telle que diagnostiquée par le médecin traitant.
Méthodologie:
Le jumelage des patients atteints de la maladie polykystique rénale autosomique dominante (MPRAD) avec les sujets contrôles s’est fait sur la base du score de propension dans un rapport d’un pour quatre (1:4). Les autres critères employés pour le jumelage étaient le profil démographique des patients, leurs comorbidités et leurs résultats de laboratoire. Des modèles conditionnels des risques proportionnels de Cox ont été utilisés pour évaluer le risque
i) de développer une cardiopathie (coronopathie ou insuffisance cardiaque congestive) ou
ii) une infection ou
iii) une évolution vers l’IRT, de même que
iv) le risque de mortalité (toutes causes confondues), dans les deux groupes de patients.
Résultats:
Parmi les 2 370 participants, 105 atteints de MPR ont été jumelés à 416 patients non atteints. L’âge initial moyen des participants était de 62,6 ans et leur DFGe moyen se situait à 27,8 mL/min/1,73 m2. Au cours des 1 680 années-personnes de suivi (suivi moyen de 3,8 ans par patient), on a répertorié 43 cas de cardiopathie et 117 cas d’infection; 83 patients ont développé une IRT et 39 sont décédés (toutes causes confondues). La MPR a été associée à un risque plus élevé de cardiopathie (9,5 % [MPR] c. 7,9 % [non MPR]; RR : 1,46; IC à 95 % : 1,04-2,04) et d’évolution vers l’IRT (25,7 % [MPR] c. 13,5 % [non MPR]; RR : 2,00; IC à 95 % : 1,33-3,01) par rapport aux patients non atteints. Les risques d’infection (21,9 % [MPR] c. 22,6 % [non MPR]; RR : 1,16; IC à 95 % : 0,75-1,82) et de mortalité toutes causes confondues (6,7 % [MPR] c. 7,7 % [non MPR]; RR : 0,87; IC à 95 %; 0,40-1,91) se sont toutefois avérés similaires dans les deux groupes. De plus, aucune différence n’a été observée selon le type d’infection (urinaire, respiratoire, hématologique ou autre) entre les deux groupes (P = 0,585).
Conclusion:
Les patients atteints d’un stade avancé d’IRC et d’une MPR sont plus à risque d’évoluer vers l’IRT ou de développer une cardiopathie que les patients non atteints d’une MPR. Ces résultats, s’ils sont confirmés par des études sur cohortes plus nombreuses, suggèrent que le suivi et le traitement des pathologies associés à la MPR sur la santé des patients, particulièrement la cardiopathie, pourraient s’avérer bénéfiques.
What was known before
Polycystic kidney disease (PKD) is associated with progressive, incurable chronic kidney disease (CKD). Whether the risk of complications (end-stage kidney disease [ESKD], cardiac disease, infection, and death) related to CKD are similar in patients with PKD to without PKD is unknown.
What this adds
Patients with PKD and advanced CKD in Canada have a higher risk of ESKD and cardiac events and comparable risks of infections and death compared with similar patients without PKD.
Introduction
Polycystic kidney disease is a commonly inherited disorder resulting in kidney disease, affecting roughly 1:1000 to 1:400 people worldwide.1 Polycystic kidney disease leads to a progressive, often predictable decline in kidney function over decades, ultimately resulting in ESKD.2 As such, patients with PKD may experience long periods of time with nondialysis-dependent CKD.3 Despite this, the majority of research has focused on PKD-related complications such as hematuria, pain, abdominal distension, and hypertension.4 Conversely, the risks of well-established CKD complications such as cardiac disease and infections are not as well known.
Chronic kidney disease–related adverse outcomes are well established; these include, but are not limited to, infection, ischemic heart disease, congestive heart failure (CHF), CKD progression, and death.5-8 Chronic kidney disease–related adverse outcomes confer a significant increase in the risk of mortality and morbidity in patients with ESKD, regardless of the etiology.9-11 However, not all patients with CKD appear to be at a similar risk of developing these adverse outcomes. As patients with PKD are often younger with fewer comorbid illnesses than other patients with CKD, it follows that their subsequent risk of adverse outcomes, including ESKD, may differ compared with that of patients with CKD due to other etiologies. Better characterization of these risks in patients with PKD could aid in determining appropriate monitoring and identifying potentially modifiable risk factors to reduce these risks and their burden to patients, families, and the health care system.
In this study, using the Canadian study of Prediction of Death, Dialysis and Interim Cardiovascular events (CanPREDDICT) prospective cohort, we set out to examine the risk of adverse outcomes of patients with PKD and advanced CKD relative to those with advanced CKD and without PKD. We hypothesized that for patients with PKD, the risk of adverse cardiac events, infections, and all-cause mortality would be lower, but the risk of ESKD would be higher, relative to those with CKD and without PKD.
Materials and Methods
Participants
In this study, we used data from the CanPREDDICT prospective cohort study.12 CanPREDDICT was a multicenter observational study of non-dialysis CKD patients cared for by nephrologists across Canada from 2008-2009. Patients were followed for 3 years at 6-month intervals then annually up to 5 years. Inclusion criteria were patients with an estimated glomerular filtration rate (eGFR) 15 to 45 mL/min/1.73 m2. Patients were excluded if they had a functioning kidney or other solid organ transplant, acute vasculitis requiring immunosuppressive therapy, or a life expectancy of less than 6 months. The full study description with enrollment criteria and baseline characteristics has previously been described.13 All study participants gave signed consent and regional ethics board approval was granted by the Ottawa Hospital.
Sociodemographic, clinical, and laboratory data were collected at the initial visit. These included sex, age (years), ethnicity, presence of diabetes, as well as cardiac, cerebrovascular, and peripheral vascular disease. Laboratory parameters recorded at baseline included serum eGFR (mL/min/1.73 m2 by Modification of Diet in Renal Disease (MDRD)), serum phosphate (mEQ/L), albumin (g/L), and urine albumin to creatinine ratio (ACR) (mg/mmol).14
Exposure
The exposure of interest was PKD as identified by the treating physician.
Study Outcomes
Adjudicated study outcomes were cardiac ischemia, CHF, ESKD, and mortality. Exact dates of the outcomes were available. Cardiac ischemia was defined as fatal or non-fatal myocardial infarction (MI), need for coronary revascularization (coronary artery bypass graft or percutaneous coronary intervention or percutaneous transluminal coronary angioplasty), and unstable angina (chest pain and absence of infarction shown on electrocardiogram [ECG], dynamic troponin or creatine kinase changes, or treatment with intravenous heparin). Congestive heart failure was defined as dyspnea plus 2 of the following: bibasilar rales, raised jugular venous pressure, or chest x-ray with evidence of interstitial or alveolar pulmonary edema. A panel of 3 physicians comprising a nephrologist, a cardiologist, and a neurologist independently adjudicated all cardiac outcomes based on source documentation. End-stage kidney disease initiation date was recorded at 3 months after commencing renal replacement therapy (RRT) (except in patients who suffered a death, who received a transplant <3 months of initiation of RRT, or were on their final visit) to avoid misclassification of transient dialysis use followed by recovery. Deaths were included if they occurred <3 months after commencing ESKD. The criteria of an infectious episode included one of the following: (1) positive culture, (2) prescription of antibiotics, or (3) requiring hospitalization for infection. Infections were classified by anatomical location as respiratory, urinary, bacteremia (blood culture positive), and other (including those whose anatomical location was not clear).
We censored all patients following an organ transplant, who were lost to follow-up, who withdrew from the study, and at the onset of ESKD. If no event occurred, the last visit date was taken as the end date. For patients who withdrew or were lost to follow-up, the previous visit date was taken as the censoring date.
Statistical Analyses
We used standardized differences to assess differences in baseline characteristics between patients with CKD by PKD status. Standardized differences describe differences between group means relative to the pooled standard deviation and are less sensitive to large sample sizes than traditional hypothesis testing.15 A difference of >10% was considered meaningful. We used propensity score (PS) matching followed by Cox proportional hazards model to examine the association of PKD and the outcomes of interest.16 The following variables were included in the PS model: age (per year), sex, comorbidities at baseline (diabetes, MI, coronary artery disease, peripheral vascular disease), laboratory values (eGFR, phosphate, albumin, urine ACR), systolic and diastolic blood pressure (BP), and medications (antiplatelet agents, statins, beta-blockers, angiotensin converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARB]). Patients with PKD were matched with no replacement 1:4 to patients without PKD on the logit of the PS (caliper of 0.01).
The association of PKD and each outcome of interest were examined by Cox proportional hazards models. The date of the first study visit was used as start of the follow-up time and the time to first outcome (cardiac, infection, ESKD) was modeled. Models accounted for clustering due to matching.17 In a sensitivity analysis, imbalanced covariates with standardized differences >0.10 post–PS match (diastolic BP, acetylsalicylic acid [ASA], ACE inhibitor, ARB; see Table 1) were added to the model. All statistical analyses were performed using SAS v9.4. A P value of < .05 was considered significant.
Table 1.
Baseline Characteristics of Patients With PKD Compared With Those Without for the Total Cohort and PS-Matched Cohort.
| Variable | Total cohort |
SDiff | PS matched |
SDiff | |||
|---|---|---|---|---|---|---|---|
| Total | PKD | Non-PKD | PKD | Non-PKD | |||
| N | 2370 | 105 (4.4) | 2265 (95.6) | 105 | 416 | ||
| Age (years, SD) | 68 (13) | 62.7 (13.0) | 68.3 (12.6) | 0.43 | 62.7 (13.0) | 62.5 (14.7) | 0.02 |
| Sex, n (% male) | 1477 (62) | 60 (57.1) | 1417 (62.6) | 0.11 | 60 (57.1) | 233 (56.0) | 0.02 |
| Race, n (% Caucasian) | 2108 (89) | 92 (87.6) | 2016 (89.0) | 0.04 | 92 (87.6) | 365 (87.7) | 0.00 |
| Cause of CKD | |||||||
| Diabetes, n (%) | 673 (29.7) | 40 (9.6) | |||||
| Ischemia, n (%) | 610 (26.9) | 106 (25.5) | |||||
| Glomerulonephritis, n (%) | 273 (12.1) | 98 (23.6) | |||||
| Pyelonephritis/interstitial, n (%) | 107 (4.7) | 33 (7.9) | |||||
| Other, n (%) | 281 (12.4) | 71 (17.1) | |||||
| Unknown, n (%) | 321 (14.2) | 68 (16.3) | |||||
| Diabetes, n (%) | 1133 (48%) | 15 (14.3) | 1118 (49.4) | 0.81 | 15 (14.3) | 66 (15.9) | 0.04 |
| Cardiovascular disease, n (%) | 1055 (44%) | 22 (21.0) | 1033 (45.6) | 0.37 | 22 (21.0) | 114 (27.4) | 0.04 |
| Ischemic, n (%) | 784 (33%) | 18 (17.1) | 766 (33.8) | 0.39 | 18 (17.1) | 83 (20.0) | 0.07 |
| Congestive heart failure, n (%) | 617 (26%) | 13 (12.4) | 604 (26.7) | 0.37 | 13 (12.4) | 57 (13.7) | 0.04 |
| Peripheral vascular disease, n (%) | 464 (20%) | 14 (13.3) | 450 (19.9) | 0.37 | 14 (13.3) | 50 (12.0) | 0.04 |
| No. of comorbidities (SD) | 1.1 (1.4) | 0.4 (1.0) | 1.1 (1.4) | 0.58 | 0.4 (1.0) | 0.5 (1.0) | 0.07 |
| eGFR in mL/min per 1.73 m2 (SD) | 28.2 (9) | 27.3 (9.0) | 28.2 (9.0) | 0.11 | 27.3 (9.0) | 28.0 (9.0) | 0.08 |
| Albumin in g/L (SD) | 40.0 (4) | 41.2 (3.5) | 40.4 (4.2) | 0.19 | 41.2 (3.5) | 41.5 (4.0) | 0.09 |
| Urine ACR in mg/mmol (IQR) | 79.1 (153.4) | 44.5 (80.5) | 80.7 (155.8) | 0.29 | 44.5 (80.5) | 38.3 (83.7) | 0.08 |
| Systolic BP in mm Hg (SD) | 133.7 (19.7) | 128.3 (16.1) | 134.0 (19.8) | 0.31 | 128.3 (16.1) | 129.9 (18.0) | 0.09 |
| Diastolic BP in mm Hg (SD) | 71.0 (11.8) | 74.0 (11.8) | 70.9 (11.8) | 0.27 | 74.0 (11.8) | 74.0 (10.9) | 0.12 |
| Medications | |||||||
| ASA, n (%) | 1272 (53.7) | 36 (34.3) | 1276 (54.6) | 0.42 | 36 (34.3) | 36 (40.9) | 0.14 |
| ACE inhibitors, n (%) | 1058 (44.6) | 49 (46.7) | 44.5 (53.0) | 0.42 | 49 (46.7) | 175 (42.1) | 0.14 |
| ARB, n (%) | 882 (37.2) | 29 (27.6) | 853 (37.7) | 0.22 | 29 (27.6) | 142 (34.1) | 0.14 |
| Beta-blocker, n (%) | 1082 (45.7) | 38 (36.2) | 1044 (46.1) | 0.20 | 38 (36.2) | 141 (33.9) | 0.05 |
| Statin, n (%) | 1598 (67.4) | 58 (55.2) | 1540 (68.0) | 0.26 | 58 (55.2) | 243 (58.4) | 0.06 |
Note. Bold P values denote statistically significant standardized differences (SDiff > 0.2). PKD = polycystic kidney disease; SDiff = standardized difference; PS matched = propensity score matched; eGFR = estimated glomerular filtration rate; ACR = albumin to creatinine ratio; IQR = interquartile range; BP = blood pressure; ASA = acetylsalicylic acid; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blocker.
Results
Based on inclusion and exclusion criteria, the study cohort consisted of 105 patients with PKD and 2265 without PKD. The analytic cohort consisted of 105 patients with PKD and 416 without PKD who were PS-matched (1:4). There were 4 patients with PKD for whom only 3, rather than 4, matches were identified. The median and total follow-up times were 3.8 (interquartile range [IQR]: 2.7-4.0) and 1680 years, respectively.
Baseline characteristics of the study cohort included in this study are depicted in Table 1. Prior to PS matching, there were statistically significant differences across all covariates except for race. The PKD group was younger (63 vs 68 years old), with a lower proportion of diabetes (14% vs 49%), cardiovascular disease (21 vs 45.6), peripheral vascular disease (13.3% vs 19.9%), and fewer total mean comorbidities (0.4 vs 1.1). Serum albumin (mean 41.2 vs 40.4) and diastolic BP (74 vs 70.9) were higher in the PKD group, whereas urine ACR (44.5 vs 80.7) and systolic BP (128.3 vs 134) were lower. There was lower use of all medications in the PKD group. After PS matching, there was no statistically significant difference between the age (62.7 vs 62.5), diabetes (14.3 vs 15.9%), cardiac disease (21 vs 27.4), peripheral vascular disease (13.3 vs 12%), and number of comorbidities (0.4 vs 0.5). Similarly, serum albumin, urine ACR ratio, systolic BP, as well as beta-blocker and statin usage were similar between the groups. Standardized differences greater than 0.10 persisted for diastolic BP (11.8 vs 10.9, SD = 0.12), ASA use (34.3 vs 40.9 SD = 0.14), ACE inhibitors (46.7 vs 42.1%, SD = 0.14), and ARB (27.6 vs 34.1%, SD = 0.14).
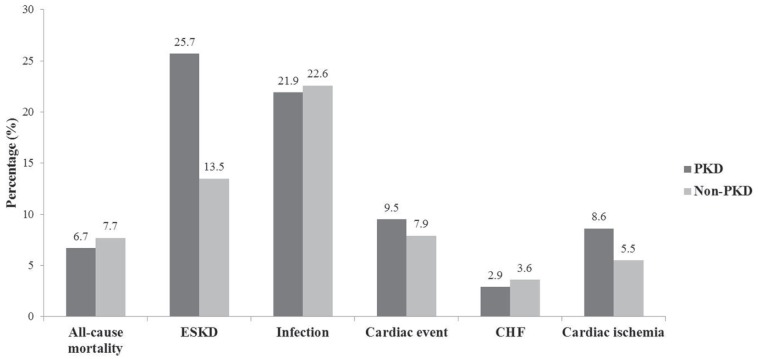
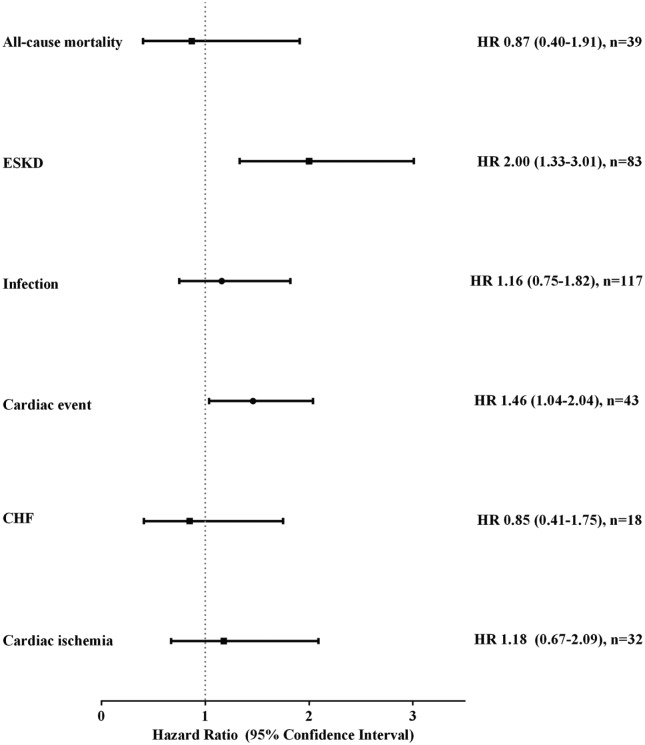
During the study period, 25.7% (n = 27) of patients with PKD developed ESKD as compared with 13.5% (n = 56) of patients without PKD (Figure 1). For patients with PKD, 9.5% had any cardiac event (8.6% [n = 9] cardiac ischemia and 2.9% [n = 3] CHF). For patients without PKD, 7.9% experienced a cardiac event (5.5% [n = 23] cardiac ischemia and 3.6% [n = 15] CHF). In total, the all-cause mortality was 6.7% of patients with PKD (n = 7 from 105 total) versus 7.7% for those without PKD patients (n = 32 from 416 total). In time to event analyses, PKD was independently associated with ESKD (hazard ratio [HR] = 2.00, 95% confidence interval [CI] = 1.33-3.01) and any cardiac event (HR = 1.46, 95% CI = 1.04-2.04). Any infection (HR = 1.16, 95% CI = 0.75-1.82), cardiac ischemia (HR = 1.18, 95% CI = 0.67-2.09), CHF (HR = 0.85, 95% CI = 0.41-1.75), and all-cause mortality (HR = 0.87, 95% CI = 0.40-1.91) were similar between the 2 groups (see Figure 2). These findings were consistent in a sensitivity analysis with adjusting for imbalance post–PS match (Supplementary Table 1).
Figure 1.
Proportion of clinical outcomes in CKD patients with PKD compared with those without.
Note. CKD = chronic kidney disease; PKD = polycystic kidney disease; ESKD = end-stage kidney disease; CHF = congestive heart failure.
Figure 2.
Proportion of types of infections in CKD patients with PKD compared with those without.
Note. CKD = chronic kidney disease; PKD = polycystic kidney disease.
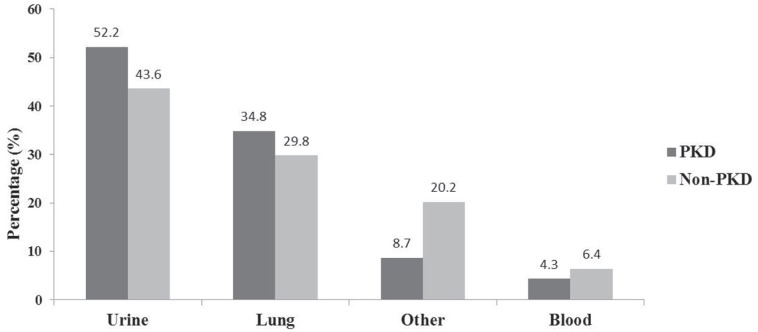
A comparison of the proportions of infection types are presented in Figure 3. Twelve patients with PKD experienced a urinary tract infection (UTI) (52.2% of total PKD infections) and 8 experienced pneumonia (34.8%). Of the 23 patients with PKD, 1 developed bacteremia. For those without PKD, 43.6% developed a UTI, 29.8% pneumonia, and 6.4% bacteremia. Other infections (non-UTI/bacteremia/pneumonia) affected 8.7% of patients with PKD (n = 2) and 20.2% of those without PKD (n = 19). There were no statistically significant differences between the 2 groups for infection types.
Figure 3.
Propensity score matched hazard ratio (95% confidence interval) of clinical outcomes among patients with PKD (Non-PKD referent).
Note. PKD = polycystic kidney disease; HR = hazard ratio; n = number of events; ESKD = end-stage kidney disease; CHF = congestive heart failure.
Discussion
In this national prospective cohort study of patients with advanced CKD being followed by a nephrologist (CanPREDDICT), we found key differences in the risks of adverse outcomes for those with PKD. After matching for a broad number of covariates, we demonstrated a higher risk of ESKD, and a comparable risk of cardiovascular events, infections, and all-cause mortality. There were no apparent differences in the types of infections that occurred. Surprisingly, the composite outcome of cardiac events (ischemic events and CHF exacerbations) was higher in patients with PKD (HR = 1.46, 95% CI = 1.04-2.04) albeit with a small number of overall events. Overall, our findings illustrate that non-ESKD adverse events are comparable between patients with advanced CKD with and without PKD.
Although patients with PKD are often younger with fewer comorbid illnesses, we observed an increased risk of cardiac events in patients with PKD compared with those without. This is consistent with evidence suggesting patients with PKD are predisposed to developing coronary artery disease, valvular heart disease, and cardiomyopathies.18 Chapman et al reported the presence of left ventricular hypertrophy in 36% of patients with PKD compared with general population controls.19 In a self-reported survey study with a low response rate, Helal et al found cardiovascular disease was highly prevalent in PKD with the most common causes being arrhythmia (25.9%), peripheral vascular disease (16.5%), valvular heart disease (14.4%), and MI (6%).20 In an autopsy study consisting mostly of patients with PKD who had ESKD (77%), cardiac hypertrophy was present in 89% and coronary artery disease in 81%. Recently, in a small series of patients with PKD with echocardiographic data, idiopathic dilated cardiomyopathy was observed in 5.8% and hypertrophic obstructive cardiomyopathy in 2.5%.21 On dialysis, cardiac causes are the most common cause of death for both patients with and without PKD; however, cardiac mortality has been reported to be declining steadily among those with PKD.22 Our findings further support the notion that patients with PKD may be at a particularly increased risk of cardiac events compared with those with CKD due to other etiologies.
As cardiac complications are emerging as a major cause of morbidity and mortality in patients with PKD, aggressive treatment of hypertension, early diagnosis, and clinical intervention for such risks are beneficial. Early detection of hypertension may play a role as its onset precedes the loss of kidney function in the majority of patients with PKD.23 The role of screening echocardiography and ECG early in the course of disease and serially needs to be defined through further study. Finally, the role of prophylactic cardiovascular medications may offset the burden of cardiac disease. Patients with PKD in our cohort were less likely to be on ASA, ARB, and/or statins post-match suggesting the risk of cardiac disease may be under recognized by physicians.
The development of ESKD is a significant and predictable complication of PKD.3,24,25 Hateboer et al investigated the difference between the age of onset of ESKD or death in ADPKD individuals with PKD1 or PKD2 mutations as compared with healthy family members (controls).26 The median age at death or onset of ESKD was 53.0 years in individuals with PKD1 (35% of their cohort), 69.1 years in those with PKD2 (14%), and 78.0 years in controls. Recent reports suggest a higher prevalence of ESKD among patients with PKD compared with our cohort of 21.9%. Possibilities for this discrepancy include shorter follow-up time of the CanPREDDICT cohort study (5 years) or the inclusion of patients with less severe types of PKD.22,27
Kidney infection is a common reported occurrence in PKD (30%-50% lifetime risk) and often leads to serious complications, including perinephric abscess, acute kidney injury (AKI), septicemia, and death.28-30 Hwang et al examined 49 patients with ADPKD (19 men and 30 women) and reported the occurrences of UTI as 26% in PKD compared with 0% in healthy controls. Interestingly, we did not find any difference in the risk or type of infections in our study population as compared with other patients with non-PKD. We previously reported that infectious episodes in CKD were common and conferred a high risk of subsequent adverse outcomes.6 Possible reasons for the lack of difference observed in our study include the limited number of infectious episodes and its subtypes. In summary, while the outcomes of infections in patients with PKD may be more deleterious, we did not find any difference in the overall incidence of infections in patients with CKD with or without PKD.
We found no difference in all-cause mortality between the 2 groups. To date, there are few reports investigating mortality in patients with PKD and CKD. A study from Taiwan analyzed an adult cohort of PS matched 1:3 patients with ESKD due to ADPKD compared with those with non-ADPKD.31 The aim of this study was to compare the outcomes between patients on peritoneal dialysis (PD) with ADPKD and without ADPKD. They found no difference in overall mortality. Peritoneal dialysis patients with ADPKD were found to be comparable with PD patients without ADPKD in terms of risk of death and hospitalization in the present study. In another study that looked at 501 Taiwanese patients, there was no significant difference found in survival rates between patients with ESKD with and without ADPKCD.32 While there is a lower incidence of ADPKD in Taiwan compared to North America, these 2 studies do support our findings that there is no difference in all-cause mortality between patients with CKD with and without ADPKD.
Limitations of our study include the small number of events in certain categories, the inability to distinguish types of PKD, and the lack of other relevant PKD-related outcomes such as hematuria or pain. Although our PS-matched groups were well balanced, there is the possibility of residual confounding as important variables such as functional status and eGFR changes over time were not captured. Although we had access to a large Canadian cohort of CKD patients with PKD, the absolute numbers of patients in each outcome group was small, making statistical significance of our findings limited. Despite this limitation, as the outcomes were adjudicated, we are confident in their accuracy. We were not able to determine the severity of an infection or cardiac event. ESKD outcomes did not include patients conservatively managed for ESKD as opposed to initiating dialysis; thus, we have also technically underestimated the number of patients in whom ESKD developed and this may have differed between patients with PKD and those without. Future studies involving more patients will be needed to confirm our findings. Other limitations of our study were related to our study design being a prospective observational (cohort) analysis: there was the potential for selection bias and incomplete data collection.
In conclusion, in a large, prospective cohort of patients with PKD and advanced CKD, we found a higher risk of ESKD and cardiac events and similar risk of all-cause mortality and infection risk between matched patients. Future areas of research should focus on validating these results in other cohorts. Our findings underscore the high risk of cardiac events in patients with PKD. These findings are particularly surprising in the context of the current recommendations for aggressive BP reduction in patients with PKD using ACE inhibitors as first line therapy. Further research is needed to understand the pathophysiological underpinnings of this association. In addition, our findings suggest that an increased emphasis on the prevention, monitoring, and treatment of cardiac disease in patients with PKD may be warranted.
Supplementary Material
Supplementary Material, Supplementary_table_and_appendix for The Risk of Adverse Events in Patients With Polycystic Kidney Disease With Advanced Chronic Kidney Disease by Sonali de Chickera, Ayub Akbari, Adeera Levin, Mila Tang, Pierre Brown, Ognjenka Djurdev, Mohan Biyani, Edward G. Clark and Manish M. Sood in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: All study participants gave signed consent and regional ethics board approval was granted by the Ottawa Hospital.
Consent for Publication: Consent for publication was obtained from all authors.
Availability of Data and Materials: Not available
Authors’ Note: The investigators have complete control of study design, data, and analytics. All analyses were conducted at the University of Ottawa. This paper was presented in abstract form at American Society for Nephrology Kidney Week 2017.
Authors’ Contributions: Research idea: MMS AA; study design: MMS AA; statistical analysis: MMS; writing of introduction and discussion: SD; writing of methods, results, figures and tables: SD MMS; mentorship: MMS; editing and review: AA, AL, MT, PB, OD, MB, EC.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Manish M Sood is supported by the Jindal Research Chair for the Prevention of Kidney Disease.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding of this study was provided by Otsuka Pharmaceuticals and for CanPREDDICT by Janssen Canada Inc, both as unrestricted grants.
References
- 1. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287-1301. [DOI] [PubMed] [Google Scholar]
- 2. Cornec -Le Gall E, Audrézet M-P, Rousseau A, et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;27:942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choukroun G, Itakura Y, Albouze G, et al. Factors influencing progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;6(6):1634-1642. [DOI] [PubMed] [Google Scholar]
- 4. Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):153-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17-28. [DOI] [PubMed] [Google Scholar]
- 6. Cheikh Hassan HI, Tang M, Djurdjev O, Langsford D, Sood MM, Levin A. Infection in advanced chronic kidney disease leads to increased risk of cardiovascular events, end-stage kidney disease and mortality. Kidney Int. 2016;90(4):897-904. [DOI] [PubMed] [Google Scholar]
- 7. Cai Q-Z, Lu X-Z, Lu Y, Wang AY. Longitudinal changes of cardiac structure and function in CKD (CASCADE study). J Am Soc Nephrol. 2014;25(7):1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050-1065. [DOI] [PubMed] [Google Scholar]
- 9. Sud M, Tangri N, Pintilie M, Levey AS, Naimark D. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation. 2014;130:458-465. [DOI] [PubMed] [Google Scholar]
- 10. Sud M, Tangri N, Pintilie M, Levey AS, Naimark DMJ. ESRD and death after heart failure in CKD. J Am Soc Nephrol. 2015;26(3):715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034-2047. [DOI] [PubMed] [Google Scholar]
- 12. Langsford D, Tang M, Cheikh Hassan HI, Djurdjev O, Sood MM, Levin A. The association between biomarker profiles, etiology of chronic kidney disease, and mortality. Am J Nephrol. 2017;45(3):226-234. [DOI] [PubMed] [Google Scholar]
- 13. Levin A, Rigatto C, Brendan B, et al. Cohort profile: Canadian study of prediction of death, dialysis and interim cardiovascular events (CanPREDDICT). BMC Nephrol. 2013;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470. [DOI] [PubMed] [Google Scholar]
- 15. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat-Simul C. 2009;38(6):1228-1234. [Google Scholar]
- 16. Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84(1):151-161. [Google Scholar]
- 17. Harrell FE. Multivariable modeling strategies. In: Harrell FE, ed. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Cham, Switzerland: Springer; 2015:63-102. [Google Scholar]
- 18. Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr Hypertens Rev. 2013;9(1):2-11. [DOI] [PubMed] [Google Scholar]
- 19. Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8(8):1292-1297. [DOI] [PubMed] [Google Scholar]
- 20. Helal I, Reed B, Mettler P, et al. Prevalence of cardiovascular events in patients with autosomal dominant polycystic kidney disease. Am J Nephrol. 2012;36(4):362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5(12):2048-2056. [DOI] [PubMed] [Google Scholar]
- 22. Spithoven EM, Kramer A, Meijer E, et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(suppl 4):iv15-iv25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):153-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7(3):479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schrier RW, Brosnahan G, Cadnapaphornchai MA, et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol. 2014;25(11):2399-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hateboer N, v Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353(9147):103-107. [DOI] [PubMed] [Google Scholar]
- 27. Hwang Y-H, Conklin J, Chan W, et al. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(6):1861-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwab SJ, Bander SJ, Klahr S. Renal infection in autosomal dominant polycystic kidney disease. Am J Med. 1987;82(4):714-718. [DOI] [PubMed] [Google Scholar]
- 29. Neuville M, Hustinx R, Jacques J, Krzesinski JM, Jouret F. Diagnostic algorithm in the management of acute febrile abdomen in patients with autosomal dominant polycystic kidney disease. PLoS One. 2016;11(8):e0161277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sklar AH, Caruana RJ, Lammers JE, Strauser GD. Renal infections in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1987;10(2):81-88. [DOI] [PubMed] [Google Scholar]
- 31. Yang JY, Chen L, Chao CT, et al. Outcome comparisons between patients on peritoneal dialysis with and without polycystic kidney disease: a nationwide matched cohort study. Medicine (Baltimore). 2015;94(48):e2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee PW, Chien CC, Yang WC, Wang JJ, Lin CC. Epidemiology and mortality in dialysis patients with and without polycystic kidney disease: a national study in Taiwan. J Nephrol. 2013;26(4):755-762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material, Supplementary_table_and_appendix for The Risk of Adverse Events in Patients With Polycystic Kidney Disease With Advanced Chronic Kidney Disease by Sonali de Chickera, Ayub Akbari, Adeera Levin, Mila Tang, Pierre Brown, Ognjenka Djurdev, Mohan Biyani, Edward G. Clark and Manish M. Sood in Canadian Journal of Kidney Health and Disease