Abstract
Estrogen receptor α (ERα) is essential for male fertility. Its activity is responsible for maintaining epithelial cytoarchitecture in efferent ductules and the reabsorption of fluid for concentrating sperm in the head of the epididymis. These discoveries and others have helped to establish estrogen's bisexual role in reproductive importance. Reported here is the molecular mechanism to explain estrogen's role in fluid reabsorption in the male reproductive tract. It is shown that estrogen regulates expression of the Na+/H+ exchanger-3 (NHE3) and the rate of 22Na+ transport, sensitive to an NHE3 inhibitor. Immunohistochemical staining for NHE3, carbonic anhydrase II (CAII), and aquaporin-I (AQP1) was decreased in ERα knockout (αERKO) efferent ductules. Targeted gene-deficient mice were compared with αERKO, and the NHE3 knockout and CAII-deficient mice showed αERKO-like fluid accumulation, but only the NHE3 knockout and αERKO mice were infertile. Northern blot analysis showed decreases in mRNA for NHE3 in αERKO and antiestrogen-treated mice. The changes in AQP1 and CAII in αERKO seemed to be secondary because of the disruption of apical cytoarchitecture. Ductal epithelial ultrastructure was abnormal only in αERKO mice. Thus, in the male, estrogen regulates one of the most important epithelial ion transporters and maintains epithelial morphological differentiation in efferent ductules of the male, independent of its regulation of Na+ transport. Finally, these data raise the possibility of targeting ERα in developing a contraceptive for the male.
Targeted disruption of the murine homologue of the estrogen receptor α (ERα) gene results in dilation of rete testis and efferent ductules in the head of the epididymis, with subsequent accumulation of fluid in seminiferous tubules of the testis, dilution of sperm, and infertility (1–3). Similar to responses following occlusion of the reproductive tract (4), a transient increase in testis weight followed by a gradual decrease was noted in ERα knockout (αERKO) mice, suggesting that fluid accumulation induced testicular atrophy (2, 5). Thus, αERKO mice provided conclusive evidence that estrogen, or more specifically, a functional ERα, regulates fluid transport in the male reproductive tract.
Efferent ductules of the testis are small, coiled tubules that form the proximal epididymal head (4) and conduct sperm from rete testis to the epididymal duct. Their primary function is to reabsorb nearly 90% of the luminal fluid (6), thereby increasing concentration of sperm before entering the epididymis (7). Functionally, the efferent ductules are similar to proximal tubules of the kidney (7). Reabsorption of fluid in efferent ductules requires Na+/K+-ATPase along the basolateral membranes and the rate-limiting step of electroneutral Na+ absorption mediated by an apically located Na+/H+ exchanger (NHE) (6, 8–11).
Recent studies have shown that the NHE isoform NHE3 is expressed on the apical membrane of nonciliated cells in efferent ductules and is the only functional isoform for Na+ reabsorption in this region (10–13). NHE3 is found also in caput and corpus epididymis (14). NHE1 is expressed in cultured efferent ductules, epithelial cells, and along the basolateral membrane of epididymal epithelium, where it is likely to carry out housekeeping functions in the maintenance of intracellular pH and regulation of cell volume (10). The function of NHE2, which is expressed in the cytoplasm of the ciliated cells of the efferent ductules, is not clear (10). Proton export by NHE3 largely depends on H+ generation by either spontaneous or catalyzed reversible hydration of CO2 by carbonic anhydrase (CA) activity, which is abundant in the efferent ductules (15). CAII is the primary isoform located in the head of the epididymis of the rat (16). Therefore, Na+ flux depends on both of these proteins. Water transit through the efferent ductal epithelium follows several pathways, including the free movement of water through aquaporin (AQP) water channels (17), a leaky cell junctional complex (18), and endocytosis (6, 19). AQP1 has been localized in the efferent ductule epithelium (17), and its expression was modulated by estrogen exposure during neonatal development (20). Therefore, we hypothesized that estrogen's function in the adult male reproductive tract is to control water and ion transport, with three potential candidate genes for regulation: NHE3, CAII, and AQP1.
Data reported here show that ERα function is responsible for the expression of NHE3 in efferent ductules of the male reproductive system and, thus, influences Na+ reabsorption and passive water transport. Moreover, it is shown that estrogen action is required for maintenance of the apical cytoarchitecture in these epithelial cells independent of its effects on Na+ transport.
Materials and Methods
Animals and Antiestrogen Treatments.
Animal experiments were approved by the Institutional Animal Care and Use Committees of the respective universities and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Wild-type (WT), αERKO, and NHE3 knockout (NHE3KO) mice were obtained from the University of Missouri (2, 21); the AQP1 knockout (AQP1KO) from the University of California-San Francisco (22); and CAII-deficient mice (CAIIdef) from the University of Arizona Health Sciences Center (23). WT and αERKO adult male mice (30 pairs that were 90–120 days old) were used in Northern blot analysis. For antiestrogen treatment, 25 male C57BL/10 mice (6–8 weeks old) received single s.c. injections of antiestrogen ICI 182,780 (5 mg in 0.1 ml of the Faslodex, long-acting formulation by AstraZeneca, Macclesfield, U.K.) once per week. Controls (25 mice) received weekly s.c. injections of the castor oil vehicle. Tissues were collected for morphology or total RNA analysis on day 8 after the first injection.
Immunohistochemistry and Histology.
The male reproductive tract was fixed by vascular perfusion and processed for immunohistochemistry, glycol methacrylate light microscopy, or transmission electron microscopy (2, 5). Immunohistochemistry was performed on tissues fixed by vascular perfusion with Bouin's fluid or neutral buffer formalin, embedded in paraffin, and stained by using standard methods of microwave antigen retrieval. Antibodies included rabbit anti-human CA-II (Chemicon) diluted 1:1,000, rabbit anti-rat NHE3 (Chemicon) diluted 1:1,000, and rabbit anti-rat AQP1 diluted 1:1,000. Sections were incubated with primary antibody for 12 h at 4°C. Sections were incubated with secondary antibody (biotinylated anti-rabbit IgG, diluted 1:100; Dako) for 45 min at room temperature and then incubated with the ABC Kit (Vector Laboratories) for 30 min at room temperature. Reactivity was visualized by using diaminobenzidine chromogen and counterstained with hematoxylin.
Northern Blot Analysis.
Total RNA was isolated by the guanidinium isothiocynate/phenol chloroform method from mouse efferent ductules. After electrophoresis of total RNA (20 μg) on a 1.5% formaldehyde-denaturing agarose gel and blotting to Duralon-UV membranes (Stratagene), the membranes were prehybridized in QuikHyb solution (Stratagene) for 2 h, then hybridized with denatured 32P-labeled probes and sonicated salmon sperm DNA at 58°C overnight. After hybridization, the filters were washed twice for 5 min each at room temperature in 2 × SSC/0.1% SDS, followed by a 10-min wash in 0.1 × SSC/0.1% SDS at 65°C. Hybridization probes for CAII and AQP1 were made by reverse transcription–PCR of total RNA from adult mouse efferent ductules. Primers for CAII were 5′-TGGGGATACAGCAAGCACAA-3′ and 5′-TTTCAGCACTGCATTGTCCT-3′ (GenBank accession no. NM009801, nucleotides 13–228). Primers for AQP1 were 5′-TAATCTGTCAGGCACAAAAGG-3′ and 5′-TTCGCTAAGCTGCTCTTCAA-3′ (GenBank Accession no. NM007472, nucleotides 1509–2269). The NHE3 probe was a PCR product from rat NHE3 cDNA plasmid. Primers for NHE3 were 5′-TGGATTTCCTGCTATTTGGC-3′ and 5′-TCGCTCCTCTTCACCTTCA-3′ (GenBank accession no. M85300, nucleotides 615-1485). The same membrane was used to hybridize with all genes examined in each replication study. All Northern blots were normalized for variations in loading by a final hybridization with the 36B4 probe, which is not influenced by estrogen treatment (24). Relative quantification was made by digitizing images using un-scan-it (Silk Scientific, Orem, UT).
NHE3 Activity.
Isolated efferent ductules were suspended in 0.1% collagenase (Collagenase B, Roche Molecular Biochemicals) in sterile Hanks' balanced salt solution (HBSS) containing 1 mM CaCl2 and 1 mM MgCl2 (HBSS) at 37°C for 15 min. After rinsing in fresh HBSS, the ductules were resuspended in HBSS containing 0.01% DTT for dissection from extraneous connective tissue. The ductules were cut into ≈50-μm segments and allowed to incubate for 10 min, during which time the ductule sections everted to expose the luminal surface. The 22Na uptake by the efferent ductule segments was performed by using a modification of a method previously described (25). The ductule sections were suspended in the defined medium to clamp intracellular pH near 6.3. The nigericin solution contained 1 mM ouabain to inhibit the Na+/K+-ATPase and 10 μM amiloride to inhibit Na+ uptake via NHE1 and NHE2 proteins and the epithelial Na+ channel. After 3 min at room temperature, ≈1 μCi of 22Na and 2 μCi of 3H-mannitol (for estimation of extracellular fluid) ± 100 μM 5-(N-ethyl-N-isopropyl) amiloride (EIPA), an NHE inhibitor to block NHE3 activity, were added to the incubation tube. The ductules were incubated during the linear phase of uptake (1 min) at 37°C before adding the cold stop at pH 7.4. The ductule sections were separated by vacuum filter (nylon 60 μm mesh) followed by three washings with the cold stop solution, solubilized in 0.5 M HCl at 55°C for 10 min and then neutralized with 0.5 N NaOH. Aliquots were taken for protein determination (Bradford assay) and radioisotope counting. The samples were counted sequentially on a gamma counter (for 22Na) and a liquid scintillation counter (for 3H). The beta dpm were corrected for 22Na spillover into the 3H window. Sample 22Na dpm were corrected for differences in the specific activity of the uptake solution and extracellular 22Na activity. Samples were normalized to their protein concentrations.
Results
Morphology of the Male Reproductive Tract and Fertility.
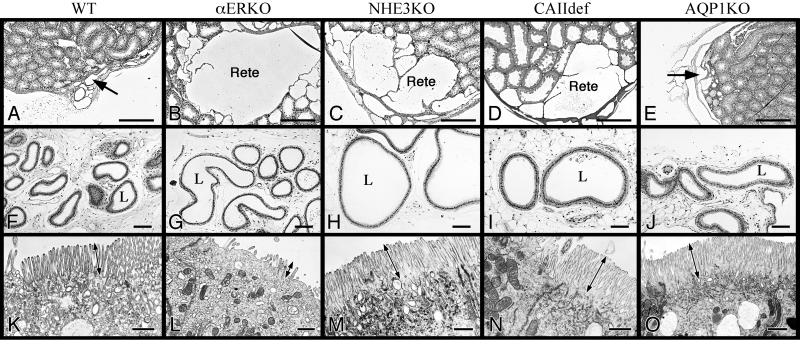
In efferent ductules of adult αERKO mice, essentially all immunohistochemical staining for NHE3 was absent, whereas its expression was abundant along the brush border of nonciliated cells in WT efferent ductules (Fig. 1 A and B). CAII was expressed in both cytoplasm and brush border of efferent ductule epithelium in WT, but showed no immunostaining along the brush border in αERKO and reduced staining in the cytoplasm (Fig. 1 C and D). AQP1 showed intense staining along the brush border of nonciliated cells in WT mice, but staining was reduced significantly in αERKO efferent ductules (Fig. 1 E and F). The proximal efferent ductule epithelia show no immunostaining for AQP1, but in the more distal regions staining was observed. In the conus region, intermittent staining of some nonciliated cells showed total absence of AQP1, whereas other cells showed prominent staining in αERKO (Fig. 1F). The common ductule showed no change in staining in αERKO mice. To further resolve the protein(s) that is primarily responsible for fluid reabsorption in the male reproductive tract, we examined the histology of efferent ductules and rete testis from mice deficient in the following individual genes: ERα (1), NHE3 (26), CAII (23), and AQP1 (22). Both the NHE3KO (Fig. 2 C and H) and CAIIdef (Fig. 2 D and I) mice had efferent ductules and rete testes that were dilated compared with WT, with equal or greater volume than in αERKO males. WT tissue from all gene-deficient littermates was comparable, so only αERKO littermate WT tissue is shown. The AQP1KO revealed no major pathological changes, with the exception of a slight dilation of rete testis lumen (Fig. 2 E and J). Only the NHE3KO and αERKO mice were infertile, although the CAIIdef males showed reduced fertility with aging (data not shown). Epithelia in NHE3KO (Fig. 2M), CAIIdef (Fig. 2N), and AQP1KO (Fig. 2O) appeared normal, with a densely populated microvillus border (Fig. 2K). WT tissue from all gene-deficient littermates was comparable, so only αERKO littermate WT epithelium is shown (Fig. 2K). There was no flattening of the epithelium or loss of cytoplasmic organelles, as seen in αERKO (Fig. 2L) and ICI-treated mice (27).
Figure 1.
Immunohistochemical staining for NHE3, CAII, and AQP-1 in efferent ductules of WT and αERKO mice. (A and B) NHE3 is abundant along the brush border of WT (αERKO littermate) nonciliated cells (arrow) but is absent in αERKO (B). (C and D) CAII staining found in the cytoplasm (arrowhead) and along the apical border of the epithelial cells (arrow) in WT, but CAII in αERKO is reduced in the cytoplasm (arrowhead) and absent along the apical border. (E and F) AQP1 is abundant along the brush border of WT nonciliated cells (arrow), but AQP1 in αERKO staining is absent (arrowhead) in the proximal ductules (prox) or intermittent in the more distal regions (arrow). (Bar = 25 μm.)
Figure 2.
Histological comparison of rete testis (A–E), efferent ductule lumen (F–J), and electron microscopy of apical epithelium (K–O) in gene-deficient and WT mice (αERKO). The rete testis (arrow) is small and narrow in WT (A), whereas there are large dilations in αERKO (B), NHE3KO (C), and CAIIdef (D) mice. AQP1KO has a slight dilation of the rete (E). The efferent ductule lumen (L) has a small diameter in WT (F) and AQP1KO (J) mice, but is dilated in αERKO (G), NHE3KO (H), and CAIIdef (I). The lumen in some NHE3KO ductules was 2- to 3-fold greater in diameter than in αERKO. Ultrastructure of apical cytoplasm in nonciliated cells of the efferent ductules reveals a loss or shortening of microvilli (double arrow) and a decreased number of endocytotic vesicles in αERKO (L) compared with WT (K). Microvilli in NHE3KO (M), CAIIdef (N), and AQP1KO mice (O) are taller than WT. [Bar = 25 μm (A–E), 100 μm (F–J), and 10 μm (K–O).]
RNA Expression in the Efferent Ductules.
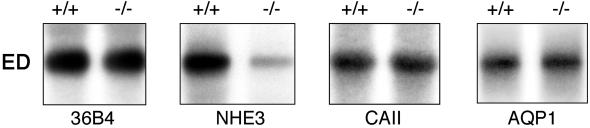
Based on morphological data and animal fertility, we tested the hypothesis that estrogen primarily regulates NHE3 gene expression, whereas effects on CAII and AQP1 could be secondary to loss of Na+ transport or the disruption of apical cytoplasmic architecture. The expression of NHE3 was 6-fold less in αERKO than in WT ductules, whereas mRNA for CAII and AQP1 was not altered (Fig. 3).
Figure 3.
Alterations in mRNA expression in efferent ductules (ED) of the αERKO (−/−) mice compared with WT (+/+). NHE3 (5.9 kb) is reduced nearly 6-fold in αERKO, whereas CAII (1.7 kb) and AQP1 (3.2 kb) show no change in mRNA expression. All Northern blots were normalized for loading by a final hybridization with the 36B4 probe, which is not responsive to estrogen (24).
NHE3 Activity.
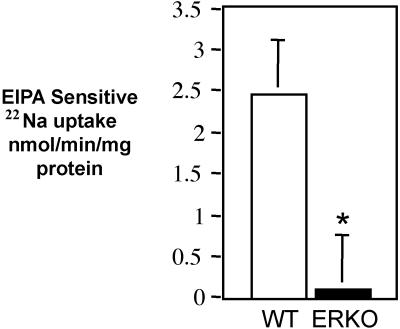
The quantity of 22Na uptake, sensitive to EIPA, an NHE inhibitor, was measured in ductule segments taken from αERKO and paired WT littermate mice. In the αERKO efferent ductules, the EIPA-sensitive 22Na uptake was reduced 4-fold, compared with uptake in WT ductules (Fig. 4).
Figure 4.
The rate of 22Na uptake sensitive to 100 μM EIPA in efferent ductule segments is reduced nearly 4-fold in αERKO mice compared with WT male littermates. Means ± SEM; n = 7 pairs. Significant difference (*) was determined by the paired t test, P < 0.01.
Effects of Antiestrogen Treatment on NHE3, CAII, and AQP1.
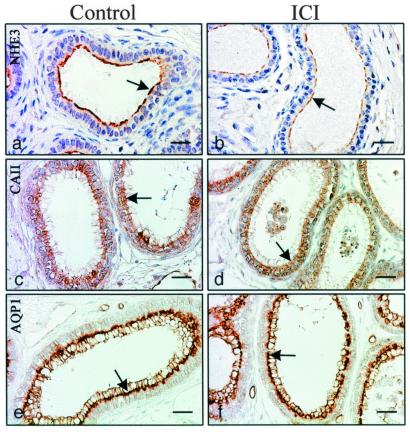
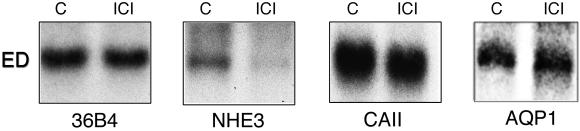
Adult control mice were treated with ICI 182,780 for 8 days to determine which candidate protein(s) would show the first response to an inhibition of ER function. After treatment, immunohistochemistry revealed a decrease in NHE3, no change in AQP1, and possibly a slight decrease in CAII along the microvillus border (Fig. 5). Northern blot analysis corroborated the immunohistochemical data, as NHE3 mRNA was decreased by nearly 63%, but there was no change in CAII and AQP1 mRNA expression (Fig. 6).
Figure 5.
Effects of ICI 182,780 treatment for 8 days in adult males. Immunohistochemical staining for NHE3 (A and B), CAII (C and D), and AQP-1 (E and F) in efferent ductules of control and treated mice. NHE3 is greatly reduced in staining after ICI treatment (arrow), whereas CAII may be reduced slightly (arrow) and AQP1 (arrow) shows no change compared with controls (C and E, respectively). (Bar = 25 μm.)
Figure 6.
NHE3 mRNA from efferent ductules is decreased nearly 63% on day 8 after ICI 182,780 treatment. CAII and AQP1 mRNAs show no change.
Discussion
The current study evaluated candidate genes for estrogen regulation in efferent ductules of the epididymis and provides a molecular mechanism to explain the accumulation of fluid in αERKO and antiestrogen-treated mice, resulting in infertility. These findings include (i) a decrease in immunoreactivity for NHE3, CAII, and AQP1 in both αERKO and antiestrogen-treated mice; (ii) dilation of rete testis and efferent ductules in NHE3KO and CAIIdef mice; (iii) a 6-fold decrease in mRNA for NHE3, but no change in expression for CAII and AQP1 in αERKO; (iv) a 4-fold decrease in Na+ uptake, sensitive to EIPA, in αERKO ductule epithelium; (v) a decrease in NHE3 immunoreactivity and its mRNA after an 8-day antiestrogen (ICI 182,780) treatment of adult control males but no change for CAII and AQP1; and (vi) the presence of abnormal epithelial ultrastructure only in αERKO efferent ductules, whereas epithelial cells in other gene-deficient mice exhibited normal appearing microvilli and apical cytoplasm.
NHE3, CAII, and AQP1 are among the critical proteins involved in fluid transport in this absorptive epithelium of the male reproductive tract (10, 11, 17). Loss of any one of these proteins could have been a primary cause of fluid accumulation in αERKO efferent ductules. Therefore, we hypothesized that an examination of mice deficient in these individual genes should reveal the more important protein(s) regulating fluid reabsorption. A decrease in NHE3 would inhibit Na+ transport across the epithelium from lumen to interstitium, and a decrease in CAII would further reduce NHE3 activity by limiting available protons for exchange. Although the volume of water transported via AQP1 in efferent ductules is not known, by analogy with proximal renal tubules, transcellular water movement through AQP1 may be the primary route of water reabsorption (28). Both NHE3KO and CAIIdef mice showed severe dilation of the rete testis and efferent ductules; however, only the NHE3KO mice were infertile. The CAIIdef mice were fertile at puberty but showed reduced fertility with aging, and their seminiferous epithelium was abnormal during puberty (unpublished data). In contrast, αERKO spermatogenesis was normal during early adulthood but regressed as the males aged (2, 3). Thus, unidentified factors in addition to estrogen receptors must be considered when comparing the CAIIdef and αERKO mice. In AQP1KO efferent ductules, no dilation was observed, and these mice were fertile. Therefore, these data suggest that NHE3 is the more critical gene for fluid reabsorption in the efferent ductules and that lack of fluid transport in this region is associated with male infertility.
The lack of effect in efferent ductules of AQP1KO mice was surprising because osmotic water permeability in the kidney is reduced 8-fold in the AQP1 knockout mouse (29). This may indicate that another isoform is compensating for the loss of AQP1. The efferent ductules are capable of reabsorbing a large volume of fluid, ≈96% of the rete testis fluid (6); therefore, an alternative explanation for the AQP1KO response is compensatory increase in reabsorption via the endocytotic and paracellular pathways.
Northern blot analysis revealed that only the NHE3 mRNA was decreased significantly in αERKO. The 4-fold reduction in 22Na+ uptake via the NHE3 pathway was consistent with estrogen's regulation of NHE3 expression and demonstrated a physiological response in the efferent ductule epithelium. An examination of the published sequence for the rat NHE3 promoter region (GenBank accession no. S83406) revealed a palindromic estrogen response element with only 1-bp change from the consensus (GtTCAgtcTGACC), plus an additional consensus estrogen response element half-site. Thus, transcriptional regulation of NHE3 by estrogen would be expected.
These αERKO data, however, are based on a mouse that lacks ERα throughout development. Therefore, the observed abnormalities could represent developmental defects rather than direct regulation of ERα in the adult. Previously, this hypothesis was tested by treating adult WT rodents with a pure antiestrogen, ICI 182,780 (27, 30). The results demonstrated that ERα is required for adult function, as ICI-induced pathological changes were nearly identical to those in αERKO efferent ductules (5, 27). In the present study, the first protein to respond to ICI treatment was detected by treating adult control males for a shorter time period. After treatment for 8 days, only NHE3 showed a substantial decline in immunostaining, and Northern blot analysis confirmed that its mRNA was also decreased.
Results in this study explain the accumulation of fluid in efferent ductules and testes of αERKO and ICI-treated mice. However, the ductal epithelium in both of these animal models was decreased in height, and the endocytotic apparatus, including apical vesicles and PAS+ lysosomal granules, was also greatly reduced (2, 5, 27). These organelles are normally prominent in all reabsorbing epithelia and are typical in nonciliated cells of WT efferent ductules (4, 7). Therefore, it was hypothesized that epithelial dysmorphogenesis in αERKO and the ICI-treated mice resulted from excessive stretching that occurred as luminal fluid accumulated in the absence of Na+ transport. However, electron microscopic examination of the epithelium in αERKO and the other gene-deficient mice showed that only the αERKO epithelium was abnormal. Efferent ductule epithelia in NHE3KO, AQP1KO, and CAIIdef showed no flattening or loss of microvilli and cytoplasmic organelles, effects that are obvious in αERKO and ICI-treated mice (2, 5, 27). Instead, microvilli and the absorptive apical cytoplasm appeared normal. Therefore, we must reject the previous hypothesis, which supposed that excessive stretching by fluid accumulation would cause the loss of microvilli and flattening of the epithelium. The data presented here suggest that estrogen plays a critical role in the maintenance of normal morphology of these epithelial cells independent of its regulation on fluid transport. The decreased immunostaining for CAII and AQP1 proteins in αERKO mice likely represents a secondary effect resulting from the loss of microvilli, as there appeared to be CAII staining in the cytoplasm and reduced AQP1 staining along the apical border of many nonciliated cells.
In conclusion, the basic molecular mechanism by which estrogen controls fluid reabsorption in efferent ductules of the male reproductive tract has been uncovered. Its primary function is expression of the NHE3 gene, which regulates the exchange of Na+ and H+ in mediating water transport and the concentration of sperm in the epididymis, and thus fertilizing ability of sperm. Interference with this physiological process or inhibition of the expression and function of this critical gene leads to male infertility. In addition to this physiological role in ion transport, estrogen seems to have a separate function in maintaining epithelial morphology, particularly the apical cytoarchitecture of nonciliated cells. The accumulation of luminal fluids or occlusion of these ductules, two opposing results of ductule dysfunction, may lead to seminiferous tubule degeneration, testicular atrophy, and infertility (4, 31). Thus, an understanding of these pathophysiological processes is fundamental to diagnosis and treatment of male infertility and diseases that target the head of the epididymis. Finally, if studies into the effectiveness and reversibility of ERα antagonist were pursued, it may be possible to develop a novel male contraceptive by selectively inhibiting NHE3 function by means of an antiestrogen such as ICI 182,780, which does not cross the blood–brain barrier (32), and therefore should not inhibit testosterone synthesis and libido.
Acknowledgments
We acknowledge AstraZeneca for providing ICI 182,780. We recognize Dr. Hyun Wook Cho for originally showing that ICI produces effects in 8 days and Drs. Gary Shull and Patrick Schultheis (University of Cincinnati) for the NHE3KO mice. We also thank Leslie Newton for helping to maintain αERKO mice and Ms. Tracy Gerrling and Dr. Masaaki Nakai for expert technical assistance. This work was supported by the Kenneth A. Scott Charitable Foundation (to R.A.H.) and National Institutes of Health Grants R01HD35126 (to R.A.H. and L.L.C.) and RO1 52358 (to Y.H.L.).
Abbreviations
- ER
estrogen receptor
- NHE
Na+/H+ exchanger
- CA
carbonic anhydrase
- AQP1
aquaporin-I
- αERKO
ERα knockout
- WT
wild type
- NHE3KO
NHE3 knockout
- AQP1KO
knockout
- CAIIdef
CAII deficient
- EIPA
5-(N-ethyl-N-isopropyl) amiloride
Footnotes
References
- 1.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess R A, Bunick D, Lee K H, Bahr J, Taylor J A, Korach K S, Lubahn D B. Nature (London) 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddy E M, Washburn T F, Bunch D O, Goulding E H, Gladen B C, Lubahn D B, Korach K S. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 4.Ilio K Y, Hess R A. Microsc Res Tech. 1994;29:432–467. doi: 10.1002/jemt.1070290604. [DOI] [PubMed] [Google Scholar]
- 5.Hess R A, Bunick D, Lubahn D B, Zhou Q, Bouma J. J Androl. 2000;21:107–121. [PubMed] [Google Scholar]
- 6.Clulow J, Jones R C, Hansen L A, Man S Y. J Reprod Fertil. 1998;53,Suppl.:1–14. [PubMed] [Google Scholar]
- 7.Robaire B, Hermo L. In: The Physiology of Reproduction. Knobil E, Neill J, editors. Vol. 1. New York: Raven; 1988. pp. 999–1080. [Google Scholar]
- 8.Ilio K Y, Hess R A. Anat Rec. 1992;234:190–200. doi: 10.1002/ar.1092340206. [DOI] [PubMed] [Google Scholar]
- 9.Hansen L A, Clulow J, Jones R C. Exp Physiol. 1999;84:521–527. [PubMed] [Google Scholar]
- 10.Leung G P H, Tse C M, Cheng Chew S B, Wong P Y D. Biol Reprod. 2001;64:482–490. doi: 10.1095/biolreprod64.2.482. [DOI] [PubMed] [Google Scholar]
- 11.Bagnis C, Marsolais M, Biemesderfer D, Laprade R, Breton S. Am J Physiol. 2001;280:F426–F436. doi: 10.1152/ajprenal.2001.280.3.F426. [DOI] [PubMed] [Google Scholar]
- 12.Chew S B, Leung G P, Leung P Y, Tse C M, Wong P Y. Biol Reprod. 2000;62:755–758. doi: 10.1095/biolreprod62.3.755. [DOI] [PubMed] [Google Scholar]
- 13.Biemesderfer D, Reilly R F, Exner M, Igarashi P, Aronson P S. Am J Physiol. 1992;263:F833–F840. doi: 10.1152/ajprenal.1992.263.5.F833. [DOI] [PubMed] [Google Scholar]
- 14.Pushkin A, Clark I, Kwon T H, Nielsen S, Kurtz I. J Androl. 2000;21:708–720. [PubMed] [Google Scholar]
- 15.Cohen J P, Hoffer A P, Rosen S. Biol Reprod. 1976;14:339–346. doi: 10.1095/biolreprod14.3.339. [DOI] [PubMed] [Google Scholar]
- 16.Hermo L, Adamali H I, Andonian S. J Androl. 2000;21:376–391. [PubMed] [Google Scholar]
- 17.Brown D, Verbavatz J M, Valenti G, Lui B, Sabolic I. Eur J Cell Biol. 1993;61:264–273. [PubMed] [Google Scholar]
- 18.Suzuki F, Nagano T. Anat Rec. 1978;191:503–520. doi: 10.1002/ar.1091910409. [DOI] [PubMed] [Google Scholar]
- 19.Hermo L, Oko R, Morales C R. Int Rev Cytol. 1994;154:106–189. [PubMed] [Google Scholar]
- 20.Fisher J S, Turner K J, Fraser H M, Saunders P T, Brown D, Sharpe R M. Endocrinology. 1998;139:3935–3945. doi: 10.1210/endo.139.9.6213. [DOI] [PubMed] [Google Scholar]
- 21.Schultheis P J, Clarke L L, Meneton P, Harline M, Boivin G P, Stemmermann G, Duffy J J, Doetschman T, Miller M L, Shull G E. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, Yang B, Gillespie A, Carlson E J, Epstein C J, Verkman A S. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 23.Lien Y H, Lai L W. Am J Physiol. 1998;274:L301–L304. doi: 10.1152/ajplung.1998.274.2.L301. [DOI] [PubMed] [Google Scholar]
- 24.Ediger T R, Kraus W L, Weinman E J, Katzenellenbogen B S. Endocrinology. 1999;140:2976–2982. doi: 10.1210/endo.140.7.6885. [DOI] [PubMed] [Google Scholar]
- 25.Kurashima K, D'Souza S, Szaszi K, Ramjeesingh R, Orlowski J, Grinstein S. J Biol Chem. 1999;274:29843–29849. doi: 10.1074/jbc.274.42.29843. [DOI] [PubMed] [Google Scholar]
- 26.Schultheis P J, Clarke L L, Meneton P, Miller M L, Soleimani M, Gawenis L R, Riddle T M, Duffy J J, Doetschman T, Wang T, et al. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 27.Lee K H, Hess R A, Bahr J M, Lubahn D B, Taylor J, Bunick D. Biol Reprod. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- 28.Verkman A S, Yang B, Song Y, Manley G T, Ma T. Exp Physiol. 2000;85:233S–241S. doi: 10.1111/j.1469-445x.2000.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 29.Schnermann J, Chou C L, Ma T, Traynor T, Knepper M A, Verkman A S. Proc Natl Acad Sci USA. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira C A, Carnes K, França L R, Hess R A. Biol Reprod. 2001;65:913–920. doi: 10.1095/biolreprod65.3.913. [DOI] [PubMed] [Google Scholar]
- 31.Hess R A, Nakai M. Histol Histopathol. 2000;15:207–224. doi: 10.14670/HH-15.207. [DOI] [PubMed] [Google Scholar]
- 32.Wade G N, Blaustein J D, Gray J M, Meredith J M. Am J Physiol. 1993;265:R1392–R1398. doi: 10.1152/ajpregu.1993.265.6.R1392. [DOI] [PubMed] [Google Scholar]