In the January-February 2016 issue of the Canadian Urological Association Journal, a multidisciplinary committee published a white paper entitled, “Recommendations for the improvement of bladder cancer quality of care in Canada: A consensus document reviewed and endorsed by Bladder Cancer Canada (BCC), Canadian Urologic Oncology Group (CUOG), and Canadian Urological Association (CUA), December 2015.”1 The paper was produced in response to concerns regarding the variability in management and outcomes of patients with bladder cancer throughout centres and geographical areas in Canada. The final paper contents were the result of consensus deliberations during a two-day meeting that took place in late 2014. In November 2016, another multidisciplinary committee consisting largely of the same members convened the second “BCC-CUA-CUOG Bladder Cancer Quality of Care Meeting 2016.” The focus was on patient journey and optimizing management. The following document is a summary of the proceedings of this meeting. The objectives for the meeting were as follows:
-
Patient journey
- To discuss unmet needs in bladder cancer care from the patient perspective.
-
Optimizing management
- To select the top 10–15 indicators of bladder cancer quality of care and establish benchmarks;
- To develop a score card for measurement of bladder cancer quality of care;
- To address complex bladder cancer management from a training perspective;
- To identify bladder cancer centres of expertise across Canada using refined criteria;
- To discuss the establishment of a bladder cancer research network of excellence.
1. Unmet needs in bladder cancer: The patient perspective
Patient representatives from Bladder Cancer Canada (BCC) presented their perspective on unmet needs in bladder cancer care. These perspectives were gathered by patients from BCC through the BCC website/discussion forum, BCC patient-to-patient emails and phone calls, as well as a patient needs survey conducted at the Princess Margaret Hospital in Toronto.
Patient needs were subdivided into four timeframes across the patient journey: beginning the journey with signs/symptoms (pre-diagnosis); from diagnosis to treatment; during treatment; and after treatment: living “the new normal.”
A. Pre-diagnosis
The key unmet need identified in the pre-diagnosis phase is a desire for greater support in coping with the emotional impact of uncertainty while waiting for examination/test results. Long wait time for many patients amplifies the stress during this phase.
B. Time of diagnosis/pre-treatment
At the time of diagnosis, patients have reported several areas of concern, including coping with the emotional impact of the diagnosis, confusion and fear regarding future treatments and prognosis, and a lack of clear knowledge of where to go for more information and resources for education and support. Further, lack of empathy and personal connection by doctors has been identified as a perceived issue from the patient perspective. Patients have expressed frustration that their physicians are often rushed, do not take the time to explain the diagnosis or the full treatment plan/prognosis, and focus on the disease rather than the patient. One of the suggestions to help overcome the knowledge transfer gap is the creation of a patient-friendly interpretation of the pathology report (including explanation of the stage, grade, and histology) to be discussed in the physician’s office, with a copy provided for the patient to take home. Participants at the meeting suggested that a patient-friendly explanation of the report could be developed and added as a resource to the BCC website. Other suggestions included having a model of the bladder or similar visual aids showing depth of invasion, layers of the bladder, and other anatomical features available in offices to be used as teaching tools. Such visuals could also include the ability to show patients where they are on the disease spectrum, what treatments may be needed, and how the disease is likely to evolve.
Patients with non-muscle-invasive bladder cancer (NMIBC) have reported that their care teams may not convey the appropriate degree of concern in response to their level of fear and apprehension. They have received a diagnosis of cancer and are not necessarily able to distinguish or appreciate the difference between NMIBC and muscle-invasive disease (MIBC) and the ramifications of this distinction. The emotional impact of a cancer diagnosis may be similar regardless of the extent of disease.
With respect to investigations, patients (particularly women) have expressed concern with a lack of respect for personal dignity, modesty, and stress during cystoscopy appointments. Women in particular have reported personal discomfort due to the hospital-supplied gowns and the amount of body these garments expose while sitting in the waiting area prior to a cystoscopy. They have voiced their opinion that their physicians could be more considerate about this issue, particularly given that most of the patients in these waiting areas are men.
Finally, patients have expressed frustration over the lack of uniformity in treatments from centre to centre for management of high-grade NMIBC. For those with MIBC, patients have also indicated confusion and uncertainty about which treatment plan and diversion plan to follow. Healthcare professionals should be aware that patients talk to each other — in patient forums online and other means of interaction. Differing approaches from centre to centre can create confusion and apprehension.
C. During treatment
For treatment, patients want to have the post-treatment followup plan clearly explained upfront, including what they should be looking out for before their next appointment. Patients have also expressed a desire for their treatment team to have an understanding of the impact of the treatment plan on work and family life, including the financial impact of travel, work interruption, and out-of-pocket expenses to attend treatments. This is of particular concern for patients from rural areas who live further away from their treatment centre. There is a frustration among patients that the healthcare system is not set up to provide and coordinate all the necessary services and elements of patient care at the beginning of treatment and they are too often left to fend for themselves.
In general, patients would also like to engage more with their doctor during the examinations themselves. For example, discussion of the solutions and gels being applied as the scope enters and interpretation of the display (e.g., what may be of concern and why).
D. Living with bladder cancer (the new normal)
Perceptions of unmet needs in this phase of the disease are mostly specific to particular patient situations. For those who undergo radical cystectomy, concerns include the emotional support of living with this new normal, including the potential value of psychologist care, discussion of exercise, sexual function (women raise the topic more than men), and diet. Understanding of ureter blockage and reduced flow has also been highlighted as an unmet need. Among women who undergo oophorectomy and hysterectomy, discussion of how to deal with hormonal and emotional issues is an additional need. Support for caregivers is important, including how to live with patients and provide support.
For individuals who receive a neobladder, education on bladder control, bowel problems, pain, urinary frequency, sexual function, and diet are key concerns, as is the desire for counselling on how to handle and reduce mucus buildup. For those with an incontinent urostomy, patients have expressed a need for better training on how to manage and troubleshoot common issues, such as changing the ostomy bag and maintaining healthy skin around the stoma. Again, in this setting, adequate counselling on quality of life issues, such as bowel function, sexual activity, diet, and exercise has emerged as an unmet need.
Patients with a continent cutaneous diversion have reported similar concerns, with insufficient education provided regarding common issues, such as leaks, stoma care, bowel problems, pain, lack of sleep during the “pouch expanding” stage, and management of mucus buildup. Instructions on how to locate and use the correct medical supplies would be appreciated. Further, patients have asked for healthcare professionals to provide them with reassurance about life returning to normal and a timeline for return to normal activities.
Some patients, and particularly patients with MIBC who have been treated at smaller centres, have reported that they are not offered the same treatment options as patients at major cancer centres. The smaller centres, in many cases, may not refer these patients to major centres where trials are being conducted or newer treatments may be available. Further, patients have reported that they are not aware that these options may be available to them at a major cancer centre. This issue may be more apparent with increased clinical trial activity, as we have seen since the introduction of immuno-oncology trials, which have been/are being conducted at the major centres.
Lastly, three common challenges were highlighted from patients with metastatic disease: 1) a lack of information on immunotherapy options and trials for treatment; 2) difficulty in navigating through the process of getting eligible for open trials, and the disappointment of not being accepted for those who did not qualify; and 3) out-of-pocket expense for certain treatments.
E. Resources provided by BCC
Healthcare providers are encouraged to direct their patients with bladder cancer to the resources offered by BCC, including the website (including the discussion forum), peer support program, educational meetings, patient videos, and patient guidebooks. Participant suggestions to expand the library of available resources included information on the decision-making process between neobladders and conduits, the impact of stomas on activities and required lifestyle adjustments, information on bladder-sparing options vs. surgery, more information on radiotherapy as a treatment alternative, information on the new wave of immunooncology drugs, and further information on diet and smoking cessation. Other valuable resources available include BCAN (U.S.) and FightBladderCancer (U.K.).
2. Review and selection of quality indicators in bladder cancer
Observations from the consensus panel, as well as data from the literature, have highlighted a need for a list of meaningful quality indicators in bladder cancer care in Canada. To date, there has been no such list developed in this field.
At the first BCC-CUA-CUOG Bladder Cancer Quality of Care Meeting in 2014, participants were asked to develop a list of potential indicators. Over the ensuing two years, members of the panel undertook a Delphi process to identify a consensus list of the 60 most important indicators.2 At the second Quality of Care Meeting, the results of this Delphi process were presented and the participants were asked to further narrow the list to a manageable number to be used for a quality-of-care scorecard for Canadian centres.
A. Background: Identification of the need for quality indicators in bladder cancer
Researchers have published numerous reports highlighting a need for improved care of bladder cancer in Canada. An evaluation of practice and referral patterns for patients with microscopic or gross hematuria reported data from 599 primary care physicians in Quebec.3 Among the key findings of the survey was suboptimal evaluation of patients with hematuria. The importance of these findings is illustrated by another Canadian study investigating the impact of referral delay from primary care physicians on survival after radical cystectomy for bladder cancer.4 There was a significant detrimental impact on survival associated with delay in referral (age-adjusted hazard ratio [HR]: 1.29; 95% confidence interval [CI]: 1.10–1.52).
The referral delay is not the only delay along the trajectory of bladder cancer treatment that can have an impact on outcomes. Indeed, delays can exist at every step along the way. Research has also suggested that these delays have increased over time in certain areas:5 first general practitioner (GP) visit to first urologist visit: 32 days; first urologist visit to cystoscopy: 22 days; cystoscopy to transurethral resection of bladder tumour (TURBT): 18 days; TURBT to computed tomography (CT) scan: 17 days; CT scan to radical cystectomy: 34 days; total delay from first GP visit to radical cystectomy: 116 days.6,7
The delay from TURBT to cystectomy has also been associated with an increased mortality risk in patients not receiving neoadjuvant therapy. Using data from 2535 patients who underwent cystectomy for bladder cancer in the province of Ontario from 1992–2004, investigators assessed mortality risk by duration of this delay.8 Similar observations were made among patients in Quebec.9 Based on these observations, shortening wait times is a key goal of improving quality of care for patients and improving overall survival for patients with bladder cancer in Canada.
The above highlights the impact of bladder cancer diagnostic and treatment delays within the healthcare system. However, the problems that may underlie the suboptimal overall management of patients with bladder cancer are multifactorial, including a shortage of proper healthcare facilities, non-adherence to published guidelines, diagnostic and treatment delays, suboptimal/absence of therapy, and underuse of multidisciplinary management teams.
B. Development of the quality indicators for bladder cancer in Canada
The objectives of the standardized quality of care assessment initiative are to track performance and subsequent impact on clinical outcomes across the healthcare system, and to quantify adherence to best practices and provide data for benchmarking and quality improvement. We would anticipate that performance measurement of common bladder cancer quality indicators will encourage the advancement of practice standards, promote performance comparison across jurisdictions in efforts to improve care, and stimulate sharing of best practices. The Delphi process was used by our group to produce an evidence- and consensus-based list of quality indicators spanning the bladder cancer care continuum with input from a multidisciplinary expert panel. Referral to the recent publication is recommended for more details about the process and results.2 In brief, each indicator was evaluated using a Likert scale ranging from 0 (extremely inappropriate) to 4 (extremely appropriate) according to the following criteria: 1) is the indicator relevant and important to quality of care?; 2) is the indicator scientifically sound (valid and reliable)?; 3) does the indicator allow for comparison across jurisdictions?; and 4) is the indicator amenable to action (is it under your control to change)? Finally, the feasibility assessment specified whether the indicators would be feasible for data collection.
The final list included 60 potential indicators, including all phases of care (Table 1, adapted from reference 2). The developed quality indicators span practice disciplines (surgery, pathology, medical oncology, radiation oncology, and enterostomal therapy), as well as phases of the cancer care continuum. Some indicators, such as for systemic therapy in metastatic disease (including toxicity of chemotherapy) and Enhanced Recovery After Surgery (ERAS®), were evaluated but did not meet the criteria for selection. One of the limitations of the list discussed at the meeting included the fact that there were no indicators dealing with psychosocial or palliative care. However, quality indicators in both areas have been previously described for cancer patients in general and could be added to the list if the participants decide to measure them in the future.
Table 1.
Evidence- and consensus-based quality indicators for bladder cancer
| Phase of care | Indicator | Supporting evidence |
|---|---|---|
| Diagnosis | For patients with gross hematuria, percent who saw urology within 2 weeks of the date of request for consultation | Retrospective |
| For patients with microhematuria and >40 years of age, percent who saw urology within 6 weeks of the date of request for consultation | Retrospective | |
| For patients with gross hematuria, percent who had complete workup within 4 weeks of the urology visit | Expert opinion | |
| For patients with asymptomatic microhematuria and >40 years of age, percent who had complete workup within 12 weeks of the urology visit | Expert opinion | |
| Percent of patients with documented performance status and comorbidities at time of bladder cancer diagnosis | Suggested by expert panel | |
| Staging | Percentage of newly diagnosed patients who had TURBT within 3 weeks of diagnosis | Expert opinion |
| Percentage of newly diagnosed patients who received upper tract imaging within 1 month peri-TURBT | Retrospective/guideline | |
| Percentage of patients for whom there was surgical report documentation on visual completeness of TUR, depth of TUR, and EUA findings | Recommendation/guideline | |
| Percentage of pathology reports available within 1 week of TURBT | Expert opinion | |
| Percentage of newly diagnosed, high-risk patients who were informed of pathology within 2 weeks of TURBT | Expert opinion | |
| Percentage of patients with T1–2 tumours for whom pathology was reviewed by a genitourinary pathologist | Retrospective | |
| Percentage of pathology reports noting detrusor muscle in the pathologic specimen (indicating completeness of resection) | Meta-analysis | |
| For patients with T1 disease and whose pathology report noted absence of detrusor muscle, percentage who were restaged by TUR within 6 weeks of initial resection | RCT | |
| Percentage of patients with MIBC undergoing chest imaging (chest radiograph/chest computed tomography) as part of staging | Suggested by expert panel | |
| For RC, percentage of patients who have preoperative cTNM recorded | Suggested by expert panel | |
| For RC, percentage of patients who have pTNM recorded postoperatively | Suggested by expert panel | |
| Treatment | For patients who were referred to medical or radiation oncology, percent who were seen within 2 weeks of the date of request for referral | Expert opinion |
| Percent of patients undergoing concurrent chemoradiotherapy who have a complete TURBT prior to therapy | Suggested by expert panel | |
| Percent of patients who initiated TMT within 6 weeks of last TURBT | Expert opinion | |
| Percent of patients undergoing radiotherapy who have image-guided radiation therapy | Suggested by expert panel | |
| For patients indicated for radiation, percent who received chemosensitizer with radiation | RCT | |
| Percent of patients who started first cycle of NAC within 4 weeks of date of request to consult medical oncology | Recommendation | |
| Percent of patients without NAC who had RC within 6 weeks of last TURBT | Retrospective | |
| Percent of patients with NAC who had RC within 16 weeks of initiation of NAC | Retrospective | |
| For patients who underwent cystectomy, percent who had a preoperative consultation with the enterostomal therapist | Recommendation/guideline | |
| Percent of patients with adequate lymph node dissection defined as >16 nodes | Systematic review | |
| For patients who underwent cystectomy and <70 years of age, percent who received continent diversion | Retrospective | |
| Percent of patients who had soft tissue positive margin at cystectomy | Retrospective | |
| For patients with MIBC, percent who received any definitive therapy (RC or TMT) | RCT | |
| Percent of patients with MIBC who initiated curative intent therapy (NAC, TMT, or RC) within 6 weeks of TURBT | Suggested by expert panel | |
| Percent of eligible patients with MIBC on TURBT being referred to medical oncology preoperatively for consideration of NAC | Suggested by expert panel | |
| For patients with MIBC and normal estimated glomerular filtration rate, ECOG of 0–1, and <80 years of age, percent who received NAC | RCT | |
| For patients with MIBC and receiving NAC, percent who completed a minimum of 3 cycles of cisplatin-based combination therapy | RCT | |
| For patients with MIBC and pT3–4 or pN+, percent for whom there was a consult to medical oncology postoperatively | RCT | |
| For patients with MIBC, percent who were managed by a multidisciplinary team (e.g., a multidisciplinary bladder cancer clinic, presentation at a genitourinary tumour board, arranged consultations with medical and radiation oncology when appropriate) | Expert opinion | |
| For patients NMIBC, percent who received postoperative instillation of intravesical chemotherapy | Meta-analysis of RCTs | |
| Percent of patients with high risk NMIBC receiving BCG | RCT | |
| Percent of patients with high risk NMIBC who initiated intravesical BCG within 4 weeks of TURBT | Suggested by expert panel | |
| For patients with high-risk NMIBC, percent who had intravesical BCG induction course with at least 1-year maintenance | RCT | |
| Percent of patients with metastatic or unresectable bladder cancer receiving cisplatin-based systemic chemotherapy | Suggested by expert panel | |
| Prophylactic measures | Percent of patients who received intravenous antibiotics within 60 minutes prior to incision | RCT |
| Percent of patients who received pharmacological thrombosis prophylaxis perioperatively | Recommendation/guideline | |
| Percent of patients for whom a pneumatic compression device was used intraoperatively | Recommendation/guideline | |
| Percent of patients who received pharmacological thrombosis prophylaxis post discharge for a period of 4 weeks | Recommendation/guideline | |
| Organizational process and outcomes | Presence of quality assurance rounds for discussion of complications post-cystectomy | Suggested by expert panel |
For patients undergoing TMT, percent who had:
|
Recommendation/guideline | |
| Percent of patients who had a length of stay of ≤10 days post-cystectomy | Retrospective | |
Transfusion rate during hospital admission for post-RC patients who:
|
Suggested by expert panel | |
| Percent of patients who had a severe (Clavien Grade III/IV) postoperative complication within 90 days of surgery (e.g., requiring surgical, endoscopic, or radiological intervention OR requiring ICU management for life-threatening complications) | Recommendation | |
| Percent of patients who were readmitted within 90 days of cystectomy | Prospective studies | |
| Percent of patients deceased within 90 days post-cystectomy | Prospective studies | |
| Percent of patients with ureteroenteric anastomotic stricture within 1 year of RC | Suggested by expert panel | |
| Stage (at diagnosis)-specific 5-year recurrence-free, disease-specific and overall survival following RC or TMT | Suggested by expert panel | |
| Followup | Percent of patients who had cystoscopy 3 months after TURBT | Prospective studies |
| For patients who underwent cystectomy with ileal conduit, percent who had follow-up with the enterostomal therapist within 1 month post-discharge | Expert opinion | |
| For patients with MIBC, percent with upper tract imaging and metastatic workup within 1 year of definitive therapy | Suggested by expert panel | |
| Case volume | Annual hospital volume of TMT | Expert opinion |
| Annual hospital volume of RC | Meta-analysis | |
| Annual surgical volume of RC by each surgeon performing this procedure | Retrospective | |
| Annual hospital volume of neobladders | Retrospective |
Taken from Khare SR, et al. Urol Oncol 2017;35:328–34. Reprinted with permission. BCG: Bacillus Calmette-Guerin; ECOG: Eastern Cooperative Oncology Group; EUA: examination under anesthesia; ICU: intensive care unit; MIBC: muscle-invasive bladder cancer; NAC: neo-adjuvant chemotherapy; NMIBC: non-muscle-invasive bladder cancer; RC: radical cystectomy; RCT: randomized controlled trial; RTOG: Radiation Therapy Oncology Group; TURBT: transurethral resection of the bladder tumour; TMT: trimodality therapy.
C. Rationale for and development of a quality of care scorecard
The concept of a scorecard to measure performance and drive positive change was first put forth by Kaplan and Norton in 199210 and represents a management system that organizations use to: communicate what they are trying to accomplish; align the day-to-day work that everyone is doing with strategy; prioritize projects, products, and services; and measure and monitor progress towards strategic targets.11,12 The following quotation nicely sums up the goals of scorecard implementation: “Better to have a mediocre strategic plan well-implemented and measured than an outstanding corporate strategy fail due to poor execution.” The concept of a balanced scorecard has recently been adapted to healthcare settings. Scorecards evaluating the performance of healthcare professionals and centres are currently in use in other disciplines, but none yet exist for genitourinary (GU) cancers in Canada.
Many lessons have been learned regarding the successful implementation of a balanced scorecard.11,12 Some of these include:
– Be flexible in choosing performance measures, as the measures should reflect the critical performance issues of the day and these may change over time;
– Some indicator compromises due to lack of data are inevitable while steps are put in place to collect more appropriate data;
– Data quality is a major concern and needs to be addressed for credibility;
– Form — how the data are presented — is as important as substance;
– Comparisons are valuable when the data is reliable and often leads to a fresh appreciation that something needs to be changed;
– Expert advice is not an option — it is essential that there is buy-in;
– Build data linkages early on, as it is much harder later;
– Information is political — for example, when obtained by local media without understanding how it should be interpreted;
– Real variation, even after case mix and other adjustments are made, exists between hospitals/providers; and
– While there can be some overlap between ‘operational’ measures and ‘strategic’ measures, it is important that the balanced scorecard measures are limited and strategic, as there is usually strong pressure for ‘measurement creep.’
For the purposes of the development of a bladder cancer-specific scorecard, the participants agreed that the list of indicators should be narrowed to as small a list as possible, preferably in the range of 10–15 items, with established benchmarks for each item. Centres are scored based on how close they are to achieving these benchmarks (e.g., equal to, better or worse than target) and the kinetics (i.e., moving toward target or away from target). The measures should reflect the critical performance issues of the day. Given that these may change over time, a scorecard has to be a flexible and dynamic tool. It was recognized that some desirable indicators would need to be left off a scorecard due to lack of high-quality, reliable data.
Any quality indicators selected, particularly for such an endeavour structured to improve the care of bladder cancer patients, would need to be strictly defined such that there is no ambiguity or misinterpretation of what is being measured. Furthermore, there was much discussion around the consideration to facilitate the acceptance of potential indicators to reflect concepts from the Donabedian framework of examining health services and evaluating quality of healthcare. 13 According to the Donabedian model, information about quality of care can be drawn from three categories: structure, process, and outcomes. Structure describes the context in which care is delivered, including hospital buildings, staff, and equipment. Process includes the transactions between patients and providers throughout the delivery of healthcare. Outcomes refer to the effects of healthcare on the health status of patients and populations.
After acceptance of quality indicators, corresponding benchmarks can be made in several different ways, including expert opinion/evidence-based, relative to best performers, or other data-driven processes. For example, for the indicator of annual hospital volume for radical cystectomy, evidence has shown that patients treated at centres with more than 20 cases per year have better outcomes than those treated in lower-volume centres.14 This represents a potential evidence-based benchmark for a quality of care scorecard.
With respect to the target centres for the scorecard, the participants agreed that they should be all hospitals that treat bladder cancer. With respect to the audience for the results of the scorecard, there was some debate as to whether the result should be immediately made available and shared first among the GU oncology community, including the CUA and CUOG, to allow for improvement.
D. Consensus selection of quality indicators for inclusion in the scorecard
The participants deliberated over the list of potential quality indicators to include in the bladder cancer scorecard. They narrowed down the list to a core group of 13 items:
Annual surgical volume of radical cystectomy by each surgeon performing this procedure (structure);
Time from cystoscopy to TURBT (process);
Time from TURBT to pathology report (process);
Percent of patients without neoadjuvant chemotherapy who had radical cystectomy within six weeks of last TURBT (process);
For patients with high-risk NMIBC, percent who had intravesical bacillus Calmette-Guerin (BCG) induction course with at least one year of maintenance (process);
For patients with MIBC, percent who received any curative-intent definitive therapy (radical cystectomy or radiation-based therapy) (process);
Percent of patients with adequate lymph node dissection defined as >14 nodes (process);
Percent of patients with MIBC being referred to medical oncology preoperatively for consideration of neoadjuvant chemotherapy (process);
For patients with MIBC and receiving neoadjuvant chemotherapy, percent who completed a minimum of three cycles of cisplatin-based combination therapy (process);
Percent of metastatic patients offered second-line systemic therapy after receiving first-line chemotherapy (process);
Percent of patients with MIBC on TURBT being referred to radiation oncology preoperatively for consideration of radiotherapy (process);
Percent with positive soft tissue margin at radical cystectomy (outcome); and
Percent of patients deceased within 90 days post-cystectomy (outcome).
With respect to benchmarking these 13 items, there was some discussion among the participants, but consensus on defining each benchmark will be reached at a later date.
Other items that received support among the meeting participants but were ultimately not included in the list were: annual hospital volume of radical cystectomy; percent of pathology reports noting detrusor muscle in the pathological specimen (indicating completeness of resection); percent of patients with metastatic or unresectable bladder cancer receiving systemic chemotherapy; and time from TURBT to physician assessment.
3. Update on the Canadian Bladder Cancer Information System (CBCIS)
The CBCIS is a prospective information system and database that is collecting de-identified information about current bladder cancer practice patterns and patient outcomes across 14 academic health centres in Canada. It will provide up-to-date information on all aspects of patient care from demographics, tumour histology and staging, to treatment, complications and survival. The CBCIS is a not-for-profit joint venture between BCC (the founding sponsor) and the Research Institute of McGill University Health Centre (RI-MUHC, the coordinating institution).
A. Objectives
Some of the objectives of the CBCIS are to capture comprehensively and prospectively data on all high-grade bladder cancer patients to:
Evaluate the outcomes of bladder cancer patients in Canada;
Identify the differences across Canada in the treatment of bladder cancer;
Identify the strengths and weaknesses in the management of bladder cancer in Canada in order to develop quality improvement programs;
Understand the regional needs to provide adequate care to bladder cancer patients;
Support the development of centres of excellence in bladder cancer research; and
Understand the impact of novel therapeutic strategies on outcome of bladder cancer patients.
B. Inclusion criteria
Patients will be included in the CBCIS if they have been diagnosed with a high-grade bladder cancer within the last 12 months, regardless of prior disease history. A new diagnosis can include a new tumour in a patient with a prior history of disease, new metastatic diagnosis, or a completely new diagnosis.
C. Governance
The CBCIS is governed by a steering committee of three urologists, one medical oncologist, one radiation oncologist, and up to two members of BCC who act as patient advocates. An operations committee consists of two principle investigators from different disciplines from each participating site, as well as the CBCIS project manager, CBCIS implementation specialist, an outcome specialist, and a privacy advisor. One representative from each sponsor is allowed to join the operations committee as a non-voting member if approved by the steering committee.
Data is entered into a specially designed secure web portal based on the REDCap software platform hosted at McGill University. REDCap software is used by medical institutions throughout the world. Each participating centre will have access to its own data but not the data from other sites. All participants will be able to query data through the central data coordinator according to predefined rules.
D. Patient information to be captured
The CBCIS will capture the following characteristics: ethnicity; date of diagnosis; date of appointment or surgery; family history of urinary tract cancers; staging information of bladder tumour, including biopsy and medical imaging results; medications used to treat the cancer; other treatments used to treat the cancer, including surgery and radiation; and information about tissue and/or fluid samples collected during treatment.
E. Ongoing health assessments
The CBCIS will capture the date of initial visit and followup visits with the treating physician; vital signs taken at each visit; blood test results; medication(s) taken or changes to dosing; general health status, including other conditions the patient may have; and changes to health status, including new or changed diagnosis or accidents.
F. Outcome topics of interest
Among the outcomes being considered for analysis using the CBCIS database are: overall survival, disease-free survival, and recurrence-free survival stratified by stage; treatments for localized and metastatic bladder cancer; impact of lymph node dissection and diversion; role of neoadjuvant therapy; impact of surgery in metastatic or locally recurrent disease; and outcome of rare histological variants; outcome and toxicity of first-, second-, and third-line therapies; and surveillance and complications.
In the context of the discussion of quality of care indicators, the meeting participants agreed that the CBCIS might be useful to help identify and measure some of the quality indicators that will be used for the performance scorecard.
4. Update on care of the bladder cancer patient
Training perspective
Research has demonstrated a wide variability in outcomes for patients with bladder cancer, suggesting considerable variability in the quality of care being delivered for these patients. The perception, supported by the literature, is that residency programs graduating general urologists may not provide sufficient training to handle all complex oncology patients. A review of the literature reveals some of the key areas of concern.
i. Variability in care
Recurrence rate at first followup cystoscopy after TURBT is a marker for the quality of TURBT. An analysis of 2410 patients from seven EORTC phase 3 trials demonstrated significant variability across institutions, with tumour detection at first followup cystoscopy varying between 3% and 46%.15
Research has also demonstrated that patients are not being considered for radical cystectomy in a timely fashion following failure of intravesical therapy. In a report from the Canadian Bladder Cancer Network, approximately 50% of 306 patients with cT1G3 disease had non-organ-confined disease at cystectomy and 26% were pN+.
Other Canadian research has suggested that there are unacceptably high rates of inappropriate surgery. In a population-based study using billing records of all partial and radical cystectomies performed for bladder cancer in Quebec from 1983–2005, a total of 714 patients were identified as having undergone partial cystectomy.16 More than 25% of patients with invasive bladder cancer received partial cystectomy rather than radical cystectomy, the majority (65%) of whom were treated in non-academic institutions; 13% of patients undergoing partial cystectomy required ureteral reimplantation, and only 23% received pelvic lymphadenectomy. An Ontario-based study of 2802 patients also reported substantial variability in the proportion of patients receiving lymphadenectomy, with 30% overall not receiving any lymph node dissection.17 Continent diversions are underused. Even at academic centres, the Canadian Bladder Cancer Network reported that only 20% of patients treated with radical cystectomy from 1998–2008 received a continent diversion.18
Suboptimal surgery for patients with bladder cancer in Canada almost certainly has an impact on the overall survival rates of these patients. Five-year survival rates for Ontario (1994–2008) and Quebec (2000–2009) have been estimated at 36% and 45%, respectively.19,20 Furthermore, data from more than 8000 patients who died from bladder cancer in Ontario show that the majority (61%) of these individuals never received definitive therapy to the bladder.21
Analysis of mortality rates after radical cystectomy in Quebec showed wide variability in 90-day mortality rates across centres, ranging from 2.6% to 18.5%.20 In this analysis, mortality rates were higher among hospitals that performed fewer cystectomies. Researchers examining data from Ontario have also observed higher mortality rates among hospitals and surgeons with lower surgical volumes compared to those with higher volumes.22 Further elucidation of surgeon details reveals that mortality rates are significantly lower among patients treated by surgeons whose focus is bladder cancer compared to those who are not bladder-cancer focused (adjusted HR for overall survival 0.68; 95% CI 0.55–0.85; for bladder-cancer specific survival 0.63; 95% CI 0.41–0.96).23
Another key area in which bladder cancer patients may be undertreated is in the proportion of patients with MIBC who receive either neoadjuvant chemotherapy, for which there is Level 1 evidence, or adjuvant chemotherapy. In an Ontario study of 2044 patients with MIBC who underwent radical cystectomy,24 the proportion receiving adjuvant chemotherapy did increase over time (16% from 1994–1998, 18% from 1999–2003, and 22% from 2004–2008), but also remained underused up to that time. More recently, however, a single-centre, retrospective study of patients treated at the Alberta Urology Institute showed much higher usage of neoadjuvant chemotherapy for MIBC (57%).25 Neoadjuvant chemotherapy was considered the standard of care for MIBC by 96% of medical oncologists and 88% of urologists.26
A. Perspectives on urology training
The adequacy of residency training in urology may contribute to the variable treatment of bladder cancer in Canada. An email-based survey completed by 100% of graduating Canadian chief residents in 2012 demonstrated deficient training across all categories of procedures (Royal College classification A/B/C where A is most competent and C least competent).27 Of the 42 category A procedures listed in the survey, 100% of the respondents believed they were deficient in at least one procedure. Further, 53.6% of the respondents believed that they were deficient in at least 10 of the 42 procedures. A perceived deficiency was indicated by one-third of respondents for radical cystoprostatectomy and almost two-thirds for anterior pelvic exenteration.
A separate survey of Canadian urology training faculty, to which 95 of 217 faculty members responded, included a question asking which procedures should be listed as category A, B, or C.28 Only 25%, 32%, and 44% of faculty stated that graduating residents did not achieve category A proficiency in radical cystectomy, extended pelvic lymphadenectomy, and anterior exenteration, respectively.
Further illustration of discrepancies among Canadian urologists with respect to core surgical procedures was provided by a survey of members of the CUA, conducted from August to October 2014.29 Of the 536 members who received the survey, 138 responded (40.6% community and 59.4% academic urologists). The key question was: “After completion of residency training in Canada, a urologist should be proficient in…” There were 16 procedures identified with 90–100% agreement and a total of 30 core procedures with ≥75% agreement. However, there was significant discrepancy between community and academic urologists on 27 procedures (including 11 core procedures). Cystectomy, for example, was rated as a core procedure by 88.5% of community urologists and only 67.1% of academic urologists (p=0.002).
Participants discussed the areas of bladder cancer treatment that required specific experience and/or extra training. These included:
High-risk NMIBC if one of the following is found: initial tumour is T1HG (with or without variant histology), high-grade tumour in a diverticulum, or high-grade recurrence;
Urethral cancer (primary or concomitant prostatic urethral disease); and
MIBC
All of these patients require specific bladder cancer expertise, which can be defined by either a fellowship in urologic oncology or a bladder cancer-focused practice.
B. Medical oncology training perspective
Medical oncologists are integral members of the multidisciplinary team that manages bladder cancer. To date, there is no data in regard to bladder cancer correlating medical oncology practitioner experience with treatment outcomes. To appropriately treat patients with MIBC and metastatic disease, medical oncologists require training in several key areas.
i. MIBC
Level 1 evidence supports the use of cisplatin-based combination regimens for MIBC.30 Node-positive patients should also be treated with induction chemotherapy with consideration of consolidation if there is a good response.
Medical oncologists need to be available to see and start chemotherapy in a timely manner. Time to consultation with a medical oncologist is one of the many potential bottlenecks in the care of a patient with bladder cancer that can delay appropriate treatment. Whenever possible, a medical oncologist with a specific GU focus should be consulted for patients with MIBC (expert opinion).
Medical oncologists dealing with bladder cancer also need to be aware of common and less common side effects of chemotherapy (e.g., pulmonary embolism, deep vein thrombosis) and be able to manage these during chemotherapy.
The medical oncologist also needs to be aware of the risk of progression during neoadjuvant chemotherapy and to know when to refer back to the uro-oncologist or radiation oncologist for local management.
Since bladder-sparing approaches are an established alternative to radical cystectomy, the medical oncologist should also be trained on how to administer concurrent regimens, including both standard regimens and alternates to consider for patients with impaired renal function. A referral to a medical oncologist with a specific GU focus should be considered, especially for those patients with a creatinine clearance below 60 mL/min.
ii. Metastatic disease
In this setting, medical oncologists should be trained on when to start systemic therapy and when disease could be followed closely. Knowledge and understanding of standard regimens both in first-line and second-line is critical, and in this respect, medical oncologists need to also be aware of ongoing and upcoming trials and to refer their patients for trials wherever possible. Finally, the medical oncologist should be involved in referring to palliative care at the most appropriate time.
C. GU radiation oncology training perspective
i. Clinical assessment
Particular note needs to be taken of irritable bladder symptoms that affect baseline bladder capacity and function. Radiation oncologists should be trained in the interpretation of investigations and other evaluations, including the results of cystoscopy, examination under anesthesia (EUA), and TURBT with bladder mapping and “random” biopsies. The importance of the extent of carcinoma in situ (CIS) and presence of hydronephrosis should also be appreciated.
ii. Patient selection
It is pivotal that the radiation oncologist be trained in the appropriate selection of patients for bladder-conserving therapy and to advise cystectomy prudently where organ preservation is inappropriate. Competent care of the patient requires an understanding of the toxicity associated with bladder cancer radiotherapy, specifically with respect to the rectum, small bowel, bone marrow, and the bladder itself. The radiation oncologist should also be familiar with the other modalities used in the treatment of bladder cancer, including surgical and systemic options, and their role relative to radiation therapy.
iii. Technical issues
One of the key areas of focus for radiation oncologists is the interpretation of diagnostic imaging and in particular as it relates to the radiation treatment planning. This includes the concept of identifying the gross tumour volume (GTV), which is derived from information gleaned at cystoscopy and bladder mapping and complemented by various imaging techniques (e.g., CT, magnetic resonance imaging [MRI]). The clinical target volume (CTV) includes the GTV, plus a margin for subclinical disease that cannot be imaged. Finally, a planning target volume is derived that combines the GTV and relevant CTV and additionally accounts for variability in anatomical position and bladder filling. Contemporary radiotherapy may require consideration of modifying treatment plans to accommodate anatomical daily changes by employing image-guided radiotherapy. The trainee needs to be aware of the role of partial bladder irradiation either solely or as a boost after initial whole bladder treatment. Familiarity with 1) the controversies associated with elective pelvic nodal irradiation; 2) the evolving place of radiation therapy in treating high-grade T1 bladder cancer; 3) the historical data concerning the use of radiation therapy in the pre- and postoperative settings; 4) the crucial integration with systemic treatment; and 4) the recognition of the important palliative benefits of radiotherapy using different fractionation schedules is important in the clinical armamentarium that allows the radiation oncologist to offer the bladder cancer patient the most appropriate use of radiation.
iv. Impact of radiation oncology training on outcomes
To date, there is no data correlating radiation oncology practitioner experience with treatment outcomes for bladder cancer patients, be it tumour control or toxicity. However, investigation in other tumour sites has shown that increasing radiotherapy practitioner volumes are correlated with better outcomes. For example, in head and neck cancers (HNC), a population-based study in the U.S. evaluated the influence of radiation oncologist experience on outcomes in patients with HNC treated with intensity-modulated radiation therapy (IMRT) compared with patients with HNC treated with conventional radiation therapy.31 There was no significant correlation between provider volume and patient survival among patients treated with simpler, less sophisticated conventional radiotherapy. However, among patients receiving IMRT, those treated by higher-volume radiation oncologists had improved survival compared with those treated by low-volume providers (HR 0.79; 95% CI 0.67–0.94). For HNC-specific mortality, the risk was even more dramatically reduced (HR 0.68; 95% CI 0.50–0.91). In terms of toxicity, rates of aspiration pneumonia were also significantly lower among more experienced providers (HR 0.72; 95% CI 0.52–0.99). It is plausible that not only sound clinical decision making, but also technical bladder cancer radiotherapy delivery is improved with increasing patient volumes.
D. GU pathology training perspective
i. New recommendations
In the time since the first Bladder Cancer Quality of Care Meeting in 2014, there have been three major pathology-related works undertaken: The eighth edition of the American Joint Committee on Cancer (AJCC) staging manual;32 the fourth edition of the World Health Organization (WHO)’s Classification of Tumours of the Urinary System and Male Genital Organs;33 and the ongoing publication of pathology reporting datasets of the International Collaboration on Cancer Reporting (ICCR).34 The ICCR was founded by major pathology organizations from around the world, including the College of American Pathologists and the Canadian Association of Pathologists, in order to produce internationally standardized and evidence-based datasets for the pathology reporting of cancer. Using the ICCR datasets, pathology elements are divided into mandatory/core elements, those being validated and important for clinical management, staging and prognosis, and recommended/non-core elements, which may be clinically important and recommended as good practice but are not yet validated or regularly used in patient management. Although only minor differences are anticipated between the upcoming ICCR and the adopted 2014 BCC-CUOG-CUA bladder cancer TURBT datasets, potential alignment of the latter to the ICCR recommendations may be needed, especially in historically controversial issues, such as reporting the percentage of variant histology, the definition of CIS in the context of a concomitant papillary high-grade lesion, and the optimal method of substaging T1 disease.
ii. Role of subspecialized pathologist
Existing data show that obtaining a second opinion on TURBT from a dedicated GU pathologist leads to treatment changes in a non-negligible proportion of bladder cancer patients.35 One study involved a second review of 1191 transurethral biopsies of the bladder by dedicated GU pathologist.35 Of the 1191 biopsies reviewed, the second opinion provided a pathological change in 27.4% of patients, including change in grade, stage, presence of muscularis propria, presence of CIS, lymphovascular invasion, variant histology, or non-urothelial tumour type (Table 2). Treatment alterations based on the findings were also relatively common, occurring in 182 of the 1191 patients (Table 2). A similar recent study showed a change in management in 35% of bladder cancer patients post-TURBT revision, with some of the changes being major (initiation or avoidance of radical cystectomy), mainly based on differences in stage assessment.36 In fact, a compilation of seven studies looking into the value of second pathology opinion in bladder cancer patients shows a significant upstaging (T1 to T2) in 4.8% of cases and significant downstaging (T2 to <T2) in 14% of patients.37–43
Table 2.
Change in pathology report and treatment based on pathological second opinion from a GU-focused pathologist
| n (%) | |
|---|---|
| Pathological changes on review | |
| Stage | 115/1191 (9.7) |
| Grade | 62/1191 (5.2) |
| Presence or absence of CIS | 34/1191 (2.9) |
| Presence of lymphovascular invasion | 35/620 (5.6) |
| Mixed, variant, or nonurothelial histology | 114/212 (53.8) |
| Treatment alterations | |
| Any treatment alteration | 182/1191 (15.3) |
| Major alteration in treatment | 141/1191 (11.8) |
| Change for radical cystectomy | 82/1191 (6.8) |
| Change in primary tumour type | 38/1191 (3.2) |
| Change in systemic chemotherapy regimen | 21/1191 (1.8) |
| Minor alteration in treatment | 41/1191 (3.4) |
CIS: carcinoma in situ; GU: genitourinary.
At the second Bladder Cancer Quality of Care Meeting, participants discussed the feasibility of having all specimens reviewed by a dedicated GU pathologist. It was decided that this was too time-consuming, would introduce further delays, and would be too costly. Instead, a set of scenarios in which an expert pathology opinion be considered were identified, some related to tumour type, and others related to grade and stage.
Among the scenarios related to tumour type that were discussed is the confirmation of the presence of variant histology, especially those with prognostic implications (e.g., micropapillary; plasmacytoid). Since variant histologies are more likely to be identified by GU-focused pathologists than general pathologists, the latter are encouraged to seek a second opinion whenever this possibility is suspected.35,44 In addition, new variants such as the “large nested” variant of invasive urothelial carcinoma (UC), have been described. This deceptive variant, which behaves similar to usual invasive UC, can be easily mistaken for low-grade Ta (TaLG) with inverted growth pattern due its large nests’ sizes, its bland appearance, and the lack of surrounding stromal reaction.33,45 Therefore, pathologists and urologists are encouraged to ask for a second opinion whenever a Ta tumour shows extensive inverted growth pattern or if the possibility of this variant is considered. Another important distinction that may warrant an expert’s opinion, as it impacts the preoperative management, is between UC with extensive squamous differentiation and pure squamous-cell carcinoma — a distinction that may not always be possible in a TURBT specimen.
A GU pathologist report may be of value in relation to specific questions of tumour grading. The AJCC, WHO, and ICCR recommend the mandatory reporting of grade according to the modified 2004 WHO system for any Ta tumour. The inclusion of other grading systems, such as the WHO 1973 system, is considered optional.32–34 The 1973 three-tier grading system is valid and still in use at the institutional or regional levels, especially in Europe, where a 2011 study showed that 43.4% of pathologists were still using the 1973 criteria.46 However, since both systems (2004 and 1973) do not overlap perfectly at the morphological level, and since most management guidelines followed in Canada are based on the 2004 system classification (TaLG vs. TaHG), it was felt that Ta cases in which the grading is only reported using the 1973 criteria should undergo an expert review.
Sometimes the pathologist is uncertain about the appropriate grade of a Ta tumour because the high-grade areas are focal or because the cytological features are borderline between low-grade and high-grade. Due to the limited data available, the prevailing approach that is currently advocated by the WHO is to grade a lesion based on the highest grade present regardless of its percentage, but to mention the percentage of the high-grade component, especially if it is focal.33 With respect to staging, and in addition to the routine review of all T1 and T2 tumours previously advocated by our group, it was felt that all cases should be reviewed if there is any challenges related to histological staging (e.g., Ta vs. T1 on TUR-BT, or T2 vs. T3 on radical cystectomy).
The growing complexity of the diagnostic and therapeutic fields in bladder cancer coupled with the continuous histological refinement of known entities, as well as the emergence of new entities and classifications, make the integration of the latest knowledge in GU pathology training a necessity. An additional important question that needs to be addressed — independent from the recommendations related to the value of a GU pathologist’s second opinion — is the definition of an expert in GU pathology. In light of the absence of subspecialized surgical pathology certification examinations and diplomas, at least in Canada and the U.S., what constitutes a GU pathologist becomes a complex issue in which multiple elements should be taken into account. These elements include the completion of fellowship training, the extent and duration of exposure to subspecialized pathology material, the recognition within the pathology community as an expert and/or a consultant, as well as academic achievements and contribution to the field.
5. Update on bladder cancer Centres of Expertise
The concept of bladder cancer Centres of Excellence was first discussed at the First Bladder Cancer Quality of Care Meeting.1 Before and during that event, the participants developed and proposed the defining characteristics of a Centre of Excellence.1
Since that first meeting, the criteria agreed upon were circulated as a brief online survey to members of the working group representing most academic centres across Canada to evaluate the feasibility of the definitions proposed. The results of the survey were presented and discussed during the second Bladder Cancer Quality of Care meeting held in November 2016.
The survey highlighted several points of discussion regarding the definitions, thresholds, and subjectivity of some of the criteria. It was agreed that the term Centres of Excellence be replaced by Centres of Expertise. Furthermore, it was suggested that two categories of such centres be distinguished; namely those with both a clinical and an academic expertise with personnel dedicated to bladder cancer care, clinical innovation, and research (comprehensive centre of expertise in bladder cancer), and those with mainly clinical expertise (clinical centre of expertise in bladder cancer). With respect to the important criteria of interdisciplinary clinical care, the participants observed that true integrated interdisciplinary care in a common clinical setting is rare in Canada, and thus it was recommended that this criterion be more inclusive to any type of interdisciplinary activity focused around clinical care, including tumour boards, for example. Refined sets of criteria for the two types of centres are illustrated in Table 3.
Table 3.
Defining bladder cancer Centres of Expertise
| A. Comprehensive centre of expertise | ||
|---|---|---|
| Criteria | Method of evaluation | |
| A. A Level 1 Centre of Expertise in bladder cancer in Canada is defined as a healthcare institution that provides comprehensive clinical care for patients diagnosed with all stages of bladder cancer, with the following: | ||
| i. | Availability of a team of health professionals dedicated to bladder cancer (defined as postgraduate training, majority of practice, or academic focus), including one or more of each of the following: | CV |
| a. Urologic oncologist | ||
| b. Radiation oncologist | ||
| c. Medical oncologist | ||
| d. Genitourinary pathologist | ||
| e. Genitourinary radiologist | ||
| f. Nurse practitioner, navigator, or pivot nurse | ||
| With availability of the following professional services | ||
| g. Colorectal, vascular, gynecologic, plastic surgeons with expertise in reconstruction | Letters of attestation | |
| h. Intensive/critical care | ||
| i. Interventional radiology | ||
| j. Advanced imaging (MRI, PET) | ||
| k. Stoma therapy | ||
| l. Clinical psychology/sexology | ||
| m. Social work | ||
| n. Supportive and palliative care | ||
| ii. | Provides guidance and support to a regional network of primary and secondary care urologists and other physicians | Documentation, letters of attestation |
| iii. | Serves as a referral centre for complex genitourinary cancer patient care | |
| iv. | Provides care in an interdisciplinary fashion | Letters of attestation clinic schedules, minutes of interdisciplinary team meetings |
| v. | Establishes or adopts, and adheres to evidence-based standards | Guidelines, institutional |
| vi. | Conducts regular multidisciplinary tumour boards or conferences with the presence at minimum of urologist(s), medical oncologist(s), radiation oncologist(s), radiologist, and GU pathologist | Minutes, schedules |
| vii. | Conducts clinical trial research in bladder cancer | Documentation of active trials in past 3 years |
| viii. | Publishes clinical and/or laboratory-based research in bladder cancer | List of publications over last 3 years |
| ix. | Measures and reports several indicators of clinical performance, including outcomes, compliance to guidelines, etc. that can be benchmarked | Quality of care audits with benchmarks over past 2 years |
| x. | Provides education to trainees, nurses, and continued medical education | Documentation |
| xi. | Royal College-accredited training program in urology, radiation oncology and medical oncology in good standing | Letters of attestation |
| xii. | Promotes bladder cancer public awareness, early diagnosis, and prevention | Documentation |
| xiii. | Actively participates in a nationwide network of bladder or genitourinary cancer Centres of Expertise and in patient groups | Documentation |
| xiv. | The centre manages greater than the annual minimum case load in the following: | Case logs or equivalent over past 3 years |
| a. Radical cystectomy: 25 | ||
| b. Continent urinary diversion: 5 | ||
| c. Radiation-based definitive treatment: 5 | ||
| B. Clinical Centre of Expertise | ||
| Criteria | Method of evaluation | |
| B. A Level 2 Centre of Expertise in bladder cancer in Canada is defined as a healthcare institution that provides comprehensive clinical care for patients diagnosed with all stages of bladder cancer, with the following: | ||
| i. | Availability of a team of health professionals dedicated to urologic oncology (defined as postgraduate training, majority of practice, or academic focus), including one or more of each of the following: | CV |
| a. Urologic oncologist | ||
| b. Radiation oncologist | ||
| c. Medical oncologist | ||
| d. Genitourinary pathologist | ||
| e. Nurse practitioner, navigator, or pivot nurse | ||
| With availability of the following professional services | ||
| f. Colorectal, vascular, gynecologic, plastic surgeons with expertise in reconstruction | Letters of attestation | |
| g. Intensive/critical care | ||
| h. Interventional radiology | ||
| i. Advanced imaging (MRI, PET) | ||
| j. Stoma therapy | ||
| k. Clinical psychology/sexology | ||
| l. Social work | ||
| m. Supportive and palliative care | ||
| ii. | Provides care in an interdisciplinary fashion | Letters of attestation, clinic schedules, minutes of interdisciplinary team meetings |
| iii. | Adheres to evidence-based standards of practice and guidelines | Guidelines |
| iv. | Conducts regular multidisciplinary tumour boards or conferences with the presence of urologist(s), medical oncologist(s), radiation oncologist(s), radiologist, and pathologist | Minutes, schedules |
| v. | Measures and reports several indicators of clinical performance, including outcomes, compliance to guidelines, etc. | Quality of care audits with benchmarks over past 2 years |
| vi. | Provides education to trainees, nurses, and continued medical education | Documentation |
| vii. | Promotes bladder cancer public awareness, early diagnosis, and prevention | Documentation |
| viii. | Actively participates in a nationwide network of bladder or genitourinary cancer Centres of Expertise and in patient groups | Documentation |
| ix. | The centre manages greater than the annual minimum case load in the following: | Case logs or equivalent over past 3 years |
| a. Radical cystectomy: 25 | ||
| b. Continent urinary diversion: 5 | ||
| c. Radiation-based definitive treatment: 5 | ||
The next step for this initiative is to validate each of the criteria and develop a two-tier approach for Centres of Expertise. Additionally, the quality indicators currently in development (see above, section 2) should be linked to this initiative to assess the performance of centres across Canada. Participants suggested that each of the centres across Canada be invited to participate in a more elaborate and formal survey assessing compliance to the criteria for designation.
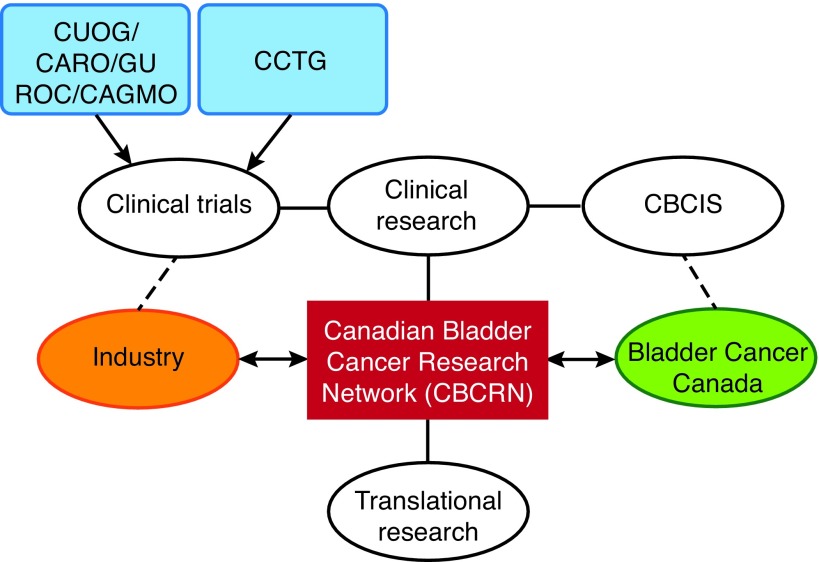
6. Bladder Cancer Research Network of Excellence
Another topic discussed at the 2016 Bladder Cancer Quality of Care Meeting was the desirability and feasibility of developing and implementing a formal Canadian Bladder Cancer Network (Fig. 1). It was discussed that the current environment is ripe for this initiative, as bladder cancer is evolving rapidly, there is a critical mass of bladder cancer expertise and research across Canada, there is a strong and growing advocacy in the field with BCC, and there is interest from the pharmaceutical industry (with potential arms-length funding of translational and clinical research, as well as investigator-initiated trials).
Fig. 1.
Conceptual framework of a Canadian Bladder Cancer Research Network. CAGMO: Canadian Association of Genitourinary Medical Oncologists; CARO: Canadian Association of Radiation Oncology; CBCIS: Canadian Bladder Cancer Information System; CCTG: Canadian Cancer Trials Group; CUOG: Canadian Urologic Oncology Group; GUROC: Genitourinary Radiation Oncologists of Canada.
The potential for the research network to develop a collaborative strategy to maximize Canadian input in key bladder cancer trials was discussed. This would include especially trials on therapies developed in Canada and Canadian-led investigator initiated trials, followed by those of the Canadian Cancer Trials Group (CCTG), and finally industry trials.
The clinical and translational research projects of the network would be collaborative efforts across Canadian centres, building on the strengths and core infrastructures unique to each centre. Translational initiatives could include investigations of the immunology of bladder cancer, a national collaborative to investigate molecular subtyping of MIBC to predict response to neoadjuvant chemotherapy, correlative studies related to clinical trials, and the establishment of a virtual biobank. The network would also serve to attract PhD researchers to the bladder cancer field.
There are multiple grant opportunities available that could be accessed by the network, including those offered by the Terry Fox Research Institute (e.g., the New Frontiers Program Project and Translational Program), the U.S. Congressionally Directed Medical Research Program, and Genome Canada, which could fund large-scale applied research projects.
The path forward towards the establishment of the research network will involve: formalizing the structure (e.g., using other research networks as a template); establishing the mission, objectives, governance, and steering committee; when to begin implementing the network; securing funding; and determining the area(s) of focus.
During the deliberations at the 2016 Bladder Cancer Quality of Care Meeting, the participants suggested that the initiative could begin immediately, with CUOG and CUA members performing their upcoming research projects using a common name (e.g., the Canadian Bladder Cancer Network).
7. Next steps
The committee members concluded the session with a discussion of the next steps towards implementation of the Bladder Cancer Quality of Care initiative.
The first step is to establish benchmarks and measure the 13 selected quality of care indicators, which, it was estimated, would take up to two years to complete. The panelists agreed that this process should be as inclusive as possible, targeting all centres that treat bladder cancer in Canada, but in a stepwise manner, starting with the larger institutions and working towards smaller centres.
With respect to who would lead this initiative, the participants recommended a multi-organizational approach, with members of this working group soliciting and obtaining the endorsement of the CUA, CUOG, Genito-Urinary Radiation Oncologists of Canada (GUROC), and Genito-Urinary Medical Oncologists of Canada (GUMOC). It was suggested that CUOG should be at the core of this group.
With respect to data acquisition, the panelists discussed using the CBCIS infrastructure to capture some of the information on the selected quality indicators. Funding for this initiative might be available from the existing CBCIS funding structure, but the panelists also thought that approaching government for additional funding would be worthwhile. However, it was acknowledged that the funding levels would likely vary widely from province to province. In making funding requests, the participants agreed that there needs to be a specific budget requested from a united front. It was suggested that a letter be prepared in support of the CBCIS and the Quality of Care initiative and signed by the CUOG executive board. The desirability of approaching pharmaceutical industry partners was also discussed, and some panelists felt that this could lead to a sustainable funding solution for the next five to seven years.
The panelists also discussed what to do once the indicators were measured. They debated how to move from a model of care where there are many smaller centres with small volumes of patients with bladder cancer to the model where care is centralized in fewer, higher-volume institutions. Some participants believed that this would happen organically, as professionals at some low-volume centres will voluntarily shed their smaller number of cases to larger centres. Other participants pointed out that this has already largely been done in some regions with respect to MIBC patients in particular. Even in those areas, however, care of patients with complex NMIBC has yet to be centralized.
It was agreed that part of the process needs to be an educational push, making it clear that changing patterns of care to a more centralized model is in the best interest of the patients. Part of this educational effort would be to disseminate to Canadian healthcare professionals the data discussed in this summary, which show that treatment in higher-volume expert centres with multidisciplinary care leads to better outcomes.
It was pointed out that the process of improving care in bladder cancer might not simply be a shift to established high-volume expert centres, but may also include the creation of new local networks and enhancing care at an existing lower-volume centre or newly establishing a high-volume expert centre.
There was also some discussion regarding the feasibility and desirability of changing urology training going forward such that the care of complex bladder cancer is removed from general training and restricted to subspecialty training. The panelists held a variety of opinions on this topic, with no consensus emerging.
Acknowledgment
Dr. Kassouf is a recipient of a Research Scholar Award from the FRSQ. Financial support for the consensus meeting was provided by BCC, CUOG, and CUA.
Footnotes
Competing interests: Dr. Kassouf has received grants/honoraria from Amgen, Astellas, and Janssen. Dr. Aprikian has received grants/honoraria from Abbvie, Amgen, Astellas, and Janssen; and has participated in clinical trials supported by Astellas. Dr. Saad has attended advisory boards for and received payment/honoraria from AbbVie, Amgen Astellas, Bayer, Janssen, and Sanofi; and has participated in clinical trials supported by Amgen Astellas, Bayer, Janssen, and Sanofi. Dr. Izawa has received grants/honoraria from Abbott, AstraZeneca, Astellas, Janssen, Sanofi, and Pfizer. Dr. Eapen has received grants/honoraria from Abbott and AstraZeneca; and has participated in numerous clinical trials. Dr. Fairey has received speaker honoraria from J&J and Roche. Dr. So has been a speaker for Amgen, Astellas, and Janssen. Dr. North has attended advisory boards for Astellas; has received grants/honoraria from Astellas, Janssen, and Sanofi; and has participated in clinical trials supported by Astellas, Janssen, and Sanofi. Dr. Rendon has attended advisory boards and has been a speaker for Amgen, Astellas, Ferring, and Janssen. Dr. Sridhar has attended advisory boards for Astellas; has received grants/honoraria from Astellas, Janssen, and Sanofi; and has participated in clinical trials for Agenisys, Imclone, OGX, Roche, and Sanofi Aventis. Dr. Chung has received grants/honoraria from Sanofi. Dr. Fradet has attended advisory boards for Amgen, Astellas, AstraZeneca, DiagnoCure, and Janssen; has received grants/honoraria from Amgen, Astellas, AstraZeneca, GammaDynacare, and Janssen; and has participated in clinical trials supported by Abbott. Dr. Morash has attended advisory boards for AbbVie, Astellas, Ferring, Janssen, and Sanofi. Dr. Shayegan has received grants/honoraria from AbbVie, Astellas, Janssen, and Sanofi; and has participated in clinical trials supported by Astellas and Janssen. Dr. Gotto has attended advisory boards for Amgen, Astellas, and Janssen; has received honoraria from Amgen, Astellas, Janssen, and Novartis; and has participated in the clinical trials SPARTAN, ENZAMET, and COSMiC. Dr. Siemens has participated in clinical trials supported by Amgen, Astellas, Ferring, and Janssen. Dr. Black has attended advisory boards for AbbVie, Amgen, Astellas, Biocancell, Cubist, Janssen, Novartis, and Sitka; has been a speaker for AbbVie, Janssen, Ferring, Novartis, and Red Leaf Medical; has received grants/honoraria from Pendopharm; has participated in clinical trials supported by Amgen, Astellas, Ferring, Janssen, and Roche; and has received research funding from GenomeDx, iProgen, Lilly, and New B Innovation. The remaining authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Kassouf W, Aprikian A, Black P, et al. Recommendations for the improvement of bladder cancer quality of care in Canada: A consensus document reviewed and endorsed by Bladder Cancer Canada (BCC), Canadian Urologic Oncology Group (CUOG), and Canadian Urological Association (CUA), December 2015. Can Urol Assoc J. 2016;10:E46–80. doi: 10.5489/cuaj.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khare SR, Aprikian A, Black P, et al. Quality indicators in the management of bladder cancer: A modified Delphi study. Urol Oncol. 2017;35:328–34. doi: 10.1016/j.urolonc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Yafi FA, Aprikian AG, Tanguay S, et al. Patients with microscopic and gross hematuria: Practice and referral patterns among primary care physicians in a universal healthcare system. Can Urol Assoc J. 2011;5:97–101. doi: 10.5489/cuaj.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos F, Dragomir A, Kassouf W, et al. Urologist referral delay and its impact on survival after radical cystectomy for bladder cancer. Curr Oncol. 2015;22:e20–6. doi: 10.3747/co.22.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos F, Dragomir A, Kassouf W, et al. Predictors of preoperative delays before radical cystectomy for bladder cancer in Quebec, Canada: A population-based study. BJU Int. 2015;115:389–96. doi: 10.1111/bju.12742. [DOI] [PubMed] [Google Scholar]
- 6.Mahmud SM, Fong B, Fahmy N, et al. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: A population-based study. J Urol. 2006;175:78–83. doi: 10.1016/S0022-5347(05)00070-4. [DOI] [PubMed] [Google Scholar]
- 7.Fahmy NM, Mahmud S, Aprikian AG. Delay in the surgical treatment of bladder cancer and survival: Systematic review of the literature. Eur Urol. 2006;50:1176–82. doi: 10.1016/j.euru-ro.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni GS, Urbach DR, Austin PC, et al. Longer wait times increase overall mortality in patients with bladder cancer. J Urol. 2009;182:1318–24. doi: 10.1016/j.juro.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Fahmy N, Kassouf W, Jeyaganth S, et al. An analysis of preoperative delays prior to radical cystectomy for bladder cancer in Quebec. Can Urol Assoc J. 2008;2:102–8. doi: 10.5489/cuaj.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan RS, Norton DP. The balanced scorecard — measures that drive performance. Harv Bus Rev. 1992;70:71–9. [PubMed] [Google Scholar]
- 11.Kaplan RS, Norton DP. Using the Balanced Scorecard as a strategic management system. Harv Bus Rev. 1996;74:75–85. [Google Scholar]
- 12.Kaplan RS, Norton DP. The balanced scorecard: Translating strategy into action. Harvard Business School Press; Boston, MA: 1996. [Google Scholar]
- 13.Donabedian A. The quality of care: How can it be assessed? JAMA. 1988;260:1743–8. doi: 10.1001/jama.1988.03410120089033. [DOI] [PubMed] [Google Scholar]
- 14.Siemens DR, Mackillop WJ, Peng Y, et al. Processes of care and the impact of surgical volumes on cancer-specific survival: A population-based study in bladder cancer. Urology. 2014;84:1049–57. doi: 10.1016/j.urology.2014.06.070. [DOI] [PubMed] [Google Scholar]
- 15.Brausi M, Collette L, Kurth K, et al. Variability in the recurrence rate at first followup cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: A combined analysis of seven EORTC studies. Eur Urol. 2002;41:523–31. doi: 10.1016/S0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 16.Fahmy N, Aprikian A, Tanguay S, et al. Practice patterns and recurrence after partial cystectomy for bladder cancer. World J Urol. 2010;28:419–23. doi: 10.1007/s00345-009-0478-x. [DOI] [PubMed] [Google Scholar]
- 17.Siemens DR, Mackillop WJ, Peng Y, et al. Lymph node counts are valid indicators of the quality of surgical care in bladder cancer: A population-based study. Urol Oncol. 2015;33:425.e15–23. doi: 10.1016/j.urolonc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Yafi FA, Aprikian AG, Chin JL, et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: A Canadian multicentre experience. BJU Int. 2011;108:539–45. doi: 10.1111/j.1464-410X.2010.09912.x. [DOI] [PubMed] [Google Scholar]
- 19.Booth CM, Siemens DR, Li G, et al. Curative therapy for bladder cancer in routine clinical practice: A population-based outcomes study. Clin Oncol (R Coll Radiol) 2014;26:506–14. doi: 10.1016/j.clon.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Zakaria AS, Santos F, Dragomir A, et al. Postoperative mortality and complications after radical cystectomy for bladder cancer in Quebec: A population-based analysis during the years 2000–2009. Can Urol Assoc J. 2014;8:259–67. doi: 10.5489/cuaj.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Personal communication from Dr. Chris Booth. Jan, 2016.
- 22.Kulkarni GS, Urbach DR, Austin PC, et al. Higher surgeon and hospital volume improves long-term survival after radical cystectomy. Cancer. 2013;119:3546–54. doi: 10.1002/cncr.28235. [DOI] [PubMed] [Google Scholar]
- 23.Bhindi B, Yu J, Kuk C, et al. The importance of surgeon characteristics on impacting oncological outcomes for patients undergoing radical cystectomy. J Urol. 2014;192:714–9. doi: 10.1016/j.juro.2014.02.093. [DOI] [PubMed] [Google Scholar]
- 24.Booth CM, Siemens DR, Li G, et al. Perioperative chemotherapy for muscle-invasive bladder cancer: A population-based outcomes study. Cancer. 2014;120:1630–8. doi: 10.1002/cncr.28510. [DOI] [PubMed] [Google Scholar]
- 25.El-Gehani F, North S, Ghosh S, et al. Improving the outcome of patients with muscle-invasive urothelial carcinoma of the bladder with neoadjuvant gemcitabine/cisplatin chemotherapy: A single institution experience. Can Urol Assoc J. 2014;8:e287–93. doi: 10.5489/cuaj.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu T, Black PC, Chi KN, et al. Treatment of muscle-invasive bladder cancer in Canada: A survey of genitourinary medical oncologists and urologists. Can Urol Assoc J. 2014;8:309–16. doi: 10.5489/cuaj.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachir BG, Aprikian AG, Kassouf W. Are Canadian urology residency programs fulfilling the Royal College expectations?: A survey of graduated chief residents. Can Urol Assoc J. 2014;8:109–15. doi: 10.5489/cuaj.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakaria AS, Haddad R, Dragomir A, et al. Royal College surgical objectives of urological training: A survey of faculty members from Canadian training programs. Can Urol Assoc J. 2014;8:167–72. doi: 10.5489/cuaj.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rourke KF, MacNeily AE. Mapping a competency-based surgical curriculum in urology: Agreement (and discrepancies) in the Canadian national opinion. Can Urol Assoc J. 2016;10:161–6. doi: 10.5489/cuaj.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seah J, Sridhar S, Blais N, et al. Neoadjuvant chemotherapy should be administered to fit patients with newly diagnosed, potentially resectable muscle-invasive urothelial cancer of the bladder: A 2013 CAGMO consensus statement and call for a streamlined referral process. Can Urol Assoc J. 2013;7:312–8. doi: 10.5489/cuaj.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boero IJ, Paravati AJ, Xu B, et al. Importance of radiation oncologist experience among patients with head-and-neck cancer treated with intensity-modulated radiation therapy. J Clin Oncol. 2016;34:684–90. doi: 10.1200/JCO.2015.63.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amin MB, Edge S, Greene F, et al., editors. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017. [DOI] [Google Scholar]
- 33.Moch H, Humphrey PA, Ulbright TM, et al., editors. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. 2016. [DOI] [PubMed] [Google Scholar]
- 34.International Collaboration on Cancer Reporting. Updated recommendations. 2016. [Accessed November 2016]. http://www.iccr-cancer.org/
- 35.Luchey AM, Manimala NJ, Dickinson S, et al. Change in management based on pathological second opinion among bladder cancer patients presenting to a comprehensive cancer centre: Implications for clinical practice. Urology. 2016;93:130–4. doi: 10.1016/j.urology.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 36.Traboulsi SL, Brimo F, Yang Y, et al. Pathology review impacts clinical management of patients with T1–T2 bladder cancer. Can Urol Assoc J. 2017;11:188–93. doi: 10.5489/cuaj.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Der Meijden A, Sylvester R, Collette L, et al. The role and impact of pathology review on stage and grade assessment of stages Ta and T1 bladder tumours: A combined analysis of 5 European Organization for Research and Treatment of Cancer trials. J Urol. 2000;164:1533–7. doi: 10.1016/S0022-5347(05)67022-X. [DOI] [PubMed] [Google Scholar]
- 38.Coblentz TR, Mills SE, Theodorescu D. Impact of second opinion pathology in the definitive management of patients with bladder carcinoma. Cancer. 2001;91:1284–90. doi: 10.1002/1097-0142(20010401)91:7<1284::AID-CNCR1130>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 39.Witjes JA, Moonen PM, van der Heijden AG. Review pathology in a diagnostic bladder cancer trial: Effect of patient risk category. Urology. 2006;67:751–5. doi: 10.1016/j.urology.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Lee MC, Levin HS, Jones JS. The role of pathology review of transurethral bladder tumour resection specimens in the modern era. J Urol. 2010;183:921–7. doi: 10.1016/j.juro.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 41.Wayment RO, Bourne A, Kay P, et al. Second opinion pathology in tertiary care of patients with urological malignancies. Urol Oncol. 2011;29:194–8. doi: 10.1016/j.urolonc.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Giunchi F, Panzacchi R, Capizzi E, et al. Role of inter-observer variability and quantification of muscularis propria in the pathological staging of bladder cancer. Clin Genitourin Cancer. 2016;14:e307–12. doi: 10.1016/j.clgc.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 43.van Rhijn BW, van der Kwast TH, Kakiashvili DM, et al. Pathological stage review is indicated in primary pT1 bladder cancer. BJU Int. 2010;106:206–11. doi: 10.1111/j.1464-410X.2009.09100.x. [DOI] [PubMed] [Google Scholar]
- 44.Shah RB, Montgomery JS, Montie JE, et al. Variant (divergent) histologic differentiation in urothelial carcinoma is under-recognized in community practice: Impact of mandatory central pathology review at a large referral hospital. Urol Oncol. 2013;31:1650–5. doi: 10.1016/j.urolonc.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Cox R, Epstein JI. Large nested variant of urothelial carcinoma: 23 cases mimicking von Brunn nests and inverted growth pattern of non-invasive papillary urothelial carcinoma. Am J Surg Pathol. 2011;35:1337–42. doi: 10.1097/PAS.0b013e318222a653. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Beltran A, Algaba F, Berney DM, et al. Handling and reporting of transurethral resection specimens of the bladder in Europe: A web-based survey by the European Network of Uropathology (ENUP) Histopathology. 2011;58:579–85. doi: 10.1111/j.1365-2559.2011.03784.x. [DOI] [PubMed] [Google Scholar]