The respiratory community generally defines respiratory health as the absence of overt lung disease. This overly simplistic definition has limited the development of a robust approach to the prevention of chronic lung disease. In contrast, the cardiovascular community has productively conceptualized “ideal cardiovascular health,” a set of factors, including several directly along the causal pathway of transitions from health to disease, that protect against the development of cardiac disease (1, 2). The fundamental concept of “ideal health” suggests the existence of a complementary concept of “impaired health” that is an intermediate point on the continuum from health to disease. Considering cardiovascular health along such a continuum has facilitated identification of risk factors for loss of health, has enabled delineation of intermediate endotypes such as heightened inflammation and hypercholesterolemia that are deployed in clinical practice as screening tools, and has led to a rapid expansion of increasingly effective preventive measures to promote maintenance of cardiovascular health. Cardiovascular researchers and clinicians have used this conceptual framework to inform public health policy, integrate prevention into primary care, and educate communities.
In recent years, several potential risk factors for developing lung disease apart from smoking and environmental exposures have been identified. These investigations, however, have not been longitudinal across the lifespan, making it challenging to construct a timeline for how such risk factors influence respiratory health. Furthermore, these factors have frequently been identified either in the context of “healthy” control subjects in disease-focused studies (3–5) or using limited information about respiratory symptoms, physiology, and imaging derived from cohort studies focused on cardiovascular conditions (6–9). Although the Framingham Heart Study, for example, represents data collected from three generations of subjects followed over 70 years of observation, it was not designed as a respiratory cohort study and did not collect detailed phenotypic information for the respiratory system (10). The respiratory cohorts that do exist typically encompass no more than 10 years of observation (11).
Given the absence of life-course studies focused exclusively on respiratory health, it is a challenge to conceptualize how an individual might transition from ideal respiratory health to an intermediate phenotype of impaired respiratory health as a potential, albeit not universal, precursor to chronic respiratory disease such as chronic obstructive pulmonary disease (COPD) or interstitial lung disease. Although it is plausible that the transition from health to disease occurs with the accumulation of risk factors that lead to impaired lung health in some individuals, there are no empirical data demonstrating this pathway across the lifespan.
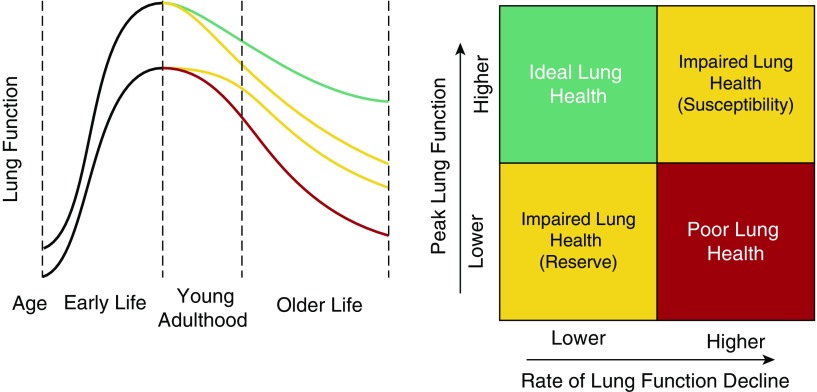
In this Pulmonary Perspective, we propose a conceptual model of respiratory health across the lifespan incorporating definitions of ideal respiratory health, impaired respiratory health, and chronic lung disease. Central to our working definition of respiratory health are the dual concepts of pulmonary reserve, as reflected by peak lung function in young adulthood, and susceptibility, as reflected by risk for future accelerated decline in lung function after the attainment of this peak (Figure 1). Because peak lung health is attained in young adulthood, we portray this as a critical period for differentiation between ideal and impaired respiratory health and a key time in the life-course to evaluate respiratory health. Lung function is the most widely recognized indicator of respiratory health; however, its actual measurement outside of established respiratory disease is rare. Below, we review several other possible contributors to and indicators of impaired (versus ideal) respiratory health and identify areas for leveraging novel techniques that could be used in the prediction of respiratory health.
Figure 1.
A conceptual depiction of respiratory health trajectories over the lifespan (adapted from the seminal work of Fletcher and Peto [33] and updated to incorporate work of Kalhan and colleagues [13] and Lange and colleagues [12]). We believe efforts toward developing a comprehensive definition of respiratory health will be aided by conceptualizing the major contributors to the development of impaired respiratory health as influencing either peak lung function in young adulthood (pulmonary “reserve”) or the rate of decline in lung function (pulmonary “susceptibility”). Multiple risk factors are associated with an individual’s reserve and susceptibility, which may ultimately contribute to the development of chronic respiratory disease (Table 1).
Factors Associated with Impaired Peak Lung Function (Poor Pulmonary Reserve)
The Clinical Significance of Impaired Peak Lung Function
Low peak lung function in young adulthood, reflective of poor pulmonary reserve, portends impaired future respiratory health. In an analysis of three independent cohorts in both the United States and Europe (the Framingham Offspring Cohort, the Copenhagen City Heart Study, and the Lovelace Smokers Cohort), it was documented that participants with lower lung function (defined as an FEV1 < 80% of predicted) at cohort inception were at greater risk of COPD after 22 years of observation than those who had normal lung function at baseline. Furthermore, among those with COPD at the end of observation, approximately one-half had an FEV1 less than 80% in early adulthood (12). These findings reinforced a prior report from the CARDIA (Coronary Artery Risk Development in Young Adults) cohort, which revealed that baseline low FEV1 and FEV1/FVC between 18 and 30 years of age predicted airflow obstruction 20 years later independent of smoking status (13). Most of the individuals in this study with lower baseline lung function still had values that would be considered in the “normal” range (i.e., FEV1 > 80% predicted and FEV1/FVC greater than the lower limit of normal), did not report a diagnosis of any underlying lung disease, and typically would not have undergone spirometric testing in the context of routine clinical care.
Beyond the risks associated with development of chronic lung disease, many studies have shown associations between lower lung function (even when still within an accepted normal range) and nonrespiratory adverse health outcomes. As early as the 1960s, analyses of the Framingham and Tecumseh cohorts demonstrated an increased risk of cardiovascular mortality associated with even modest reductions in FVC (6, 14). In an analysis that controlled for smoking status, lower lung function as measured by FVC, FEV1, or the FEV1/FVC ratio was associated with an increased risk of death from cardiovascular causes in the Copenhagen City Heart Study independent of blood pressure or serum cholesterol level (7). Heart failure hospitalizations were significantly increased in participants with lower FVC and FEV1 in the Malmö Preventive Project independent of smoking (15). In the Seven Countries Study, after adjusting for smoking, the hazard ratio of death attributable to dementia was lower in individuals with higher FVC, and lower FVC was associated with a higher risk of completed suicide. More recently, an analysis that combined CARDIA and the Framingham Offspring and Generation 3 cohorts documented that an FEV1 less than 80% predicted in early adulthood was associated with earlier development of respiratory, metabolic, and cardiovascular conditions, lending further credence to the idea of peak lung function as a risk factor for a variety of future health consequences (16).
Early-Life Factors Associated with Peak Lung Function
Notably, most of the studies described above determined only the approximate “peak” lung function in early adulthood, not the factors in the prenatal period and childhood that influence an individual’s maximum lung growth and development. Multiple factors have been identified that influence respiratory health before an individual reaches adulthood (17). These include risk factors pertaining to an individual’s parents, such as parental diagnosis of asthma or allergic rhinitis, as well as perinatal risk factors, including birth weight and gestational age in preterm infants (18–20). Tobacco smoke exposure during pregnancy and during childhood is a critical risk factor for childhood asthma, respiratory infections, and poor measured lung function (21). Other environmental factors, including exposure to air pollution and poor socioeconomic status, contribute to the increased prevalence and severity of childhood asthma (22, 23). Childhood asthma, in turn, increases an individual’s risk of having asthma and poor lung function as an adult (24, 25).
Undoubtedly, a complete profile of respiratory health in adulthood will include elements of an individual’s family history, birth history, and childhood medical history. In a recent study from the Tucson Children’s Respiratory Study, the authors took advantage of the repeated measures of lung function in this nonselected birth cohort (26). In a latent class model, two distinct trajectories of FEV1 from age 11 to 32 years old were discovered: a low lung function trajectory and a normal trajectory. The participants in the low trajectory attained a mean maximum FEV1 of 85.6% predicted in adult life compared with 94.5% predicted among those in the normal trajectory (which would still be considered normal by current interpretation criteria if measured in a clinical setting [27]). Factors associated with the low trajectory included history of maternal asthma, early-life respiratory syncytial virus infection, and physician-diagnosed asthma. These findings magnify the need to study interventions that promote respiratory health from the prenatal period through childhood and adolescence before peak respiratory health is attained as part of maximizing pulmonary reserve in adult life and as a key component of preventing chronic lung diseases.
Cardiorespiratory fitness, or an individual’s maximal ability to perform work in a standardized exercise test, is a well-accepted predictor of heart disease and mortality (28) and is considered to be an important element of cardiovascular health (1). Physical activity and cardiorespiratory fitness are also associated with peak lung function. Several studies have suggested that increased physical activity among children is associated with higher lung function (29, 30). At the baseline examination of the CARDIA study (mean age, 25 years; range, 18–30 years), lower cardiorespiratory fitness (as measured by a symptom-limited, graded treadmill exercise test) was associated with lower lung function (albeit still in the normal range) (31). A recent paper evaluated the association between fitness and lung function in two population-based cohorts in Denmark and New Zealand among children and young adults (32). Objectively measured aerobic fitness was associated with better lung function between the ages of 9 and 38 years, and improvements in fitness during childhood and adolescence were associated with greater increases in lung volumes into early adulthood. Maximizing fitness through childhood and adolescence then appears to be associated with better pulmonary reserve in young adulthood in our conceptual framework of respiratory health.
Factors Associated with Accelerated Decline in Lung Function (Pulmonary Susceptibility)
The Clinical Significance of Accelerated Decline in Lung Function
For many years it was believed that lung function defined by FEV1 peaked in the third decade of life and steadily declined over the remainder of the lifespan, and that this decline could be accelerated by noxious exposures such as smoking (33). It stands to reason that for an individual with adequate pulmonary reserve (manifested by adequate baseline lung function) to transition from health to disease, that person would have to experience accelerated decline in lung function to meet a threshold indicating disease. However, few adult life-course studies have attempted to understand different patterns of change in lung function. We conceptualize risk factors associated with decline in lung function as a hallmark of susceptibility to future lung disease and a deviation from ideal to impaired respiratory health, even when the decline does not reach a threshold that has traditionally defined disease (Figure 1).
Accelerated decline in lung function occurred in approximately half of individuals by the conclusion of the three-cohort study published by Lange and colleagues, including in many whose lung function was normal (FEV1 > 80% predicted) (12). Several other studies have supported the conclusion that rapid decline in lung function over time is a key feature of susceptibility to poor respiratory and overall health. In the Atherosclerosis Risk in Communities study of adults aged 44 to 66 years (including individuals both with and without COPD), those with the most rapid decline in lung function over a 3-year period were at greatest risk for COPD-related hospitalization as well as mortality (34).
More recently, findings from CARDIA suggested that specific patterns of lung function decline are associated with the development of distinct cardiac phenotypes (35). On the basis of serial spirometry over 20 years after initial study enrollment, individuals with FVC decline were more likely to have larger left ventricular mass, greater cardiac output, and diastolic dysfunction, whereas individuals with decline in FEV1/FVC ratio were at greater risk of having smaller left atrial measurements and lower cardiac output. Data from the COPDGene study suggest that accelerated lung function decline in COPD, for instance, is preceded by development of radiographically evident small airways abnormalities, even among individuals without classically defined airflow obstruction (36). These findings suggest that accelerated decline in lung function is associated with pathology, even among individuals who do not meet the standard thresholds of lung function impairment that define disease (such as an FEV1/FVC < 70% or the lower limit of normal to define COPD).
Lifestyle Factors Associated with Decline in Lung Function
Cardiorespiratory fitness and physical activity are associated not just with attainment of peak lung function but also with the rate of lung function decline. Among participants in CARDIA, lung function decline measured at 20 years of follow-up was predicted by cardiorespiratory fitness at Year 0 (31). Furthermore, sustaining higher fitness and achieving an increased fitness level were associated with slower decline in FEV1. Active smokers who reported higher levels of physical activity in the Copenhagen City Heart Study experienced less decline in FVC and FEV1 and were less likely to develop COPD over 10 to 13 years than those who reported low levels of activity (37).
Diet may also be associated with decline in lung function in adult life. In the NHANES (National Health and Nutrition Examination Surveys) cycle of 2009 to 2010, higher dietary fiber intake was associated with higher FEV1 and FVC and a lower rate of obstructive lung physiology among adults sampled from the general population (38). In a study based on the ECRHS (European Community Respiratory Health Survey), greater dietary antioxidant consumption was associated with slower FEV1 and FVC decline over a 10-year period (39). Although the respiratory research community has long debated whether preventive care can alter the rate of lung function decline in at-risk individuals (40), the work described above suggests that lifestyle interventions aimed at modifying aspects of an individual’s cardiorespiratory fitness level and diet may alter that individual’s susceptibility to developing impaired respiratory health. When considering opportunities to test interventions to promote respiratory health, both fitness and dietary interventions are worth considering.
Respiratory Symptoms as Predictors of Susceptibility to Declining Lung Function
Respiratory symptom burden is an easily ascertainable factor that may be independently associated with poor health outcomes (41). The prevalence of respiratory symptoms in the general population, particularly earlier in life among nonsmokers, is not known. In the NHANES 2007 to 2010 cycle, the presence of chronic respiratory symptoms in 40- to 79-year-old participants was associated with worse health-related quality of life compared with those without symptoms (42). Current smokers in the COPDGene study were more likely than former smokers to have respiratory symptoms (43). An analysis of ever smokers aged 40 to 80 years with preserved lung function by the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) group found that 50% of participants had respiratory symptoms (defined as a score > 10 out of 40 on the eight-question COPD Assessment Test) and that the presence of symptoms was associated with activity limitation, lower pulmonary function, and thickened airway walls on computed tomography (CT) imaging (44). The CARDIA group found, in an analysis that controlled for smoking status, that the presence of respiratory symptoms at baseline was associated with more rapid decline in FEV1 and FVC over 30 years of follow-up as well as greater risk of emphysema (45). Prospective study of the relationship between respiratory symptom burden and health outcomes, controlling for the presence of asthma, smoking, or other known exposures, will better delineate to what extent the presence of respiratory symptoms should be incorporated into a survey of an individual’s overall respiratory health status.
Detection of Lung Injury via CT Imaging—An Early Indicator of Susceptibility?
Imaging studies of the lung represent a potentially underutilized tool for assessing respiratory health. Nishimura and colleagues observed that severity of emphysema on CT imaging was associated with more rapid FEV1 decline in patients with COPD (46). The COPDGene investigators used parametric response mapping, a novel technique for analysis of CT imaging, to assess the degree of emphysema and functional small airways disease in ever smokers with COPD and subsequently to demonstrate that this was associated with decline in FEV1 (36). Parametric response mapping was also used to analyze CT imaging obtained in the SPIROMICS cohort, where in individuals without airflow obstruction, functional small airway disease was associated with increased age, increased FVC, and lower FEV1/FVC ratio, independent of respiratory symptoms (47). Another study by the COPDGene investigators found evidence of gas trapping in areas of normal-appearing lung on CT scans of smokers after comparing end-expiratory and end-inspiratory lung density (48). The MESA (Multiethnic Study of Atherosclerosis) study group has published several analyses of cardiac CT scans performed on participants demonstrating that subtle lung imaging findings can reflect key aspects of an individual’s respiratory health (49–52). In two analyses, among participants without airflow obstruction, increased presence of low-attenuation areas representing emphysema-like regions on a CT scan performed at the baseline study visit was associated with increased all-cause mortality after adjusting for potential confounders and was associated with increased respiratory and lung cancer mortality (49, 50). In a follow-up study, this CT finding of low-attenuation areas was associated with increased odds of developing spirometric obstructive lung physiology after 5 years of follow-up (51). In a fourth study, the presence of high-attenuation areas on baseline CT scans was a risk factor for hospitalization and mortality attributable to interstitial lung disease (52).
Profiling Respiratory Health during a Critical Period in Early Adulthood
As reviewed above, multiple factors have been identified as potential contributors to the dual facets of respiratory health: reserve and susceptibility (Table 1). Numerous studies have investigated “birth cohorts” to identify early-life risk factors for chronic lung disease that exert influence primarily on an individual’s respiratory health reserve. Studies that investigate early-life interventions to influence peak respiratory health or pulmonary reserve are critically important. Such studies can be designed around outcomes related to the determination of lung function at the time it peaks in young adulthood and certainly would inform both the promotion of respiratory health and disease prevention. Studies focused on enhancing peak lung function (reserve) need to be supplemented, however, with studies focused on identifying factors associated with accelerated decline in lung function (susceptibility) to promote health as it relates not only to maximizing peak lung function but also to attenuating age-related decline in lung function.
Table 1.
Risk Factors Associated with Two Aspects of Respiratory Health Presented in Figure 1: Pulmonary “Reserve” and “Susceptibility”
| Factors associated with peak lung function (pulmonary “reserve”) |
| Early-life factors |
| Maternal exposures |
| Perinatal exposures |
| Gestational age at birth |
| Birth weight |
| Childhood factors |
| Environmental exposures |
| Respiratory infections |
| Physical activity and fitness |
| Biomarkers |
| CC16 |
| Factors associated with decline in lung function (pulmonary “susceptibility”) |
| Environmental exposures, including smoking |
| Physical activity and fitness |
| Diet |
| Respiratory symptoms |
| CT imaging measurements |
| Low-attenuation areas |
| High-attenuation areas |
| Functional small airways disease |
| Biomarkers |
| Systemic inflammation (CRP, fibrinogen) |
| CC16 |
Definition of abbreviations: CC = club cell protein; CRP = C-reactive protein; CT = computed tomography.
Existing investigations of respiratory health factors in adulthood have largely been focused on older individuals and oriented around established respiratory disease. We believe that a research agenda around the study of respiratory health is needed to determine factors associated with susceptibility to future decline in lung function and the key point of deviation from ideal to impaired respiratory health. Therefore, we argue that a key focus of future studies must be the period encompassing early adulthood, when peak lung function is achieved and when susceptible individuals diverge from ideal to impaired respiratory health. Determination of factors that predict susceptibility would enable identification of surrogate markers of ideal respiratory health in adult life. Furthermore, such markers would be informative in an effort to intercept the path toward disease at an early time point in its pathobiology when, unlike in established chronic lung disease such as COPD and interstitial lung disease, the process may still be amenable to resolution of injury and regeneration.
Developing Novel Predictors of Impaired Respiratory Health
No biomarker has been identified that can be considered an essential component of respiratory health in the way that serum cholesterol is used to define cardiovascular health. In contrast to cholesterol, which is on the causal pathway for atherosclerotic plaque formation, existing candidate respiratory health biomarkers are nonspecific and largely have not been proven to have a direct causal biologic relationship with lung injury or remodeling. Nevertheless, a number of serum biomarkers can predict future outcomes related to declining respiratory health. In CARDIA, two markers of systemic inflammation measured at age 25 to 32 years, plasma fibrinogen and C-reactive protein, were independently associated with greater lung function decline by spirometry between Year 0 and Year 20 of the study (53). In a pooled cohort analysis, lower concentrations of the antiinflammatory protein CC16 were associated with reduced lung function in childhood (diminished pulmonary reserve), accelerated decline in lung function in adult life (enhanced susceptibility), and greater risk of obstructive lung physiology. In MESA, higher soluble ICAM-1 (intercellular adhesion molecule 1) levels were associated with progression of low-attenuation (emphysema-like) areas on CT imaging (54). Also in MESA, having a higher level of serum MMP-7 (matrix metalloproteinase 7) at baseline between 45 and 84 years of age predicted greater FVC decline and exertional dyspnea after 5 years and was associated with increased odds of interstitial lung abnormalities and all-cause mortality after 10 years (55).
A clear definition of impaired respiratory health and the discovery and validation of biomarkers to detect it will enable enhanced population-based screening and more targeted interventions that may mitigate the transition from ideal to impaired respiratory health (and later in life to disease) or, in an ideal scenario, may even promote transition back to ideal respiratory health. Existing research points to several promising approaches for predicting the development of impaired respiratory health aligned with the current model of cardiovascular disease, where markers on the causal pathway to disease inform robust strategies for disease interception and health promotion.
Genomic techniques applied to lung tissue have shown promise for diagnosing usual interstitial pneumonia or characterizing common patterns of gene expression in COPD and idiopathic pulmonary fibrosis (56, 57). However, generalizing these approaches to characterization of healthy individuals is challenging because of the risks associated with obtaining lung tissue samples. Promisingly, gene expression profiling by whole-transcript array of bronchial epithelial cells obtained by brushings from current and former smokers with and without COPD successfully identified genes associated with COPD (58). Furthermore, gene expression profiling of nasal epithelium by the same group was also sufficient for distinguishing patients with COPD from control subjects, and a subset of the differentially expressed genes overlapped with those identified in the earlier study of bronchial epithelial gene expression (59). The value of this kind of approach has been demonstrated in the field of oncology, where genomic techniques have already entered clinical practice for cancer prevention, screening, and diagnosis (60).
Although careful analysis of CT imaging data quantifying known abnormalities such as regions of emphysema-like lung and high-attenuation areas can be useful for predicting respiratory health outcomes, machine learning approaches to analyzing the vast quantities of data collected in a single CT scan may yield even more diagnostic and prognostic information. Such an approach using convolutional neural network analysis, using a training dataset of 7,983 CT scans in 2,672 smokers, was successful in classifying more than 70% of participants within one stage of COPD and predicted the development of acute respiratory disease events and mortality (61). A similar approach used earlier in adult life may be able to detect the radiographic phenotypes associated with impaired respiratory health and susceptibility to future disease.
Designing Studies to Define Respiratory Health
Although the paradigm of ideal cardiovascular health can serve as a useful paradigm for developing a definition of respiratory health, the body of evidence available to the cardiovascular research community is richer than that available to the respiratory research community. New forward-thinking epidemiologic studies are needed to better delineate how the various factors outlined in this Pulmonary Perspective can be incorporated into a comprehensive definition of respiratory health. These studies should be designed to prospectively assess associations between novel aspects of the respiratory health phenotype, such as genomic biomarkers or machine learning–based imaging assessments, with more well-accepted aspects of respiratory health, such as pulmonary function parameters or respiratory symptoms. A recently published conceptual computational model of the natural history of COPD on the basis of integrating key aspects of disease exemplified how different factors contributing to respiratory health could ultimately be brought together into a comprehensive model of respiratory health spanning ideal health, impaired health, and disease (62).
Conclusions
Incorporating health promotion into respiratory medicine as a pillar of disease prevention is mandated by a contemporary approach to public health. To satisfy this mandate, we believe the respiratory research community must prioritize developing a comprehensive definition of respiratory health. Key factors pertaining to respiratory health will belong to two broad categories: factors affecting peak respiratory health or pulmonary reserve and factors affecting the rate of lung function decline in adulthood or susceptibility. Achieving a consensus about these key respiratory health factors will facilitate identification of surrogate outcomes for use as practical endpoints in trials aimed at slowing or reversing the onset of impaired respiratory health and preventing chronic respiratory disease. Novel precision medicine approaches, including genomic profiling and imaging-based analyses, are promising for inclusion as surrogate outcomes of respiratory health. Although health promotion is a key goal to prevent chronic lung disease, enhancing our ability to detect impaired respiratory health at the earliest possible stage would also allow for the development of strategies to intercept chronic respiratory disease before it becomes clinically apparent.
Footnotes
Supported by NHLBI grants HL122477 and HL136111.
Author Contributions: P.A.R. conceived the paper and drafted and revised the manuscript. G.R.W. conceived the paper and revised the manuscript. M.T.D. conceived the paper and revised the manuscript. A.S. conceived the paper and revised the manuscript. M.K.H. conceived the paper and revised the manuscript. R.K. conceived the paper and drafted and revised the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201801-0120PP on April 6, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 2.Gooding HC, Shay CM, Ning H, Gillman MW, Chiuve SE, Reis JP, et al. Optimal lifestyle components in young adulthood are associated with maintaining the ideal cardiovascular health profile into middle age. J Am Heart Assoc. 2015;4:e002048. doi: 10.1161/JAHA.115.002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. ECLIPSE investigators. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 4.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawber T, Kannel WB, Friedman GD. Vital capacity, physical activity and coronary heart disease. In: Raab W, editor. Prevention of ischemic heart disease: principles and practice. Springfield, IL: Charles C. Thomas; 1966. pp. 254–265. [Google Scholar]
- 7.Lange P, Nyboe J, Jensen G, Schnohr P, Appleyard M. Ventilatory function impairment and risk of cardiovascular death and of fatal or non-fatal myocardial infarction. Eur Respir J. 1991;4:1080–1087. [PubMed] [Google Scholar]
- 8.Gidding SS, Xie X, Liu K, Manolio T, Flack JM, Gardin JM. Cardiac function in smokers and nonsmokers: the CARDIA study: the Coronary Artery Risk Development in Young Adults Study. J Am Coll Cardiol. 1995;26:211–216. doi: 10.1016/0735-1097(95)00118-j. [DOI] [PubMed] [Google Scholar]
- 9.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Marco R, Accordini S, Marcon A, Cerveri I, Antó JM, Gislason T, et al. European Community Respiratory Health Survey (ECRHS) Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183:891–897. doi: 10.1164/rccm.201007-1125OC. [DOI] [PubMed] [Google Scholar]
- 12.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 13.Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later. Am J Med. 2010;123:468, e1–7. doi: 10.1016/j.amjmed.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins MW, Keller JB. Predictors of mortality in the adult population of Tecumseh. Arch Environ Health. 1970;21:418–424. doi: 10.1080/00039896.1970.10667260. [DOI] [PubMed] [Google Scholar]
- 15.Engström G, Melander O, Hedblad B. Population-based study of lung function and incidence of heart failure hospitalisations. Thorax. 2010;65:633–638. doi: 10.1136/thx.2010.135392. [DOI] [PubMed] [Google Scholar]
- 16.Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5:935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 17.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 18.Grabenhenrich LB, Gough H, Reich A, Eckers N, Zepp F, Nitsche O, et al. Early-life determinants of asthma from birth to age 20 years: a German birth cohort study. J Allergy Clin Immunol. 2014;133:979–988. doi: 10.1016/j.jaci.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Henderson-Smart DJ, Hutchinson JL, Donoghue DA, Evans NJ, Simpson JM, Wright I Australian and New Zealand Neonatal Network. Prenatal predictors of chronic lung disease in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2006;91:F40–F45. doi: 10.1136/adc.2005.072264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke RW. Factors associated with chronic lung disease in preterm infants. Arch Dis Child. 1991;66:776–779. doi: 10.1136/adc.66.7_spec_no.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168:897–905. doi: 10.1007/s00431-009-0967-3. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, et al. Early-life air pollution and asthma risk in minority children: the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;188:309–318. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forno E, Celedón JC. Health disparities in asthma. Am J Respir Crit Care Med. 2012;185:1033–1035. doi: 10.1164/rccm.201202-0350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 25.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194:607–612. doi: 10.1164/rccm.201604-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 28.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 29.Berntsen S, Wisløff T, Nafstad P, Nystad W. Lung function increases with increasing level of physical activity in school children. Pediatr Exerc Sci. 2008;20:402–410. doi: 10.1123/pes.20.4.402. [DOI] [PubMed] [Google Scholar]
- 30.Menezes AM, Wehrmeister FC, Muniz LC, Perez-Padilla R, Noal RB, Silva MC, et al. Physical activity and lung function in adolescents: the 1993 Pelotas (Brazil) birth cohort study. J Adolesc Health. 2012;51:S27–S31. doi: 10.1016/j.jadohealth.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benck LR, Cuttica MJ, Colangelo LA, Sidney S, Dransfield MT, Mannino DM, et al. Association between cardiorespiratory fitness and lung health from young adulthood to middle age. Am J Respir Crit Care Med. 2017;195:1236–1243. doi: 10.1164/rccm.201610-2089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hancox RJ, Rasmussen F. Does physical fitness enhance lung function in children and young adults? Eur Respir J. 2018;51:1701374. doi: 10.1183/13993003.01374-2017. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med. 2006;173:985–990. doi: 10.1164/rccm.200508-1344OC. [DOI] [PubMed] [Google Scholar]
- 35.Cuttica MJ, Colangelo LA, Shah SJ, Lima J, Kishi S, Arynchyn A, et al. Loss of lung health from young adulthood and cardiac phenotypes in middle age. Am J Respir Crit Care Med. 2015;192:76–85. doi: 10.1164/rccm.201501-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. COPDGene Investigators. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175:458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 38.Hanson C, Lyden E, Rennard S, Mannino DM, Rutten EP, Hopkins R, et al. The relationship between dietary fiber intake and lung function in the National Health and Nutrition Examination Surveys. Ann Am Thorac Soc. 2016;13:643–650. doi: 10.1513/AnnalsATS.201509-609OC. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Larsen V, Potts JF, Omenaas E, Heinrich J, Svanes C, Garcia-Aymerich J, et al. Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur Respir J. 2017;50:1602286. doi: 10.1183/13993003.02286-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry CE, Drummond MB. The horse-racing effect and lung function: can we slow the fastest horse? Am J Respir Crit Care Med. 2017;195:1134–1135. doi: 10.1164/rccm.201703-0540ED. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Roisin R, Han MK, Vestbo J, Wedzicha JA, Woodruff PG, Martinez FJ. Chronic respiratory symptoms with normal spirometry: a reliable clinical entity? Am J Respir Crit Care Med. 2017;195:17–22. doi: 10.1164/rccm.201607-1376PP. [DOI] [PubMed] [Google Scholar]
- 42.Wheaton AG, Ford ES, Thompson WW, Greenlund KJ, Presley-Cantrell LR, Croft JB. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States--National Health and Nutrition Examination Survey 2007-2010. BMC Public Health. 2013;13:854. doi: 10.1186/1471-2458-13-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JHM, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalhan R, Dransfield MT, Colangelo LA, Cuttica MJ, Jacobs DR, Jr, Thyagarajan B, et al. Respiratory symptoms in young adults and future lung disease: the CARDIA Lung Study Am J Respir Crit Care Med [ahead of print] 25 Jan 2018; DOI:10.1164/rccm.201710-2108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura M, Makita H, Nagai K, Konno S, Nasuhara Y, Hasegawa M, et al. Hokkaido COPD Cohort Study Investigators. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 47.Martinez CH, Diaz AA, Meldrum C, Curtis JL, Cooper CB, Pirozzi C, et al. SPIROMICS Investigators. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in SPIROMICS. Am J Respir Crit Care Med. 2017;195:464–472. doi: 10.1164/rccm.201604-0871OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bodduluri S, Reinhardt JM, Hoffman EA, Newell JD, Jr, Nath H, Dransfield MT, et al. COPDGene Investigators. Signs of gas trapping in normal lung density regions in smokers. Am J Respir Crit Care Med. 2017;196:1404–1410. doi: 10.1164/rccm.201705-0855OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161:863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oelsner EC, Carr JJ, Enright PL, Hoffman EA, Folsom AR, Kawut SM, et al. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort study. Thorax. 2016;71:624–632. doi: 10.1136/thoraxjnl-2015-207822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oelsner EC, Smith BM, Hoffman EA, Folsom AR, Kawut SM, Kaufman JD, et al. Associations between emphysema-like lung on CT and incident airflow limitation: a general population-based cohort study. Thorax. 2018;73:486–488. doi: 10.1136/thoraxjnl-2017-210842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Podolanczuk AJ, Oelsner EC, Barr RG, Bernstein EJ, Hoffman EA, Easthausen IJ, et al. High attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults. Am J Respir Crit Care Med. 2017;196:1434–1442. doi: 10.1164/rccm.201703-0555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalhan R, Tran BT, Colangelo LA, Rosenberg SR, Liu K, Thyagarajan B, et al. Systemic inflammation in young adults is associated with abnormal lung function in middle age. PLoS One. 2010;5:e11431. doi: 10.1371/journal.pone.0011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aaron CP, Schwartz JE, Bielinski SJ, Hoffman EA, Austin JH, Oelsner EC, et al. Intercellular adhesion molecule 1 and progression of percent emphysema: the MESA Lung Study. Respir Med. 2015;109:255–264. doi: 10.1016/j.rmed.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong HF, Podolanczuk AJ, Barr RG, Oelsner EC, Kawut SM, Hoffman EA, et al. MESA (Multi-Ethnic Study of Atherosclerosis) Serum matrix metalloproteinase-7, respiratory symptoms, and mortality in community-dwelling adults. Am J Respir Crit Care Med. 2017;196:1311–1317. doi: 10.1164/rccm.201701-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pankratz DG, Choi Y, Imtiaz U, Fedorowicz GM, Anderson JD, Colby TV, et al. Usual interstitial pneumonia can be detected in transbronchial biopsies using machine learning. Ann Am Thorac Soc. 2017;14:1646–1654. doi: 10.1513/AnnalsATS.201612-947OC. [DOI] [PubMed] [Google Scholar]
- 57.Kusko RL, Brothers JF, II, Tedrow J, Pandit K, Huleihel L, Perdomo C, et al. Integrated genomics reveals convergent transcriptomic networks underlying chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194:948–960. doi: 10.1164/rccm.201510-2026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steiling K, van den Berge M, Hijazi K, Florido R, Campbell J, Liu G, et al. A dynamic bronchial airway gene expression signature of chronic obstructive pulmonary disease and lung function impairment. Am J Respir Crit Care Med. 2013;187:933–942. doi: 10.1164/rccm.201208-1449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boudewijn IM, Faiz A, Steiling K, van der Wiel E, Telenga ED, Hoonhorst SJM, et al. Nasal gene expression differentiates COPD from controls and overlaps bronchial gene expression. Respir Res. 2017;18:213. doi: 10.1186/s12931-017-0696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beane J, Campbell JD, Lel J, Vick J, Spira A. Genomic approaches to accelerate cancer interception. Lancet Oncol. 2017;18:e494–e502. doi: 10.1016/S1470-2045(17)30373-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.González G, Ash SY, Vegas Sánchez-Ferrero G, Onieva Onieva J, Rahaghi FN, Ross JC, et al. COPDGene and ECLIPSE Investigators. Disease staging and prognosis in smokers using deep learning in chest computed tomography. Am J Respir Crit Care Med. 2018;197:193–203. doi: 10.1164/rccm.201705-0860OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agustí A, Compte A, Faner R, Garcia-Aymerich J, Noell G, Cosio BG, et al. The EASI model: a first integrative computational approximation to the natural history of COPD. PLoS One. 2017;12:e0185502. doi: 10.1371/journal.pone.0185502. [DOI] [PMC free article] [PubMed] [Google Scholar]