To the Editor:
Intact CFTR (cystic fibrosis transmembrane conductance regulator) function is crucial for proper airway, pancreatic, and reproductive tract functions by regulating chloride and bicarbonate transport at the epithelial surface (1). Cystic fibrosis, an autosomal-recessive genetic disorder, is a consequence of dysfunctional CFTR function and results in defective respiratory host defense, including abnormal mucus, diminished mucociliary clearance, and a propensity for bacterial infections, ultimately leading to bronchiectasis (1). Even in the absence of genetic CFTR mutations, individuals with smoking-related illnesses such as chronic obstructive pulmonary disease can develop acquired CFTR dysfunction, which is associated with chronic bronchitis, disease severity, and bronchiectasis (2).
Compelling data indicate that acquired CFTR dysfunction is demonstrable in the nasal epithelium (3), lungs (4), and even sweat glands (5) of smokers with and without chronic obstructive pulmonary disease compared with healthy control subjects, and can partially (but not completely) reverse with smoking cessation (6). Elevated sweat chloride levels underscore the fact that cigarette smoking causes systemic CFTR dysfunction beyond the respiratory tract. Moreover, in vitro evidence demonstrates that plasma from smokers can transmit CFTR dysfunction to healthy human bronchial epithelial cells (5), indicating that reactive constituents such as acrolein (5), cadmium (7), and other oxidants (1) have the potential to reduce CFTR function.
Maternal smoking confers a risk of infection to the infant, including otitis media, upper respiratory infections, and pneumonia; however, the mechanistic basis of this heightened risk is not well established (8). The consequences of smoking for the respiratory health of infants have been postulated to be partially induced in utero through transplacental exposure to circulating cigarette smoke toxins during the key stages of neonatal development (8, 9). However, to our knowledge, there have been no studies investigating the effects of smoking on the epithelial function of the infant or fetus. We hypothesized that cigarette smoking by pregnant mothers could induce acquired CFTR dysfunction and transmit this to the fetus, ultimately inducing a risk of respiratory infection. A portion of this work has been presented in the form of an abstract (10).
Methods
Pregnant rodent model
All studies were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (20342). Pregnant Sprague Dawley rats (10 wk old, n = 12) were exposed to mainstream cigarette smoke for 10 days before fertilization and throughout their 21- to 23-day gestation using a whole-body exposure chamber and smoking apparatus (InExpose, SCIREQ). Smoke exposure was applied 4 hours daily, 5 days a week, with an average of 800 μg/L particulate matter per day. Air-exposed pregnant control rats (n = 10) were placed in identical chambers for the same duration. Tracheas were excised from neonates at Day 0 (within 12 h of delivery) and Day 3 of life. There was no ongoing cigarette smoke exposure to neonates postpartum.
CFTR expression and function
CFTR activity was measured ex vivo in excised tracheas from Day 0 and Day 3 neonates using short-circuit current (Isc) analysis under voltage-clamp conduits as previously described (11). CFTR mRNA was extracted with the RNeasy Mini Kit (Qiagen). qRT-PCR was performed using the QuantStudio 3 system (Life Technologies) with the TaqMan RNA-to-Ct 1-Step Kit and TaqMan gene expression assays (Thermo Scientific) (β-actin and CFTR). PCR amplifications were performed in triplicate and gene expression was determined by the comparative cycle threshold (ΔΔCt) method using β-actin as an internal control as described previously (4).
Statistics
Descriptive statistics (mean, SD, and SEM) were compared using Student’s t test or ANOVA as appropriate. All statistical tests were two-sided and were performed at a 5% significance level using GraphPad Prism (GraphPad Software Inc.).
Results
Maternal smoking reduced the weight of rat neonates at birth (Day 0) by 14.1% compared with air control neonates (P < 0.0001), consistent with known effects on growth (8). Reduced weight persisted by 12.5% at Day 3 (P < 0.0001) despite cessation of smoke exposure postpartum (Figure 1F).
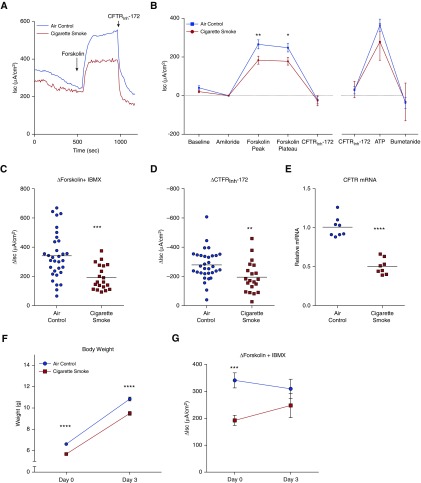
Figure 1.
CFTR (cystic fibrosis transmembrane conductance regulator) dysfunction in tracheas from Day 0 neonatal rats born from cigarette smoke–exposed mothers. Tracheas were excised, opened longitudinally along the dorsal surface, and microscopically dissected to remove extraneous connective tissue. Segments were mounted as flat sheets in modified Ussing chambers. Short-circuit current (Isc) measurements were performed under voltage-clamp conditions before and after addition of channel modulators in symmetrical chloride solutions as described previously (12). (A) Representative Isc tracing of neonatal rats from cigarette smoke–exposed mothers compared with air control mothers, demonstrating a depressed forskolin-sensitive and CFTRInh-172 (selective CFTR inhibitor 172) response. (B) Mean neonatal tracheal Isc treated with sequential addition of Ringer’s solution (baseline), amiloride (100 μM), forskolin (10 μM) + IBMX (100 μM), and CFTRInh-172 (10 μM). Tracings were normalized to postamiloride Isc for clarity of comparisons. After a delay (baseline), a subset of tracings were subjected to ATP (10 μM) and bumetanide (100 μM). n = 20 smoke pups from 5 litters; n = 32 control pups from 7 litters. The results were analyzed by two-way ANOVA with Bonferroni multiple comparisons. (C) Forskolin + IBMX–dependent change in tracheal Isc to detect cAMP-dependent ion channel function. (D) CFTRInh-172 (10 μM)-dependent changes in Isc; n = 20 smoke and 32 control. (E) Normalized tracheal CFTR mRNA levels as compared with the internal β-actin control; n = 8 smoke and n = 8 control. The results were analyzed by unpaired t test. (F) Body weights at Day 0 and Day 3 of neonates. Day 0: n = 115 smoke and n = 107 control. Day 3: n = 27 smoke and n = 20 control. Smoke pups from 12 litters; control pups from 10 litters. (G) CFTR-dependent changes in tracheal Isc as reflected by changes with forskolin + IBMX, as also shown in C. Day 0: n = 20 smoke and n = 32 control. Day 3: n = 13 smoke and n = 9 control. Symbols with error bars in B, F, and G represent mean ± SEM. Horizontal bars in C–E represent mean. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. IBMX = 3-isobutyl-1-methylxanthine.
CFTR function was studied in smoke-exposed and air control neonates at birth via Isc analysis of excised tracheas (Figures 1A and 1B). As opposed to postpartum females (32.4 + 36.9 μA/cm2) or historic mixed-sex adult rats (38.9 + 73.4 μA/cm2) (11), neonates had minimal endogenous CFTR preactivation under voltage-clamp conditions, and thus a preserved Isc response to cAMP stimulation, permitting traditional estimates of CFTR activity by forskolin stimulation (followed by CFTR inhibitor response). On Day 0, neonates from smoke-exposed mothers had a mean (± SEM) CFTR-dependent forskolin-stimulated ∆Isc of 192.3 ± 18.9 μA/cm2, a 43.6% reduction compared with air control neonates (341.1 ± 28.1 μA/cm2; P < 0.001; Figure 1C). These results were substantiated by a 30.1% reduction in CFTRInh-172 (selective CFTR inhibitor 172) currents (−192.6 ± 23.7 μA/cm2 smoke vs. −275.4 ± 18.5 μA/cm2 control; P < 0.01; Figure 1D). Although there were important trends, maternal smoking did not significantly affect the amiloride-sensitive sodium current, a measure of ENaC (epithelial sodium channel) function (∆Isc −12.3 ± 4.6 μA/cm2 smoke vs. −28.0 ± 8.5 μA/cm2 control; P > 0.05), or ATP-dependent Cl− currents (∆Isc 246.8 ± 65.5 μA/cm2 smoke vs. 340.1 ± 25.1 μA/cm2 control; P > 0.05), illustrating the relative specificity of circulating cigarette smoke toxins for CFTR. Neither baseline current (249.5 ± 49.2 μA/cm2 and 277.8 ± 31.1 μA/cm2; P > 0.05) nor transepithelial electrical resistance (1.3 ± 0.1 Ω · cm2 and 1.1 ± 0.1 Ω · cm2; P > 0.05) were significantly different between neonates of smoke-exposed mothers and controls, respectively. As seen in nasal (3) and lung (4) specimens from humans, the relative levels of tracheal CFTR mRNA expression by real-time RT-PCR were significantly reduced in Day 0 smoke-exposed neonates (0.5-fold ± 0.04-fold) compared with controls (P < 0.0001; Figure 1E). Finally, examination of time-dependent changes revealed that the negative effect of maternal smoking on CFTR-dependent Isc dissipated by Day 3 in the absence of ongoing exposure (Figure 1G).
Discussion
Systemic acquired CFTR dysfunction is attributable to circulating cigarette toxins in the blood of individuals who smoke (5). Likewise, this has significant ramifications for a developing fetus subjected to maternal smoking in utero, especially given our understanding of the numerous respiratory and infectious sequelae that occur in neonates postpartum (8). Our study demonstrates that maternal smoking impairs airway CFTR-dependent epithelial ion transport in neonates immediately at birth. There were nonsignificant effects on amiloride-sensitive and ATP-dependent currents, highlighting the specificity of these toxins for the CFTR channel. Furthermore, this significant effect on CFTR dissipated by Day 3 without ongoing smoke exposure postpartum, indicating the potential reversibility of this acquired CFTR dysfunction in neonates with a high regenerative capacity. However, although this scenario is promising and helpful to ascertain causality, it is distinct from reality in that the majority of infants exposed to maternal smoking in utero have continued passive environmental smoke exposure throughout infancy. Compared with adult rats, neonatal rats exhibit a more robust response to forskolin owing to less cAMP preactivation, and understanding the underlying cause of this could reveal new information regarding CFTR regulation.
Emerging data on maternal smoking now underscore the role of epigenetic modifications in fetal respiratory development and neonatal illness (9, 12). Our data also suggest a mechanistic link for neonatal-acquired CFTR dysfunction in that relative CFTR mRNA levels were reduced in tracheas from smoke-exposed mothers compared with controls. This observation is consistent with literature on genome-wide fetal DNA methylation secondary to maternal smoking (12). Our study represents the first report of maternal smoking impairing fetal epithelial physiology and may indicate why infants of smokers are predisposed to respiratory disorders. Additionally, given the substantial role of CFTR potentiators in the treatment of cystic fibrosis, our results indicate a potential therapeutic target for CFTR dysfunction in infants of mothers who smoke.
Footnotes
Supported by NIH grants P30 DK072482 and R35HL135816 (S.M.R.), an Alpha Omega Alpha Carolyn L. Kuckein Research Fellowship (L.L.M.), and a UAB Russell Cunningham Memorial Research Internship (L.L.M.). S.V.R. received funding from Celgene and a Young Clinical Scientist Award from the Flight Attendant Medical Research Institute. The sponsors had no role in the design of the study, the collection and analysis of data, or in the preparation of the manuscript.
Author Contributions: S.M.R., S.V.R., and L.L.M conceived of the experiments. L.L.M., S.E.P., N.K., L.P.T., L.R., and S.A.B. conducted the research. L.L.M., S.M.R., and S.E.P. analyzed the data. L.L.M. and S.M.R. wrote the manuscript. S.M.R. and S.V.R. supervised the project. S.M.R. is the guarantor of the manuscript and takes responsibility for the integrity of the data and accuracy of the data analysis.
Originally Published in Press as DOI: 10.1164/rccm.201805-0827LE on July 6, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raju SV, Solomon GM, Dransfield MT, Rowe SM. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis and other diseases of mucus clearance. Clin Chest Med. 2016;37:147–158. doi: 10.1016/j.ccm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courville CA, Raju SV, Liu B, Accurso FJ, Dransfield MT, Rowe SM. Recovery of acquired cystic fibrosis transmembrane conductance regulator dysfunction after smoking cessation. Am J Respir Crit Care Med. 2015;192:1521–1524. doi: 10.1164/rccm.201502-0396LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, et al. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol Sci. 2010;116:349–358. doi: 10.1093/toxsci/kfq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEvoy CT, Spindel ER. Pulmonary effects of maternal smoking on the fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev. 2017;21:27–33. doi: 10.1016/j.prrv.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotroff DM, Joubert BR, Marvel SW, Håberg SE, Wu MC, Nilsen RM, et al. Maternal smoking impacts key biological pathways in newborns through epigenetic modification in utero. BMC Genomics. 2016;17:976. doi: 10.1186/s12864-016-3310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick L, Kaza N, Tang LP, Rasmussen L, Jackson P, Byzek S, et al. Maternal smoking induces acquired CFTR dysfunction in neonatal rats [abstract] Am J Respir Crit Care Med. 2017;195:A2650. doi: 10.1164/rccm.201805-0827LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One. 2014;9:e91253. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KW, Richmond R, Hu P, French L, Shin J, Bourdon C, et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect. 2015;123:193–199. doi: 10.1289/ehp.1408614. [DOI] [PMC free article] [PubMed] [Google Scholar]