Abstract
Case series
Patient: Female, 37 • Female, 2
Final Diagnosis: Mycobacterium tuberculosis
Symptoms: Positive PPD
Medication: —
Clinical Procedure: —
Specialty: Pediatrics and Neonatology
Objective:
Challenging differential diagnosis
Background:
In 2017, in New York City (NYC), 86% of the cases of tuberculosis (TB) occurred in patients who were born outside the United States (US). This case report illustrates the importance of the use of the tuberculin skin test (TST), and other tests for TB infection (TTBI), in screening high-risk groups, the challenges of diagnosing TB in young children, and highlights the importance of preventing a delay in the diagnosis of TB in family members.
Case Report:
Following a routine TST in an asymptomatic 10-year-old girl, a diagnosis of TB was made, which was confirmed on chest X-ray (CXR) and by the presence of acid-fast bacilli (AFB) in the sputum. Her family had emigrated from China to NYC ten years previously. All the family was screened using the TST, which was positive in her 2-year-old sister and her 37-year-old pregnant mother, and pulmonary TB was confirmed on CXR and by AFBs in the sputum. All three family members and the newborn baby were treated according to current guidelines, with a good clinical outcome.
Conclusions:
This case report raises awareness about the lack of symptoms in childhood TB and the importance of screening high-risk patients in an urban immigrant population. In children under 5 years of age, a diagnosis of TB can indicate a sentinel event, suggesting a potential undiagnosed or untreated source case, which is usually an adult family member. This report highlights the challenges of diagnosing TB in children, who may be asymptomatic with negative laboratory findings.
MeSH Keywords: Latent Tuberculosis; Pediatrics; Pregnancy Complications, Infectious; Tuberculin
Background
Tuberculosis (TB) is an airborne disease caused by infection with Mycobacterium tuberculosis that affects 10.4 million people annually worldwide, including 9,500 people living in the United States (US). The mode of transmission of pulmonary TB is through inhalation of airborne organisms, transmitted by sneezing or coughing from a person with active TB [1]. The global fatality rates for TB range from between 7–35%, with almost 1.8 million deaths occurring in 2015 [1]. Risk factors for mortality from TB include non-infective co-morbidities, co-infection with human immunodeficiency virus (HIV), and multidrug-resistant tuberculosis (MDRTB) [1]. Tests for TB include the tuberculin skin test (TST), the detection of acid-fast bacilli (AFB) in sputum or tissues, and molecular methods of identification of M. tuberculosis, which are collectively now known as tests for TB infection (TTBI).
In 2017, New York City (NYC) experienced the largest increase in cases of TB since 1992, with a 10% increase in cases from the previous year [2]. In 2017, the rate of TB in NYC was 7.5 per 100,000 persons, which was twice the US national rate of 2.8 per 100,000 persons [2]. The incidence of MDRTB in NYC also increased from 11% in 2016 to 14% in 2017 [2]. Of the cases of TB reported in NYC in 2017, 86% of cases occurred in people who were born outside the USA and resident in the USA [2]. According to the Center for Disease Control and Prevention (CDC), the national incidence of TB among people born outside the USA is 15 times higher than among the US-born population [3]. Between 2013–2015, two-thirds of newly diagnosed cases of TB were in people who had had been born outside the US, with people having emigrated from Asia having the highest rate (28.2 cases per 100,000 persons), and Caucasian immigrants having the lowest rate (0.5 cases per 100,000) [3].
The American Thoracic Society (ATS), the CDC, and the Infectious Diseases Society of America (IDSA) recommends screening high-risk individuals and their contacts, including family and household contacts, and children less than 5 years of age, with the goal of beginning treatment before the TB infection progresses to active disease [4]. Children less than 5 years of age who are infected with TB may indicate a sentinel or source case, and their diagnosis should prompt testing for TB in family members who are close contacts at high risk of infection due to the duration of contact to with index case [4]. Therefore, the screening of household members and other close contacts is highly recommended in order to diagnose new cases of TB as early as possible and to prevent further transmission [4].
Delay in the diagnosis of TB also increases the risk of spread of infection within families, communities, and populations. According to World Health Organization (WHO), globally, an estimated three million people remain undiagnosed for TB and untreated by health systems [5]. A ‘missed’ diagnosis of TB is defined as the gap between the estimated number of people who become ill with TB in a year and the number of people who were notified to national TB programs [5]. Populations that are particularly vulnerable to being ‘missed’ for a diagnosis of TB include pregnant women, due to the non-specific symptoms related to the physiological changes that occur in pregnancy, and children because they can present with non-specific signs and symptoms, including extrapulmonary manifestations of TB [6]. Even if they present with typical signs and symptoms, only 40% of children with TB have positive laboratory tests for TB [7]. Missed cases of TB may also be attributed to disparities in health care and disease prevention due to race, disability, access to resources, and varying quality of care. These ‘missed’ cases have significant clinical consequences, including the continued spread of TB infection, a higher burden of illness, and increased mortality [5].
This case report illustrates the importance of the use of the tuberculin skin test (TST), and other TTBIs in screening high-risk groups, the challenges of diagnosing TB in young children, and highlights the importance of preventing a delay in the diagnosis of TB in family members. Also, the report highlights the importance of screening and diagnosing TB in family members that include a pregnant mother with active disease.
Case Reports
As part of routine medical screening for summer camp enrolment, a 10-year-old girl with no significant past medical history was found to have a positive tuberculin skin test (TST) (11 mm) by her primary care physician. The 10-year-old girl had no history of a cough, fever, weight loss, or night sweats. She had a chest X-Ray (CXR) which showed right middle lobe pneumonia and she was prescribed a course of azithromycin for a presumed atypical pneumonia. She was also started on isoniazid by her primary care physician, for a presumed diagnosis of latent tuberculosis infection (LTBI), but without additional workup for a diagnosis of TB.
Subsequently, a tuberculin skin test (TST) was requested by the primary care physician for the 2-year-old sister of the 10-year-old girl. The 2-year-old child had no symptoms of a cough, night sweats, weight loss, or fever. The 2-year-old sister had a positive TST test with 10 mm of induration and was referred to a pediatric pulmonologist who initially interpreted her CXR as unremarkable. She was treated with isoniazid for LTBI and referred to a pediatric infectious disease specialist. Of note, neither of the two sisters had ever traveled outside the US, but both children were living with parents who had emigrated to the US from China, 10 years previously. Also, both children were frequently visited by their grandparents, who still resided in China.
The 2-year-old girl was accompanied to the pediatric infectious disease appointments by her mother, a 37-year-old woman who was 19 weeks pregnant (G3 P2). During the first pediatric outpatient visit, the mother was noted to be coughing. On questioning, the mother reported a worsening productive cough for the previous six months, night sweats, and a ten-pound weight loss during the preceding three months. Of note, the mother had a non-reactive TST and a negative CXR two months previously, and her symptoms had been treated with antihistamines, for a presumed post-nasal drip, by her primary care physician, a course of unknown antibiotics for had been prescribed for her cough, and ranitidine had been prescribed for a presumed diagnosis of gastric reflux. Because of the mother’s symptoms of a cough and the history of positive TST in her two daughters, a TST was requested by her primary care physician, which was positive with a reaction measuring 30 mm in diameter. She was then referred to a pulmonolo-gist and she was started on a five-day course of azithromycin for presumed pneumonia (Figure 1), and although she was referred to the emergency room for further workup, she elected not to go to the emergency room at that time.
Figure 1.
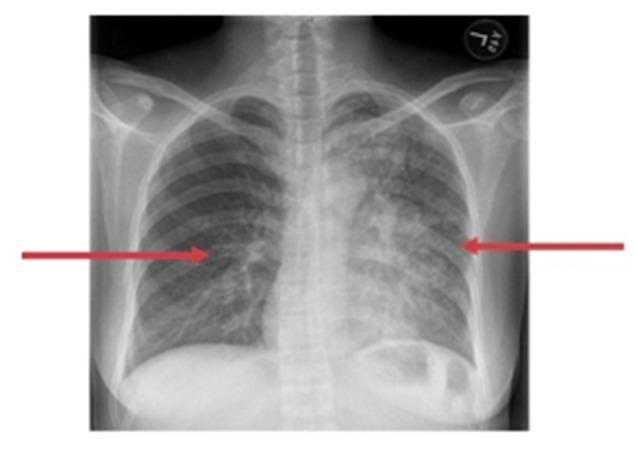
Chest X-ray of a 37-year-old woman. The chest X-ray (CXR) shows reticular nodular infiltrates throughout the lung.
At the pediatric infectious disease appointment, both the mother and her 2-year-old daughter were admitted directly to the hospital for further evaluation and treatment of tuberculosis. All smears and cultures from three aspirates were negative for acid-fast bacilli (AFB). On re-evaluation of the previous CXR of the 2-year-old, right hilar lymphadenopathy was noted (Figure 2). During hospitalization, the 2-year-old child began treatment with isoniazid, 125 mg once daily, rifampin 125 mg once daily, pyrazinamide 250 mg once daily, pyridoxine 50 mg once daily.
Figure 2.
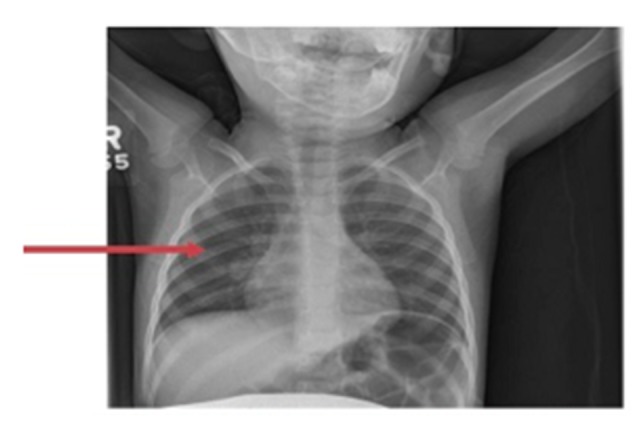
Chest X-ray of a 2-year-old female child. The chest X-ray (CXR) shows a prominent right hilum and right hilar lymphadenopathy.
The initial examination of a sputum sample from the mother showed numerous AFBs. She was treated with isoniazid 300 mg four times daily, ethambutol 400 mg once daily, and rifampin 300 mg once daily. She remained in isolation for 27 days until three consecutive sputum smears were negative for AFBs, as recommended by the New York City Department of Health (NYC DOH) [2].
Because the clinical history, CXR and laboratory findings were consistent with a diagnosis of active TB in the mother, and a diagnosis of probable TB was made the 2-year-old daughter, the 10-year-old daughter was recalled and admitted to the pediatric inpatient ward and placed under isolation. Her repeat CXR was unremarkable, and three sputum specimens were negative for AFB. However, one of the cultures grew acid-fast bacilli two weeks later (non-M. tuberculosis complex) as detected by polymerase chain reaction (PCR) testing, which is recommended by current guidelines, including those of the NYC DOH, as being an indication for treatment as pulmonary TB [2]. Even though the right middle lobe infiltrates had resolved by the time of her hospital admission, and it was possible that the 10-year-old girl could have had atypical pneumonia and concurrent LTBI, she was started on treatment for TB infection, including isoniazid, 300 mg once daily, ethambutol 400 mg once daily, rifampin 450 mg once daily, pyrazin-amide 750 mg once daily, and pyridoxine 50 mg once daily.
After 27 days, the mother was cleared for active TB and was discharged home but continued treatment with isoniazid, ethambutol, and rifampin for the following two months, followed by continued on isoniazid and rifampin for the remaining seven months, as recommended by the CDC guidelines for the treatment TB in pregnant women [4].
On discharge from the hospital, all three patients, the 37-year-old mother and her 2-year-old and 10-year-old daughters, continued treatment through the NYC DOH Direct Observed Therapy Program for pulmonary TB [2]. Both the 2-year-old and 10-year-old daughters were sent home on isoniazid, rifampin, and pyrazinamide for two months, followed by isoniazid and rifampin for a further four months. The mother gave birth to a healthy infant who was treated with isoniazid at birth. A TST was performed at 3 months-of-age, and because the result was non-reactive, isoniazid treatment of the newborn infant was discontinued. At follow-up at age 1 year and 6 months, the infant was developing normally. All four patients did well and recovered fully. Follow up CXRs were unremarkable for all patients.
A contact investigation was carried out for family members who lived with the mother and the children. The father and grandparents had positive TSTs but unremarkable CXRs and were treated for LTBI. There were no more cases of active TB detected among other family members or contacts.
Discussion
In 2017, the national incidence of tuberculosis (TB) in the United States (US) was reported to be decreasing, apart from in New York, California, Florida, and Texas, with these four states accounting for half of the reported cases of TB in that year [2]. According to the 2018 advisory recommendations issued by the New York City Department of Health (NYC DOH) Health Alert Network (HAN), in 2017 the city experienced the largest increase in cases of TB since 1992, and cases of multidrug-resistant TB (MDRTB) also increased [2]. The majority of cases of TB in NYC (86%) occurred in people born outside the USA, with the rate of TB among people born outside the USA and living in the USA being 15 times greater than the incidence of TB among people who were born and lived in the USA [2]. Urban populations may have a higher risk of TB due to the large immigrant populations that exist in cities. Medical practitioners and health service workers in areas with high immigrant populations should be aware of the increased incidence of TB in people born outside the USA and be prepared to initiate screening for TB in populations at high risk.
In 2014, the Centers for Disease Control and Prevention (CDC) recommended the implementation of new TB screening requirements for the 450,000 immigrants who arrive in the US annually [9]. The recommendations include that before entering the US, screening for TB should be performed [9,10]. The elimination of TB in the US would require that these recommendations are followed, and also require that increasing effort is made in the US to detect and treat latent TB infection (LTBI), and to improve detection and treatment of TB within the US and globally [8–10].
In 2017, the American Thoracic Society (ATS), the Infectious Diseases Society of America (ISDA) and the CDC issued clinical practice guidelines for the diagnosis of TB in adults and children that included the use of targeted tuberculin skin tests (TSTs) and the treatment of LTBI in immigrants [4]. These recommendations vary among different age groups. The CDC recommends that for applicants who wish to enter the US and who are aged 15 years or older, a medical history, physical examination, and chest X-ray (CXR) are required, and if the individual has symptoms, abnormal physical examination or CXR, or is HIV-positive, then TST is recommended [4,9,10]. Applicants aged between 2–14 years must also provide a medical history and undergo a physical examination. Applicants residing in countries with a WHO estimated TB incidence rate of ≥20 cases per 100,000 people, are mandated to have a TST. If the TST induration diameter is ≥10 mm, a CXR must be done [4,9,10]. Applicants for immigration who are <2 years of age, and living in countries with a World Health Organization (WHO)-estimated tuberculosis incidence rate of ≥20 cases per 100,000 require a medical history and physical examination [4,9,10]. If these applicants show symptoms of TB, or are HIV-positive, they must have a TST, a CXR, and provide three sputum specimens that will be examined for acid-fast bacilli (AFB) [10].
Interferon-gamma release assays (IGRAs) are now often used instead of TST [11]. IGRAs are whole-blood tests that can help diagnose immune reactivity to TB infection [11]. The advantages of IGRAs are that they require a single patient visit and results are available in 24 hours [11]. However, limitations include timely collection of blood samples and limited data on the use of IGRA in children less than 5 years of age [11].
Infection with TB in immunocompetent adults differs from that in children, as 90% of adults will control further replication of the Mycobacterium bacilli, which can enter a latent phase; the remaining 10% of adults will develop TB pneumonia presenting with hilar adenopathy and/or disease at more distant sites, which may include cervical lymphadenopathy, meningitis, pericarditis, or miliary dissemination [12]. In contrast, children less than 5 years of age are at an increased risk of developing active TB [13]. Older children, including adolescents, have a clinical course similar to that of adults. In neonates, congenital TB is more likely to disseminate, with high mortality rates [14,15]. Children may not show symptoms of fever and weight loss, which are usually classically seen in adults. A diagnosis of TB in children is often made based on a positive TST, a positive CXR, and evidence of contact with cases of infectious TB [15]. Only 40% of children with TB will have positive laboratory findings [15].
Contact investigations should be initiated for active TB, and the highest priority should be given to contacts who have pulmonary, laryngeal or pleural disease [4]. Once contact investigations have begun, contacts are prioritized in order to determine which contacts should be investigated and in what order, and contact investigations should occur immediately and in person (within 24 hours) after an index case of TB is identified [4]. For all contacts who are symptomatic, a full evaluation of TB should occur immediately [4].
For index patients with pulmonary, laryngeal, or pleural disease and a positive test for AFB and/or positive nucleic acid amplification tests, the high priority contacts include household contacts, contacts aged <5 years old, contacts with medical risk factors, contacts with exposure during a medical procedure (bronchoscopy, sputum induction, or autopsy), and contacts with exposure in family settings or of prolonged duration [4]. Medium priority contacts are those aged between 5–15 years who do not fall into the high priority contact category [4].
For children aged <5 years who are asymptomatic, a CXR should be performed, which if abnormal will lead to a full evaluation [4]. If the CXR is normal, and a TST has an induration diameter <5 mm, the patient is treated for LTBI. If the TST induration diameter is >5 mm, then the child is treated for LTBI until the re-testing is non-reactive at 8 weeks. For immuno-compromised contacts, the evaluation is the same as for children <5 years, except that they may be treated for LTBI even if the TST induration diameter is <5 mm, since this may represent a false-negative result [4].
For all other contacts who are >5 years of age, the evaluation involves a TST at initial screening [4]. If the TST induration diameter at initial presentation and at between 8–10 weeks after the last exposure is <5 mm, then a CXR is not needed. If the TST induration diameter is >5 mm, then a CXR is obtained, which if abnormal necessitates a full evaluation for TB, and if the CXR is normal, the patient is treated for LTBI [4].
Contact investigations are generally not needed for young children since they are not considered infectious unless they have pulmonary forms of disease as reported in adults involving the larynx or cavitating lesions [4,15]. However children <5 years old who are diagnosed with active TB are considered to represent a sentinel event and a source-case investigation is carried out as this represents a new infection. For children with LTBI, a source contact investigation is recommended if the child is <2 years old, since it represents a recent infection [4]. Also, genetic factors have been implicated for host susceptibility to TB, as there is a suggestion that TB may have a genetic predisposition, based on twin studies [16]. These findings reinforce the importance of source case investigation, especially of household contacts in patients with active TB [18].
Currently, routine prenatal care does not include testing for TB [18]. Physicians responsible for the care of pregnant women should routinely conduct TST testing in high-risk women, including relatives from a country where TB is endemic, immigrants from an endemic country, or women with exposure to relatives or contacts who have spent time in prison [18]. TB risk assessment questionnaires may aid in the screening and identification of high-risk groups [19]. Ozuah et al., conducted a prospective study among 2,920 children in South Bronx, New York, to determine the validity of the New York City Department of Health (NYC DOH) risk-assessment questionnaire for identifying children who should receive a TST; the screening tool had a negative predictive value of 99.8%, and positive predictive value of 5.4% [19]. There is no evidence that the immunosuppression that occurs in pregnancy leads to a false-negative TST result [20]. However, having a recent TB infection during pregnancy may lead to false-negative TST result [20]. This false-negative TST associated with TB infection in early pregnancy was demonstrated in the mother with TB described in this case report.
Conclusions
This report has described the presentation, diagnosis, and management of tuberculosis (TB) in a 10-year-old girl, her 2-year-old sister, and their pregnant mother, in a family that were immigrants from China to New York City (NYC) in the United States (US). This case report raises awareness about the lack of symptoms in childhood TB and the importance of screening high-risk patients in an urban immigrant population. In children under 5 years of age, a diagnosis of TB can indicate a sentinel event, suggesting a potential undiagnosed or untreated source case, which is usually an adult family member. This report highlights the challenges of diagnosing TB in children, who may be asymptomatic with negative laboratory findings. The presentation of these cases highlights the importance of adhering to the current guidelines for testing for TB, including those from the Centers for Disease Control and Prevention (CDC), regarding the investigation of individuals who are at high risk from TB, as well as testing their contacts and family members. US urban settings, with high immigrant populations, have a higher incidence of TB compared with the national average. In NYC, physicians should maintain a high level of suspicion for the possibility of a diagnosis of TB in children and pregnant women, and they should be aware that children with TB who are under 5 years of age can represent a sentinel case that should alert clinicians to the possibility of TB in other family members. Screening mothers and contact family members for TB could lead to a reduction in secondary cases in children, and reduce morbidity and mortality from TB in this vulnerable population.
Footnotes
Conflict of interest
None.
References:
- 1.World Health Organization (WHO) Tuberculosis. 2018. Feb 16, Available from: URL: http://www.who.int/en/news-room/fact-sheets/detail/tuberculosis.
- 2.Bassett MT, New York City Department of Health and Mental Hygiene. Health Alert Network (HAN). 2018 Advisory #5: Largest Increase in Tuberculosis Cases Since 1992. 2018. Mar 26, Available from: URL: https://www1.nyc.gov/assets/doh/downloads/pdf/han/advisory/2018/advisory-5-tuberculosis.pdf.
- 3.Salinas J, Mindra G, Haddad M, et al. Leveling of tuberculosis incidence – United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65(11):273–78. doi: 10.15585/mmwr.mm6511a2. [DOI] [PubMed] [Google Scholar]
- 4.Lewinsohn D, Leonard M, Philip A, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):1–33. doi: 10.1093/cid/ciw778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Gains in tuberculosis control at risk due to 3 million missed patients and drug resistance. 2014. May 26, Available from: URL: http://www.who.int/mediacentre/news/releases/2013/tuberculosis-report-2013/en/ [PubMed]
- 6.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8(5):636–47. [PubMed] [Google Scholar]
- 7.Lobato MN, Mohle-Boetani JC, Royce SE. Missed opportunities for preventing tuberculosis among children younger than five years of age. Pediatrics. 2000;106(6):E75. doi: 10.1542/peds.106.6.e75. [DOI] [PubMed] [Google Scholar]
- 8.Shanmugalakshmi S, Pitchappan RM. Genetic basis of tuberculosis susceptibility in India. Indian J Pediatr. 2002;69:S25–28. [PubMed] [Google Scholar]
- 9.Posey DL, Naughton MP, Willacy EA, et al. Centers for Disease Control and Prevention (CDC) Implementation of New TB Screening Requirements for U.S.-Bound Immigrants and Refugees – 2007–2014. Morb Mortal Wkly Rep. 2014. Available from: URL: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6311a3.htm. [PMC free article] [PubMed]
- 10.Centers for Disease Control and Prevention (CDC) Disease during the Domestic Medical Examination for Newly Arrived Refugees. Mar 29, 2012. Available from: URL: https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/tuberculosis-guidelines.html.
- 11.Mazurek GH, Jereb J, Vernon A, et al. IGRA Expert Committee. Centers for Disease Control and Prevention (CDC) Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 12.Hunter RL. Pathology of post primary tuberculosis of the lung: An illustrated critical review. Tuberculosis (Edinburgh) 2011;91(6):497–509. doi: 10.1016/j.tube.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013:828939. doi: 10.1155/2013/828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loto OM, Awowole I. Tuberculosis in pregnancy: A review. J Pregnancy. 2012;2012:379271. doi: 10.1155/2012/379271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz AT, Starke JR. Clinical manifestations of tuberculosis in children. Paediatr Respir Rev. 2007;8(2):107–17. doi: 10.1016/j.prrv.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Curtis AB, Ridzon R, Vogel R, et al. Extensive transmission of Mycobacterium tuberculosis from a child. N Engl J Med. 1999;341(20):1491–95. doi: 10.1056/NEJM199911113412002. [DOI] [PubMed] [Google Scholar]
- 17.Comstock GW. Tuberculosis in twins. Am Rev Respir Dis. 1978;117(4):621–24. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Technical Instructions for Panel Physicians and Civil Surgeons. Tuberculosis Screening and Treatment Technical Instructions (TB TIs) using Cultures and Directly Observed Therapy (DOT) for Panel Physicians. Oct 1, 2013. Available from: URL: https://www.cdc.gov/immigrantrefugeehealth/exams/ti/panel/tuberculosis-panel-technical-instructions.html.
- 19.New York City Department of Health (NYC DOH) and Mental Hygiene Tuberculosis Clinical Policies and Protocols. 4th Edition. Section X. Testing for Latent Tuberculosis Infection. Available from: URL: https://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb-manual-section10.pdf.
- 20.Ozuah PO, Ozuah TP, Stein RE, et al. Evaluation of a risk assessment questionnaire used to target tuberculin skin testing in children. JAMA. 2001;285(4):451–53. doi: 10.1001/jama.285.4.451. [DOI] [PubMed] [Google Scholar]


