Abstract
Rationale: Cystic fibrosis (CF) is characterized by dietary antioxidant deficiencies, which may contribute to an oxidant–antioxidant imbalance and oxidative stress.
Objectives: Evaluate the effects of an oral antioxidant-enriched multivitamin supplement on antioxidant concentrations, markers of inflammation and oxidative stress, and clinical outcomes.
Methods: In this investigator-initiated, multicenter, randomized, double-blind, controlled trial, 73 pancreatic-insufficient subjects with CF 10 years of age and older with an FEV1 between 40% and 100% predicted were randomized to 16 weeks of an antioxidant-enriched multivitamin or control multivitamin without antioxidant enrichment. Endpoints included systemic antioxidant concentrations, markers of inflammation and oxidative stress, clinical outcomes (pulmonary exacerbations, anthropometric measures, pulmonary function), safety, and tolerability.
Measurements and Main Results: Change in sputum myeloperoxidase concentration over 16 weeks, the primary efficacy endpoint, was not significantly different between the treated and control groups. Systemic antioxidant (β-carotene, coenzyme Q10, γ-tocopherol, and lutein) concentrations significantly increased in the antioxidant-treated group (P < 0.001 for each), whereas circulating calprotectin and myeloperoxidase decreased in the treated group compared with the control group at Week 4. The treated group had a lower risk of first pulmonary exacerbation requiring antibiotics than the control group (adjusted hazard ratio, 0.50; P = 0.04). Lung function and growth endpoints did not differ between groups. Adverse events and tolerability were similar between groups.
Conclusions: Antioxidant supplementation was safe and well tolerated, resulting in increased systemic antioxidant concentrations and modest reductions in systemic inflammation after 4 weeks. Antioxidant treatment was also associated with a lower risk of first pulmonary exacerbation.
Clinical trial registered with www.clinicaltrials.gov (NCT01859390).
Keywords: cystic fibrosis, inflammation, oxidative stress, antioxidant, pulmonary exacerbation
At a Glance Commentary
Scientific Knowledge on the Subject
Cystic fibrosis (CF) lung disease is characterized by oxidant/antioxidant imbalance and oxidative stress. Optimizing antioxidant status in CF is an important clinical goal and may positively influence health outcomes.
What This Study Adds to the Field
Supplementation with an oral antioxidant-enriched multivitamin over 4 months was safe and well tolerated and led to increased systemic antioxidant concentrations and a transient decrease in systemic inflammation. Although the primary endpoint (change in sputum myeloperoxidase concentration) was not achieved, a clinically meaningful increased time to first pulmonary exacerbation was observed in the antioxidant-treated group.
Cystic fibrosis (CF) lung disease is characterized by chronic bacterial infection and neutrophil-dominated inflammation that leads to the release of vast amounts of proteases and reactive oxygen species (ROS) into the airways (1). Normally, ROS and other oxidants are neutralized by the body’s antioxidant defenses. In CF, however, exocrine pancreatic insufficiency and diminished bile acids cause malabsorption of important dietary antioxidants, including carotenoids such as β-carotene, tocopherols (vitamin E), coenzyme Q10 (CoQ10), and selenium. Despite treatment with pancreatic enzymes and supplementation with CF-specific multivitamins, dietary antioxidant deficiencies have been repeatedly demonstrated in individuals with CF, particularly in those with pancreatic insufficiency (2–7). Increased ROS in combination with deficient antioxidant concentrations result in an oxidant–antioxidant imbalance and oxidative stress in CF (8, 9).
Optimizing antioxidant status in CF is an important clinical goal and may positively influence health outcomes. The results of several trials of oral antioxidant formulations in CF have been previously reviewed (10, 11). In these studies, circulating antioxidant concentrations were consistently low before supplementation and improved with oral treatment. The evidence for clinical effectiveness, however, was mixed and not compelling across studies. A recent Cochrane Review, which examined the evidence for dietary antioxidant micronutrients (vitamins E and C, β-carotene, and selenium) as treatment for CF lung disease, concluded there has not been a single well-designed randomized controlled trial in this area (11).
A panel of vitamin and antioxidant experts at a CF Foundation–sponsored antioxidant workshop recommended conducting carefully controlled trials, using antioxidant “cocktails,” and including measurements of biomarkers of inflammation and oxidative stress in such interventional studies (10). In two previous pilot studies, an oral formulation containing several vitamins and antioxidant micronutrients, specifically designed for individuals with malabsorptive disorders including those with CF, safely increased systemic antioxidant levels (12, 13). Although these were not controlled clinical trials, modest improvements in weight and pulmonary function and small yet significant reductions in airway inflammation were observed over 8 to 12 weeks, supporting further clinical study of this antioxidant-enriched multivitamin supplement. Therefore, this investigator-initiated, multicenter, randomized, controlled (phase II) study was conducted to further evaluate the effects of an oral antioxidant-enriched multivitamin supplement on antioxidant concentrations, markers of inflammation and oxidative stress, and clinical outcomes in CF. Some of the results of this study have been previously reported in abstract form (14).
Methods
Study Design and Population
This was a multicenter, randomized, double-blind, controlled trial conducted from September 2013 to October 2015 at 15 accredited CF care centers across the United States and coordinated by the CF Foundation Therapeutics Development Network Coordinating Center (ClinicalTrials.gov identifier: NCT01859390). Institutional review boards at each participating center approved the study, and each subject and/or his/her parent provided written informed consent (assent when applicable). Pancreatic-insufficient subjects with CF (documented by having a spot fecal elastase ≤ 100 μg/g in a stool sample done either historically or at the screening visit) 10 years of age and older with an FEV1 between 40% and 100% predicted were eligible to participate. Exclusion criteria were: unwilling or unable to discontinue current oral vitamin and antioxidant supplementation, use of dietary supplements with antioxidants, treatment with ivacaftor/oral corticosteroids/anticoagulant medications, liver enzymes greater than three times the upper limits of normal, use of antibiotics for respiratory symptoms within 2 weeks of randomization, recent initiation of new chronic therapy, active treatment for allergic bronchopulmonary aspergillosis or nontuberculous mycobacteria, severe malnutrition (body mass index [BMI] less than fifth percentile for subjects < 18 yr; BMI < 18 kg/m2 for subjects ≥ 18 yr), poorly controlled CF-related diabetes (Hb A1c ≥ 7.5%), current tobacco smoker, and enrollment in another interventional trial.
The trial entailed four study visits (see Figure E1 in the online supplement). After the screening visit, participants were instructed to discontinue their current vitamin supplementation and take a control multivitamin without antioxidant enrichment (Table E1), two softgel capsules once daily for 4 to 8 weeks. At visit 2 (baseline visit), subjects were randomized 1:1 to receive either the antioxidant-enriched multivitamin (treated group) or continue on the control multivitamin (control group), two softgels once daily for 16 weeks (identical in appearance and taste to ensure blinding). An adaptive randomization algorithm was used based on stratification factors for: age (10–17 yr, ≥18 yr), FEV1% predicted (40–70%, >70–100%), chronic use of inhaled antibiotics, and chronic use of azithromycin. A fasting blood draw for antioxidant and vitamin concentrations and markers of inflammation and oxidative stress, anthropometric measures, and pulmonary function were obtained at baseline and at 4 and 16 weeks. Subjects were instructed to hold their vitamin formulations and fast for at least 8 hours before these study visits and blood draws, so that the most recent dose of the vitamin formulations was taken the prior day and more than 12 hours before the scheduled blood draw. Induced sputum and urine specimens were collected at baseline and Week 16 for markers of inflammation and oxidative stress. Biochemical measurements were performed in the CF Foundation Therapeutics Center for Biochemical Markers at the University of Colorado (detailed in the online supplement).
Primary and Secondary Outcomes
The primary endpoint was the difference between treatment groups in the change in sputum myeloperoxidase (MPO) from baseline to Week 16. Secondary endpoints included change from baseline to Weeks 4 and 16 in systemic antioxidant concentrations, systemic and sputum markers of inflammation and oxidative stress, vitamin levels, change from baseline in FEV1% predicted, weight, BMI, time to first protocol-defined pulmonary exacerbation, and number of exacerbations. Safety and tolerability were monitored throughout the study.
Statistical Analyses
The study was designed to enroll 80 participants to detect a log10 difference between groups of 0.60 ng/ml (SD, 0.87) in sputum MPO with 80% power on the basis of a two-sided α of 0.05, assuming an attrition rate of 15% (12). All analyses were based on a modified intent-to-treat population, defined as all randomized participants who received at least one dose of study drug. The primary endpoint was assessed using linear regression to model 16-week change in log10 sputum MPO, adjusting for treatment group and randomization stratification factors. Secondary endpoint models for biochemical markers were adjusted similarly. Cox proportional hazards models were fit to estimate hazard ratios for time to first protocol-defined pulmonary exacerbation and graphically displayed using Kaplan-Meier estimates. Two sample t tests were used to compare all continuous variables by study group, whereas differences in proportions were tested using the Fisher exact test with confidence intervals (CIs) estimated using the Newcombe-Wilson method without continuity correction. Poisson regression with an offset for follow-up time was used to calculate rate ratios, CIs, and corresponding P values. No adjustments for multiple comparisons were made, and all testing was performed using a two-sided, 0.05 significance level; all analyses were performed with SAS version 9.4 (SAS Institute Inc.) or R version 3.0.3 (R Foundation for Statistical Computing).
Results
Subjects
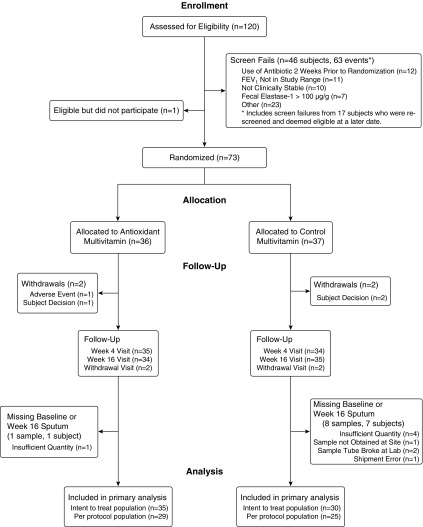
Of the 120 screened participants, 74 met the eligibility criteria and 73 were randomized, 36 to the treated group and 37 to the control group (Figure 1). Reasons that the goal of randomizing 80 subjects was not achieved are provided in the online supplement. All randomized subjects received at least one dose of study drug, constituting the modified intent-to-treat population. Baseline demographics and clinical characteristics for the randomized subjects are shown in Table 1. The two groups were well matched. Two participants from each group withdrew from the study. The two control subjects and one treated subject withdrew per subject decision, and one treated subject withdrew because of flatulence/abdominal pain. The per-protocol population excluded 19 patients: 2 had a major protocol violation, 16 had study drug compliance less than 80%, 8 had sputum MPO missing at baseline or Week 16, and 6 had more than one of these criteria.
Figure 1.
Flow diagram of the study participants.
Table 1.
Clinical Characteristics of the Study Participants at Randomization
| Antioxidant (n = 36) | Control (n = 37) | |
|---|---|---|
| Female sex, n (%) | 20 (55.6) | 20 (54.1) |
| Age, yr, mean (SD) | 22.3 (8.9) | 22.9 (9.5) |
| Age distribution, yr, n (%) | ||
| ≥10–18 | 13 (36.1) | 16 (43.2) |
| ≥18–30 | 17 (47.2) | 11 (29.7) |
| >30 | 6 (16.7) | 10 (27.0) |
| Race, n (%) | ||
| White | 34 (94.4) | 30 (81.1) |
| Hispanic | 1 (2.8) | 5 (13.5) |
| African American | 1 (2.8) | 1 (2.7) |
| Unknown/other | 0 (0) | 1 (2.7) |
| Genotype, n (%) | ||
| F508del homozygous | 23 (63.9) | 16 (43.2) |
| F508del heterozygous | 9 (25.0) | 18 (48.6) |
| Other/unknown* | 4 (11.1) | 3 (8.1) |
| BMI, kg/m2, mean (SD) | 21.9 (4.4) | 21.0 (3.2) |
| FEV1% predicted,† mean (SD) | 74.9 (15.4) | 76.3 (13.4) |
| FEV1% predicted distribution, n (%) | ||
| ≤70% | 11 (30.6) | 10 (27.0) |
| >70 to ≤90% | 18 (50.0) | 22 (59.5) |
| >90% | 7 (19.4) | 5 (13.5) |
| History of meconium ileus, n (%) | 4 (11.1) | 4 (10.8) |
| Chronic inhaled antibiotics,‡n (%) | 20 (55.6) | 20 (54.1) |
| Chronic azithromycin,‡n (%) | 16 (44.4) | 18 (48.6) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis.
Other refers to subjects with either two known, non-F508del CF mutations, or one known, non-F508del CF mutation and one unidentified allele that has not been classified as a CF mutation.
FEV1% predicted is calculated using the Wang (female < 16 yr, male < 18 yr) or Hankinson equations (female ≥ 16 yr, male ≥ 18 yr).
Chronic is defined as initiated 8 or more weeks before randomization.
Compliance and Study Drug Discontinuation
Mean study drug compliance for the treated and control groups were 86% and 87%, respectively (P = 0.82; Table E2). Eighty-one percent of the treated group had compliance greater than or equal to 80%, as compared with 76% of the control group. Two subjects (5.6%) in the treated group permanently discontinued study drug, as compared with four (10.8%) in the control group.
Primary Endpoint
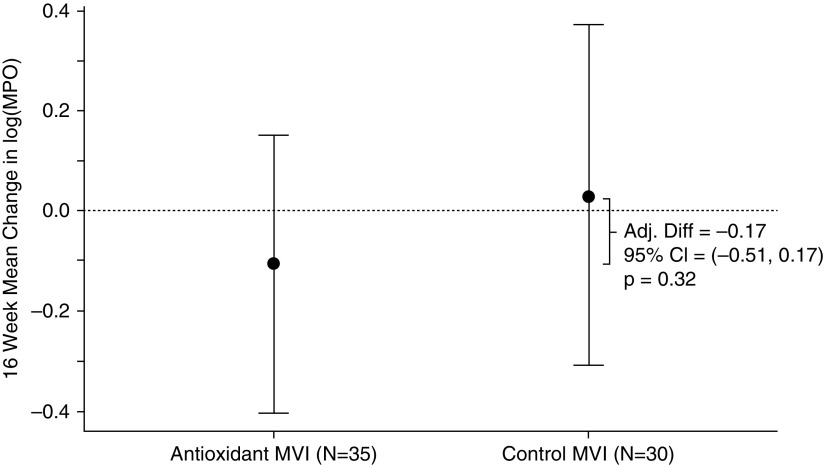
The change in sputum MPO over the 16-week treatment period was not significantly different between groups. The adjusted mean difference between groups was −0.17 log10(ng/ml) (95% CI, −0.51 to 0.17; P = 0.32) (Figure 2).
Figure 2.
Mean change in sputum myeloperoxidase (MPO) over 16 weeks by treatment group. The change in sputum MPO over the 16-week treatment period was not significantly different between groups. The adjusted mean difference (Adj. Diff) between groups was −0.17 log10(ng/ml) (95% confidence interval [CI], −0.51 to 0.17; P = 0.32). Error bars show 95% CIs. MVI = multivitamin.
Clinical Efficacy Endpoints
Pulmonary exacerbations
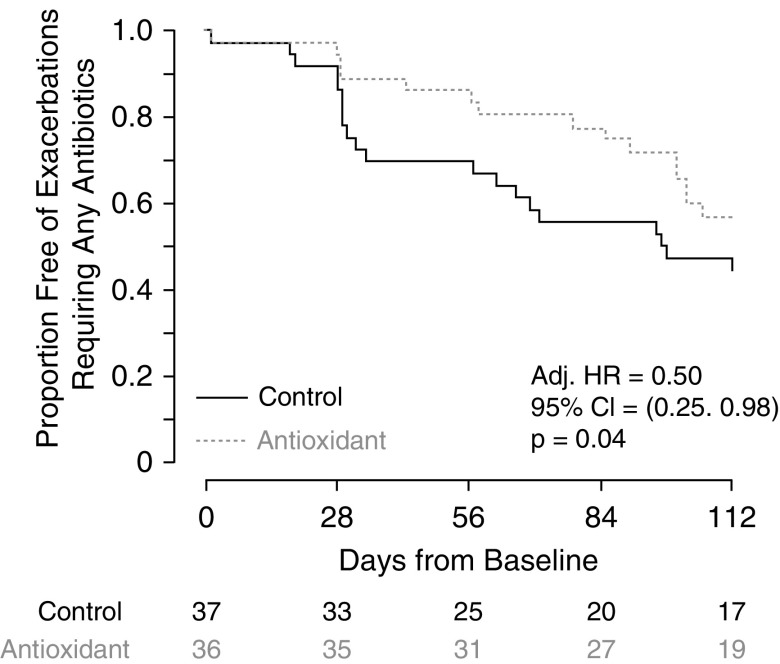
The time to first pulmonary exacerbation and rate of pulmonary exacerbations were more favorable in the antioxidant-treated group. The antioxidant-treated group had a significantly lower risk of first pulmonary exacerbation requiring antibiotics than the control group (covariate-adjusted hazard ratio, 0.50; 95% CI, 0.25–0.98; P = 0.04) (Figure 3). To account for the possibility that a higher number of older subjects (>30 yr) in the control group might be contributing to the difference in time to first exacerbation between the two groups, we included this age category in our adjusted model and found similar results (adjusted hazard ratio, 0.47; 95% CI, 0.24–0.91; P = 0.03). Nineteen out of 36 antioxidant-treated subjects (53%) experienced 28 protocol-defined exacerbations, as compared with 25 out of 37 control subjects (68%) who experienced 39 exacerbations (difference, −15%; 95% CI, −35% to 7%; P = 0.24; rate ratio, 0.72; P = 0.17). Although the hospitalization event rate was lower in the treated group, it was not significantly different compared with the control group (0.60 hospitalizations/participant-year in the treated group vs. 1.08 in the control group; rate ratio, 0.54: 95% CI, 0.20–1.31; P = 0.17).
Figure 3.
Time to first pulmonary exacerbation by treatment group. The antioxidant-treated group had a significantly lower risk of first pulmonary exacerbation requiring antibiotics than the control group (covariate-adjusted hazard ratio [HR], 0.50; 95% confidence interval [CI], 0.25–0.98; P = 0.04).
Lung function
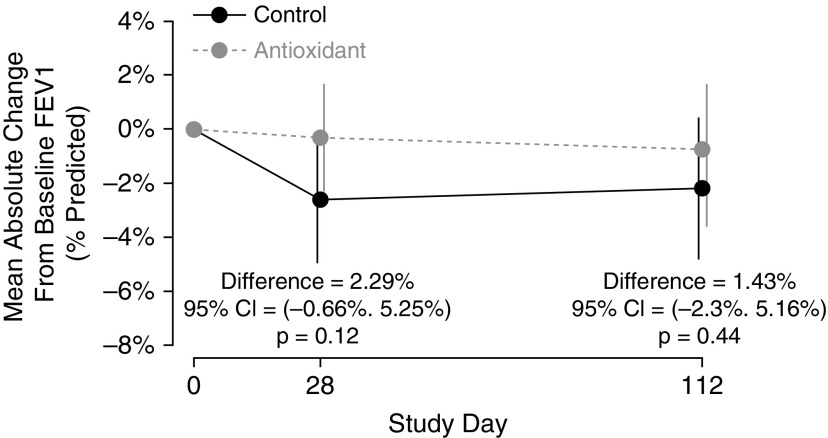
At Week 4, the absolute change in mean FEV1% predicted was −0.31% in the treated group and −2.60% in the control group (difference, 2.29%; 95% CI, −0.66 to 5.25; P = 0.12). The 16-week absolute change in mean FEV1% predicted in the treated group was −0.76%, compared with −2.20% in the control group (difference, 1.43%; 95% CI, −2.30 to 5.16; P = 0.44) (Figure 4). Similarly, no significant differences between groups were observed for absolute or relative changes of FVC and forced expiratory flow in the midexpiratory phase.
Figure 4.
Mean absolute change in FEV1% predicted from baseline by treatment group. The 16-week absolute change in mean FEV1% predicted in the treated group was −0.76%, compared with −2.20% in the control group (difference, 1.43%; 95% confidence interval [CI], −2.30 to 5.16; P = 0.44). Error bars show 95% CIs.
Weight
No significant differences between groups were observed for 16-week change in weight or BMI. The 16-week difference in unadjusted weight z-score was 0.07 (95% CI, −0.10 to 0.25; P = 0.41) (Figure E2).
Circulating Antioxidant and Vitamin Concentrations
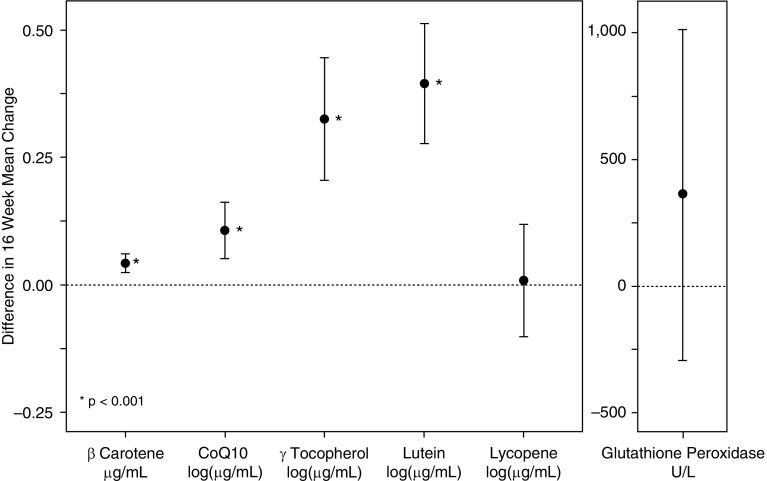
At baseline, circulating antioxidant and vitamin concentrations were similar between the two groups. At Week 16, the following antioxidants were significantly higher in the antioxidant-treated group than in the control group (adjusted mean difference; P < 0.001 for each): β-carotene (0.04 μg/ml), CoQ10 (0.11 log10[μg/ml]), γ-tocopherol (0.38 log10[μg/ml]), and lutein (0.37 log10[μg/ml]) (Figure 5). Pre–post changes in lycopene and glutathione peroxidase activity did not differ between groups. During the treatment period, the circulating vitamin levels (retinol, 25-hydroxy vitamin D, α-tocopherol, PIVKA-II [protein induced by vitamin K absence-II]) were not different between groups (Table E3).
Figure 5.
Difference in 16-week change in mean circulating antioxidant levels between the antioxidant and control groups with corresponding 95% confidence intervals. At Week 16, β-carotene, coenzyme Q10 (CoQ10), γ-tocopherol, and lutein were significantly higher in the antioxidant-treated group compared with the control group (adjusted mean difference; *P < 0.001 for each). Pre–post changes in lycopene and glutathione peroxidase activity did not differ between groups. Error bars show 95% confidence intervals.
Biomarkers of Inflammation and Oxidative Stress
Several markers of inflammation and oxidative stress were assessed in blood, sputum, and urine specimens. None of these measures were different between groups at baseline (Tables E4 and E5). At Week 4, there were significant reductions in circulating calprotectin (mean difference, −0.13 log10[μg/ml]; P = 0.03) and MPO (mean difference, −0.13 log10[ng/ml]; P = 0.04) in the antioxidant-treated group relative to the control group (Table E4). At Week 16, however, changes in systemic (Table E4) and sputum (Table E5) biomarkers were not significantly different between the two groups.
Adverse Event Profile
The incidence of adverse and serious adverse events was similar between the two groups (Table E6). All serious adverse events were deemed unrelated to study treatment.
Discussion
In this multicenter, randomized, controlled trial in subjects with CF, antioxidant supplementation was safe and well tolerated. Although changes in lung function and weight did not differ significantly between groups, the antioxidant-treated group had a significantly lower risk of first pulmonary exacerbation requiring antibiotics. Adherence to study medication was high (approaching 90%), and circulating antioxidant (β-carotene, CoQ10, γ-tocopherol, and lutein) concentrations increased in the treated subjects, demonstrating absorption of this antioxidant-enriched multivitamin supplement. Significant reductions in circulating calprotectin and MPO were observed in the antioxidant-treated group relative to the control group at Week 4, although no significant reductions in sputum MPO (primary outcome measure) or any of the secondary systemic and sputum markers of inflammation and oxidative stress were found at Week 16.
This specific formulation, which uses micelle-like particles to enhance absorption of fat-soluble nutrients (15), is a “cocktail” containing several vitamins and micronutrients with antioxidant properties, including carotenoids (particularly β-carotene), CoQ10, mixed tocopherols (particularly γ-tocopherol), and selenium. Numerous studies have shown that children and adults with CF are deficient in vitamin E (4, 16), β-carotene and other carotenoids (2, 3, 17, 18), CoQ10 (6, 19), and selenium (7, 20). The improvements in systemic antioxidant levels we found in this trial were analogous to previous pilot studies (12, 13). We can only conclude that our findings are due to using the exact formulation investigated in this trial and cannot make presumptions about other combinations and formulations of antioxidants and vitamins. Improving antioxidant status alone may be sufficient justification to add antioxidants to commercially available vitamin or nutritional supplements. However, a key question we were trying to determine in this trial was whether improving antioxidant status in turn leads to reduction in inflammation and oxidative stress and positively affects health outcomes in CF.
There is strong rationale to normalize antioxidant concentrations in CF. Epidemiologic surveys among the general population have found that higher dietary antioxidant vitamin consumption (including β-carotene and vitamin E) and higher circulating antioxidant levels are associated with better pulmonary function and lower rates of lung function decline (21–24). Similarly, in CF, correlations between antioxidant concentrations and lung function have been reported (20, 25). Low circulating concentrations of vitamins A and E have also been associated with an increased number of pulmonary exacerbations in CF (26). Circulating antioxidant levels have been reported to decrease during acute exacerbations and increase after antibiotic treatment (27).
The effect of this antioxidant formulation on time to first pulmonary exacerbation was significant and clinically meaningful. Consistent with our findings, previous studies have reported that β-carotene supplementation in CF normalizes β-carotene concentrations, leading to a reduced need for antibiotic therapy (18, 28). The 50% reduced risk of time to first exacerbation observed in our trial compares to that observed in previous trials of recombinant human DNase (rhDNase) (29), azithromycin (30), and lumacaftor–ivacaftor (31). Although the time to first pulmonary exacerbation is a more feasible endpoint than frequency (total number) of exacerbations, these two exacerbation endpoints are linked (32). Shorter time intervals between exacerbations are associated with more frequent exacerbations, and both are risk factors for lung function decline (32). In addition, when designing a study to detect a difference in pulmonary exacerbation frequency or treatment, it is important to account for the varying risk of exacerbations across the CF population, as lower lung function, female sex, prior exacerbation history, and Pseudomonas aeruginosa infection are associated with increased pulmonary exacerbations requiring treatment (33).
Although subjects in the control arm experienced an acute decline in lung function at 28 days relative to participants in the treatment arm, there were not obvious differences between the groups at baseline. Per study inclusion criteria, participants had to be clinically stable, with no significant changes in health status within 2 weeks before randomization. The groups were very well matched in terms of clinical characteristics at the time of randomization, including the comparable use of chronic inhaled antibiotics and azithromycin therapy. These data do not indicate that the control group was at more risk for experiencing decline in lung function and more frequent pulmonary exacerbations. One potential explanation for the decrease in FEV1 observed in the control group was that the 4-week run-in/wash-out period was an insufficient length of time to allow for β-carotene and other antioxidants to be depleted from the body of control subjects. Most of the commercially available CF multivitamins that study subjects were taking at the time of enrollment had added antioxidants, and control subjects may not have reached their antioxidant nadir at the end of the run-in period but rather during the first 4 weeks of the active treatment period. We also cannot exclude the possibilities that the respiratory health of control participants was more optimally controlled relative to the subjects receiving antioxidant treatment at the time of study entry or that control subjects were more adherent to treatments leading to short-term improvements in lung function during the screening and run-in period. These reasons might explain an acute decline in lung function observed in the control group as they regressed to their mean values. Furthermore, because pulmonary exacerbations are associated with lung function decline (32, 34), this could also account for the difference in time to first exacerbation between the groups.
It is important to recognize the effect of this antioxidant supplementation as an “add-on” therapy in intensely treated participants with CF, approximately half of whom were prescribed inhaled antipseudomonal antibiotics and chronic azithromycin therapy. This trial was performed before the approval of lumacaftor–ivacaftor, and treatment with these CFTR modulator therapies was an exclusion criterion for participation in this study. It is unknown whether the benefits of antioxidant supplementation would be observed on top of CFTR modulator treatment or whether modulator therapies might actually enhance the efficacy of antioxidants. But in contrast to these mutation-specific therapies, antioxidant therapy holds the promise of benefiting all patients with CF.
Despite a significant increase in circulating antioxidant concentrations, this dietary antioxidant supplement did not appear to exert sustained antiinflammatory or antioxidative effects, as only modest reductions in circulating calprotectin and MPO occurred at Week 4. One consideration is that higher antioxidant concentrations are needed to effect a change in inflammation. The transient reductions in circulating inflammatory markers also raises the question as to whether treatment efficacy of the antioxidant-enriched multivitamin decreases over time. The lack of biologic activity in sputum may also be due in part to the relatively short study duration versus the intrinsic variability, particularly between-subject variance, of sputum biomarkers (35). It is possible that other standard-of-care CF treatments hamper an ability to detect an antiinflammatory signal. For instance, rhDNase, chronic cycled inhaled antibiotics, and azithromycin therapy have known effects on inflammatory markers (36–38). Similarly, oral and intravenous antibiotics are often prescribed, and these may affect biomarker measurements and treatment effects in CF clinical trials (39). If these antioxidants are in fact working through an antiinflammatory mechanism, it does call into question the value of biomarkers of inflammation and oxidative stress measured in this study. Another possibility is that the antioxidants are mediating their effects through an alternative mechanism (e.g., improved muscle function or energy metabolism) or by enhancing immune cell function, which has been observed as β-carotene concentrations increase (40).
In designing this study, we had considered whether to proceed with a larger, longer-term study targeting a clinical endpoint. However, recent antioxidant trials of inhaled glutathione and oral N-acetylcysteine in CF, both of 6 months’ duration, provided caution, in that they did not demonstrably affect inflammation or oxidative stress or unequivocally improve clinical outcomes (41, 42). Instead, we decided to perform a phase II study and selected sputum MPO as the primary efficacy endpoint, making sample size determinations on the basis of this endpoint. We chose MPO rather than another marker of airway inflammation, such as neutrophil elastase, because MPO generates reactive oxidant species as part of its function in innate host defense mechanisms and is considered by many a marker of oxidative stress. However, sputum MPO may not be the most appropriate marker to demonstrate the efficacy of this dietary antioxidant. Although we were surprised by the lack of clear antiinflammatory effects, the antioxidant supplement provided a benefit in an important clinical outcome. Suffice it to say, developing safe and effective antiinflammatory treatments remains a key priority to the CF community (43).
Optimizing antioxidant status in CF is an important clinical goal. In this clinical trial, we showed that an antioxidant-enriched multivitamin supplement safely increased circulating antioxidant concentrations and positively influenced a clinically relevant outcome in CF pulmonary exacerbations. This treatment resulted in a transient decrease in systemic inflammation without having a sustained effect on oxidative, proteolytic, or inflammatory measures.
Acknowledgments
Acknowledgment
The authors thank Meg Anthony, the lead research coordinator for this study, who helped to prepare study-related documents and manuals, and the study site research coordinators listed in the online supplement for helping to enroll patients and coordinating study visits at their sites; Liebe Antoine, Arthur Baines, Jill VanDalfsen, and the entire team at the CFF Therapeutics Development Network Coordinating Center (Pharmacy, Regulatory, Data Safety Monitoring Committee) for helping to manage and oversee the execution of this study; Gus Papas and Callion Pharma for manufacturing the antioxidant-enriched and control multivitamins and providing them at no charge for this study; Peggy Emmett, Hobie Harrington, and the lab technicians in the CF Foundation Therapeutics Center for Biochemical Markers for processing the biospecimens and completing the measurements of inflammation and oxidative stress; and all of the study participants and their families.
Footnotes
Supported by Cystic Fibrosis Foundation Therapeutics grant AQUADEK12K1 and by NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards grant UL1 TR001082.
Author Contributions: S.D.S. designed the study. S.D.S., R.J., G.G., C.L.D., J.M.D., D.B., D.M.O., I.A., J.N., J.P.C., B.S., M.J.R., K.S.M., S.S., and F.R.L. were the lead site investigators, recruited and studied patients at their sites, and reviewed the manuscript. U.K. and M.L.S. performed statistical analyses. S.D.S. and U.K. prepared the initial draft of the manuscript and incorporated comments and edits from the other study authors. K.A.P. oversaw the manufacturing and stability of the antioxidant-enriched and control multivitamins used in the study and critically reviewed the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201801-0105OC on April 24, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol. 2015;50:S39–S56. doi: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 2.Homnick DN, Cox JH, DeLoof MJ, Ringer TV. Carotenoid levels in normal children and in children with cystic fibrosis. J Pediatr. 1993;122:703–707. doi: 10.1016/s0022-3476(06)80008-9. [DOI] [PubMed] [Google Scholar]
- 3.Kawchak DA, Sowell AL, Hofley PM, Zemel BS, Scanlin TF, Stallings VA. Longitudinal analysis shows serum carotenoid concentrations are low in children with cystic fibrosis. J Am Diet Assoc. 1999;99:1569–1572. doi: 10.1016/S0002-8223(99)00386-7. [DOI] [PubMed] [Google Scholar]
- 4.Feranchak AP, Sontag MK, Wagener JS, Hammond KB, Accurso FJ, Sokol RJ. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J Pediatr. 1999;135:601–610. doi: 10.1016/s0022-3476(99)70059-4. [DOI] [PubMed] [Google Scholar]
- 5.Wood LG, Fitzgerald DA, Gibson PG, Cooper DM, Collins CE, Garg ML. Oxidative stress in cystic fibrosis: dietary and metabolic factors. J Am Coll Nutr. 2001;20:157–165. doi: 10.1080/07315724.2001.10719028. [DOI] [PubMed] [Google Scholar]
- 6.Laguna TA, Sontag MK, Osberg I, Wagener JS, Accurso FJ, Sokol RJ. Decreased total serum coenzyme-Q10 concentrations: a longitudinal study in children with cystic fibrosis. J Pediatr. 2008;153:402–407. doi: 10.1016/j.jpeds.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin B, Newman LJ, Berezin S, Rosenthal WS, Schwarz SM, Weiss L. Low blood selenium levels in patients with cystic fibrosis compared to controls and healthy adults. JPEN J Parenter Enteral Nutr. 1987;11:38–41. doi: 10.1177/014860718701100138. [DOI] [PubMed] [Google Scholar]
- 8.Winklhofer-Roob BM. Oxygen free radicals and antioxidants in cystic fibrosis: the concept of an oxidant-antioxidant imbalance. Acta Paediatr Suppl. 1994;83:49–57. doi: 10.1111/j.1651-2227.1994.tb13229.x. [DOI] [PubMed] [Google Scholar]
- 9.Galli F, Battistoni A, Gambari R, Pompella A, Bragonzi A, Pilolli F, et al. Working Group on Inflammation in Cystic Fibrosis. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta. 2012;1822:690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis: conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic Biol Med. 2007;42:15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciofu O, Lykkesfeldt J. Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2014;8:CD007020. doi: 10.1002/14651858.CD007020.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Papas KA, Sontag MK, Pardee C, Sokol RJ, Sagel SD, Accurso FJ, et al. A pilot study on the safety and efficacy of a novel antioxidant rich formulation in patients with cystic fibrosis. J Cyst Fibros. 2008;7:60–67. doi: 10.1016/j.jcf.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Sagel SD, Sontag MK, Anthony MM, Emmett P, Papas KA. Effect of an antioxidant-rich multivitamin supplement in cystic fibrosis. J Cyst Fibros. 2011;10:31–36. doi: 10.1016/j.jcf.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Sagel SD, Baines A, Abdulhamid I, Borowitz D, Clancy JP, Daines CL, et al. Effects of an antioxidant-enriched multivitamin supplement on inflammation and oxidative stress in CF [abstract] Pediatr Pulmonol Suppl. 2016;45:283. [Google Scholar]
- 15.Papas K, Kalbfleisch J, Mohon R. Bioavailability of a novel, water-soluble vitamin E formulation in malabsorbing patients. Dig Dis Sci. 2007;52:347–352. doi: 10.1007/s10620-006-9489-2. [DOI] [PubMed] [Google Scholar]
- 16.Lancellotti L, D’Orazio C, Mastella G, Mazzi G, Lippi U. Deficiency of vitamins E and A in cystic fibrosis is independent of pancreatic function and current enzyme and vitamin supplementation. Eur J Pediatr. 1996;155:281–285. doi: 10.1007/BF02002713. [DOI] [PubMed] [Google Scholar]
- 17.Lepage G, Champagne J, Ronco N, Lamarre A, Osberg I, Sokol RJ, et al. Supplementation with carotenoids corrects increased lipid peroxidation in children with cystic fibrosis. Am J Clin Nutr. 1996;64:87–93. doi: 10.1093/ajcn/64.1.87. [DOI] [PubMed] [Google Scholar]
- 18.Rust P, Eichler I, Renner S, Elmadfa I. Long-term oral beta-carotene supplementation in patients with cystic fibrosis: effects on antioxidative status and pulmonary function. Ann Nutr Metab. 2000;44:30–37. doi: 10.1159/000012818. [DOI] [PubMed] [Google Scholar]
- 19.Oudshoorn JH, Lecluse AL, van den Berg R, Vaes WH, van der Laag J, Houwen RH. Decreased coenzyme Q10 concentration in plasma of children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2006;43:646–650. doi: 10.1097/01.mpg.0000233193.77521.66. [DOI] [PubMed] [Google Scholar]
- 20.Wood LG, Fitzgerald DA, Lee AK, Garg ML. Improved antioxidant and fatty acid status of patients with cystic fibrosis after antioxidant supplementation is linked to improved lung function. Am J Clin Nutr. 2003;77:150–159. doi: 10.1093/ajcn/77.1.150. [DOI] [PubMed] [Google Scholar]
- 21.Hu G, Cassano PA. Antioxidant nutrients and pulmonary function: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Epidemiol. 2000;151:975–981. doi: 10.1093/oxfordjournals.aje.a010141. [DOI] [PubMed] [Google Scholar]
- 22.Schünemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, et al. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163:1246–1255. doi: 10.1164/ajrccm.163.5.2007135. [DOI] [PubMed] [Google Scholar]
- 23.Gilliland FD, Berhane KT, Li YF, Gauderman WJ, McConnell R, Peters J. Children’s lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol. 2003;158:576–584. doi: 10.1093/aje/kwg181. [DOI] [PubMed] [Google Scholar]
- 24.Guénégou A, Leynaert B, Pin I, Le Moël G, Zureik M, Neukirch F. Serum carotenoids, vitamins A and E, and 8 year lung function decline in a general population. Thorax. 2006;61:320–326. doi: 10.1136/thx.2005.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown RK, Wyatt H, Price JF, Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J. 1996;9:334–339. doi: 10.1183/09031936.96.09020334. [DOI] [PubMed] [Google Scholar]
- 26.Hakim F, Kerem E, Rivlin J, Bentur L, Stankiewicz H, Bdolach-Abram T, et al. Vitamins A and E and pulmonary exacerbations in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2007;45:347–353. doi: 10.1097/MPG.0b013e31804069e5. [DOI] [PubMed] [Google Scholar]
- 27.Lagrange-Puget M, Durieu I, Ecochard R, Abbas-Chorfa F, Drai J, Steghens JP, et al. Longitudinal study of oxidative status in 312 cystic fibrosis patients in stable state and during bronchial exacerbation. Pediatr Pulmonol. 2004;38:43–49. doi: 10.1002/ppul.20041. [DOI] [PubMed] [Google Scholar]
- 28.Renner S, Rath R, Rust P, Lehr S, Frischer T, Elmadfa I, et al. Effects of beta-carotene supplementation for six months on clinical and laboratory parameters in patients with cystic fibrosis. Thorax. 2001;56:48–52. doi: 10.1136/thorax.56.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. The Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 30.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 31.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40:61–66. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 33.Vandevanter DR, Yegin A, Morgan WJ, Millar SJ, Pasta DJ, Konstan MW. Design and powering of cystic fibrosis clinical trials using pulmonary exacerbation as an efficacy endpoint. J Cyst Fibros. 2011;10:453–459. doi: 10.1016/j.jcf.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46:393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 35.Chmiel JF, Konstan MW, Accurso FJ, Lymp J, Mayer-Hamblett N, VanDevanter DR, et al. Assessment of Induced Sputum in Cystic Fibrosis Study Group. Use of ibuprofen to assess inflammatory biomarkers in induced sputum: implications for clinical trials in cystic fibrosis. J Cyst Fibros. 2015;14:720–726. doi: 10.1016/j.jcf.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Paul K, Rietschel E, Ballmann M, Griese M, Worlitzsch D, Shute J, et al. Bronchoalveolar Lavage for the Evaluation of Antiinflammatory Treatment Study Group. Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. Am J Respir Crit Care Med. 2004;169:719–725. doi: 10.1164/rccm.200307-959OC. [DOI] [PubMed] [Google Scholar]
- 37.Noah TL, Ivins SS, Abode KA, Stewart PW, Michelson PH, Harris WT, et al. Inhaled versus systemic antibiotics and airway inflammation in children with cystic fibrosis and Pseudomonas. Pediatr Pulmonol. 2010;45:281–290. doi: 10.1002/ppul.21176. [DOI] [PubMed] [Google Scholar]
- 38.Ratjen F, Saiman L, Mayer-Hamblett N, Lands LC, Kloster M, Thompson V, et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest. 2012;142:1259–1266. doi: 10.1378/chest.12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer-Hamblett N, Saiman L, Lands LC, Anstead M, Rosenfeld M, Kloster M, et al. Impact of acute antibiotic therapy on the pulmonary exacerbation endpoint in cystic fibrosis clinical trials. Contemp Clin Trials. 2013;36:99–105. doi: 10.1016/j.cct.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Prabhala RH, Garewal HS, Hicks MJ, Sampliner RE, Watson RR. The effects of 13-cis-retinoic acid and beta-carotene on cellular immunity in humans. Cancer. 1991;67:1556–1560. doi: 10.1002/1097-0142(19910315)67:6<1556::aid-cncr2820670616>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Griese M, Kappler M, Eismann C, Ballmann M, Junge S, Rietschel E, et al. Glutathione Study Group. Inhalation treatment with glutathione in patients with cystic fibrosis: a randomized clinical trial. Am J Respir Crit Care Med. 2013;188:83–89. doi: 10.1164/rccm.201303-0427OC. [DOI] [PubMed] [Google Scholar]
- 42.Conrad C, Lymp J, Thompson V, Dunn C, Davies Z, Chatfield B, et al. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects: a phase II randomized placebo-controlled trial. J Cyst Fibros. 2015;14:219–227. doi: 10.1016/j.jcf.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Torphy TJ, Allen J, Cantin AM, Konstan MW, Accurso FJ, Joseloff E, et al. Antiinflammatory Therapy Working Group. Considerations for the conduct of clinical trials with antiinflammatory agents in cystic fibrosis: a Cystic Fibrosis Foundation Workshop report. Ann Am Thorac Soc. 2015;12:1398–1406. doi: 10.1513/AnnalsATS.201506-361OT. [DOI] [PubMed] [Google Scholar]