To the Editor:
Pulmonary arterial hypertension (PAH; World Health Organization group 1) is a disease of the small pulmonary arteries, characterized by vasoconstriction, vascular proliferation, and remodeling. Although at present there are 14 drugs approved by the U.S. Food and Drug Administration for the treatment of PAH available on the U.S. market, the morbidity and mortality of PAH remain high. Pulmonary hypertension associated with heart failure with preserved ejection fraction (PH-HFpEF) (also referred to as combined pre- and postcapillary PH or CpcPH; World Health Organization group 2) is known to occur secondary to the left ventricular diastolic dysfunction and is recognized as a clinical complication of the metabolic syndrome (1). At present, there are no approved therapies for PH-HFpEF. We have recently reported that metformin, the first-line antidiabetic drug and the canonical AMP-activated protein kinase (AMPK) activator, exhibits therapeutic efficacy in the early treatment of PH-HFpEF in preclinical rat models (2). Because PH-HFpEF shares many pathophysiological characteristics with PAH, and many patients with PAH exhibit signs of insulin resistance and glucose intolerance in the absence of obesity and diabetes (3, 4), we evaluated metformin in the treatment of group 1 PH.
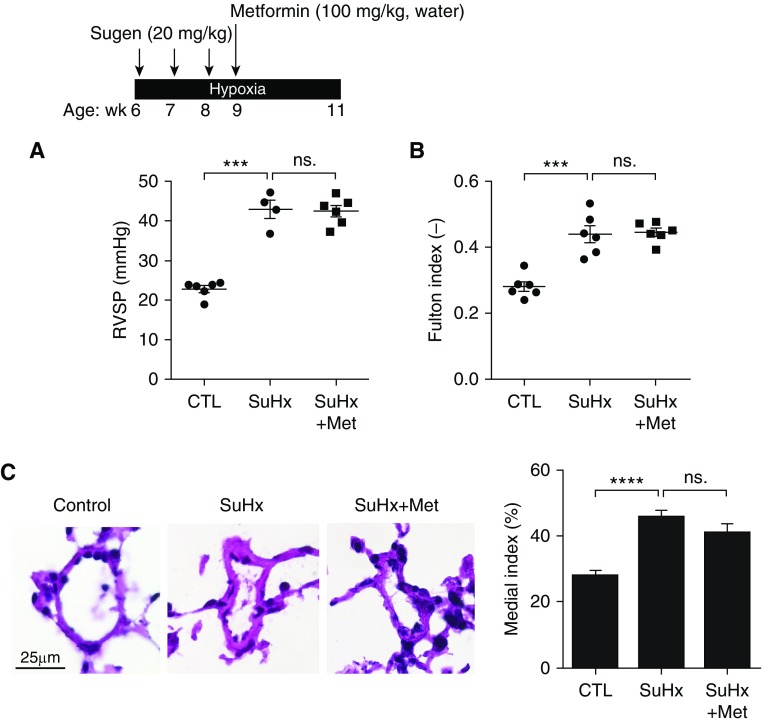
Although metformin has been found to prevent the development of PAH in hypoxia and monocrotaline rat models (5) and reverse PAH in SU5416/hypoxia (SuHx) rats (6), and is currently in a phase 2 clinical trial for the treatment of PAH (NCT01352026), our data showed that metformin treatment failed to reverse pulmonary pressures and vascular remodeling in mice with SuHx-induced PAH (Figure 1). In this experimental model, 6-week-old male C57BL/6J mice were injected subcutaneously with SU5416 (20 mg/kg) or vehicle buffer once per week during the first 3-week exposure to hypoxia (10% oxygen). Metformin (100 mg/kg) was given in drinking water and continued for 2-week exposure to hypoxia (Figure 1A). We observed no changes in right ventricular systolic pressure (RVSP) in metformin-treated SuHx mice compared with untreated animals (Figure 1A), nor did we detect changes in right ventricular hypertrophy (Figure 1B) or pulmonary vascular remodeling in metformin-treated SuHx mice (Figure 1C).
Figure 1.
(A) Right ventricular systolic pressure, (B) Fulton index [weight of right ventricle/(weight of left ventricle + septum)], and (C, right) medial index [(mean medial wall thickness/mean external diameter) × 100] were measured in mice with SuHx-induced pulmonary arterial hypertension. (C, left) Representative images of hematoxylin and eosin–stained pulmonary arteries. Data are mean ± SEM. Statistical significance was assessed by one-way ANOVA followed by a Tukey post hoc test. ***P < 0.001; ****P < 0.0001. CTL = control; Met = metformin; ns. = not significant; RVSP = right ventricular systolic pressure; SuHx = SU5416/hypoxia.
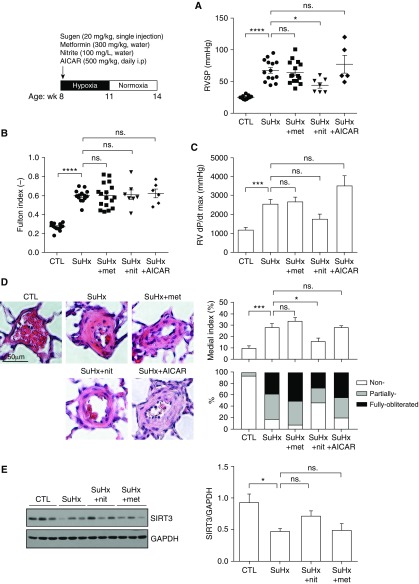
To further validate these unexpected findings, we evaluated the preventative effect of metformin in the conventional SuHx rat model of PAH with a higher dose of metformin (300 mg/kg, the effective dose used in the rat model of PH-HFpEF, drinking water, starting at Day 1). In this experimental model, 8-week-old male Sprague Dawley rats were single-injected with SU5416 (20 mg/kg), followed by 3 weeks of exposure to hypoxia (10% oxygen) and an additional 3 weeks of exposure to normoxia. We further compared the effect of metformin with the effect of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR, an AMPK agonist) and nitrite (a drug that can be reduced to nitric oxide under hypoxia and that has been shown to improve hypoxia- and monocrotaline-induced PAH) (7) in the SuHx rat model of PAH. In line with our observations in mice with SuHx-induced PAH, no significant difference in RVSP (Figure 2A) was observed in metformin-treated SuHx rats compared with untreated animals. Right ventricular hypertrophy, fastest rate of pressure change in the right ventricle (RV dP/dt max), and pulmonary vascular remodeling were not affected in metformin-treated SuHx rats compared with untreated animals (Figures 2B–2D). In addition, the AMPK agonist AICAR (500 mg/kg, intraperitoneally, daily, starting at Day 1) did not reduce RVSP in SuHx rats, whereas nitrite (100 mg/L, drinking water, starting at Day 1) treatment significantly lowered RVSP and pulmonary vascular remodeling in rats with SuHx-induced PAH (Figures 2A and 2D). Collectively, these data demonstrate a lack of efficacy of metformin in prevention or reversal of PAH induced by SuHx, in contrast to clear beneficial effects in PH-HFpEF rat models with metabolic syndrome. These data suggest that metformin therapy for PH may be limited to PH associated with metabolic syndrome.
Figure 2.
(A) Right ventricular systolic pressure, (B) Fulton index, (C) RV dP/dt max, (D) representative images of hematoxylin and eosin–stained pulmonary arteries and medial index, and (E) Western blot for SIRT3 activation levels in skeletal muscle (n = 6) obtained from SuHx rats treated or untreated with metformin, nitrite, or AICAR. Data are mean ± SEM. Statistical significance was assessed by one-way ANOVA followed by a Tukey post hoc test. *P < 0.05; ***P < 0.001; ****P < 0.0001. AICAR = 5-aminoimidazole-4-carboxamide ribonucleotide; CTL = control; met = metformin; nit = nitrite; ns. = not significant; RV dP/dt = fastest rate of pressure change in the right ventricle; RVSP = right ventricular systolic pressure; SuHx = SU5416/hypoxia.
Recently, we have also reported that metformin limits PH-HFpEF by a mechanism involving, at least in part, activation of SIRT3 in skeletal muscle (2). SIRT3 is a member of the sirtuin family of protein deacetylases that is preferentially localized in mitochondria and known to regulate reactive oxygen species levels and global respiration. Dysregulation of SIRT3 has been implicated in the development of insulin resistance and diabetes both in humans and rodents (8). In addition, SIRT3 deficiency in pulmonary artery smooth muscle cells has been shown to promote PAH (4). Decreased levels of SIRT3 in skeletal muscle have been associated with the development of insulin resistance and diabetes, whereas caloric restriction- and exercise-induced increase in skeletal muscle SIRT3 leads to multiple health benefits within the cardiovascular and the musculoskeletal system (9). Our previously published data demonstrated that in rats with PH-HFpEF, the levels of activated SIRT3 are decreased specifically in skeletal muscle (not in the pulmonary vasculature and the heart), concomitant with robust increase in pulmonary pressures and vascular remodeling (2). Restoration of SIRT3 in skeletal muscle with nitrite and metformin improved insulin sensitivity and reduced pulmonary pressures (2). Because insulin resistance and exercise intolerance are key features of PAH (3, 4, 10), we next assessed the activation levels of skeletal muscle SIRT3 in SuHx rats. Consistent with our previous findings, our new data show that skeletal muscle SIRT3 activation is reduced in rats with SuHx-induced PAH (Figure 2E), accompanied by increased pulmonary pressures (Figure 2A). Chronic oral nitrite supplementation, in turn, trended toward increasing SIRT3 activation in skeletal muscle with reduced pulmonary pressures, whereas metformin treatment failed to induce SIRT3 activation in SuHx rats (Figure 2E).
Collectively, our data suggest that reduction of skeletal muscle SIRT3 levels may be, at least in part, a unifying mechanism in the regulation of both PAH and PH-HFpEF. Notably, a recent report by Dean and colleagues showed the beneficial effect of metformin (100 mg/kg, daily oral gavage) on reversing PAH induced by SuHx in Wistar Kyoto female rats (6), in contrast to our studies performed using male mice and rats. Sex differences in the response to metformin have been described previously with respect to aging, life span, spontaneous tumorigenesis, and obesity (11, 12). In conclusion, our data suggest that metformin treatment may be preferentially beneficial in the treatment of PH-HFpEF, with limited efficacy for PAH.
Acknowledgments
Acknowledgment
The authors thank Dr. Sergei Snovida for helpful comments on the manuscript.
Footnotes
This work was supported by NIH grants 2R01HL098032, 1R01HL125886-01, and P01HL103455 (M.T.G.) and R01HL113178 and R01HL130261 (E.A.G.) and by American Heart Association grant 17SDG33400233 (Y.-C.L.).
Originally Published in Press as DOI: 10.1164/rccm.201801-0022LE on May 4, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Dixon DD, Trivedi A, Shah SJ. Combined post- and pre-capillary pulmonary hypertension in heart failure with preserved ejection fraction. Heart Fail Rev. 2016;21:285–297. doi: 10.1007/s10741-015-9523-6. [DOI] [PubMed] [Google Scholar]
- 2.Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, et al. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133:717–731. doi: 10.1161/CIRCULATIONAHA.115.018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 4.Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014;20:827–839. doi: 10.1016/j.cmet.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, et al. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol. 2009;158:1285–1294. doi: 10.1111/j.1476-5381.2009.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean A, Nilsen M, Loughlin L, Salt IP, MacLean MR. Metformin reverses development of pulmonary hypertension via aromatase inhibition. Hypertension. 2016;68:446–454. doi: 10.1161/HYPERTENSIONAHA.116.07353. [DOI] [PubMed] [Google Scholar]
- 7.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, et al. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 8.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, III, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra AM, Arcopinto M, Bossone E, Ehlken N, Cittadini A, Grünig E. Pulmonary arterial hypertension-related myopathy: an overview of current data and future perspectives. Nutr Metab Cardiovasc Dis. 2015;25:131–139. doi: 10.1016/j.numecd.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010;2:945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan H, Zhang H, Wei W, Fang T. Gender-related different effects of a combined therapy of Exenatide and Metformin on overweight or obesity patients with type 2 diabetes mellitus. J Diabetes Complications. 2016;30:686–692. doi: 10.1016/j.jdiacomp.2016.01.013. [DOI] [PubMed] [Google Scholar]