Abstract
Introduction
Prognosis in patients with cancer is influenced by underlying tumour biology and also the host inflammatory response to the disease. There is limited evidence to suggest that an elevated neutrophil-lymphocyte ratio (NLR) predicts a poorer prognosis in patients undergoing nephrectomy for renal cell carcinoma (RCC). The aim of this paper is to investigate if patients undergoing nephrectomy for RCC with NLR ≤4 have a better overall and recurrence-free survival than patients with NLR >4.
Methods
All patients who underwent nephrectomy at a single centre between January 1, 2011 and December 31, 2014 were identified. Patients were included if postoperative histology demonstrated RCC and if preoperative NLR was available. Patients were excluded if nephrectomy was not curative intent (i.e., cytoreductive nephrectomy), if primary tumour was graded to be T3b–4 disease, if there was presence of nodal or metastatic disease on preoperative staging, or if adequate followup notes were not available. Primary and secondary outcomes were overall survival and recurrence-free survival, respectively.
Results
A total of 154 patients were included in analysis of overall survival; 146 patients were included in analysis of recurrence-free survival. Patients with NLR ≤4 had a much better overall survival than patients with NLR >4 (95% vs. 78%; p=0.0219). Patients with NLR >4 also had higher rates of recurrence (p=0.0218).
Conclusions
NLR may be a useful tool in identifying patients who may benefit from more frequent surveillance in the early postoperative period and may allow clinicians to offer surveillance schemes tailored to the individual patient.
Introduction
The incidence of renal cancer has increased over the past 35 years from 7 per 100 000 to 20 per 100 000;1 this increase in incidence is expected to continue to 32 per 100 000 by 2035.2 Renal cancer currently has the seventh highest incidence of all cancers in the U.K.2 Renal cell carcinoma (RCC) accounts for approximately 85% of all renal malignancies.3
Over three-quarters of patients will have localized disease at time of diagnosis4,5 and may be candidates for nephrectomy with curative intent. Despite resection of localized disease, a significant proportion of patients will subsequently develop metastatic disease. This has been reported to occur in 10–30% patients,4,6–8 and so the majority of patients undergo long-term postoperative radiological surveillance.
The European Association of Urology currently recommends that surveillance should be based on an individual patient’s risk of recurrence; however, there is insufficient evidence to recommend any one surveillance scheme to be optimal.9 As there is currently no gold-standard surveillance protocol, prognostic indicators based on patient factors may contribute to determining a patient’s risk of recurrence and, therefore guide intensity of postoperative surveillance.
The neutrophil-lymphocyte ratio (NLR) has been demonstrated to predict prognosis in patients undergoing treatment for a variety of malignancies.10,11 The reason biomarkers that measure for inflammatory processes may be considered a prognostic tool in patients with malignancy is that prognosis for these patients depends not only on underlying tumour biology, but also the patient’s immune response to this disease process. An elevated NLR usually corresponds with a poorer prognosis; this is seen as a reflection of an inflammatory response to the underlying malignant process.
NLR has been investigated as a prognostic indicator in RCC, however, the evidence is limited. Several studies investigating the role of NLR in patients undergoing treatment for metastatic RCC demonstrated patients with elevated NLR to have poorer prognosis.3,12 The role of NLR in predicting prognosis in patients undergoing nephrectomy for RCC was investigated in a recent review13 and only four studies were identified. Only two of these studies reported on overall survival (OS), but both demonstrated elevated NLR to predict poorer prognosis.14,15 Cancer-specific survival and rate of recurrence were also reported, but a statistically significant difference was not demonstrated in any study.14–16 Many different cutoff values have been described when comparing elevated with non-elevated NLR, but there does not appear to be an optimum value.
The aim of this paper is to investigate the role of NLR in predicting OS and recurrence-free survival (RFS) in patients undergoing nephrectomy for localized RCC.
Methods
A retrospective electronic case note review was conducted on all patients who underwent nephrectomy at a single centre between January 1, 2011 and December 31, 2014. All patients had full staging imaging preoperatively in the form of computed tomography (CT) of chest, abdomen, and pelvis, and disease was recorded in accordance with TNM classification. Patients identified were included in this study if inclusion and exclusion criteria were met.
Inclusion criteria included:
– Postoperative histology demonstrated RCC
– Disease was localized (T1–T3a)
– Preoperative NLR was available; this was based on blood samples taken at a preoperative assessment clinic when patients were well (i.e., no concurrent acute illness). Where blood tests were abnormal and this was thought to be due to a transient acute illness, blood tests were repeated preoperatively during the same admission as elective surgery.
– Adequate followup notes available
Exclusion criteria included:
– Nephrectomy for benign disease
– Malignancy was not RCC
– Nephrectomy was not with curative intent (i.e., cytoreductive nephrectomy)
– Locally advanced disease (T3b–T4)
– Presence of nodal or metastatic disease
– Other significant pathology that would impact survival
The primary outcome studied was OS, defined as time from nephrectomy to time of death (or July 1, 2016 if death did not occur). Secondary outcome was RFS, defined as time from nephrectomy to radiological diagnosis of recurrence, or most recent radiological investigation excluding recurrence. NLR ≤4 was taken as normal and NLR >4 was considered to be elevated. Kaplan-Meier curves were generated for each cohort and OS and RFS were compared using log rank test.
Results
A total of 253 patients were identified during the study period, 99 of whom were excluded for the following reasons:
– 45 patients had benign disease
– 12 patients had malignancy other than RCC
– 19 patients had nodal or metastatic disease
– 7 patients had T3b disease
– 5 patients had significant concurrent pathology
– 11 patients had insufficient data available
After exclusions, 154 patients were assessed in the study. Their characteristics are summarized in Table 1.
Table 1.
Summary of patients included in study
| NLR ≤4 | NLR >4 | |
|---|---|---|
| Number | 126 | 28 |
| Male/female | 61/65 | 20/8 |
| Median age (range) | 64.5 (26–86) | 65 (34–88) |
| T1/T2/T3a | 87/14/25 | 14/4/10 |
| Partial/radical | 42/84 | 5/23 |
NLR: neutrophil-lymphocyte ratio.
Of the 154 patients included in the study, all had sufficient followup data to determine OS, however, eight patients had insufficient followup data for inclusion in RFS and so only 146 patients were included in the RFS analysis. OS and RFS were plotted on Kaplan-Meier curves comparing NLR≤4 with NLR >4 (Figs. 1, 2).
Fig. 1.
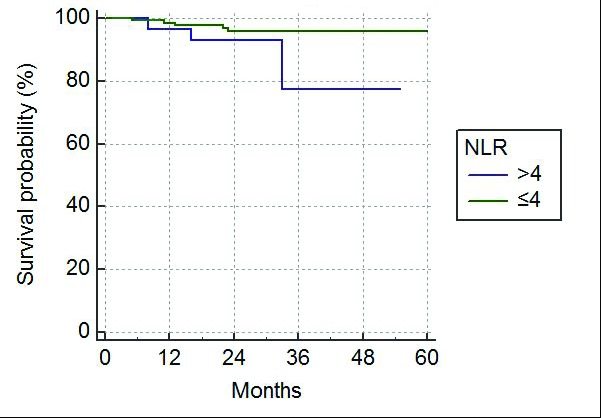
Kaplan-Meier curve for overall survival following nephrectomy. NLR: neutrophil-lymphocyte ratio.
Fig. 2.
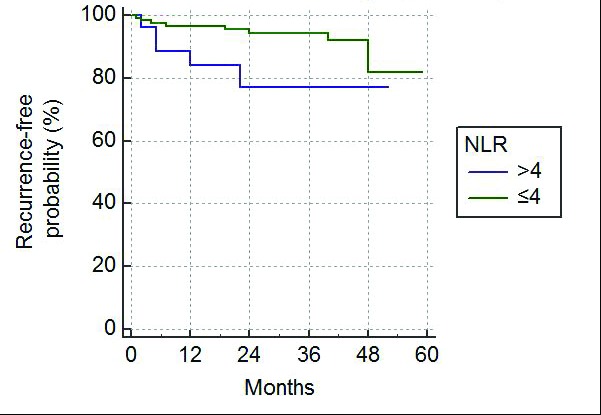
Kaplan-Meier curve for recurrence-free survival following nephrectomy. NLR: neutrophil-lymphocyte ratio.
Patients with a normal NLR had a better OS than patients with NLR >4. While OS was similar in the first two years following surgery, the survival curves diverged after this, with three-year OS being 95% and 78% for patients with NLR≤4 and NLR >4, respectively (p=0.0219).
An NLR >4 also predicted a poorer RFS, particularly in the early postoperative period compared with patients with NLR ≤4 (p=0.0218). One-, two- and three-year RFS for NLR ≤4 vs. NLR >4 were 97% vs. 88%, 95% vs. 78%, and 92% vs. 78%, respectively. This difference in RFS decreased by the fourth postoperative year (82% for NLR≤4 vs. 78% for NLR >4).
Discussion
These results support that an elevated NLR is associated with poorer OS and RFS in patients with RCC undergoing nephrectomy with curative intent. Within the first two postoperative years, OS was comparable between patients with NLR ≤4 and NLR >4; however, the survival curves diverged significantly after this point. Interestingly, the greatest difference in RFS between the two cohorts was within three years, with a smaller difference at four years after resection. This poor RFS in the early stage following resection may explain the difference in OS after three years, as this may correspond with the time period that patients who had developed early recurrence died from their disease. An NLR >4 may be a useful indicator for which patients are suitable for more intensive postoperative surveillance in the first few years following resection so that should there be recurrent disease, it can be recognized at the earliest possible stage and be considered for treatment.
There is no one particular cutoff value when considering NLR and this makes comparing studies in this subject difficult, as there is significant variation reported in the literature. Pichler et al15 investigated the role of NLR in patients undergoing nephrectomy for clear-cell RCC (NLR <3.3 n=398; NLR ≥3.3 n=280) and demonstrated that a cutoff of 3.3 showed a statistically significant difference in OS, but not cancer-specific survival or metastasis-free survival. Viers et al14 also compared outcomes in patients undergoing nephrectomy for clear-cell RCC using 4 as a cutoff and found that there was a statistically significant difference in OS and cancer-specific survival, but not metastasis-free survival. Other studies have investigated the prognostic value of NLR without assigning a particular cutoff representing elevated or non-elevated NLR, but have noted that patients who develop metastatic disease within five years of nephrectomy tend to have a higher preoperative NLR than those who develop metastases longer than five years after nephrectomy.17 In this study, a cutoff of 4 was used, as this has been demonstrated to be useful as a prognostic tool in various settings, including prognosis of patients being treated for localized RCC, patients with metastatic RCC,18 and in other malignancies.19
Another issue that makes comparing studies on this subject difficult is patient inclusion. Previously published papers include patients with T1–T4 disease,15 and others included patients with nodal disease.14 This study only included patients with localized disease, defined as T1–T3aN0M0. Patients with T3b–T4 disease were excluded, as they would require surgery more extensive than radical nephrectomy (some may require significant vascular surgery) and had these patients been included, their outcomes may have skewed the results of their cohort. Patients with nodal disease were also excluded on the basis that the possible role for NLR may be in guiding intensity of postoperative followup. Patients who have positive nodes on histology will already be undergoing more intense followup, as they would be expected to have a higher risk of recurrent disease, regardless of what their preoperative NLR may have been; NLR is not relevant for this group of patients.
While this study highlights the possible benefit of NLR as a prognostic tool, it also demonstrates that it would not be of use as a diagnostic tool in distinguishing benign from malignant disease. Over 80% of the patients included in this study had a normal NLR. While the use of preoperative NLR as a predictor of final histology was not investigated in this study, with such a high proportion of patients with malignant disease having a normal ratio, it wouldn’t be of any diagnostic use in the investigation of a patient with a renal lesion.
One limitation of this study is that it is a retrospective analysis of patients who had already undergone nephrectomy. Another limitation is the duration of followup after nephrectomy; results were not available past five years. Furthermore, with patients being included until December 31, 2014 and final followup data being collected on July 1, 2016, a significant number of patients included in this study will only have had a short period of postoperative followup and, therefore, these patients do not have robust long-term followup outcome data. While this study suggests there is a poorer OS and PRS survival associated with NLR >4, a longer followup period would be required to investigate if this trend is maintained in the long-term.
Conclusion
The results of this study support that an elevated preoperative NLR is associated with poorer prognosis in patients undergoing nephrectomy for RCC with curative intent. These results are in keeping with currently available evidence. Preoperative NLR may be a useful tool in identifying patients at higher risk of early recurrence following nephrectomy and as such, may be used to identify patients who may benefit from more frequent postoperative surveillance.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Cancer Research UK. Kidney cancer incidence statistics. [Accessed Jan. 19, 2017]. Available at http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer/incidence#heading-Two.
- 2.Cancer Research UK. Kidney cancer statistics. [Accessed Jan. 19, 2017]. Available at http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer#heading-Zero.
- 3.Dirican A, Kucukzeybek Y, Somali I, et al. The association of hematologic parameters on the prognosis of patients with metastatic renal cell carcinoma. J BUON. 2013;18:413–9. [PubMed] [Google Scholar]
- 4.Bukowski RM. Prognostic factors for survival in metastatic renal cell carcinoma: Update 2008. Cancer. 2009;115:2273–81. doi: 10.1002/cncr.24226. [DOI] [PubMed] [Google Scholar]
- 5.Jagdev SP, Gregory W, Vasudev NS, et al. Improving the accuracy of preoperative survival prediction in renal cell carcinoma with C-reactive protein. Br J Cancer. 2010;103:1649–56. doi: 10.1038/sj.bjc.6605973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 7.Park YH, Baik KD, Lee YJ, et al. Late recurrence of renal cell carcinoma >5 years after surgery: Clinicopathological characteristics and prognosis. BJU Int. 2012;110:E553–8. doi: 10.1111/j.1464-410X.2012.11246.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim SP, Weight CJ, Leibovich BC, et al. Outcomes and clinicopathological variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology. 2011 Nov;78:1101–6. doi: 10.1016/j.urology.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 9.European Association of Urology. Renal cell carcinoma. [Accessed Jan. 19, 2017]. Available at: http://uroweb.org/guideline/renal-cell-carcinoma.
- 10.Ishizuka M, Nagata H, Takagi K, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109:401–7. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 12.Dirican A, Kucukzeybek Y, Erten C, et al. Prognostic and predictive value of hematological parameters in patients with metastatic renal cell carcinoma: Second-line sunitinib treatment following IFN-alpha. Asian Pac J Cancer Prev. 2013;14:2101–5. doi: 10.7314/APJCP.2013.14.3.2101. [DOI] [PubMed] [Google Scholar]
- 13.Grimes N, Tyson M, Hannan C, et al. A systematic review of the prognostic role of hematologic scoring systems in patients with renal cell carcinoma undergoing nephrectomy with curative intent. Clin Genitourin Cancer. 2016;14:271–6. doi: 10.1016/j.clgc.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Viers BR, Houston Thompson R, et al. Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear-cell renal carcinoma undergoing nephrectomy. Urol Oncol. 2014;32:1277–84. doi: 10.1016/j.urolonc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Pichler M, Hutterer GC, Stoeckigt C, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer. 2013;108:901–7. doi: 10.1038/bjc.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Martino M, Pantuck AJ, Hofbauer S, et al. Prognostic impact of preoperative neutrophil-tolymphocyte ratio in localized non-clear-cell renal cell carcinoma. J Urol. 2013;190:1999–2004. doi: 10.1016/j.juro.2013.06.082. [DOI] [PubMed] [Google Scholar]
- 17.Sejima T, Iwamoto H, Morizane S, et al. The significant immunological characteristics of peripheral blood neutrophil-to-lymphocyte ratio and Fas ligand expression incidence in nephrectomized tumour in late recurrence from renal cell carcinoma. Urol Oncol. 2013;31:1343–9. doi: 10.1016/j.urolonc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Baum Y, Patil D, Huang J, et al. Elevated preoperative neutrophil-to-lymphocyte ratio may be associated with decreased overall survival in patients with metastatic clear-cell renal cell carcinoma undergoing cytoreductive nephrectomy. Asian J Urol. 2016;3:20–5. doi: 10.1016/j.ajur.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutro-lymphocyte vs. platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]


