Summary
In order to determine the impact of fermentation on protein quality, pea protein concentrate (PPC) was fermented with Lactobacillus plantarum for 11 h and total phenol and tannin contents, protease inhibitor activity, amino acid composition and in vitro protein digestibility were analyzed. Phenol levels, expressed as catechin equivalents (CE), increased on dry mass basis from 2.5 at 0 h to 4.9 mg CE per 1 g of PPC at 11 h. Tannin content rose from 0.14 at 0 h to a maximum of 0.96 mg CE per 1 g of PPC after 5 h, and thereafter declined to 0.79 mg/g after 11 h. After 9 h of fermentation trypsin inhibitor activity decreased, however, at all other fermentation times similar levels to the PPC at time 0 h were produced. Chymotrypsin inhibitor activity decreased from 3.7 to 1.1 chymotrypsin inhibitory units (CIU) per mg following 11 h of fermentation. Protein digestibility reached a maximum (87.4%) after 5 h of fermentation, however, the sulfur amino acid score was reduced from 0.84 at 0 h to 0.66 at 11 h. This reduction in sulfur content altered the in vitro protein digestibility-corrected amino acid score from 67.0% at 0 h to 54.6% at 11 h. These data suggest that while fermentation is a viable method of reducing certain non-nutritive compounds in pea protein concentrate, selection of an alternative bacterium which metabolises sulfur amino acids to a lesser extent than L. plantarum should be considered.
Key words: pea protein concentrate, fermentation, non-nutritive compounds, protein digestibility, protein quality
Introduction
Pulses represent a nutritionally and economically viable protein source in developing countries where the consumption of animal proteins is scarce and expensive (1). In developed countries, pulses are often considered as alternative sources of protein, particularly as key ingredients in vegan foods. The classes of pulse crops include peas, chickpeas, lentils, faba beans and dry beans. Pulses contain significant quantities of proteins, carbohydrates and micronutrients, and are generally low in fat. With respect to amino acid content, pulses tend to have limited amount of thiol-containing amino acids (i.e. cysteine and methionine) and are rich in lysine compared to human nutritional requirements, whereas cereals have limited lysine content and high sulfur amino acid content (2). For this reason, pulse crops are often consumed alongside cereal grains as the two provide complementary amino acid profiles. Currently, the FAO/WHO defines protein quality in terms of the amino acid profile and digestibility of a protein source based on an in vivo bioassay (3). This definition of protein quality could be broadened further to include protein functionality, as in addition to their nutritional attributes, it is essential for protein ingredients to perform well in food product design.
Dehulling, soaking and thermal treatments (e.g. cooking) are often used in the preparation of pulses for human consumption. In addition to improving their palatability, cooking can reduce non-nutritive compounds that can have a negative effect on protein digestion and nutrient absorption. After cooking pulses for 3 h, the methionine, tyrosine and threonine content of beans has been shown to decrease (4). Although not new technology, fermentation is re-gaining popularity as consumers seek out everyday foods with improved nutritional value, and can be used in either commercial/industrial environments or at the household level in developing countries as a means of improving food security. Fermentation has been used on pulses involving the raw seed (5-7), flour (8-12) or protein isolates (13, 14). This may involve a solid-state batch fermentation or a submerged fermentation process involving protease-producing bacteria or fungi. Fermentation can improve the protein digestibility of pulses by reducing the levels of non-nutritive compounds that inhibit digestive enzymes (e.g. trypsin and chymotrypsin inhibitors) and promote protein crosslinking (e.g. phenolic and tannin compounds), as well as through the production of microbial proteases, which partially degrade and release some of the proteins from the matrix (9, 15, 16). Hemalatha et al. (17) reported that fermentation also improved mineral bioavailability, as microbial metabolism generates organic acids, which then form soluble complexes with mineral compounds preventing the formation of insoluble mineral-phytate complexes. In addition, fermentation can be employed with or without heating; thus it can bypass the loss of nutrients seen in cooking processes.
The overall goal of the present study is to examine the impact of Lactobacillus plantarum fermentation of pea protein concentrate on protein digestibility as well as the quantity and activity of certain non-nutritive compounds. While the recommended method for determining protein quality is a rodent bioassay (3), there is a tendency for reducing animal experimentation. For that reason, an in vitro method for determining protein digestibility, which has shown good correlation with in vivo values, was used (18). Pea protein is considered an emerging alternative to soy protein, due to it not being genetically modified and having lower allergenicity issues than soy. Development of technology for producing more nutritious fermented pea protein products is highly desirable by industry and the consumer.
Materials and Methods
Materials
Pea protein concentrate (PPC) was kindly donated by Parrheim Foods (Saskatoon, SK, Canada), De Man, Rogosa, Sharpe (MRS) broth was purchased from Oxoid Co. (Nepean, ON, Canada), whereas all other chemicals used were of reagent grade and purchased from Thermo Fisher Scientific (Ottawa, ON, Canada). Water used in this research was produced using a Milli-Q® water purification system (Millipore, Etobicoke, ON, Canada). Lactobacillus plantarum NRRL B-4496 was obtained from the Agricultural Research Service Culture Collection, USDA (Peoria, IL, USA).
Fermentation
Fermentation of PPC ingredients was performed at 32 °C over 11 h under anaerobic conditions using L. plantarum NRRL B-4496. In brief, L. plantarum cells were cultivated until the late exponential phase of growth (approx. 10 h), collected by centrifugation (centrifuge model 5810R; Eppendorf, Mississauga, ON, Canada) at 10 000´g for 20 min at 4 °C, and then washed twice with sterile peptone solution. The resulting pellet was used to inoculate an Erlenmeyer flask containing 25% by mass per volume of PPC solution (400 mL), at a concentration of 7 log CFU per g of PPC, and thereafter incubated under anaerobic conditions at 32 °C for 11 h. Anaerobic conditions were maintained by placing the experiments within a rectangular jar with AnaeroGen™ anaerobic gas-generating kits (Thermo Fisher Scientific, Miami, FL, USA). Aliquots (60 mL) were taken at times 0, 1, 5, 9 and 11 h of fermentation, and then freeze-dried for 48 h using a freeze dryer (Labconco, FreeZone 12, Kansas City, MO, USA). All dried samples were then ground using a coffee grinder (Custom Grind™, model 80365; Hamilton Beach, Glen Allen, VA, USA) and stored under refrigeration temperature (4 °C) for further use. Fermentation experiments were run in triplicate, yielding three separately fermented PPC powders for each time point.
Non-nutritive compounds
Trypsin inhibitory activity
Trypsin inhibitory activity (TIA) was determined colorimetrically using an UV-visible spectrophotometer (Genesys™ 10S UV-Vis; Thermo Fisher Scientific, Wilmington, DE, USA) in accordance with the AACC International method 22-40.01 (19) with slight modification. In brief, 0.25 g of fermented sample was placed in a 50-mL centrifuge tube to which 25 mL of 0.01 M NaOH were added. Tubes were then vortexed for 1 min and stirred on a mechanical stirrer at 500 rpm (RT 5; IKA® Works Inc., Wilmington, NC, USA) for 3 h. The mixture was centrifuged (model 5810R; Eppendorf) at 14 000´g for 10 min at 4 °C. Aliquots (0, 0.6, 1.0, 1.4 and 1.8 mL) of supernatant were then pipetted into test tubes and each volume was adjusted to 2.0 mL with Milli-Q® water. The tubes were then incubated with 2 mL of trypsin solution (4 mg of trypsin in 200 mL of 0.001 M HCl) for 5 min at 37 °C in a water bath. A volume of 5 mL of pre-warmed substrate solution (40 mg of N-α-benzoyl-d,l-arginine 4-nitroanilide hydrochloride (d,l-BAPNA) dissolved with 1 mL of dimethyl sulfoxide, then diluted to 100 mL with Tris-HCl buffer (0.05 M, pH=8.2)) was added into each test tube to initiate the reaction. The reaction was stopped by the addition of 1 mL of acetic acid after exactly 10 min. The mixed solution was then filtered through Whatman no. 2 filter paper (GE Healthcare UK Limited, Buckinghamshire, UK). One trypsin inhibitory unit (TIU) was equivalent to an increase of 0.01 absorbance unit at 410 nm per 10 mL of reaction mixture compared to the blank sample (addition of trypsin solution after acetic acid). Trypsin inhibitor activity (TIA) was defined as the number of trypsin units inhibited per mg of sample and expressed in trypsin inhibitory units (TIU) per mg of dry sample, calculated using the following equation (20):
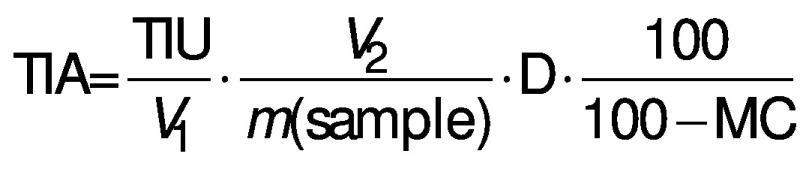 |
where V1 is the volume of extract taken in mL, V2=25 mL of extract, m(sample)=500 mg, D is the dilution factor and MC is the moisture content of PPC samples. The trypsin inhibitor extract was diluted to fall within 40-60% of trypsin inhibition at 1 mL.
Chymotrypsin inhibitory activity
Chymotrypsin inhibitory activity (CIA) was assayed according to the method described by Makkar et al. (21) with the following modifications. A mass of 1 g of PPC sample was placed in a 50-mL centrifuge tube to which 10 mL of borate buffer (0.1 M, pH=7.6) were added and vortexed for 1 min. The mixture was then stirred on a mechanical stirrer at 500 rpm for 1 h. The slurry was centrifuged at 3000´g (model 5810R; Eppendorf) for 10 min at 4 °C. In test tubes, 0, 0.25, 0.5 and 0.75 mL of the extract were added and diluted to 1 mL with borate buffer. These mixtures were incubated with 1 mL of stock chymotrypsin solution containing 4 mg of chymotrypsin in 100 mL of 0.001 M HCl at 37 °C for 10 min. Next, 2 mL of pre-warmed casein solution (1%, by mass per volume) in borate buffer at pH=7.6 were added, samples were mixed and incubated at 37 °C for 10 min. The reaction was then stopped by the addition of 6 mL of trichloroacetic acid reagent (containing 18 g of trichloroacetic acid, 18 g of anhydrous sodium acetate and 20 mL of glacial acetic acid and diluted up to 1 L with distilled water). The suspension was held at room temperature for at least 30 min and then filtered using Whatman no. 2 filter paper. The absorbance of the filtrate was recorded at 275 nm using a Genesys™ 10S UV-Vis spectrophotometer (Thermo Fisher Scientific) against the appropriate blank. A blank contained 6 mL of trichloroacetic acid reagent and 2 mL of casein solution. One chymotrypsin unit was defined as an increase of 0.01 absorbance unit at 275 nm of the reaction mixture. Chymotrypsin inhibitory activity is defined as the number of inhibited chymotrypsin units and the results are expressed in chymotrypsin inhibitory units (CIU) per milligram of the sample, and calculated using the following equation:
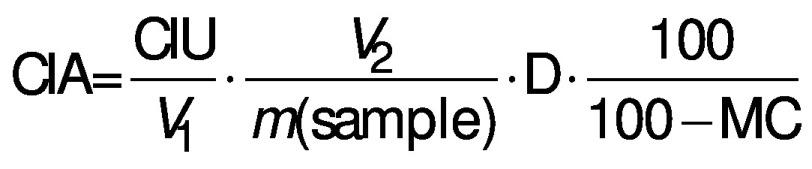 |
where V1 is the volume of extract taken in mL, V2=10 mL of extract, m(sample)=1000 mg, D is the dilution factor, and MC is the moisture content of PPC samples.
Total phenolic content
The total phenolic content in the fermented samples was determined using the Folin-Ciocalteu assay according to Waterman and Mole (22). Briefly, 1 g of sample was extracted with 15 mL of solvent (1% HCl in methanol) for 2 h, and then centrifuged for 10 min at 1510´g (model 5810R; Eppendorf) and 25 °C. The resulting supernatant was decanted and kept in a different tube. A volume of 5 mL of solvent was again added to the residue after removal of supernatant and vortexed every 5 min for 20 min, centrifuged (as above) and the supernatant was removed. This procedure was repeated once more, and all three supernatants were then pooled. A 0.5-mL aliquot of the extract from the pooled supernatants was then mixed with 2.5 mL of Folin-Ciocalteu reagent. The solution was kept at 25 °C for 5-8 min before adding 7.5 mL of sodium carbonate solution (20%, by mass per volume) and adjusting the volume to 50 mL with Milli-Q® water. After 2 h, the absorbance was measured at 760 nm using a Genesys™ 10S UV-Vis spectrophotometer (Thermo Fisher Scientific). Catechin (0-1 mg/mL) was used as a standard for the calibration curve. The total phenolic content was expressed in mg of catechin equivalents (CE) per 1 g of dry matter.
Total tannins
Tannins were quantified as the difference between the total phenols before and after tannin removal from the extract using insoluble polyvinylpolypyrrolidone (PVPP) according to the method of Makkar et al. (23). In brief, 1 mL of extract was mixed with 100 mg of PVPP. After vortexing, the extract was left for 15 min at 4 °C and then centrifuged at 1110´g (model 5810R; Eppendorf) for 10 min. The supernatant was then used to determine the phenolic content (having no tannin) as performed by the aforementioned method. The tannin content was determined as the difference between the total phenolic content before and after precipitation with PVPP.
Protein quality
Amino acid analysis
Amino acid analysis was carried out on PPC from one fermentation run at POS Bio-Sciences Corp. (Saskatoon, SK, Canada) utilizing acid/heat hydrolysis followed by quantification using chromatographic techniques. In brief, approx. 20 mg of each protein concentrate sample were weighed into separate 20´150 mm screw cap Pyrex® tubes containing 15 mL of 6 M HCl. Each tube was then flushed with N2 gas. The tubes were then capped and placed into an oven at 110 °C for 20 h. After acid hydrolysis, the individual amino acids were quantified by high-performance liquid chromatography using the Pico Tag amino acid analysis system (Waters Corporation, Milford, MA, USA) (24-26). The amino acid score was calculated as the ratio of individual amino acids in 1 g of PPC to the FAO/WHO/UNU recommended reference protein (27). The amino acid composition of the reference protein was as follows (amino acid in mg per g of protein): histidine 19, isoleucine 28, leucine 66, lysine 58, methionine+cysteine 25, phenylalanine+tyrosine 63, threonine, 34, tryptophan 11 and valine 35. The amino acid score of the protein concentrate was the lowest ratio value among the 18 amino acids.
In vitro protein digestibility and in vitro protein digestibility-corrected amino acid score
The in vitro protein digestibility (IVPD) was determined by the pH drop of the solution after digestion by a multi-enzyme solution (28, 29). This solution was prepared fresh daily by mixing 31 mg of chymotrypsin (bovine pancreas ≥40 units per mg of protein), 16 mg of trypsin (porcine pancreas 13 000-20 000 BAEE units per mg of protein) and 13 mg of protease (Streptomyces griseus ≥15 units/mg solid) in 10 mL Milli-Q® water and kept at 37 °C and pH adjusted to 8.0 using 0.1 M NaOH and HCl. Approximately 155.9 mg of PPC (to meet 62.5 mg protein in 10 mL of water) was mixed with 10 mL of pre-warmed Milli-Q® water in a 50-mL beaker. The mixture was stirred for 1 h at 37 °C, and pH adjusted to 8.0 using 0.1 M NaOH and HCl before adding 1 mL of the multi-enzyme solution. The pH of the protein solution was recorded every 30 s for 10 min and the in vitro protein digestibility (IVPD) was calculated using the following equation:
| IVPD=65.66+18.10 · ∆pH10min /3/ |
where ΔpH10 min refers to the change in pH from initial 8.0 to the end of 10 min. The in vitro protein digestibility-corrected amino acid score (IVPDCAAS) was calculated as the product of the amino acid score and in vitro protein digestibility.
Statistical analysis
A one-way ANOVA with Tukey’s post-hoc test was used to detect statistical differences in response to fermentation time of protein quality data (IVPD and IVPDCAAS) and for the non-nutritive compounds. All statistical analyses were performed with Systat v. 10 software (30).
Results and Discussion
Effect of fermentation on the levels of non-nutritive compounds
Non-nutritive compounds that are known to negatively impact protein quality such as the presence of trypsin and chymotrypsin inhibitors, as well as total phenolics and tannins (31-33) were examined as a function of fermentation time and are reported in Table 1. Trypsin inhibitory activity (TIA) of unfermented PPC (t=0 h) was 2.3 TIU/mg and was in the range of what has been reported for pea (Pisum sativum L.) (2.22-7.66 TIU/mg) (34). After 9 h of fermentation, TIA reached a low (1.1 TIU/mg) value and was significantly different from t=0 h (p<0.01). Chandra-Hioe et al. (16) reported no significant change in TIA values after 16 h of fermentation of chickpea (desi/kabuli) and faba bean flour. Starzyńska-Janiszewska and Stodolak (35) found that solid-state fermentation of grass pea flour resulted in a 99% reduction in TIA levels. Reduction in TIA values was also reported by Coda et al. (36) of faba bean flour by L. plantarum VTT E-133328. The chymotrypsin inhibitory activity (CIA) of PPC as a function of fermentation time is given in Table 1. In contrast to the TIA, CIA continuously declined over the 11-hour fermentation process (p<0.01). At t=0 h, the PPC had 3.7 CIU/mg but this decreased to 1.9 CIU/mg after 1 h of fermentation, a reduction of 49%. The CIA values proceeded to decrease until reaching 1.1 CIU/mg after the 11-hour fermentation time course.
Table 1. Non-nutritive content on a dry mass basis of pea protein concentrate fermented for different times with L. plantarum.
| t(fermentation)/h | w(total phenol)*/(mg/g) | w(tannin)*/(mg/g) | CIA/(CIU/mg) | TIA/(TIU/mg) |
|---|---|---|---|---|
| 0 | (2.5±0.3)a | (0.14±0.04)a | (3.7±0.2)a | (2.3±0.0)ab |
| 1 | (3.1±0.2)ab | (0.41±0.06)ab | (1.9±0.4)b | (2.6±0.2)a |
| 5 | (3.9±0.5)bc | (0.96±0.19)c | (1.7±0.3)bc | (1.9±0.1)b |
| 9 | (4.7±0.3)c | (0.55±0.16)abc | (1.7±0.3)bc | (1.1±0.0)c |
| 11 | (4.9±0.4)c | (0.79±0.16)bc | (1.1±0.5)c | (1.9±0.1)b |
*Expressed as catechin equivalent (mg) on a dry mass basis. Data represent the mean value±standard deviation (N=3). Data with different superscript letters in the same column indicate significant differences (p<0.01). TIA=trypsin inhibitory activity defined as the number of trypsin units (TIU) inhibited per mg of sample, CIA=chymotrypsin inhibitory activity defined as the number of chymotrypsin inhibitory units (CIU) per mg of sample
Phenolic compounds (including tannins) cross-link proteins, making them less susceptible to enzyme action during digestion. The total phenolic and tannin levels of PPC, as a function of fermentation time, are given in Table 1. A one-way ANOVA found that the effect of fermentation time on the level of total phenolic content was significant (p<0.01). At t=0 h, total phenolic content expressed as catechin equivalent (CE) mass fraction on a dry mass basis was 2.5 mg per g of PPC, which then continually increased over the 11-hour fermentation period to a final level of 4.9 mg per g of PPC. This increase may be due to the release of soluble phenolic compounds as polymeric phenolics are degraded during fermentation (37, 38). It is also likely that loosening of the lignocellulosic matrix via fermentation liberates phenolic compounds from an inaccessible state. The effects of fermentation on total phenolic content in different legumes have been reported in several previous studies. For instance, Starzyńska-Janiszewska and Stodolak (35) reported total phenolic content of grass pea flour to increase after 24 h of fermentation with L. plantarum and that a higher percent inoculum resulted in a higher amount of total phenolics. Fernandez-Orozco et al. (39) also observed that fermentation with L. plantarum increased total phenolic levels by approx. 200% in soybean flour and by approx. 310% when the soybean flour was naturally fermented. Additionally, a Bacillus subtilis-based solid-state fermentation significantly increased the phenolic content of kidney beans by 96 and 126% after 48 and 96 h, respectively (40). Overall, the total tannin levels significantly increased from approx. 0.14 mg/g at t=0 h to a maximum of approx. 0.96 mg/g after 5 h, and then declined to approx. 0.79 mg/g after 11 h. The increase was likely caused by the same factors that affected the phenolic content increase, e.g. liberation from the lignocellulosic matrix. The observed decrease in tannin levels after 5 to 9 h of fermentation may be related with an increase in tannase activity by L. plantarum during fermentation (41, 42). Ramachandran et al. (6) and Onwurafor et al. (10) reported a decrease in total tannin content after fermentation of grass pea seed meal and mung bean flour, respectively.
Effect of fermentation on protein quality
The amino acid composition (in g/100 g sample) of PPC and fermented PPC is given in Table 2. All amino acid mass fractions increased with fermentation time except arginine and tryptophan, which remained similar. To calculate the amino acid score, the amino acid composition (in mg per g protein) must be compared to the recommended FAO/WHO amino acid requirements (3) (Table 3). Each essential amino acid mass fraction declined with fermentation time when reported in mg per g of protein (vs. per g of sample). While both tryptophan and the sulfur amino acids (SAA) were limiting, when compared to the reference pattern, the SAA had lower scores at all fermentation times investigated. This is similar to previous findings where pea protein was first limiting in SAA (2). During fermentation, the reduction in SAA content resulted in the amino acid score changing from 0.84 at 0 h to 0.66 at 11 h (Table 3).
Table 2. Amino acid (AA) composition of pea protein concentrate as a function of fermentation time, and of the bacterial pellet (BP).
| AA | w(AA)/(g/100 g) | |||||
|---|---|---|---|---|---|---|
| t(fermentation)/h | ||||||
| 0 | 1 | 5 | 9 | 11 | BP | |
| Essential | ||||||
| Cysteine | 0.47 | 0.51 | 0.50 | 0.44 | 0.44 | 0.14 |
| Histidine | 1.06 | 1.14 | 1.20 | 1.18 | 1.09 | 0.42 |
| Isoleucine | 1.79 | 1.80 | 1.90 | 1.87 | 1.88 | 0.62 |
| Leucine | 3.40 | 3.37 | 3.59 | 3.51 | 3.56 | 0.92 |
| Lysine | 3.33 | 3.90 | 3.45 | 3.42 | 3.51 | 0.94 |
| Methionine | 0.37 | 0.37 | 0.37 | 0.42 | 0.35 | 0.22 |
| Phenylalanine | 2.26 | 2.24 | 2.39 | 2.35 | 2.40 | 0.48 |
| Threonine | 1.73 | 1.88 | 1.92 | 1.86 | 1.81 | 0.51 |
| Tryptophan | 0.41 | 0.41 | 0.42 | 0.41 | 0.42 | 0.12 |
| Valine | 2.03 | 2.08 | 2.13 | 2.19 | 2.19 | 0.77 |
| Non-essential | ||||||
| Alanine | 1.97 | 2.01 | 2.14 | 2.08 | 2.09 | 1.21 |
| Arginine | 3.81 | 3.87 | 4.10 | 3.97 | 3.90 | 0.76 |
| Aspartic acid | 5.40 | 5.70 | 5.88 | 5.78 | 5.68 | 1.33 |
| Glutamic acid | 7.96 | 8.41 | 8.69 | 8.54 | 8.40 | 1.69 |
| Glycine | 2.02 | 2.10 | 2.17 | 2.14 | 2.14 | 0.84 |
| Proline | 2.03 | 2.05 | 2.24 | 2.11 | 2.12 | 0.81 |
| Serine | 2.59 | 2.75 | 2.83 | 2.77 | 2.72 | 0.64 |
| Tyrosine | 1.53 | 1.61 | 1.68 | 1.65 | 1.63 | 0.40 |
Table 3. Essential amino acid (AA) mass fraction and score of pea protein concentrate as a function of fermentation time, and of the bacterial pellet (BP).
| t(fermentation)/h | AA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| THR | VAL | MET+ CYS |
ILE | LEU | PHE + TRY |
HIS | LYS | TRP | |
| w(essential amino acid)/(mg/g) | |||||||||
| 0 | 43 | 51 | 21 | 45 | 85 | 95 | 26 | 83 | 10 |
| 1 | 44 | 48 | 21 | 42 | 79 | 90 | 27 | 91 | 10 |
| 5 | 41 | 46 | 19 | 41 | 77 | 87 | 26 | 74 | 9 |
| 9 | 40 | 47 | 19 | 40 | 76 | 86 | 25 | 74 | 9 |
| 11 | 38 | 46 | 16 | 39 | 74 | 84 | 23 | 73 | 9 |
| BP | 23 | 34 | 16 | 28 | 41 | 39 | 19 | 42 | 5 |
| Reference pattern (3) |
34 | 35 | 25 | 28 | 66 | 63 | 19 | 58 | 11 |
| AA score | |||||||||
| 0 | 1.27 | 1.45 | 0.84* | 1.59 | 1.28 | 1.50 | 1.39 | 1.43 | 0.93 |
| 1 | 1.29 | 1.39 | 0.82* | 1.50 | 1.19 | 1.42 | 1.40 | 1.57 | 0.87 |
| 5 | 1.21 | 1.31 | 0.75* | 1.46 | 1.17 | 1.39 | 1.36 | 1.28 | 0.82 |
| 9 | 1.18 | 1.35 | 0.74* | 1.44 | 1.15 | 1.37 | 1.34 | 1.27 | 0.80 |
| 11 | 1.11 | 1.30 | 0.66* | 1.40 | 1.12 | 1.33 | 1.19 | 1.26 | 0.79 |
| BP | 0.67 | 0.98 | 0.64 | 0.98 | 0.62 | 0.62 | 0.98 | 0.72 | 0.48* |
*Indicates the first limiting amino acid. Data represent the mean value of one processing run. THR=threonine, VAL=valine, MET=methionine, CYS=cysteine, ILE=isoleucine, LEU=leucine, PHE=phenylalanine, TYR=tyrosine, HIS=histidine, LYS=lysine and TRP=tryptophan
In vitro protein digestibility (IVPD) of all samples showed no obvious trend, with digestibility values ranging from approx. 80 to 87% depending on the fermentation time (Table 4). Chandra-Hioe et al. (16) found that desi chickpea flour fermented with a lyophilized yogurt culture (containing Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus) showed improved IVPD where values increased from appox. 70.5 to 77.2%, whereas IVPD of kabuli chickpea and faba bean flours remained unchanged. The authors suggested that the rise in IVPD may be due to a reduction in protein cross linking induced by the presence of non-nutritive compounds (e.g. phenolics and tannins), making them more susceptible to proteolytic attack. Improved IVPD was also reported in fermented grass pea flour coinciding with a 99% reduction in TIA levels (43). In the present study, the alteration of SAA content resulted in a reduced IVPDCAAS as a function of fermentation time, from 67.0% at t=0 h to approx. 54.6% after 11 h of fermentation (Table 4). This could be due to either: (i) a dilution effect caused by the approx. 16% rise in total protein from the bacterial biomass (total protein levels increased on a dry mass basis from 40 to 48% during the 11-hour fermentation time course, data not shown), (ii) the bacterial biomass protein was of lower quality than the pea as the IVPDCAAS for the bacterial pellet was 33.2% (Table 4), and (iii) the thiol amino acids were metabolized by the bacteria to produce volatiles (44).
Table 4. In vitro protein digestibility of fermented pea protein concentrate as a function of fermentation time, and of the bacterial pellet (BP).
| t(fermentation)/h | AA score (of the limiting AA) |
IVPD/% | IVPDCAAS/% |
|---|---|---|---|
| 0 | 0.84 | (80.0±1.5)b | (67.0±1.2)a |
| 1 | 0.82 | (81.1±0.5)bc | (66.6±0.4)a |
| 5 | 0.75 | (87.4±1.0)d | (65.2±0.8)a |
| 9 | 0.74 | (83.4±0.5)c | (61.9±0.4)b |
| 11 | 0.66 | (83.2±0.2)c | (54.6±0.1)c |
| BP | 0.48 | (69.6±0.7)a | (33.2±0.3)d |
Data represent the mean value±standard deviation (N=3). Data with different superscript letters in the same column indicate significant differences (p<0.01)
Conclusion
Lactobacillus plantarum fermentation of PPC influenced the non-nutritive compounds while causing a decrease in protein quality. Phenol and tannin content increased with fermentation whereas the inhibitory digestive enzyme activity decreased. Although protein digestibility was increased, the alteration of sulfur amino acid content resulted in an overall reduction in protein quality. These results indicate that while fermentation is a viable method for reducing levels of non-nutritive compounds and improving protein digestibility of PPC, exploration of an alternative bacteria which metabolise sulfur amino acids to a lesser extent than L. plantarum is necessary. Furthermore, optimization studies of fermentation conditions may offer a different outcome than found in the present study. Fermentation of pulse protein concentrates by yeast may also offer some alternative insights for future directions. To date, little work has been done on improving the nutritional value of pulse protein concentrates, however, the present study shows potential and warrants further investigation with other bacterial, fungal or yeast inocula to better understand mechanisms for enhancing their nutritional value.
Acknowledgements
Financial support for this research was provided by the Global Institute for Food Security at the University of Saskatchewan (Saskatoon, SK, Canada) and the Saskatchewan Ministry of Agriculture Development Fund (ADF#: 2014-0283).
References
- 1.Multari S, Stewart D, Russell WR. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr Rev Food Sci Food Saf. 2015;14(5):511–22. 10.1111/1541-4337.12146 [DOI] [Google Scholar]
- 2.Sarwar G, Peace RW. Comparisons between true digestibility of total nitrogen and limiting amino acids in vegetable proteins fed to rats. J Nutr. 1986;116(7):1172–84. 10.1093/jn/116.7.1172 [DOI] [PubMed] [Google Scholar]
- 3.FAO/WHO. Protein quality evaluation: Report of the joint FAO/WHO expert consultation. Rome, Italy: Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO); 1991. Available from: http://www.fao.org/ag/humannutrition/35978-02317b979a686a57aa4593304ffc17f06.pdf.
- 4.Candela M, Astiasaran I, Bello J. Cooking and warm-holding: Effect on general composition and amino acids of kidney beans (Phaseolus vulgaris), chickpeas (Cicer arietinum), and lentils (Lens culinaris). J Agric Food Chem. 1997;45(12):4763–7. 10.1021/jf9702609 [DOI] [Google Scholar]
- 5.Yigzaw Y, Gorton L, Solomon T, Akalu G. Fermentation of seeds of teff (Eragrostis teff), grass-pea (Lathyrus sativus), and their mixtures: Aspects of nutrition and food safety. J Agric Food Chem. 2004;52(5):1163–9. 10.1021/jf034742y [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran S, Bairagi A, Ray AK. Improvement of nutritive value of grass pea (Lathyrus sativus) seed meal in the formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after fermentation with a fish gut bacterium. Bioresour Technol. 2005;96(13):1465–72. 10.1016/j.biortech.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 7.Torino MI, Limón RI, Martínez-Villaluenga C, Mäkinen S, Pihlanto A, Vidal-Valverde C, et al. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013;136(2):1030–7. 10.1016/j.foodchem.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 8.Elyas SHA, El Tinay AH, Yousif NE, Elsheikh EAE. Effect of natural fermentation on nutritive value and in vitro protein digestibility of pearl millet. Food Chem. 2002;78(1):75–9. 10.1016/S0308-8146(01)00386-7 [DOI] [Google Scholar]
- 9.Reyes-Moreno C, Cuevas-Rodríguez EO, Milán-Carrillo J, Cárdenas-Valenzuela OG, Barrón-Hoyos J. Solid state fermentation process for producing chickpea (Cicer arietinum L) tempeh flour. Physicochemical and nutritional characteristics of the product. J Sci Food Agric. 2004;84(3):271–8. 10.1002/jsfa.1637 [DOI] [Google Scholar]
- 10.Onwurafor EU, Onweluzo JC, Ezeoke AM. Effect of fermentation methods on chemical and microbial properties of mung bean (Vigna radiata) flour. Niger Food J. 2014;32(1):89–96. 10.1016/S0189-7241(15)30100-4 [DOI] [Google Scholar]
- 11.Klupsaite D, Juodeikiene G, Zadeike D, Bartkiene E, Maknickiene Z, Liutkute G. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. Lebensm Wiss Technol. 2017;75:180–6. 10.1016/j.lwt.2016.08.058 [DOI] [Google Scholar]
- 12.Chawla P, Bhandari L, Sadh PK, Kaushik R. Impact of solid state fermentation (Aspergillus oryzae) on functional properties and mineral bioavailability of black eyed pea (Vigna unguiculata) seed flour. Cereal Chem. 2017;94(3):437–42. 10.1094/CCHEM-05-16-0128-R [DOI] [Google Scholar]
- 13.Meinlschmidt P, Schweiggert-Weisz U, Eisner P. Soy protein hydrolysates fermentation: Effect of debittering and degradation of major soy allergens. Lebensm Wiss Technol. 2016;71:202–12. 10.1016/j.lwt.2016.03.026 [DOI] [Google Scholar]
- 14.Meinlschmidt P, Ueberham E, Lehmann J, Schweiggert-Weisz U, Eisner P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016;205:229–38. 10.1016/j.foodchem.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 15.Shekib LA. Nutritional improvement of lentils, chick pea, rice and wheat by natural fermentation. Plant Foods Hum Nutr. 1994;46(3):201–5. 10.1007/BF01088991 [DOI] [PubMed] [Google Scholar]
- 16.Chandra-Hioe MV, Wong CHM, Arcot J. The potential use of fermented chickpea and faba bean flour as food ingredients. Plant Foods Hum Nutr. 2016;71(1):90–5. 10.1007/s11130-016-0532-y [DOI] [PubMed] [Google Scholar]
- 17.Hemalatha S, Platel K, Srinivasan K. Influence of germination and fermentation on bioaccessibility of zinc and iron from food grains. Eur J Clin Nutr. 2007;61:342–8. 10.1038/sj.ejcn.1602524 [DOI] [PubMed] [Google Scholar]
- 18.Nosworthy MG, Neufeld J, House JD. Determination of the in vivo and in vitro protein quality of pulse protein concentrates and isolates. FASEB J. 2016;30(1_suppl.):421–6. [Google Scholar]
- 19.AACC International Method 22–40.01. Measurement of trypsin inhibitor activity of soy products—Spectrophotometric method. St. Paul, MN, USA: AACC International; 2006. [Google Scholar]
- 20.Mondor M, Aksay S, Drolet H, Roufik S, Farnworth E, Boye JI. Influence of processing on composition and antinutritional factors of chickpea protein concentrates produced by isoelectric precipitation and ultrafiltration. Innov Food Sci Emerg Technol. 2009;10(3):342–7. 10.1016/j.ifset.2009.01.007 [DOI] [Google Scholar]
- 21.Makkar HPS, Siddhuraju S, Becker K. Plant secondary metabolites. In: Methods in molecular biology, vol 393. Totowa, NJ, USA: Humana Press; 2007. pp. 1-9. https://dpo.org/10.1007/978-1-59745-425-4 [DOI] [PubMed] [Google Scholar]
- 22.Waterman PG, Mole S. Analysis of phenolic plant metabolites. Oxford, UK: Wiley-Blackwell; 1994. [Google Scholar]
- 23.Makkar HP, Blümmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric. 1993;61(2):161–5. 10.1002/jsfa.2740610205 [DOI] [Google Scholar]
- 24.White JA, Hart RJ, Fry JC. An evaluation of the waters pico-tag system for the amino-acid analysis of food materials. J Automat Chem. 1986;8(4):170–7. 10.1155/S1463924686000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landry J, Delhaye S. Determination of tryptophan in feedstuffs: Comparison of sodium hydroxide and barium hydroxide as hydrolysis agents. Food Chem. 1994;49(1):95–7. 10.1016/0308-8146(94)90238-0 [DOI] [Google Scholar]
- 26.Official Method AOAC. 988.15. Tryptophan in foods and food and feed ingredients, ion exchange chromatography method. Washington, DC, USA: AOAC International; 1995. [Google Scholar]
- 27.FAO/WHO/UNU. Energy and protein requirements: Report of a joint FAO/WHO/UNU expert consultation. WHO Tech Rep Ser 724. Geneva, Switzerland: Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO); 1985. Available from: http://www.who.int/iris/handle/10665/39527. [PubMed]
- 28.Hsu HW, Vavak DL, Satterlee LD, Miller GA. A multienzyme technique for estimating protein digestibility. J Food Sci. 1977;42(5):1269–73. 10.1111/j.1365-2621.1977.tb14476.x [DOI] [Google Scholar]
- 29.Tinus T, Damour M, van Riel V, Sopade PA. Particle size-starch–protein digestibility relationships in cowpea (Vigna unguiculata). J Food Eng. 2012;113(2):254–64. 10.1016/j.jfoodeng.2012.05.041 [DOI] [Google Scholar]
- 30.Systat v. 10 software. Systat Software Inc., San Jose, CA, USA; 2001.
- 31.Hahn DH, Rooney LW, Earp CF. Tannins and phenols of sorghum. Cereal Foods World. 1984;29(12):776–9. [Google Scholar]
- 32.Gupta YP. Anti-nutritional and toxic factors in food legumes: A review. Plant Foods Hum Nutr. 1987;37(3):201–28. 10.1007/BF01091786 [DOI] [PubMed] [Google Scholar]
- 33.Oomah BD, Caspar F, Malcolmson LJ, Bellido AS. Phenolics and antioxidant activity of lentil and pea hulls. Food Res Int. 2011;44(1):436–41. 10.1016/j.foodres.2010.09.027 [DOI] [Google Scholar]
- 34.Wang X, Warkentin TD, Briggs CJ, Oomah BD, Campbell CG, Woods S. Trypsin inhibitor activity in field pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.). J Agric Food Chem. 1998;46(7):2620–3. 10.1021/jf971007b [DOI] [Google Scholar]
- 35.Starzyńska-Janiszewska A, Stodolak B. Effect of inoculated lactic acid fermentation on antinutritional and antiradical properties of grass pea (Lathyrus sativus ‘Krab’) flour. Pol J Food Nutr Sci. 2011;61(4):245–9. 10.2478/v10222-011-0027-3 [DOI] [Google Scholar]
- 36.Coda R, Melama L, Rizzello CG, Curiel JA, Sibakov J, Holopainen U, et al. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int J Food Microbiol. 2015;193:34–42. 10.1016/j.ijfoodmicro.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 37.Dueñas M, Fernández D, Hernández T, Estrella I, Muñoz R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J Sci Food Agric. 2005;85(2):297–304. 10.1002/jsfa.1924 [DOI] [Google Scholar]
- 38.McCue PP, Shetty K. Phenolic antioxidant mobilization during yogurt production from soymilk using kefir cultures. Process Biochem. 2005;40(5):1791–7. 10.1016/j.procbio.2004.06.067 [DOI] [Google Scholar]
- 39.Fernandez-Orozco R, Frias J, Muñoz R, Zielinski H, Piskula MK, Kozlowska H, et al. Fermentation as a bio-process to obtain functional soybean flours. J Agric Food Chem. 2007;55(22):8972–9. 10.1021/jf071823b [DOI] [PubMed] [Google Scholar]
- 40.Limón RI, Peñas E, Torino MI, Martínez-Villaluenga C, Dueñas M, Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015;172:343–52. 10.1016/j.foodchem.2014.09.084 [DOI] [PubMed] [Google Scholar]
- 41.Osawa R, Kuroiso K, Goto S, Shimizu A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl Environ Microbiol. 2000;66(7):3093–7. 10.1128/AEM.66.7.3093-3097.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez H, Curiel JA, Landete JM, de las Rivas B, de Felipe FL, Gómez-Cordovés C, et al. Food phenolics and lactic acid bacteria. Int J Food Microbiol. 2009;132(2-3):79–90. 10.1016/j.ijfoodmicro.2009.03.025 [DOI] [PubMed] [Google Scholar]
- 43.Stodolak B, Starzyńska-Janiszewska A. The influence of tempeh fermentation and conventional cooking on anti-nutrient level and protein bioavailability (in vitro test) of grass-pea seeds. J Sci Food Agric. 2008;88(13):2265–70. 10.1002/jsfa.3341 [DOI] [Google Scholar]
- 44.Sreekumar R, Al-Attabi Z, Deeth HC, Turner MS. Volatile sulfur compounds produced by probiotic bacteria in the presence of cysteine or methionine. Lett Appl Microbiol. 2009;48(6):777–82. 10.1111/j.1472-765X.2009.02610.x [DOI] [PubMed] [Google Scholar]


