The thoughtless person playing with penicillin treatment is morally responsible for the death of the man who finally succumbs to infection with the penicillin-resistant organism. I hope this evil can be averted.
—Alexander Fleming
Less than a century after its discovery by Alexander Fleming, antimicrobial resistance (AR) remains a global crisis. An estimated 700,000 deaths globally are attributable to AR, with cataclysmic future projections that surpass cancer-related deaths (1). Bacteria and fungi that are resistant to all antimicrobial classes have emerged. How did we get here? This systemic failure has resulted from clonal dissemination of resistance traits and unchecked antibiotic use in humans, agriculture, and livestock; inadequate stewardship; insufficient commitment of resources; and in some instances, a lack of political will. Only recently, a call to action by professional societies and federal and international leadership has boosted antibiotic discovery, prompted legislation facilitating their expedited approval, and informed the U.S. Congress’ decision to appropriate large sums to confront the crisis (2). A high-level meeting on AR was convened at the United Nations in 2016 to rally governments worldwide to prioritize antibiotic restriction. However, these laudable efforts will fall short without a grassroots effort in ICUs. At any given time, infections afflict 51% of ICU patients worldwide, and nearly 71% these patients are receiving antibiotics (3). The ICU environment is characterized by intense antibiotic selective pressure and provider-to-patient contact, and accordingly, it is notorious for more “severe” resistance phenotypes (Figure 1) (4), de novo development of multidrug resistance, and periodic outbreaks. More than two-thirds of ICU-acquired bacteremia episodes at 162 European ICUs displayed multidrug-resistant and extensively drug-resistant strains (5). Furthermore, gravely ill patients with multiple comorbidities most often acquire and succumb to difficult-to-treat pathogens (4, 6). Despite regional variation in resistance repertoires across pathogens, ICUs everywhere have become epicenters of clinically important AR. As such, critical care providers worldwide are key stakeholders in efforts to protect critically ill and injured patients from this crisis and to ensure the future availability of highly effective antimicrobial agents.
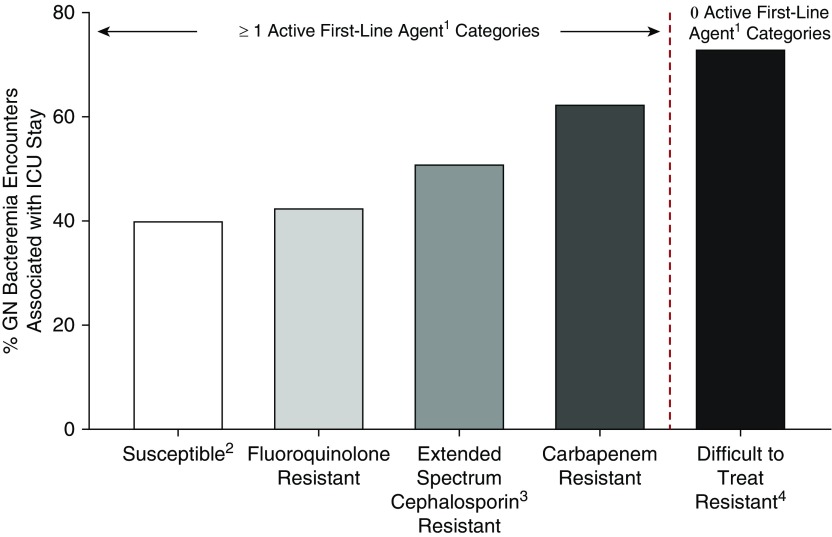
Figure 1.
Proportion of unique inpatient encounters with gram-negative bacteremia associated with an ICU stay across resistance phenotypes (29,474 unique inpatient encounters, 170 U.S. hospitals in Premier Healthcare Database, 2009–2013). The figure depicts the proportion of ICU stays associated with inpatients with bacteremia resulting from select taxa of gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., Pseudomonas aeruginosa, and Acinetobacter baumannii) across various phenotypes of resistance. The association with ICU stay was greatest for encounters with gram-negative bacteremia displaying difficult-to-treat resistance (no active first-line agent categories) at 73%. Even among isolates with one or more active first-line category, the association with ICU stay increased with increasing spectrum of the antibiotic category to which the isolate is resistant (carbapenems > extended-spectrum cephalosporins > fluoroquinolones). Susceptible: 8,547/21,410; fluoroquinolone resistant: 1,845/4,342; extended spectrum cephalosporin resistant: 1,401/2,756; carbapenem resistant: 328/526; difficult-to-treat resistant: 321/440. 1First-line agents are carbapenems, other β-lactams, and fluoroquinolones. 2Without any of the four resistance phenotypes in the figure. 3Extended-spectrum cephalosporins include third- and fourth-generation cephalosporins (for P. aeruginosa, limited to antipseudomonal cephalosporins). 4For GN bloodstream isolates, “difficult-to-treat resistant” indicates intermediate susceptibility or resistance to all carbapenems, other β-lactams, and fluoroquinolones tested. The other resistance phenotypes shown are defined using U.S. Centers for Disease Control and Prevention 2015 surveillance definitions (17). GN = gram-negative. Data from Table 2 of Reference 4.
Despite our relentless presence at the bedside, intensivists are conspicuously absent from the AR crisis at the macro level. Unlike the willful ownership of the field of sepsis by intensivists, the field of AR has largely fallen under the purview of infectious diseases (ID). Despite the substantial progress of AR advocacy and lobbying, consensus on key issues such as guidelines on antibiotic management of sepsis has suffered from this compartmentalization (7). Furthermore, the NIH-funded Antibacterial Resistance Leadership Group formulated to prioritize and implement a clinical research agenda for AR, including studies planned on critically ill patients, has no representatives from critical care in its organizational structure (8). Several clinical and research challenges related to AR today could benefit from the participation and perspective of critical care providers. For instance, for antibiotic orders, what is the correct balance between autonomy and oversight of front-line providers in the ICU? Should newly introduced antibiotics such as ceftazidime-avibactam and meropenem-vaborbactam be included in the empiric therapy repertoire for some patients? What clinical criteria, surveillance systems, and resistance prevalence thresholds will form the basis for such decisions? Should the current epidemiologic definitions of co-resistance (i.e., multidrug-resistant, extensively drug resistant, and pan-drug resistant [9]) be complemented by clinical classifiers more relevant to ICU patients (e.g., zero first-line agents available) (4) (Figure 1) to facilitate rapid empiric therapy selection? Might a nuanced understanding of acute organ dysfunction be leveraged to refine risk-adjustment approaches in AR outcomes research?
In addition to hospital-wide guidance on antibiotic stewardship and patient cohorting, ID specialists have an undeniable role in the care of patients with resistant infections. Importantly, ID involvement in ICUs has been shown to increase antibiotic appropriateness (10), improve survival, and decrease ICU days (11). However, the overwhelming volume and array of AR control and management questions encountered on morning rounds in ICUs with a high burden of resistance is unlikely to be feasibly answered using the case-by-case ID consultative model. Intensivists frequently have to make high-stakes decisions themselves, such as narrowing, maintaining, or expanding antimicrobial regimens, paying careful attention to dosage adjustments in organ failure and drug-drug interactions, and as such, perform the balancing act of managing AR infections in parallel with myriad other serious conditions. Although the public health importance of antibiotic de-escalation is well known, its direct effect on ICU patients remains controversial (12). Yet, intensivists do tend to embrace the antimicrobial stewardship mission, as noted in a Canadian survey (13). With increasing 24/7 attending coverage in ICUs nationwide, intensivists often encounter decompensating patients long before ID consultants are available to provide an informed opinion. This frequent, front-line contact with AR is reason enough to solicit the unique perspective and expertise of intensivists in every domain from research and policy to scrutiny of the safety and quality of novel management strategies. However, this argument for greater involvement also comes with an obligation for better training and more ownership of the AR problem facing ICUs.
A survey of critical care providers from 16 ICUs in New York City revealed that although >95% respondents believed in vitro antimicrobial susceptibility testing improved outcomes, only 33% were familiar with standard testing methods (14). Although emergence of rapid molecular diagnostics will better inform antimicrobial management in the future, intensivists will be expected to synthesize and implement these results in real time. Directors of ICUs need to nest the control of AR within existing ICU quality initiatives; decreasing healthcare-associated infections will in turn decrease AR. Intensivists must emphasize the importance of aseptic and barrier precautions and procedural checklists to their teams and become role models for handwashing, an inexpensive yet underutilized solution for curbing AR. The interplay between AR and sepsis is more relevant today than it was in the pre-crisis era, yet neither of the recent landmark clinical trials evaluating early goal-directed therapy (ProCESS [Protocol-based Care for Early Septic Shock], ProMISe [Protocolized Management in Sepsis], and ARISE [Australasian Resuscitation in Sepsis Evaluation]) in sepsis mention AR. A recent analysis of time to antibiotics, as part of a mandated care bundle, did not assess the appropriateness of antibiotic therapy in culture-positive sepsis (15). Antimicrobial resistance currently makes up less than 2% of the critical care fellowship curriculum posted by the American Board of Medical Specialties. Although it is not feasible to expect the intensivist to be an expert in myriad resistance mutations and their implications, knowledge of AR prevention, diagnosis, and management is a reasonable expectation on par with ventilator management, resuscitation, and extracorporeal therapies. As part of the paradigm change toward a multispecialty critical care workforce, a small but growing number of intensivists are in fact receiving fellowship training in ID (16), and programs are beginning to offer official tracks for dual-training; this dually trained group could be leveraged for clinical, policy, and academic contributions relevant to the AR crisis in our ICUs.
Acknowledgments
Acknowledgment
The author thanks his mentors Dr. Robert L. Danner, Dr. Michael Klompas, and Dr. Henry Masur for their thoughtful input on the topic, and Yi Ling (Elaine) Lai, M.P.H., for her assistance with the illustration.
Footnotes
This editorial represents the views of the author and does not necessarily represent the official position of the NIH or the U.S. Government.
Originally Published in Press as DOI: 10.1164/rccm.201805-0962ED on June 25, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Review on antimicrobial resistance Antimicrobial resistance: tackling a crisis for the health and wealth of nations. United Kingdom: Wellcome Trust and the UK Department of Health; 2014 [accessed 2018 Jul 31]. Available from: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
- 2.Centers for Disease Control and Prevention What CDC is doing: AR Solutions Initiative. Atlanta: CDC; 2017 [accessed 2018 Jul 31]. Available from: https://www.cdc.gov/drugresistance/solutions-initiative/index.html.
- 3.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4.Kadri SS, Adjemian J, Lai Y, Spaulding AB, Ricotta E, Prevots DR, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors and outcome of resistance to all first-line agents Clin Infect Dis 2018. ciy378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38:1930–1945. doi: 10.1007/s00134-012-2695-9. [DOI] [PubMed] [Google Scholar]
- 6.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 7.IDSA Sepsis Task Force. Infectious Diseases Society of America (IDSA) position statement: why IDSA did not endorse the surviving sepsis campaign guidelines. Clin Infect Dis. 2018;66(10):1631–1635. doi: 10.1093/cid/cix997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers HF, Bartlett JG, Bonomo RA, Chiou C, Cosgrove SE, Cross HR, et al. Antibacterial resistance leadership group: open for business. Clin Infect Dis. 2014;58:1571–1576. doi: 10.1093/cid/ciu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 10.Raineri E, Pan A, Mondello P, Acquarolo A, Candiani A, Crema L. Role of the infectious diseases specialist consultant on the appropriateness of antimicrobial therapy prescription in an intensive care unit. Am J Infect Control. 2008;36:283–290. doi: 10.1016/j.ajic.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt S, McQuillen DP, Nahass R, Martinelli L, Rubin M, Schwebke K, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis. 2014;58:22–28. doi: 10.1093/cid/cit610. [DOI] [PubMed] [Google Scholar]
- 12.Tabah A, Cotta MO, Garnacho-Montero J, Schouten J, Roberts JA, Lipman J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62:1009–1017. doi: 10.1093/cid/civ1199. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg M, Dresser LD, Daneman N, Smith OM, Matte A, Marinoff N, et al. A national survey of critical care physicians’ knowledge, attitudes, and perceptions of antimicrobial stewardship programs. J Intensive Care Med. 2016;31:61–65. doi: 10.1177/0885066614541922. [DOI] [PubMed] [Google Scholar]
- 14.Zhou JJ, Patel SJ, Jia H, Weisenberg SA, Furuya EY, Kubin CJ, et al. Clinicians’ knowledge, attitudes, and practices regarding infections with multidrug-resistant gram-negative bacilli in intensive care units. Infect Control Hosp Epidemiol. 2013;34:274–283. doi: 10.1086/669524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadri SS, Rhee C, Fortna GS, O’Grady NP. Critical care medicine and infectious diseases: an emerging combined subspecialty in the United States. Clin Infect Dis. 2015;61:609–614. doi: 10.1093/cid/civ360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention CDC’s antibiotic resistance patient safety atlas. Atlanta: CDC; 2016 [accessed 2018 Jul 31]. Available from: https://www.cdc.gov/hai/surveillance/ar-patient-safety-atlas.html.