Abstract
Extracorporeal life support (ECLS) was developed more than 50 years ago, initially with venoarterial and subsequently with venovenous configurations. As the technique of ECLS significantly improved and newer skills developed, complexity in terminology and advances in cannula design led to some misunderstanding of and inconsistency in definitions, both in clinical practice and in scientific research. This document is a consensus of multispecialty international representatives of the Extracorporeal Life Support Organization, including the North America, Latin America, EuroELSO, South West Asia and Africa, and Asia-Pacific chapters, imparting a global perspective on ECLS. The goal is to provide a consistent and unambiguous nomenclature for ECLS and to overcome the inconsistent use of abbreviations for ECLS cannulation. Secondary benefits are ease of multicenter collaboration in research, improved registry data quality, and clear communication among practitioners and researchers in the field.
Keywords: terminology, extracorporeal membrane oxygenation, membrane oxygenators, extracorporeal circulation, cannula
Extracorporeal therapies for temporary, non-intraoperative support of patients with cardiac and/or pulmonary dysfunction are an outgrowth of cardiopulmonary bypass. The success of cardiopulmonary bypass beginning in 1953 for short-term circulatory support did not directly translate to more prolonged support, owing in large part to the lack of biocompatibility of devices of the time (1). Long-term extracorporeal support would await the development of newer technologies and approaches over the ensuing decades. The result is a diversity of approaches to temporary support that are rapidly becoming mainstream in the management of severely ill and injured patients.
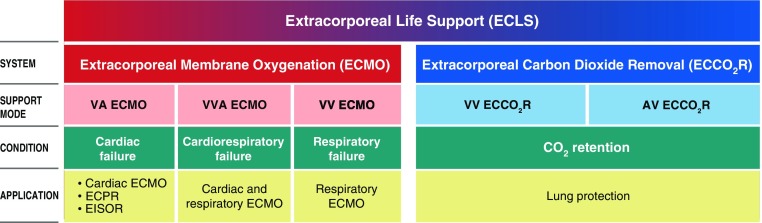
This diversity has led to the use of terms and abbreviations marked by inconsistency and ambiguity. The first reported application of extracorporeal support in the ICU setting (2) employed extracorporeal membrane oxygenation (ECMO) in post-traumatic acute respiratory failure. Although the term “ECMO” refers to a particular extracorporeal configuration and application for support of cardiopulmonary dysfunction, it became synonymous with the use of any extracorporeal system other than surgical cardiopulmonary bypass. A number of extracorporeal applications have emerged that are not considered ECMO, such as extracorporeal carbon dioxide removal for managing hypercapnic respiratory failure or supporting ultraprotective ventilation in acute respiratory distress syndrome, extracorporeal cardiopulmonary resuscitation for maintaining systemic perfusion during cardiac arrest, and extracorporeal interval support for organ recovery to provide perfusion of organs awaiting recovery after declaration of cardiac death. The term “extracorporeal life support” (ECLS) has emerged to describe the entire family of extracorporeal support modalities for long-term support (Figure 1).
Figure 1.
Relationship between ECLS systems, support modes, clinical conditions, and applications. AV = arteriovenous; ECPR = extracorporeal cardiopulmonary resuscitation; EISOR = extracorporeal interval support for organ retrieval; VA = venoarterial; VV = venovenous; VVA = venovenoarterial. Illustration by Jacqueline Schaffer.
Moreover, cannulation configurations for long-term support have evolved substantially over the years, with expanded disease indications and outcome pathways (e.g., bridge to recovery, bridge to transplant, bridge to destination), leading to novel cannulation approaches (3–10). Without a formalized approach to cannulation nomenclature, there are inconsistencies in the reporting of ECLS studies.
This document represents a consensus on terms, abbreviations, definitions, and cannulation descriptions for ECLS to establish consistency for clinical and research descriptions. The contributors represent multiple specialties performing ECLS, including cardiothoracic surgery, pediatric surgery, surgical intensive care, anesthesiology, cardiology, pulmonary medicine, medical intensive care, pediatric intensive care, neonatology, and emergency medicine. The contributors also represent the international chapters of the Extracorporeal Life Support Organization (ELSO), including North America, Latin America, EuroELSO, South West Asia and Africa, and Asia-Pacific, imparting a global perspective on ECLS. The conclusive meeting among all the contributors to finalize the document, hereby presented, was held in Maastricht, the Netherlands, on the occasion of the Sixth EuroELSO Annual Meeting, inspiring the denomination of such a nomenclature by another “Maastricht Treaty” realized for an economical/political context.
The Nomenclature Task Force was assembled by the ELSO, and the different definitions were based on review of the literature pertaining to ECLS nomenclature as well as on clinical practice. The consensus statement was determined as the most appropriate approach in the absence of studies evaluating the clarity and strength of different terms used in the setting of ECLS; all definitions were then based on expert opinion. The task force conferred by e-mail, and agreements were achieved through iterative discussion and debate. Recommendations were unanimously agreed and then approved by the task force.
This report is composed of three sections. The first gives terms, abbreviations and synonyms, and definitions used in the practice of ECLS. The second section addresses units of measurement. The third and final section is a nomenclature and taxonomy for the description of cannulas, cannulation configurations, and vascular access sites.
Principal Terms, Abbreviations, and Definitions
The terms represent concepts derived from all aspects of ECLS and are divided into sections on systems and support modes, cannulation concepts, devices, and circuit operation. An abbreviation is provided for each term if in common use. Each term is qualified by a definition. Synonyms are provided if they have been used historically, but to maintain consistency, they should not be used in lieu of the principal term. A comment may accompany a term to provide additional information or clarification.
Systems and support mode terms are given in Table E1 in the online supplement. This table provides fundamental definitions of ECLS and related therapies. A support mode is a combination of cannulation configuration and circuit operation with an intended type of organ support (Figures 2–4). Table E2 provides terminology for general concepts related to cannulation, including devices, procedures, and general approaches to cannulations. Cannulation specifics are covered later in this document. A terminology section for devices used as part of the circuit employed for ECLS is given in Table E3. Circuit operation terms and concepts are given in Table E4.
Figure 2.
Schematic of (A) venoarterial extracorporeal membrane oxygenation (ECMO), typically used for cardiac failure, and (B) venovenoarterial ECMO, typically used for combined cardiac and respiratory failure, showing common cannulation configurations and direction of blood flow. FSO2 = sweep gas inlet oxygen fraction; VA = venoarterial; Vf-Af = femoral venous drainage to femoral arterial return; Vf-VjAf = femoral venous drainage to jugular venous and femoral arterial return; VVA = venovenoarterial. Modified from Figure 1 in Reference 11. Illustration by Jacqueline Schaffer.
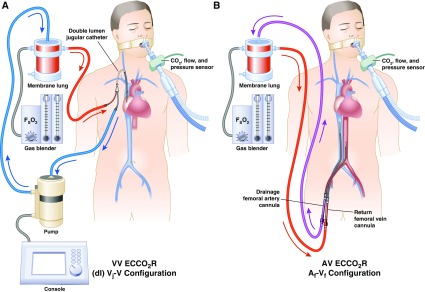
Figure 4.
Schematic of (A) venovenous extracorporeal carbon dioxide removal (ECCO2R) and (B) pumpless arteriovenous ECCO2R, typically used for hypercapnic respiratory failure or lung protection during hypoxemic respiratory failure, showing common cannulation configurations and direction of blood flow. Af-Vf = femoral arterial drainage to femoral venous return; AV = arteriovenous; (dl) Vj-V = dual-lumen jugular venous drainage to venous return; FSO2 = sweep gas inlet oxygen fraction; VV = venovenous. Modified from Figure 1 in Reference 11. Illustration by Jacqueline Schaffer.
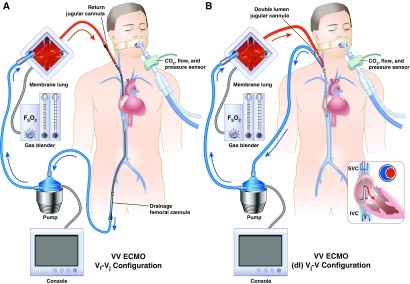
Figure 3.
Schematic of (A) venovenous extracorporeal membrane oxygenation, typically used for respiratory failure with two-site cannulation, and (B) single-site dual-lumen cannulation showing common cannulation configurations and direction of blood flow. (dl) Vj-V = dual-lumen jugular venous drainage to venous return; ECMO = extracorporeal membrane oxygenation; FSO2 = sweep gas inlet oxygen fraction; IVC = inferior vena cava; SVC = superior vena cava; Vf-Vj = femoral venous drainage to jugular venous return; VV = venovenous. Modified from Figure 1 in Reference 11. Illustration by Jacqueline Schaffer.
Units of Measurement
Several units of measurement are used for devices and patient management during ECLS. Table E5 provides the preferred measurement unit systems for ECLS. Système international d’unités units are preferred over Imperial units and are used in most cases, except where manufacturer specifications dictate the unit system.
Configurations for Peripheral Cannulation
Peripheral cannulation configurations vary in complexity from simple two-cannula configurations for traditional venovenous and venoarterial ECMO to more configurations, such as with multiple cannulas and multiple drainage or return sites. There is a need to be able to provide basic cannulation information for clinical purposes that conveys the essential configuration. To meet these objectives, a two-level classification system with increasing levels of descriptive information was developed.
Fundamental to all cannulation abbreviations is the use of a hyphen to distinguish drainage cannulas on the left of the hyphen and return cannulas on the right of the hyphen, with the membrane lung represented by the hyphen itself. In this approach, the presence of a hyphen differentiates a cannulation configuration from the support modes introduced above.
Level 1: Cannula Hierarchy
All cannulas contributing to the primary (major) draining and return circuit flow are written in upper case letters, such as “V-V” to represent venous drainage and venous return for venovenous support (Table E6). All cannulas with minor flow for secondary drainage, for unloading of specific anatomical location, or to promote distal perfusion are written in lower case letters after the major flow cannula to which side it belongs, such as “V-Aa” representing venous drainage, arterial return, and secondary arterial return (e.g., for distal perfusion). The use of a dual-lumen cannula for venovenous support would be indicated with a preceding “(dl)” abbreviation (e.g., “(dl)V-V”).
A configuration may have two major drainage or return cannulas, in which case a second upper case letter is used to the left of the first upper case letter or to the right of the second upper case letter, respectively. For example, “VV-V” would represent venovenous support with two major drainage cannulas and a single return cannula, and “V-VA” would represent venous drainage and both venous and arterial return (for venovenoarterial hybrid support).
Level 2: Cannulation Site
The next level of descriptors includes the vessel that is cannulated through the use of subscripted lowercase letters indexing the relevant drainage or return cannulation descriptor. Letters assigned to the different peripheral vessels are given in Table E6. Bifemoral cannulation for venoarterial support, for example, would be indicated as “Vf-Af.” The traditional two-cannula venovenous configuration with drainage from the femoral and return to the internal jugular would be indicated as “Vf-Vj.”
Configurations for Central Cannulation
Central cannulation involves placement of cannulas in a chamber of the heart or the proximal vena cavae through a median sternotomy or related surgical technique. The general approach for the description of peripheral cannulation is applied to central cannulation, with the exception that the major anatomical sites cannulated for are expressed as two uppercase letters, and secondary sites such as venting cannulas are expressed as a lowercase letter (Table E6). For example, the common postcardiotomy configuration for venoarterial support with right atrial drainage, a left atrial vent, and aortic return would be “RAva-AO.” Left-sided support with drainage from the left ventricle and aortic return would be “LV-AO,” and right-sided support from the right atrium to the pulmonary artery would be “RA-PA.”
Conclusions
This classification system for ECLS nomenclature provides a standardized foundation for the description of ECLS application, decreasing ambiguity and providing consistency for the comparison of clinical reports. It includes an extensive terminology of systems, support modes, devices, units of measurement, and cannulation configurations. Given the hierarchical structure of cannulation description, it provides for the inclusion of only as much detail as needed for a given purpose. Based on a defined system, it maintains flexibility to adapt to (many if not all) future developments in cannulation approaches as it supports extensibility.
This nomenclature has limitations. Although adequate for supporting descriptions for most clinical applications, it may not meet the needs for research applications where more detail for cannulation configurations, such as location of the cannula tip, additional cannulation sites, or nontraditional cannulation configurations, would be desirable. Given its systematic basis, however, it could be extended for such a purpose.
Footnotes
Supported by the National Institute of Neurological Disorders and Stroke of the NIH under award number K23NS076674 (M.M.B.).
Author Contributions: Drafting of the article: S.A.C. and L.M.B.; critical revision of the article for important intellectual content: F.S.T., R.L., M.V.M., F.P., M.D.N., M.B., L.G., R.P.B., D.M.M., V.P., D.B., M.M.B., E.F., M.M., R.D., and R.H.B.; final approval of the article: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201710-2130CP on April 3, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fortenberry JD, Lorusso R. The history and development of extracorporeal support. In: Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G, editors. Extracorporeal life support: the ELSO Red Book. 5th ed. Ann Arbor, MI: Extracorporeal Life Support Organization; 2017. pp. 1–15. [Google Scholar]
- 2.Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome): use of the Bramson membrane lung. N Engl J Med. 1972;286:629–634. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 3.Javidfar J, Brodie D, Wang D, Ibrahimiye AN, Yang J, Zwischenberger JB, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2011;91:1763–1768, discussion 1769. doi: 10.1016/j.athoracsur.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Palmér O, Palmér K, Hultman J, Broman M. Cannula design and recirculation during venovenous extracorporeal membrane oxygenation. ASAIO J. 2016;62:737–742. doi: 10.1097/MAT.0000000000000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biscotti M, Lee A, Basner RC, Agerstrand C, Abrams D, Brodie D, et al. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: a single-center experience. ASAIO J. 2014;60:635–642. doi: 10.1097/MAT.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 6.Madershahian N, Nagib R, Wippermann J, Strauch J, Wahlers T. A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation. J Card Surg. 2006;21:168–169. doi: 10.1111/j.1540-8191.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 7.Le Guyader A, Lacroix P, Ferrat P, Laskar M. Venous leg congestion treated with distal venous drainage during peripheral extracorporeal membrane oxygenation. Artif Organs. 2006;30:633–635. doi: 10.1111/j.1525-1594.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- 8.Skarsgard ED, Salt DR, Lee SK Extracorporeal Life Support Organization Registry. Venovenous extracorporeal membrane oxygenation in neonatal respiratory failure: does routine, cephalad jugular drainage improve outcome? J Pediatr Surg. 2004;39:672–676. doi: 10.1016/j.jpedsurg.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Avalli L, Maggioni E, Sangalli F, Favini G, Formica F, Fumagalli R. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J. 2011;57:38–40. doi: 10.1097/MAT.0b013e3181fe5d0b. [DOI] [PubMed] [Google Scholar]
- 10.Kim HE, Jung JW, Shin YR, Park HK, Park YH, Shin HJ. Left atrial decompression by percutaneous left atrial venting cannula insertion during venoarterial extracorporeal membrane oxygenation support. Korean J Thorac Cardiovasc Surg. 2016;49:203–206. doi: 10.5090/kjtcs.2016.49.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combes A, Pesenti A, Ranieri VM. Fifty years of research in ARDS: is extracorporeal circulation the future of acute respiratory distress syndrome management? Am J Respir Crit Care Med. 2017;195:1161–1170. doi: 10.1164/rccm.201701-0217CP. [DOI] [PubMed] [Google Scholar]