To the Editor:
Breathlessness is the cardinal symptom in people with chronic obstructive pulmonary disease (COPD) and is strongly associated with adverse health outcomes (1–3). Activity-related breathlessness, measured using the modified Medical Research Council (mMRC) breathlessness scale, is about twice as common among women as among men in the population for unclear reasons (1, 3).
Mechanistic and population-based data suggest that women are more prone to experience breathlessness in daily life owing to their lower absolute lung volumes and ventilatory capacity (3–6). No study has evaluated whether absolute lung function is related to breathlessness independent of the level of lung function impairment, and whether absolute volume may explain the higher breathlessness prevalence in women compared with men in COPD across severities of airflow limitation.
The aim of the present work was to evaluate whether the higher prevalence of breathlessness in women is related to their lower absolute spirometric lung volumes as measured by FEV1, and whether this is seen both in people with normal lung function (FEV1 ≥ 80% of predicted) and across severities of lung function impairment due to COPD.
Methods
This was a cross-sectional analysis of the COPDGene (Genetic Epidemiology of COPD) study (7). Inclusion criteria were as follows: age, 45–80 years; ethnic category, non-Hispanic white or African-American; and at least 10 years of smoking (COPD cohort) or no smoking (never-smoking control subjects), as detailed elsewhere (7). Exclusion criteria for the present analysis were as follows: missing data on spirometry (n = 63) or mMRC breathlessness score (n = 14).
Participants completed a modified American Thoracic Society Respiratory Questionnaire including smoking status, physician-diagnosed asthma, COPD, and emphysema. Activity-related breathlessness was self-rated on the mMRC scale (8). Post-bronchodilator spirometry was performed with an EasyOne spirometer (ndd Medizintechnik AG) according to American Thoracic Society standards (7). Predicted FEV1 and FVC were calculated (9).
The sex difference in breathlessness was analyzed as the odds ratio (OR) of higher mMRC scores for women compared with men, using ordinal logistic regression. All models were adjusted for age, level of chronic airflow limitation (FEV1/FVC), body mass index, pack-years of smoking, current smoking, and physician-diagnosed asthma, COPD, chronic bronchitis, emphysema, congestive heart failure, ischemic heart disease, and diabetes mellitus. No data were imputed. The impact of lung function on the sex difference in breathlessness was evaluated as the change in the sex estimate when adding each lung function measure to the fully adjusted model. Analyses were performed for the whole population, and in people with impaired lung function (FEV1 < 80% of predicted) and normal lung function (FEV1 ≥ 80% of predicted). Statistical analyses were conducted with SAS version 9.4 (SAS Institute, Inc.).
Results
In total, 10,223 participants were included, with a mean age of 59.6 years; 53.2% were men, and 4,935 (48.3%) had an FEV1 less than 80% of predicted. Despite a similar FEV1% predicted in men (76.2 ± 26.3%) and women (77.2 ± 24.8%), women had a mean 0.7-L lower absolute FEV1 (1.9 ± 0.9 vs. 2.6 ± 1.0 L).
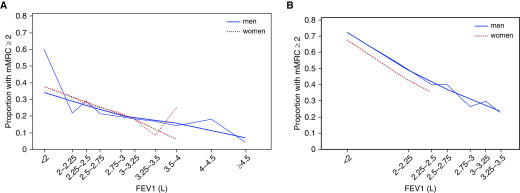
Higher absolute FEV1 was associated with lower likelihood of breathlessness (Figure 1) (adjusted OR, 0.41; 95% confidence interval [95% CI], 0.37–0.44). This was consistent in both men (OR, 0.44; 95% CI, 0.40–0.49) and women (0.33; 95% CI, 0.28–0.38), and in people with normal lung function (OR, 0.53; 95% CI, 0.46–0.61) and people with impaired lung function (OR, 0.30; 95% CI, 0.26–0.35). Women were more likely to have breathlessness than men (46.1% vs. 37.4% had mMRC ≥ 2) (adjusted OR, 1.75; 95% CI, 1.62–1.90) (Table 1). Adding FEV1% predicted to the model did not decrease the sex difference (adjusted OR, 1.72; 95% CI, 1.59–1.86). However, when instead adjusting for the absolute FEV1, sex difference disappeared (adjusted OR, 0.93; 95% CI, 0.84–1.03) (P = 0.147). Adjusting for absolute FEV1, breathlessness was similar in men and women with normal lung function, whereas breathlessness was even slightly lower among women in people with lung function impairment (Table 1). Using FVC instead of FEV1, there was no sex difference even in people with impaired lung function (OR, 0.84; 95% CI, 0.71–1.01), and all other findings were similar when using FVC compared with FEV1. Findings were robust when also adjusting for ethnicity, and when not including FEV1/FVC in the models. Analysis using multinomial and dichotomous logistic regression had similar findings as reported with ordinal logistic regression.
Figure 1.
The relationship between absolute FEV1 and breathlessness (mMRC ≥ 2) by sex in people with (A) normal FEV1 (≥80% of predicted) and (B) impaired FEV1 (<80% of predicted). Lower FEV1 was associated with more breathlessness, both among men and women. When compared at a similar absolute FEV1, breathlessness was similar between men and women. mMRC = modified Medical Research Council breathlessness scale.
Table 1.
FEV1 and Sex Difference in Breathlessness
| Models, All Adjusted for Confounders* | Sex Difference in mMRC Breathlessness Scale Score, for Women vs. Men [OR (95% CI)] |
|---|---|
| All participants (N = 10,223) | |
| Without any FEV1 measure | 1.75 (1.62–1.90) |
| With FEV1% predicted | 1.72 (1.59–1.86) |
| With FEV1, L | 0.93 (0.84–1.03) |
| Participants with FEV1 ≥ 80% predicted (n = 5,288) | |
| Without any FEV1 measure | 1.95 (1.73–2.19) |
| With FEV1, L | 1.11 (0.94–1.31) |
| Participants with FEV1 < 80% predicted (n = 4,935) | |
| Without any FEV1 measure | 1.57 (1.41–1.75) |
| With FEV1, L | 0.84 (0.73–0.95) |
Definition of abbreviations: CI = confidence interval; mMRC = modified Medical Research Council; OR = odds ratio.
All models were adjusted for age, body mass index, pack-years of smoking, current smoking, FEV1/FVC, asthma, chronic obstructive pulmonary disease or emphysema, chronic bronchitis, heart failure, cardiovascular disease, and diabetes mellitus.
Discussion: Main Findings
The key findings are that the markedly increased prevalence of breathlessness in women is related to their lower absolute lung function measured as FEV1 or FVC. People with smaller spirometric lung volumes have a higher prevalence of breathlessness, both in men and in women. When matched on absolute spirometric lung volume, men and women have similar (or near similar) likelihoods of breathlessness both in people with normal lung function and in people with lung function impairment due to COPD.
This is the first study of breathlessness in relation to sex and spirometric lung volumes across severities of chronic airflow limitation, using data from the well-characterized COPDGene study. The likely mechanism underpinning the sex difference in breathlessness is that women have smaller airways and less respiratory musculature than men, even when matched for height and lung size, resulting in a lower ventilator capacity (4, 5). For a given level of work and ventilation, women experience more breathlessness as they use a greater fraction of their ventilatory capacity than men (4, 5). Despite similar lung function impairment (% of predicted) between the sexes, women may have markedly lower absolute FEV1. This could explain the sex disparity in breathlessness seen in previous studies matching on the FEV1% predicted (10). By matching on absolute spirometric volumes, this sex bias can be overcome.
Data were unavailable on breathlessness measured using a standardized exercise test, as well as on static lung volumes and diffusion capacity. The impact on the sex difference in breathlessness has been found to be similar for FEV1 and FVC to that of static lung volumes (6).
The impact of a given lung function impairment on symptoms and function likely depends on the absolute lung volume and remaining ventilatory reserve. Relative and absolute lung volumes provide complementary information on the lung volume impairment and remaining ventilatory reserve and should be evaluated in both research and clinical care.
Acknowledgments
COPDGene Investigators: Core Units
Administrative Center: James D. Crapo (Principal Investigator), Edwin K. Silverman (Principal Investigator), Barry J. Make, and Elizabeth A. Regan.
Genetic Analysis Center: Terri Beaty, Ferdouse Begum, Robert Busch, Peter J. Castaldi, Michael Cho, Dawn L. DeMeo, Adel R. Boueiz, Marilyn G. Foreman, Eitan Halper-Stromberg, Nadia N. Hansel, Megan E. Hardin, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Margaret M. Parker, Dandi Qiao, Elizabeth A. Regan, Stephanie Santorico, Edwin K. Silverman, Emily S. Wan, and Sungho Won.
Imaging Center: Mustafa Al Qaisi, Harvey O. Coxson, Teresa Gray, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, John D. Newell, Jr., Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Douglas Stinson, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, George Washko, and Carla G. Wilson.
PFT QA Center, Salt Lake City, UT: Robert Jensen.
Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, Jim Crooks, Camille Moore, Matt Strand, and Carla G. Wilson.
Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, John Hughes, Gregory Kinney, Sharon M. Lutz, Katherine Pratte, and Kendra A. Young.
COPDGene Investigators: Clinical Centers
VA Ann Arbor Healthcare System, Ann Arbor, MI: Jeffrey L. Curtis, Carlos H. Martinez, and Perry G. Pernicano.
Baylor College of Medicine, Houston, TX: Nicola Hanania, Philip Alapat, Mustafa Atik, Venkata Bandi, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Arun Nachiappan, and Amit Parulekar.
Brigham and Women’s Hospital, Boston, MA: Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, and George Washko.
Columbia University, New York, NY: R. Graham Barr, John Austin, Belinda D’Souza, Gregory D. N. Pearson, Anna Rozenshtein, and Byron Thomashow.
Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., H. Page McAdams, and Lacey Washington.
HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy and Joseph Tashjian.
Johns Hopkins University, Baltimore, MD: Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, and Nirupama Putcha.
Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, and William Stringer.
Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, and Charlie Lan.
Minneapolis VA, Minneapolis, MN: Christine Wendt and Brian Bell.
Morehouse School of Medicine, Atlanta, GA: Marilyn G. Foreman, Eugene Berkowitz, and Gloria Westney.
National Jewish Health, Denver, CO: Russell Bowler and David A. Lynch.
Reliant Medical Group, Worcester, MA: Richard Rosiello and David Pace.
Temple University, Philadelphia, PA: Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Pharm.D., Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, and Maria Elena Vega-Sanchez.
University of Alabama, Birmingham, AL: Mark Dransfield, William Bailey, Surya Bhatt, Anand Iyer, Hrudaya Nath, and J. Michael Wells.
University of California, San Diego, CA: Joe Ramsdell, Paul Friedman, Xavier Soler, and Andrew Yen.
University of Iowa, Iowa City, IA: Alejandro P. Comellas, John Newell, Jr., and Brad Thompson.
University of Michigan, Ann Arbor, MI: MeiLan K. Han, Ella Kazerooni, and Carlos H. Martinez.
University of Minnesota, Minneapolis, MN: Joanne Billings, Abbie Begnaud, and Tadashi Allen.
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, Jessica Bon, Divay Chandra, Carl Fuhrman, and Joel Weissfeld.
University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario E. Ruiz.
Footnotes
Supported by Award No. R01 HL089897 and Award No. R01 HL089856 from the NHLBI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion. M.E. was supported by unrestricted grants from the Swedish Society of Medicine, the Swedish Respiratory Society, the Swedish Heart–Lung Foundation, the Scientific Committee of Blekinge County Council, the Wera and Emil Cornell Foundation, and the Swedish Society for Medical Research. No funding organization had a role in the design and conduct of the study, in the analysis and interpretation of data, or in the preparation or approval of the manuscript to be submitted.
Author Contributions: Conception and design: M.E.; analysis and interpretation: M.E., A.B.-H., N.W., D.C.C., and N.M.; drafting the manuscript for important intellectual content: M.E. and A.B.-H.; revising the manuscript for important intellectual content and approval of the final version to be published: M.E., A.B.-H., N.W., D.C.C., and N.M.
Originally Published in Press as DOI: 10.1164/rccm.201803-0594LE on April 27, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: for the COPDGene Investigators, James D. Crapo, Edwin K. Silverman, Barry J. Make, Elizabeth A. Regan, Terri Beaty, Ferdouse Begum, Robert Busch, Peter J. Castaldi, Michael Cho, Dawn L. DeMeo, Adel R. Boueiz, Marilyn G. Foreman, Eitan Halper-Stromberg, Nadia N. Hansel, Megan E. Hardin, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Margaret M. Parker, Dandi Qiao, Elizabeth A. Regan, Stephanie Santorico, Edwin K. Silverman, Emily S. Wan, Sungho Won, Mustafa Al Qaisi, Harvey O. Coxson, Teresa Gray, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, John D. Newell, Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Douglas Stinson, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, George Washko, Carla G. Wilson, Robert Jensen, Douglas Everett, Jim Crooks, Camille Moore, Matt Strand, Carla G. Wilson, John E. Hokanson, John Hughes, Gregory Kinney, Sharon M. Lutz, Katherine Pratte, Kendra A. Young, COPDGene Investigators: Clinical Centers, Jeffrey L. Curtis, Carlos H. Martinez, Perry G. Pernicano, Nicola Hanania, Philip Alapat, Mustafa Atik, Venkata Bandi, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Arun Nachiappan, Amit Parulekar, Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, George Washko, R. Graham Barr, John Austin, Belinda D’Souza, Gregory D. N. Pearson, Anna Rozenshtein, Byron Thomashow, Neil MacIntyre, H. Page McAdams, Lacey Washington, Charlene McEvoy, Joseph Tashjian, Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, William Stringer, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Marilyn G. Foreman, Eugene Berkowitz, Gloria Westney, Russell Bowler, David A. Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs Pharm.D, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, Maria Elena Vega-Sanchez, Mark Dransfield, William Bailey, Surya Bhatt, Anand Iyer, Hrudaya Nath, J. Michael Wells, Joe Ramsdell, Paul Friedman, Xavier Soler, Andrew Yen, Alejandro P. Comellas, John Newell, Brad Thompson, MeiLan K. Han, Ella Kazerooni, Carlos H. Martinez, Joanne Billings, Abbie Begnaud, Tadashi Allen, Frank Sciurba, Jessica Bon, Divay Chandra, Carl Fuhrman, Joel Weissfeld, Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario E. Ruiz
References
- 1. Grønseth R, Vollmer WM, Hardie JA, Ólafsdóttir IS, Lamprecht B, Buist AS, et al. Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J. 2014;43:1610–1620. doi: 10.1183/09031936.00036813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 3. Ekström M, Schiöler L, Grønseth R, Johannessen A, Svanes C, Leynaert B, et al. Absolute values of lung function explain the sex difference in breathlessness in the general population. Eur Respir J. 2017;49:49. doi: 10.1183/13993003.02047-2016. [DOI] [PubMed] [Google Scholar]
- 4. Ofir D, Laveneziana P, Webb KA, Lam YM, O’Donnell DE. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol. 2008;104:1583–1593. doi: 10.1152/japplphysiol.00079.2008. [DOI] [PubMed] [Google Scholar]
- 5. Schaeffer MR, Mendonca CT, Levangie MC, Andersen RE, Taivassalo T, Jensen D. Physiological mechanisms of sex differences in exertional dyspnoea: role of neural respiratory motor drive. Exp Physiol. 2014;99:427–441. doi: 10.1113/expphysiol.2013.074880. [DOI] [PubMed] [Google Scholar]
- 6. Ekström M, Sundh J, Schiöler L, Lindberg E, Rosengren A, Bergström G, et al. Absolute lung size and the sex difference in breathlessness in the general population. PLoS One. 2018;13:e0190876. doi: 10.1371/journal.pone.0190876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic Epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 9. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 10. de Torres JP, Casanova C, Hernández C, Abreu J, Aguirre-Jaime A, Celli BR. Gender and COPD in patients attending a pulmonary clinic. Chest. 2005;128:2012–2016. doi: 10.1378/chest.128.4.2012. [DOI] [PubMed] [Google Scholar]