To the Editor:
Pyrazinamide (PZA), which is an analog of nicotinamide, is an important first-line drug used in the short-course treatment of tuberculosis. PZA is a prodrug devoid of significant antibacterial activity. It is metabolized into its active form, pyrazinoic acid, by the amidase activity of the Mycobacterium tuberculosis nicotinamidase/pyrazinamidase, encoded by the pncA gene. Mutations in pncA that prevent activation of the prodrug represent the major mechanism of PZA resistance in M. tuberculosis (1). This antibiotic plays a key role in shortening the duration of antituberculous treatment because of its activity against the persisting tubercle bacilli at acidic pH.
Current phenotypic testing for PZA drug susceptibility is problematic. Culture-based methods such as Wayne’s method are used as screening assays with confirmation of resistant strains via the BD BACTEC MGIT 960 system (Becton Dickinson) (2). Results obtained from phenotypic laboratory testing have poor reproducibility. Sequencing of the pncA gene to determine the presence of mutations may be a more reliable method for confirmation of phenotypic PZA resistance (3). International recommendations suggest continued usage of PZA irrespective of susceptibility results, particularly in the treatment of multidrug-resistant disease (4). This is despite the adverse effects associated with PZA treatment.
Case Report
In early 2017, a 42-year-old woman, originally from Vietnam, presented with right upper lobe pneumonia; she was diagnosed with pulmonary tuberculosis. Phenotypic drug susceptibility testing identified resistance to isoniazid, rifampicin, pyrazinamide, and ethambutol. Although drug susceptibility testing suggested the patient was phenotypically resistant to PZA, consistent with World Health Organization recommendations, PZA treatment was continued as part of a multidrug-resistant tuberculosis regimen. Amplicon sequencing identified a novel frameshift mutation in the pncA gene of M. tuberculosis (c.85_86insG). Given the uncertain impact of this mutation, we went on to consider whether computational analysis of protein structure (5) could provide insight into the potential efficacy of PZA.
Methods
We have developed an in silico mutational analysis platform that is able to characterize the molecular consequences of mutations on protein structure and function (5). This has been used to preemptively identify likely resistance mutations in drug targets (6, 7). Using these tools, we assessed the biophysical changes on mutation on the structure of PncA and drug activation.
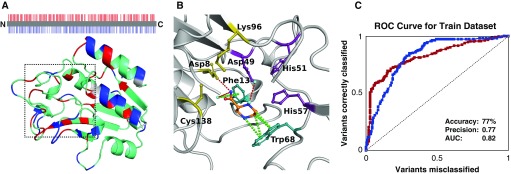
A list of 617 nonsynonymous single-nucleotide variants (nsSNVs) of pncA was obtained from the GMTV (Genome-wide Mycobacterium tuberculosis Variation) Database Project, Tuberculosis Drug Resistance Mutation Database, and saturation mutagenesis (8). Mapping nsSNVs associated with resistance onto the crystal structure of PncA revealed that they were distributed throughout the entire protein structure (Figure 1A), complicating resistance inference from sequence analysis. The structural and functional effects of these mutations were assessed using our graph-based signature pipeline (5). This provided insight into how the curated nsSNVs altered protein folding, stability, conformation, and PZA-binding affinity. This information was used to train a Random Forest (machine-learning algorithm) binary classifier, using the Weka toolkit. Random Forest is an ensemble-learning robust classification algorithm, in which multiple decision trees are included over a random subset of features and decide the output via majority voting. The model was trained by 10-fold cross-validation and performance evaluated by area under the receiver operating characteristic curve, precision, and accuracy. Further validation of the models was performed using two subsets of 93 mutations, which were nonredundant at the position-level mutations in the training set. Analysis of the final model revealed a set of structural features that distinguished between susceptible and resistant pncA point mutations.
Figure 1.
Identification of resistant and susceptible missense mutations in pncA. (A) The protein sequence and structure of PncA is colored by whether resistant (red) or susceptible (blue) variants have been observed at that location. Highlighting the difficulty of genomic analysis of pncA, both resistant and susceptible variants have been observed across many residue positions (cyan). The catalytic site in which pyrazinamide (PZA) was docked is located inside the dashed box. (B) The key molecular interactions between PZA (orange segments) and the catalytic triad (yellow), substrate-binding site (teal), and iron center (purple). Hydrogen bonds are shown as red dashes, and π interactions as green dashes. (C) The ROC curve shows that, using the structural and functional consequences of the variants, we were able to accurately identify resistant (red) and susceptible (blue) variants. AUC = area under the curve; ROC = receiver operating characteristic.
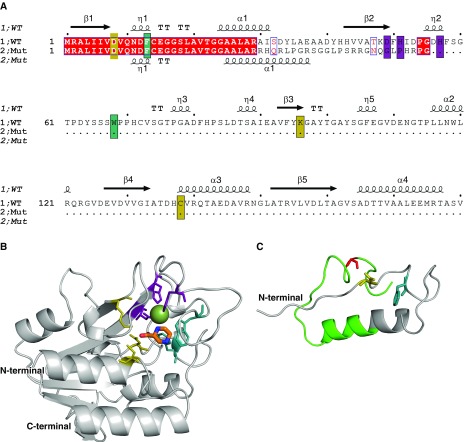
Building on this structural analysis, the functional consequence of the novel clinical frameshift mutation was analyzed in the context of the protein structure. The experimental crystal structure of holo-wild-type PncA (PDB ID: 3PL1) (9) was minimized in Prime, and PZA docked into the active site using Glide, two exclusive packages of the comprehensive homology modeling software Schrӧdinger Suites. The docking revealed that PZA formed key interactions within the pocket, including with the catalytic triad (Asp8, Lys96, and Cys138), substrate-binding residues (Trp68 and Phe13), and the iron center (Asp49, His51, His57, and Fe2+) (10). The wild-type and mutant protein sequences were manually aligned and displayed with ESPript 3.0 (Figure 2A), and the structure of the mutant (Figure 2C) was generated by homology and ab initio modeling, using the experimental structure of the wild type (Figure 2B) (Schrӧdinger Suites).
Figure 2.
Structural analysis of a novel pncA frameshift mutation. (A) The sequence alignment between the wild-type and mutant protein sequences shows that only the first 29 residues are conserved (red), and that the frameshift leads to the introduction of a premature stop codon. The catalytic triad, substrate-binding site, and iron center are highlighted in yellow, teal, and purple, respectively. The secondary structure of the wild-type PncA protein is shown above the sequence (β = β sheet, α = α helix, and η = loop). (B) The structure of the wild-type PncA protein is represented as a ribbon (gray), bound to the drug pyrazinamide (in orange segments). The Fe2+ ion is shown as a green sphere. (C) The modeled structure of the mutant PncA protein highlights that most of the catalytic site and structure of the wild-type protein is absent in the mutant. The region not conserved with the wild-type sequence is shown in green. Both wild-type and mutant structures are shown from the same perspective. Mut = mutant; WT = wild type.
Results
Using the structural and biophysical effects of the mutations on the protein structure, we were able to classify mutations as either susceptible or resistant with an accuracy of 77% (Figure 1C). This approach performed equally well in the identification of either class, correctly classifying all mutations previously associated with conferring PZA resistance at high confidence, and mutations not involved in PZA resistance (100% accuracy) (11). Strain-specific differences in variants with conflicting experimental data (12) could be detected by our tool, using homology models of the corresponding strain’s PncA protein.
Analysis of our model revealed that PncA-resistant mutations were associated with large changes in protein folding and stability (mCSM-Stability scores ≥ −1.72 kcal/mol) (P < 0.0001) or located in close proximity to the catalytic triad and substrate-binding site (<8.54 Å) (P < 0.0001). Therefore, these freely available biophysical measurements could provide useful information to help guide genomic analysis of novel pncA variants.
We next considered the patient’s frameshift mutation in light of these structural insights. As shown in Figure 2, the frameshift mutation resulted in the generation of a truncated and incomplete protein that lacked the active site pocket, including most of the catalytic residues and iron coordination residues necessary for activity. This strongly suggests that the pncA c.85_86insG frameshift mutation would lead to a total loss of catalytic activity of the protein, and hence PZA treatment would be completely ineffective in this case, as the mutant PncA could not activate the prodrug. This is reflected in the structure of the mutant protein, which is incomplete and would lack any activity (Figure 2). This result was consistent with phenotypic testing, and accordingly, pyrazinamide treatment was ceased.
Discussion
This case study demonstrates the power of using structural information to quantitatively evaluate novel variants in real time, providing invaluable insight to help guide therapy. Although existing recommendations may suggest continuing treatment of multidrug-resistant tuberculosis with pyrazinamide irrespective of phenotype testing, our approach suggests that using structural information to guide analysis of genomic sequencing may offer useful tools for clinicians to consider. These structural insights also assist in informing the mechanisms for drug activity and the development of resistance. Our approach is not limited only to analysis of variants in pncA but could be applied to any protein associated with resistance for infectious and noninfectious disease treatment.
Footnotes
Supported by a Melbourne Research Scholarship from the University of Melbourne (M.K.). D.B.A was funded by a Newton Fund RCUK-CONFAP Grant awarded by the Medical Research Council and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (MR/M026302/1), the Jack Brockhoff Foundation (JBF 4186, 2016), and a C. J. Martin Research Fellowship from the National Health and Medical Research Council of Australia (APP1072476).
Author Contributions: M.K., J.T.D., and D.B.A. were involved in the study design, execution, data analysis, and writing of all versions of this work. M.G., J.A.M.F., T.P.S., P.D.R.J., and N.E.H. were involved in clinical and laboratory aspects of investigation. All authors contributed to preparation of this manuscript and approve the final version.
Originally Published in Press as DOI: 10.1164/rccm.201712-2572LE on April 25, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Zhang Y, Shi W, Zhang W, Mitchison D. Mechanisms of pyrazinamide action and resistance. Microbiol Spectr. 2013;2:1–12. doi: 10.1128/microbiolspec.MGM2-0023-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui Z, Wang J, Lu J, Huang X, Zheng R, Hu Z. Evaluation of methods for testing the susceptibility of clinical Mycobacterium tuberculosis isolates to pyrazinamide. J Clin Microbiol. 2013;51:1374–1380. doi: 10.1128/JCM.03197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KC, Yew WW, Zhang Y. Pyrazinamide susceptibility testing in Mycobacterium tuberculosis: a systematic review with meta-analyses. Antimicrob Agents Chemother. 2011;55:4499–4505. doi: 10.1128/AAC.00630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis: 2016 update. Geneva, Switzerland: World Health Organization; 2016.
- 5.Pires DE, Chen J, Blundell TL, Ascher DB. In silico functional dissection of saturation mutagenesis: Interpreting the relationship between phenotypes and changes in protein stability, interactions and activity. Sci Rep. 2016;6:19848. doi: 10.1038/srep19848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park Y, Pacitto A, Bayliss T, Cleghorn LA, Wang Z, Hartman T, et al. Essential but not vulnerable: indazole sulfonamides targeting inosine monophosphate dehydrogenase as potential leads against Mycobacterium tuberculosis. ACS Infect Dis. 2017;3:18–33. doi: 10.1021/acsinfecdis.6b00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh V, Donini S, Pacitto A, Sala C, Hartkoorn RC, Dhar N, et al. The inosine monophosphate dehydrogenase, GuaB2, is a vulnerable new bactericidal drug target for tuberculosis. ACS Infect Dis. 2017;3:5–17. doi: 10.1021/acsinfecdis.6b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadon AN, Maharaj K, Adamson JH, Lai YP, Sacchettini JC, Ioerger TR, et al. A comprehensive characterization of PncA polymorphisms that confer resistance to pyrazinamide. Nat Commun. 2017;8:588. doi: 10.1038/s41467-017-00721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrella S, Gelus-Ziental N, Maudry A, Laurans C, Boudjelloul R, Sougakoff W. Crystal structure of the pyrazinamidase of Mycobacterium tuberculosis: insights into natural and acquired resistance to pyrazinamide. PLoS One. 2011;6:e15785. doi: 10.1371/journal.pone.0015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jubb HC, Higueruelo AP, Ochoa-Montaño B, Pitt WR, Ascher DB, Blundell TL. Arpeggio: a web server for calculating and visualising interatomic interactions in protein structures. J Mol Biol. 2017;429:365–371. doi: 10.1016/j.jmb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miotto P, Cabibbe AM, Feuerriegel S, Casali N, Drobniewski F, Rodionova Y, et al. Mycobacterium tuberculosis pyrazinamide resistance determinants: a multicenter study. MBio. 2014;5:e01819-14. doi: 10.1128/mBio.01819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baddam R, Kumar N, Wieler LH, Lankapalli AK, Ahmed N, Peacock SJ, et al. Analysis of mutations in pncA reveals non-overlapping patterns among various lineages of Mycobacterium tuberculosis. Sci Rep. 2018;8:4628. doi: 10.1038/s41598-018-22883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]