Abstract
Children whose mothers experienced childhood abuse are more likely to suffer various neurodevelopmental deficits. Whether an association exists specifically for attention deficit hyperactivity disorder (ADHD) is unknown. We examined the association of maternal experience of childhood abuse with ADHD in offspring, assessed by maternal report of diagnosis and validated with the ADHD Rating Scale-IV in a subsample, in the Nurses’ Health Study II (n = 49,497 mothers; n = 7,607 case offspring; n = 102,151 control offspring). We examined whether 10 adverse perinatal circumstances (e.g., prematurity, smoking) or socioeconomic factors accounted for a possible association. Exposure to abuse was associated with greater prevalence of ADHD in offspring (8.7% of offspring of women exposed to severe abuse vs. 5.5% of offspring of women not abused, P = 0.0001) and with greater risk for ADHD when the model was adjusted for demographic factors (male offspring, risk ratio (RR) = 1.6, 95% confidence interval (CI): 1.3, 1.9; female offspring, RR = 2.3, 95% CI: 1.7, 3.0). After adjustment for perinatal factors, the association of maternal childhood abuse with ADHD in offspring was slightly attenuated (male offspring, RR = 1.5, 95% CI: 1.2, 1.8; female offspring, RR = 2.1, 95% CI: 1.6, 2.8). We identified an association between maternal experience of childhood abuse and risk for ADHD in offspring, which was not explained by several important perinatal risk factors or socioeconomic status.
Keywords: attention deficit hyperactivity disorder, childhood abuse, longitudinal study, maternal factors, neurodevelopment, violence
Accumulating evidence suggests that maternal experience of childhood abuse may affect offspring neurodevelopment. Offspring of women exposed to childhood abuse exhibit elevated risk for internalizing (1–5), externalizing (1, 3, 4, 6–8), and social problems (4, 6), as well as autism spectrum disorder (9). In animal studies, maternal exposure to stressors is associated with anxiety (10, 11), depressive symptoms, attention problems (12), and hyperactivity (13) in offspring, with evidence for mediation through effects of maternal stress hormones on offspring hormonal and neurological stress reactivity (10, 11, 13). Thus, it is possible that maternal exposure to childhood abuse may be associated with elevated risk of attention deficit hyperactivity disorder (ADHD) in offspring.
In addition, women who experience childhood abuse are at elevated risk of many adverse perinatal circumstances that have been associated with offspring ADHD and other neurodevelopmental deficits (9, 14–30). Elevated risk of ADHD has been associated with maternal prenatal tobacco (21, 31), alcohol (20), and antidepressant use (18), prepregnancy body mass index (BMI) (26), gestational diabetes (32), preeclampsia (30), experience of stress during pregnancy (19, 23, 33), prematurity (34), and low birth weight (20, 29). It is possible that increased exposure to these circumstances may be a pathway by which offspring of women who experienced childhood abuse are at elevated risk of ADHD.
In addition, low socioeconomic status (SES) is both a risk factor for childhood abuse (35, 36) and a consequence of childhood abuse (37, 38). Low SES has been associated with increased risk of ADHD in offspring. According to a recent systematic review and meta-analysis, low maternal education, low paternal education, residence in a single-parent family, low income, and low overall SES were all associated with substantially greater risk for ADHD in children (39). However, in the largest study (n > 800,000 children), there was markedly higher ADHD risk among children living in US Census tracts with higher median household income (40), suggesting differential ascertainment by community wealth. Thus, low SES among women who experienced abuse might account for a possible increased risk of ADHD in their children.
One in 10 US children is diagnosed with ADHD, and ADHD is responsible for an estimated $143 billion to $266 billion in excess costs in the United States annually (41). Thus, identification of ADHD risk factors that could offer potential for interventions is of crucial public health significance. In this study, we examined whether maternal exposure to childhood physical, emotional, and sexual abuse is associated with offspring ADHD in a large, longitudinal cohort of women. We also examined whether 1) increased exposure to adverse perinatal circumstances, including maternal smoking, alcohol and antidepressant use, preeclampsia, gestational diabetes, pregestational BMI, prior abortion, intimate partner violence victimization, and suboptimal offspring birth weight and gestation length; and 2) lower SES might account for possible elevated risk of ADHD in offspring of women exposed to childhood abuse.
METHODS
Sample
Nurses’ Health Study II is an ongoing cohort of 116,429 US nurses enrolled in 1989 and followed biennially. Nurses were originally from 14 states and now reside in all 50 states. We included parous women who responded to a supplemental 2001 questionnaire querying about childhood abuse and who later reported in 2013 whether they had ever had a child diagnosed with ADHD (n = 49,497).
Maternal exposure to childhood abuse
Exposure to childhood abuse was assessed in a supplemental 2001 questionnaire. Physical and emotional abuse was assessed with 5 questions from the Physical and Emotional Abuse Subscale of the Childhood Trauma Questionnaire (42). Women were asked whether any of the following had happened to them up to age 11 years: 1) “people in my family hit me so hard it left bruises”; 2) “punishments I received seemed cruel”; 3) “I was punished with a belt, a board, a cord, or some other hard object”; 4) “someone in my family yelled and screamed at me”; 5) “people in my family said hurtful or insulting things to me.” Response options were given point scores as follows: 0, never; 1, rarely; 2, sometimes; 3, often; or 4, very often true. Response options were summed per scoring recommendations. The resulting scale was divided into quartiles to calculate risk ratios and to assess a possible dose-response relationship between maternal abuse and offspring ADHD.
Sexual abuse was assessed with the 2-item Sexual Maltreatment Scale of the Parent-Child Conflict Tactics Scales (43) for each of 2 time periods: up to age 11 years and ages 11–17 years. The questions were 1) “Were you ever touched in a sexual way by an adult or older child, or were you forced to touch an adult or older child in a sexual way when you did not want to?” and 2) “Did an adult or older child ever force you or attempt to force you into any sexual activity by threatening you, holding you down, or hurting you in some way?” Response options were given point scores as follows: never, 0; once, 1; and more than once, 2. Reponses were summed across all 4 questions to create a variable in 4 levels: none, mild (1–2 points), moderate (3–4 points), or severe (≥5 points) sexual abuse. Exposure to high levels of physical, emotional, or sexual abuse may be associated with increased risk of ADHD in offspring; therefore, we created a combined measure of physical, emotional, and sexual abuse by summing the categories (0–3) of each of the 2 measures (range, 0–6).
In addition, 4 items adapted from the Physical Assault Scale of the Conflict Tactics Scales (43) were asked regarding the 2 time periods, before age 11 years and ages 11–17 years. The items asked about the frequency a parent or step-parent did the following: 1) pushed, grabbed, or shoved you; 2) kick, bit, or punched you; 3) hit you with something that hurt; or 4) choked or burned you; and an additional item queried whether the respondent was attacked in some other way. Response options were given point scores as follows: never, 0; once, 1; a few times, 2; and more than a few times, 3. After scoring recommendations, a scale was created by summing these items. Because only part of this scale was queried, we examined its association with offspring ADHD in sensitivity analyses.
ADHD ascertainment
In 2005 and 2013, we asked women whether any of their children had been diagnosed with ADHD; in 2013, women were also asked the birth year of the affected child or children. After the 2005 questionnaire was returned, we randomly selected 200 women who reported having a child with ADHD to participate in a validation study; 137 women responded (68.5%). Of these, 20 women declined to participate and 9 women said their child did not have ADHD, leaving 108 participants. In the validation study, we asked women, “Who made your child’s diagnosis?”, “Has your child been evaluated by a specialist?”, and “Is your child currently taking any medications?” In addition, women were asked to return the ADHD Rating Scale-IV questionnaire. Nearly half the children were diagnosed by a neurologist or psychiatrist (48.8%), with the remainder diagnosed by a pediatrician (38.1%) or another health care provider (13.1%). Most children were currently taking medication (86.9%) and had seen a specialist (85.5%).
Eighty-six women (81.1%) completed the ADHD Rating Scale-IV regarding their child. The ADHD Rating Scale-IV is an 18-item questionnaire assessing the 2 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision factors comprising ADHD: inattention (e.g., “does not seem to listen when spoken to directly”) and hyperactivity-impulsivity (e.g., “has difficulty awaiting turn”) (44). Each response option, ranging from 0 for never to 3 for very often, was summed to create a total score. The ADHD Rating Scale-IV has been validated against the Conners Teacher Rating Scale-39 and the Conners Parent Rating Scale-48 with good correlations (45), has been found to be stable over a 4-week span, and correlates significantly with classroom behavior and task accuracy (46). Although different percentile cutoffs have been used, scores at least in the 80th, 85th, and 90th percentiles are commonly the basis for recommending ADHD diagnostic screening (44, 47). In our validation sample, all girls scored above the 90th percentile, 63.8% of boys scored above the 90th percentile, and 81.1% of boys scored above the 80th percentile. Scores on the ADHD Rating Scale among children diagnosed with ADHD did not differ by maternal abuse exposure (P = 0.88).
Selection of case and control offspring
We included as ADHD cases affected children of women who had indicated the child’s birth year in 2013 and who responded to childhood abuse questions. The ADHD control group comprised children of women who indicated in 2013 that they did not have a child with ADHD and unaffected children of women who reported they had a child with ADHD.
Adverse perinatal circumstances, SES indicators, and demographic factors
In the 2001 questionnaire, women were asked about each of their pregnancies, including the year the pregnancy ended, the outcome (e.g., live birth, miscarriage), whether they smoked or drank alcohol during the pregnancy, whether they were physically hurt by their spouse at any time during the pregnancy (response options were never, once, or more than once), the length of gestation (response options were in 7 intervals from 12–20 weeks to ≥43 weeks), and the resulting child’s sex and birth weight (response options were in 6 intervals from <5 to ≥10 lb). (One pound is equal to 0.45 kg.) Based on their association with offspring ADHD, we coded gestation length, in weeks, as follows: shorter than 37, 37–42, or longer than 42. Birth weight, in pounds, was coded as follows: less than 5.5, 5.5–9.9, or 10 or more. Because birth year was a strong predictor of offspring ADHD, we examined the association of ADHD with birth year, birth year squared, and birth year cubed (e.g., for birth year = 1990, 19902, 19903). The best-fitting model by Akaike Information Criterion included all 3 terms. Lifetime history of induced abortions and age at occurrence were assessed in 1993 and updated biennially.
History of gestational diabetes and year of diagnosis were assessed retrospectively in 1989 and updated biennially. Pregestational BMI (calculated as weight in kilograms divided by height in meters squared) was defined as BMI in the year prior to the child’s birth year. BMI at each age from 18–55 years was calculated from self-reported height in 1989; biennially reported weight, 1989–2013; retrospectively reported weight at age 18 years; and retrospectively reported somatograms for ages 20, 30, and 40 years. For ages at which weight was not reported, BMI was interpolated. Lifetime history and age at occurrence of toxemia or preeclampsia during pregnancy were assessed in 1989 and updated biennially. Mother’s age at child’s birth was categorized in 5 levels (<25, 25–29, 30–34, 35–39, and ≥40 years). Educational attainment of nurses’ parents, queried about in 2005, was used as an indicator of maternal childhood SES.
Beginning in 1989, and biennially thereafter, US Census tract data on median income and percentage college educated were obtained from nurses’ geocoded residential addresses. Marital status was queried in 1989 and every subsequent 4 years. In 1999, nurses’ husbands’ educational attainment was queried (responses were coded as less than high school, high school, some college, 4 years college, and postgraduate). In 2001, nurses reported their standing in the community and the United States by indicating where on two 10-rung ladders they would place themselves (the top rung indicating the highest standing), which are validated measures of subjective social status previously associated with health outcomes (48). Finally, pretax household income was queried in 2001.
Analyses
We first calculated prevalence of ADHD in offspring, adverse perinatal circumstances, socioeconomic indicators, and demographic factors by maternal exposure to combined childhood physical, emotional, and sexual abuse. Next, to examine risk of ADHD in offspring by maternal exposure to childhood abuse, adjusted for demographic factors, we calculated risk ratios for ADHD case status with maternal exposure to 1) childhood physical and emotional abuse, and 2) childhood sexual abuse as the independent variable in separate models. To ascertain whether joint exposure to physical, emotional, and sexual abuse was associated with offspring ADHD, we calculated risk ratios for ADHD case status with the joint measure of physical, emotional, and sexual abuse as the independent variable. To ascertain the extent to which higher prevalence of adverse perinatal circumstances in women who experienced childhood abuse might account for increased risk of ADHD in their offspring, we further adjusted this model for maternal smoking, alcohol use, antidepressant use, prior abortion, gestational diabetes, pregestational BMI, preeclampsia, gestation length, exposure to intimate partner violence, and offspring birth weight.
To ascertain whether family SES might account for an association between maternal abuse and ADHD in offspring, we further adjusted for socioeconomic indicators. Because many women chose not to report household income (n = 19,169, 17.5%), we separately examined the association of abuse with ADHD additionally adjusted for household income in the subsample with income data. For children born substantially before cohort enrollment in 1989, socioeconomic indicators may not have reflected circumstances during early childhood, when such factors might affect risk of ADHD. Therefore, we conducted additional analyses restricted to births in 1986 and later (n = 5,135 case offspring; n = 55,891 control offspring), and we adjusted for all socioeconomic indicators, using marital status, median income according to the US Census tract, and percentage college educated in the year the child was 3–4 years old. To determine whether associations of maternal abuse and ADHD differed by child’s sex, we included models a term for maternal abuse by child’s sex. This term was statistically significant in all models; therefore, we present results stratified by child’s sex.
We then assessed the extent to which adverse perinatal factors and socioeconomic indicators accounted for possible increased risk of ADHD in offspring of women exposed to childhood abuse. We first calculated the mediation percentage for each level of abuse separately (% mediation = (β estimate abusebase model − β estimate abuseadjusted for perinatal factors or socioeconomic indicators)/β estimate abusebase model), then calculated the total mediation percentage by taking a weighted average using the mediation percentage at each level weighted by the number of women exposed at that level.
We conducted sensitivity analyses examining the association of offspring ADHD with maternal experience of physical abuse measured by the Physical Assault Scale of the Conflict Tactics Scales (49). Maternal childhood abuse has been associated with offspring autism spectrum disorder in this cohort (9), so to ascertain whether associations between maternal childhood abuse and offspring ADHD were independent of offspring autism, we conducted an analysis excluding all children whose mothers reported having a child with autism (n = 414 ADHD case offspring; n = 1,603 ADHD control offspring). Participants in the Nurses’ Health Study II are predominantly non-Hispanic white (95.4%); therefore, to examine whether associations were similar among racial or ethnic minorities (n = 4,995), we conducted an analysis excluding non-Hispanic whites.
All analyses were conducted using SAS (SAS Institute, Inc., Cary, North Carolina). We calculated risk ratios using cluster-weighted generalized estimating equations implemented in PROC GENMOD to account for family clustering, using the inverse of the number of children as a weight to account for potential informative clustering; a Poisson distribution; and a log link (50). All models were adjusted for offspring birth year, the maximum of maternal parents’ education, and mother’s age at child’s birth.
RESULTS
Nearly 50,000 mothers responded to questions about childhood abuse and responded to the 2013 questionnaire querying child’s ADHD (n = 49, 497 mothers; n = 7,607 ADHD case offspring; n= 102,151 control offspring). Offspring ADHD and all adverse perinatal factors were more prevalent in women exposed to severe abuse than they were in unexposed women (Table 1). For each indicator of SES, women who experienced abuse had lower status than women who did not (Table 1).
Table 1.
Offspring Attention Deficit Hyperactivity Disorder, Demographic Factors, and Hypothesized Mediators by Maternal Exposure to Childhood Abuse (n = 49,497 Mothers; n = 109,758 Offspring), Nurses’ Health Study II, 1989–2013
| Variable | Maternal Exposure to Childhood Abuse | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (No Abuse) | 1 | 2 | 3 | 4 | 5 | 6 (Severe Abuse) | ||||||||
| % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | |
| Maternal and child characteristics | ||||||||||||||
| Mothers | 13,208 | 10,936 | 9,436 | 9,181 | 4,145 | 1,575 | 1,016 | |||||||
| Offspringa | 29,324 | 24,576 | 20,918 | 20,265 | 9,047 | 3,467 | 2,161 | |||||||
| Offspring with ADHD | 5.5 | 1,606 | 6.5 | 1,608 | 7.0 | 1,461 | 8.0 | 1,626 | 8.9 | 803 | 9.1 | 315 | 8.7 | 188 |
| Offspring per motherb | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.1 (0.9) | |||||||
| Maternal parents’ education, ≤high school | 44.4 | 5,861 | 46.8 | 5,114 | 48.6 | 4,582 | 50.7 | 4,658 | 53.2 | 2,207 | 55.4 | 873 | 54.1 | 550 |
| Male offspring | 52.1 | 15,265 | 52.0 | 12,767 | 51.9 | 10,848 | 51.7 | 10,467 | 51.7 | 4,673 | 52.8 | 1,830 | 51.6 | 1,114 |
| Perinatal factors | ||||||||||||||
| Maternal age at child’s birth, yearsb | 28.9 (4.7) | 28.8 (4.9) | 28.7 (4.9) | 28.5 (5.0) | 28.1 (5.1) | 27.5 (5.4) | 27.0 (5.5) | |||||||
| Birth weight (pounds) | ||||||||||||||
| <5.5 | 3.1 | 918 | 3.0 | 737 | 3.2 | 675 | 3.1 | 638 | 3.5 | 315 | 3.6 | 124 | 3.6 | 78 |
| ≥10 | 2.3 | 689 | 2.4 | 595 | 2.4 | 502 | 2.6 | 519 | 2.8 | 251 | 3.1 | 106 | 3.4 | 73 |
| Pregnancy length, weeks | ||||||||||||||
| <37 | 6.7 | 1,954 | 6.8 | 1,672 | 7.0 | 1,472 | 6.9 | 1,392 | 7.8 | 702 | 7.7 | 266 | 8.7 | 188 |
| ≥43 | 6.3 | 1,845 | 6.9 | 1,703 | 7.1 | 1,493 | 7.9 | 1,596 | 8.3 | 750 | 10.0 | 345 | 10.6 | 229 |
| Smoking during pregnancy | 7.9 | 2,319 | 9.2 | 2,270 | 10.5 | 2,186 | 12.1 | 2,450 | 13.7 | 1,235 | 15.8 | 547 | 15.1 | 326 |
| Gestational diabetes | 2.5 | 743 | 2.9 | 704 | 2.8 | 580 | 3.8 | 761 | 4.0 | 361 | 4.1 | 142 | 4.5 | 98 |
| Pregestational BMIc | ||||||||||||||
| 25.0–29.9 (overweight) | 10.4 | 3,040 | 11.9 | 2,926 | 12.3 | 2,577 | 13.6 | 2,758 | 13.7 | 1,242 | 14.4 | 498 | 15.4 | 333 |
| ≥30.0 (obese) | 3.0 | 875 | 3.2 | 796 | 3.8 | 799 | 4.1 | 836 | 4.6 | 418 | 4.9 | 171 | 5.9 | 127 |
| Antidepressant use | 0.5 | 138 | 0.7 | 161 | 0.9 | 195 | 1.1 | 223 | 1.5 | 134 | 1.4 | 47 | 2.0 | 44 |
| Intimate partner violence | ||||||||||||||
| Once | 0.7 | 194 | 0.9 | 220 | 1.0 | 216 | 1.6 | 324 | 2.1 | 194 | 2.6 | 91 | 3.2 | 70 |
| More than once | 0.4 | 113 | 0.6 | 153 | 0.8 | 173 | 1.4 | 279 | 2.2 | 199 | 2.7 | 94 | 5.2 | 112 |
| Preeclampsia | 3.3 | 969 | 3.7 | 901 | 3.8 | 797 | 4.7 | 958 | 5.4 | 484 | 5.1 | 177 | 7.1 | 154 |
| Prior induced abortion | 9.5 | 2,785 | 11.4 | 2,795 | 11.7 | 2,456 | 13.0 | 2,626 | 15.5 | 1,406 | 14.6 | 506 | 14.7 | 318 |
| Alcohol use during pregnancy | 11.8 | 3,470 | 13.0 | 3,189 | 14.2 | 2,971 | 14.4 | 2,913 | 14.3 | 1,297 | 14.5 | 501 | 14.5 | 314 |
| Socioeconomic indicators | ||||||||||||||
| Husband’s education, 1999, <4 years college | 33.1 | 9,701 | 34.6 | 8,503 | 34.1 | 7,114 | 34.6 | 7,018 | 35.6 | 3,220 | 37.2 | 1,288 | 38.9 | 840 |
| Maternal self-reported social standing in United States, 2001b,d | 3.8 (1.2) | 3.8 (1.3) | 3.8 (1.3) | 3.9 (1.3) | 4.0 (1.3) | 4.1 (1.4) | 4.2 (1.5) | |||||||
| Maternal self-reported social standing in community, 2001b,d | 3.9 (1.5) | 4.0 (1.5) | 4.0 (1.5) | 4.1 (1.6) | 4.2 (1.7) | 4.2 (1.7) | 4.4 (1.8) | |||||||
| Census tract median family income, 1989, $1000sb | 61.9 (22.5) | 61.3 (22.2) | 61.3 (22.4) | 61.5 (21.7) | 59.5 (21.6) | 60.2 (22.6) | 57.8 (20.0) | |||||||
| Census tract college educated, 1989, %b | 30.0 (17.1) | 29.7 (17.0) | 29.6 (17.1) | 29.5 (16.8) | 28.3 (16.7) | 28.6 (17.2) | 26.8 (15.9) | |||||||
| Income, 2001, <$50,000 | 10.3 | 3,033 | 10.8 | 2,656 | 11.6 | 2,427 | 12.4 | 2,508 | 13.8 | 1,249 | 15.7 | 543 | 18.7 | 404 |
| Marital status, 1989, divorced or separated | 5.5 | 1,609 | 5.9 | 1,443 | 6.2 | 1,304 | 7.4 | 1,493 | 9.5 | 859 | 11.5 | 397 | 13.0 | 281 |
Abbreviations: ADHD, attention deficit hyperactivity disorder; BMI, body mass index.
a Offspring birth year by maternal exposure to childhood abuse, median (interquartile range): None: 1984 (1978–1989); 1: 1984 (1979–1989); 2: 1984 (1978–1989); 3: 1984 (1978–1989); 4: 1983 (1977–1988); 5: 1982 (1976–1987); 6: 1981 (1975–1987).
b Values are expressed as mean (standard deviation).
c Weight (kg)/height (m)2.
d Lower number = higher standing.
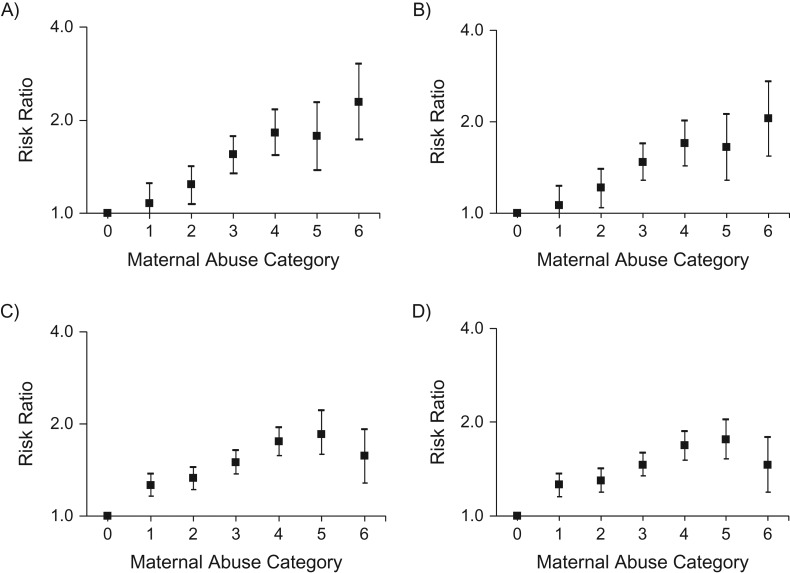
Risk of ADHD was higher in male and female offspring of mothers exposed to either childhood physical and emotional abuse or childhood sexual abuse (Table 2). At the highest levels of maternal abuse, the association with offspring ADHD was stronger among female versus male offspring. In models examining combined exposure to physical, emotional, and sexual abuse, risk of offspring ADHD was higher at each level of abuse compared with women who did not experience abuse, except the lowest level of abuse for female offspring (for severe abuse in female offspring, relative risk (RR) = 2.29, 95% confidence interval (CI): 1.73, 3.04 (Figure 1A); in male offspring, RR = 1.57, 95% CI: 1.28, 1.92 (Figure 1B). After adjustment for adverse perinatal factors, risk of ADHD remained elevated at all except the lowest level of abuse for female offspring (for female offspring, RRsevere abuse = 2.05, 95% CI: 1.54, 2.72 (Figure 1C); for male offspring, RRsevere abuse = 1.46, 95% CI: 1.19, 1.79 (Figure 1D)). Adverse perinatal factors statistically accounted for 8.6% of the increased risk of ADHD in offspring of women exposed to childhood abuse (11.6% in female and 5.5% in male offspring).
Table 2.
Maternal Exposure to Childhood Sexual or Physical and Emotional Abuse and Risk of Attention Deficit Hyperactivity Disorder in Offspring, Nurses’ Health Study II, 1989–2013a
| Exposure | Female Offspring | Male Offspring | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Offspring | No. of Case Offspring | Risk Ratio | 95% CI | No. of Offspring | No. of Case Offspring | Risk Ratio | 95% CI | |
| Model 1 | ||||||||
| Maternal childhood physical and emotional abuse, quartile | ||||||||
| First (none) | 18,380 | 606 | 1.00 | Referent | 20,200 | 1,576 | 1.00 | Referent |
| Second | 11,842 | 432 | 1.04 | 0.91, 1.19 | 12,395 | 1,208 | 1.24 | 1.15, 1.35b |
| Third | 10,492 | 447 | 1.27 | 1.12, 1.46b | 11,402 | 1,154 | 1.33 | 1.23, 1.45b |
| Fourth | 12,080 | 651 | 1.70 | 1.51, 1.92b | 12,967 | 1,533 | 1.55 | 1.44, 1.67b |
| Model 2 | ||||||||
| Maternal childhood sexual abuse | ||||||||
| None | 35,220 | 1,357 | 1.00 | Referent | 37,863 | 3,466 | 1.00 | Referent |
| Mild | 12,606 | 514 | 1.15 | 1.03, 1.28c | 13,702 | 1,411 | 1.20 | 1.12, 1.28b |
| Moderate | 3,270 | 166 | 1.40 | 1.17, 1.66d | 3,609 | 407 | 1.35 | 1.21, 1.50b |
| Severe | 1,698 | 99 | 1.69 | 1.35, 2.11d | 1,790 | 187 | 1.24 | 1.06, 1.45d |
Abbreviation: CI, confidence interval.
a The study population included 49,683 mothers, 7,607 case offspring, and 102,151 control offspring. Models were adjusted for maternal age at birth, child’s birth year, and maternal parents’ level of education.
b Wald χ2 significant at P < 0.001.
c Wald χ2 significant at P < 0.05.
d Wald χ2 significant at P < 0.01.
Figure 1.
Maternal exposure to combined physical, emotional, and sexual childhood abuse and risk of attention deficit hyperactivity disorder (ADHD) in offspring (n = 49,683 mothers; n = 7,607 ADHD case offspring; n = 102,151 control offspring), Nurses’ Health Study II, 1989–2013. A) Female offspring and B) male offspring (both models were adjusted for maternal age at child’s birth, birth year, birth year squared, birth year cubed, and maternal parents’ education). C) Female offspring and D) male offspring (both models were additionally adjusted for the following perinatal risk factors: birth weight; gestation length; preeclampsia; gestational diabetes; maternal pregestational body mass index (weight (kg)/height (m)2); maternal perinatal exposure to intimate partner violence; maternal alcohol, tobacco, and antidepressant use; and maternal prior abortion.
Adjustment for socioeconomic indicators only slightly attenuated the association of maternal abuse with ADHD risk in offspring (Table 3). Socioeconomic factors statistically accounted for 3.2% of the increased risk of ADHD in offspring of women exposed to childhood abuse (3.4% in female and 3.0% in male offspring). Further adjustment for household income in addition to other socioeconomic indicators in the subsample with income data did not notably change mediation percentage, nor did mediation differ meaningfully in analyses restricted to children born in or after 1986.
Table 3.
Association of Maternal Childhood Abuse With Attention Deficit Hyperactivity Disorder in Offspring, Base Model and Further Adjusted for Indicators of Socioeconomic Status, Nurses’ Health Study II, 1989–2013
| Category of Maternal Exposure to Childhood Abuse | Female Offspring | Male Offspring | ||||||
|---|---|---|---|---|---|---|---|---|
| Base Modela | Further Adjusted for Indicators of Socioeconomic Statusb | Base Modela | Further Adjusted for Indicators of Socioeconomic Statusb | |||||
| Risk Ratio | 95% CI | Risk Ratio | 95% CI | Risk Ratio | 95% CI | Risk Ratio | 95% CI | |
| 0 (No abuse) | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 1.08 | 0.93, 1.25 | 1.08 | 0.93, 1.25 | 1.27 | 1.16, 1.38c | 1.26 | 1.16, 1.38c |
| 2 | 1.24 | 1.07, 1.43d | 1.23 | 1.06, 1.42c | 1.33 | 1.22, 1.45c | 1.32 | 1.20, 1.44c |
| 3 | 1.55 | 1.35, 1.78d | 1.52 | 1.32, 1.75c | 1.50 | 1.37, 1.64c | 1.48 | 1.35, 1.61c |
| 4 | 1.83 | 1.54, 2.17c | 1.78 | 1.50, 2.11c | 1.75 | 1.57, 1.95c | 1.71 | 1.54, 1.90c |
| 5 | 1.78 | 1.39, 2.29c | 1.71 | 1.33, 2.20c | 1.85 | 1.59, 2.14c | 1.79 | 1.55, 2.08c |
| 6 (Severe abuse) | 2.29 | 1.73, 3.04c | 2.19 | 1.66, 2.90c | 1.57 | 1.28, 1.92c | 1.54 | 1.26, 1.88c |
Abbreviation: CI, confidence interval.
a Adjusted for child’s birth year, maternal age at birth, and educational attainment of mother’s parents.
b Further adjusted for paternal educational attainment, maternal subjective social standing in the United States, maternal subjective social standing in the community, maternal marital status, US Census tract household median income, and US Census tract percentage college educated data.
cP < 0.001.
dP < 0.01.
In sensitivity analyses excluding women who reported having a child with an autism spectrum disorder and all their children (n = 414 ADHD case offspring; n = 1,603 ADHD control offspring), results were nearly identical. Offspring ADHD was strongly associated with maternal physical abuse as measured by the Conflict Tactics Scales (girls, RRsevere abuse = 1.64, 95% CI: 1.44, 1.87; boys, RRsevere abuse = 1.37, 95% CI: 1.26, 1.49; P < 0.0001 for both; Web Table 1, available at https://academic.oup.com/aje). In analyses restricted to racial and ethnic minorities, maternal exposure to abuse remained associated with offspring ADHD, though this did not reach statistical significance (girls, RRsevere abuse = 1.95, 95% CI: 0.71, 5.41; boys, RRsevere abuse = 1.14, 95% CI: 0.51, 2.56).
DISCUSSION
In this large, nonclinical sample, maternal experience of childhood abuse was strongly associated with risk of ADHD in offspring. At the highest level of maternal abuse, we found higher risk of ADHD in female offspring, compared with male offspring, although risk for male offspring was also significantly elevated compared with offspring of women who had not experienced abuse. Given that relatively few women were exposed to this highest level of abuse and that confidence intervals for effect estimate for each sex were highly overlapping, these results should be considered tentative.
Higher prevalence of 10 adverse perinatal circumstances in pregnancies of women exposed to abuse versus those unexposed to abuse accounted for only a small portion of elevated risk of ADHD in the offspring of the former. In addition, lower SES among women who experienced abuse accounted for very little of the higher risk of ADHD in their children. Two other important pathways could account for the association between maternal exposure to childhood abuse and offspring ADHD. First, it is possible that maternal childhood abuse affects the mother’s biology during gestation in ways that increase risk of offspring ADHD. Second, it is possible that experience of childhood abuse is associated with elevated genetic risk for ADHD in mothers or their partners.
Adults who experienced childhood abuse exhibit dysregulation in the hypothalamic-pituitary-adrenal axis (51–55), the hypothalamic-pituitary-thyroid (HPT)-axis (56–61), and immune function (62–69), findings supported by animal studies (70–73). Maternal biological dysregulation of the hypothalamic-pituitary-adrenal axis, the hypothalamic-pituitary-thyroid axis, and immune function has been associated with adverse offspring neurodevelopment. In human studies, dysregulation of these systems has been linked to ADHD (74–76) as well as to delay in expressive language, poorer cognitive functioning, and externalizing behavior (74, 77–85). In animal experiments, dysregulation of these biological systems harms neurodevelopment, causes memory deficits, and induces autistic-like behavior in offspring (86–92). Thus, maternal biological dysregulation during pregnancy may be an important mechanism by which offspring of women abused are at elevated risk of ADHD.
In addition, findings from twin and adoption studies indicate that ADHD is heritable with a strong genetic component (29), and it is possible that maternal experience of childhood abuse indicates genetic loading for ADHD that increases the risk of ADHD for her child. Children with ADHD are more likely to experience abuse (in this case, perhaps the respondent) (93–96), and parents with ADHD may be more likely to perpetrate abuse (perhaps the parents of the respondent), as was found in multiple studies in which impaired parenting in persons with ADHD was reported (93, 97–104). If either of these scenarios were present between the respondent and her parents, the scenario could constitute a genetic contribution to the association between the respondent’s experience of abuse and her child’s risk of ADHD. In addition, we have shown that maternal experience of abuse is associated with mate selection, with greater maternal abuse history predicting greater autistic traits in her spouse (105). If a similar selection occurs for ADHD traits, then an association between maternal experience of abuse and child ADHD could be explained by paternal genetics as well.
Our study has important limitations. As is common in large epidemiologic studies, to limit respondent burden and due to financial constraints, we relied on maternal report of ADHD diagnosis. ADHD, like other childhood neuropsychiatric disorders, may be misdiagnosed fairly often (106–109), which may have biased our results. However, in this cohort of medical professionals, self-report of medical conditions has had good validity (111–113). In addition, women who were strongly affected by their experiences of abuse may not have finished their education and, therefore, would not be eligible for participation in this cohort, which may have biased effect estimates toward the null hypothesis.
It is of public health relevance that we did not find elevated risk of ADHD only among women exposed to severe abuse. Rather, risk was elevated at each level of abuse compared with risk in situations of no abuse. Even offspring of women who experienced the mildest level of abuse were at increased risk of ADHD. Most women in this group (82%) had had hurtful or insulting thing said to them sometimes or more often, and a large minority (31%) had 1 unwanted sexual experience. Only 3.6% of these women were ever hit hard enough to cause bruising. More than 70% of women were exposed to a level of childhood abuse that was associated with increased risk of ADHD in their offspring. Because ADHD is associated in adulthood with lower educational attainment and increased job insecurity, social isolation, criminality, and premature death (113–117), it is important to identify causal factors driving the association between maternal childhood abuse and offspring ADHD. Identifying causal factors could suggest potential interventions for alleviating this risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Andrea L. Roberts, Marc G. Weisskopf); Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, California (Zeyan Liew); A.J. Drexel Autism Institute, Drexel University, Philadelphia, Pennsylvania (Kristen Lyall); and Departments of Nutrition and Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Alberto Ascherio).
The Nurses’ Health Study II is supported by the National Institutes of Health (grant UM1CA176726). Z.L. is supported by National Institutes of Health National Institute of Environmental Health Sciences (grant K99ES026729).
Conflict of interest: none declared.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- BMI
body mass index
- CI
confidence interval
- RR
risk ratio
- SES
socioeconomic status
REFERENCES
- 1. Thompson R. Mothers’ violence victimization and child behavior problems: examining the link. Am J Orthopsychiatry. 2007;77(2):306–315. [DOI] [PubMed] [Google Scholar]
- 2. Jovanovic T, Smith A, Kamkwalala A, et al. . Physiological markers of anxiety are increased in children of abused mothers. J Child Psychol Psychiatry. 2011;52(8):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubowitz H, Black MM, Kerr MA, et al. . Type and timing of mothers’ victimization: effects on mothers and children. Pediatrics. 2001;107(4):728–735. [DOI] [PubMed] [Google Scholar]
- 4. Koverola C, Papas MA, Pitts S, et al. . Longitudinal investigation of the relationship among maternal victimization, depressive symptoms, social support, and children’s behavior and development. J Interpers Violence. 2005;20(12):1523–1546. [DOI] [PubMed] [Google Scholar]
- 5. Roberts AL, Chen Y, Slopen N, et al. . Maternal experience of abuse in childhood and depressive symptoms in adolescent and adult offspring: a 21-year longitudinal study. Depress Anxiety. 2015;32(10):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouvette-Turcot A-A, Bernier A, Meaney MJ. Intergenerational transmission of psychosocial risk: maternal childhood adversity, mother-child attachment, and child temperament. Psychol Belg. 2013;53(3):65–83. [Google Scholar]
- 7. Miranda JK, de la Osa N, Granero R, et al. . Multiple mediators of the relationships among maternal childhood abuse, intimate partner violence, and offspring psychopathology. J Interpers Violence. 2013;28(14):2941–2965. [DOI] [PubMed] [Google Scholar]
- 8. Myhre MC, Dyb GA, Wentzel-Larsen T, et al. . Maternal childhood abuse predicts externalizing behaviour in toddlers: a prospective cohort study. Scand J Public Health. 2014;42(3):263–269. [DOI] [PubMed] [Google Scholar]
- 9. Roberts AL, Lyall K, Rich-Edwards JW, et al. . Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry. 2013;70(5):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koenig JI, Elmer GI, Shepard PD, et al. . Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156(2):251–261. [DOI] [PubMed] [Google Scholar]
- 11. Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 2010;22(4):258–271. [DOI] [PubMed] [Google Scholar]
- 12. Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19(4):296–308. [DOI] [PubMed] [Google Scholar]
- 13. Deminière JM, Piazza PV, Guegan G, et al. . Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586(1):135–139. [DOI] [PubMed] [Google Scholar]
- 14. Anda RF, Felitti VJ, Bremner JD, et al. . The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Froehlich TE, Lanphear BP, Auinger P, et al. . Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124(6):e1054–e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ball SW, Gilman SE, Mick E, et al. . Revisiting the association between maternal smoking during pregnancy and ADHD. J Psychiatr Res. 2010;44(15):1058–1062. [DOI] [PubMed] [Google Scholar]
- 17. Chen Q, Sjölander A, Långström N, et al. . Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study using a sibling-comparison design. Int J Epidemiol. 2014;43(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clements CC, Castro VM, Blumenthal SR, et al. . Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2015;20(6):727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grizenko N, Shayan YR, Polotskaia A. Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. J Psychiatry Neurosci. 2008;33(1):10–16. [PMC free article] [PubMed] [Google Scholar]
- 20. Knopik VS, Sparrow EP, Madden PA, et al. . Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005;35(5):625–635. [DOI] [PubMed] [Google Scholar]
- 21. Langley K, Heron J, Smith GD, et al. . Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am J Epidemiol. 2012;176(3):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mick E, Biederman J, Faraone SV, et al. . Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002;41(4):378–385. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry. 2005;46(3):246–254. [DOI] [PubMed] [Google Scholar]
- 24. Linnet KM, Dalsgaard S, Obel C, et al. . Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160(6):1028–1040. [DOI] [PubMed] [Google Scholar]
- 25. Skoglund C, Chen Q, D´Onofrio BM, et al. . Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. J Child Psychol Psychiatry. 2014;55(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez A, Miettunen J, Henriksen TB, et al. . Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes (Lond). 2008;32(3):550–557. [DOI] [PubMed] [Google Scholar]
- 27. Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8‐and 9‐year‐olds. Child Dev. 2004;75(4):1085–1097. [DOI] [PubMed] [Google Scholar]
- 28. Polańska K, Jurewicz J, Hanke W. Exposure to environmental and lifestyle factors and attention-deficit/hyperactivity disorder in children - a review of epidemiological studies. Int J Occup Med Environ Health. 2012;25(4):330–355. [DOI] [PubMed] [Google Scholar]
- 29. Thapar A, Cooper M, Eyre O, et al. . Practitioner review: what have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord. 2011;15(8):667–673. [DOI] [PubMed] [Google Scholar]
- 31. Button T, Thapar A, McGuffin P. Relationship between antisocial behaviour, attention-deficit hyperactivity disorder and maternal prenatal smoking. Br J Psychiatry. 2005;187(2):155–160. [DOI] [PubMed] [Google Scholar]
- 32. Nomura Y, Marks DJ, Grossman B, et al. . Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med. 2012;166(4):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van den Bergh BR, Mennes M, Stevens V, et al. . ADHD deficit as measured in adolescent boys with a continuous performance task is related to antenatal maternal anxiety. Pediatr Res. 2006;59:78–82. [DOI] [PubMed] [Google Scholar]
- 34. O’Shea TM, Downey LC, Kuban KK. Extreme prematurity and attention deficit: epidemiology and prevention. Front Hum Neurosci. 2013;7:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slack KS, Holl JL, McDaniel M, et al. . Understanding the risks of child neglect: an exploration of poverty and parenting characteristics. Child Maltreat. 2004;9(4):395–408. [DOI] [PubMed] [Google Scholar]
- 36. Lee BJ, Goerge RM. Poverty, early childbearing, and child maltreatment: a multinomial analysis. Child Youth Serv Rev. 1999;21(9):755–780. [Google Scholar]
- 37. Currie J, Widom CS. Long-term consequences of child abuse and neglect on adult economic well-being. Child Maltreat. 2010;15(2):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fang X, Brown DS, Florence CS, et al. . The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl. 2012;36(2):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell AE, Ford T, Williams R, et al. . The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440–458. [DOI] [PubMed] [Google Scholar]
- 40. Getahun D, Jacobsen SJ, Fassett MJ, et al. . Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA Pediatr. 2013;167(3):282–288. [DOI] [PubMed] [Google Scholar]
- 41. Birnbaum HG, Kessler RC, Lowe SW, et al. . Costs of attention deficit–hyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Curr Med Res Opin. 2005;21(2):195–205. [DOI] [PubMed] [Google Scholar]
- 42. Bernstein DP, Fink L, Handelsman L, et al. . Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. [DOI] [PubMed] [Google Scholar]
- 43. Straus MA, Hamby SL, Finkelhor D, et al. . Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abuse Negl. 1998;22(4):249–270. [DOI] [PubMed] [Google Scholar]
- 44. DuPaul G, Power T, Anastopoulos A, et al. . ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 45. Pappas D. ADHD Rating Scale-IV: checklists, norms, and clinical interpretation. J Psychoeduc Assess. 2006;24(2):172–178. [Google Scholar]
- 46. Power TJ, Doherty BJ, Panichelli-Mindel S, et al. . The predictive validity of parent and teacher reports of ADHD symptoms. J Psychopathol Behav Assess. 1998;20:57–81. [Google Scholar]
- 47. Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales V: scales assessing attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(9):1015–1037. [DOI] [PubMed] [Google Scholar]
- 48. Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Soc Sci Med. 2003;56(6):1321–1333. [DOI] [PubMed] [Google Scholar]
- 49. Straus MA, Hamby SL. Measuring physical & psychological maltreatment of children with the Conflict Tactics Scales In: Kaufman Kanter G, Jasinski JL, eds. Out of the Darkness: Contemporary Perspectives on Family Violence. Thousand Oaks, CA: SAGE Publications; 1997. [Google Scholar]
- 50. Seaman SR, Pavlou M, Copas AJ. Methods for observed-cluster inference when cluster size is informative: a review and clarifications. Biometrics. 2014;70(2):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brand SR, Brennan PA, Newport DJ, et al. . The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35(5):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bremner JD, Vythilingam M, Vermetten E, et al. . Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28(6):733–750. [DOI] [PubMed] [Google Scholar]
- 53. Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2007;195(11):919–927. [DOI] [PubMed] [Google Scholar]
- 54. Shea A, Walsh C, MacMillan H, et al. . Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30(2):162–178. [DOI] [PubMed] [Google Scholar]
- 55. Heim C, Newport DJ, Mletzko T, et al. . The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. [DOI] [PubMed] [Google Scholar]
- 56. Sinai C, Hirvikoski T, Nordström AL, et al. . Hypothalamic pituitary thyroid axis and exposure to interpersonal violence in childhood among women with borderline personality disorder. Eur J Psychotraumatol. 2014;5(1). doi: 10.3402/ejpt.v5.23911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friedman MJ, Wang S, Jalowiec JE, et al. . Thyroid hormone alterations among women with posttraumatic stress disorder due to childhood sexual abuse. Biol Psychiatry. 2005;57(10):1186–1192. [DOI] [PubMed] [Google Scholar]
- 58. Plaza A, Garcia-Esteve L, Ascaso C, et al. . Childhood sexual abuse and hypothalamus-pituitary-thyroid axis in postpartum major depression. J Affect Disord. 2010;122(1–2):159–163. [DOI] [PubMed] [Google Scholar]
- 59. Haviland MG, Sonne JL, Anderson DL, et al. . Thyroid hormone levels and psychological symptoms in sexually abused adolescent girls. Child Abuse Negl. 2006;30(6):589–598. [DOI] [PubMed] [Google Scholar]
- 60. Girdler SS, Thompson KS, Light KC, et al. . Historical sexual abuse and current thyroid axis profiles in women with premenstrual dysphoric disorder. Psychosom Med. 2004;66(3):403–410. [DOI] [PubMed] [Google Scholar]
- 61. Machado TD, Salum GA, Bosa VL, et al. . Early life trauma is associated with decreased peripheral levels of thyroid-hormone T3 in adolescents. Int J Dev Neurosci. 2015;47(Pt B):304–308. [DOI] [PubMed] [Google Scholar]
- 62. Danese A, Moffitt TE, Pariante CM, et al. . Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65(4):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dube SR, Fairweather D, Pearson WS, et al. . Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71(2):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith AK, Conneely KN, Kilaru V, et al. . Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(6):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular immune response in women with PTSD related to childhood abuse. Am J Psychiatry. 2003;160(9):1705–1707. [DOI] [PubMed] [Google Scholar]
- 67. Danese A, Moffitt TE, Harrington H, et al. . Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163(12):1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Danese A, Pariante CM, Caspi A, et al. . Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104(4):1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Danese A, Dove R, Belsky DW, et al. . Leptin deficiency in maltreated children. Transl Psychiatry. 2014;4:e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carini LM, Nephew BC. Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Horm Behav. 2013;64(4):634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Murgatroyd CA, Nephew BC. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology. 2013;38(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. O’Mahony SM, Marchesi JR, Scully P, et al. . Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65(3):263–267. [DOI] [PubMed] [Google Scholar]
- 73. Plotsky PM, Thrivikraman KV, Nemeroff CB, et al. . Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–2204. [DOI] [PubMed] [Google Scholar]
- 74. Ghassabian A, Henrichs J, Tiemeier H. Impact of mild thyroid hormone deficiency in pregnancy on cognitive function in children: lessons from the Generation R Study. Best Pract Res Clin Endocrinol Metab. 2014;28(2):221–232. [DOI] [PubMed] [Google Scholar]
- 75. Andersen SL, Laurberg P, Wu CS, et al. . Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014;121(11):1365–1374. [DOI] [PubMed] [Google Scholar]
- 76. Päkkilä F, Männistö T, Pouta A, et al. . The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab. 2014;99(1):E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Henrichs J, Ghassabian A, Peeters RP, et al. . Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf). 2013;79(2):152–162. [DOI] [PubMed] [Google Scholar]
- 78. Haddow JE, Palomaki GE, Allan WC, et al. . Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–555. [DOI] [PubMed] [Google Scholar]
- 79. Wang P, Gao J, Zhao S, et al. . Maternal thyroxine levels during pregnancy and outcomes of cognitive development in children. Mol Neurobiol. 2016;53(4):2241–2248. [DOI] [PubMed] [Google Scholar]
- 80. Willoughby KA, McAndrews MP, Rovet JF. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid. 2014;24(3):576–584. [DOI] [PubMed] [Google Scholar]
- 81. Pop VJ, Brouwers EP, Vader HL, et al. . Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf). 2003;59(3):282–288. [DOI] [PubMed] [Google Scholar]
- 82. Pop VJ, Kuijpens JL, van Baar AL, et al. . Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149–155. [DOI] [PubMed] [Google Scholar]
- 83. Szyf M. The early life social environment and DNA methylation. Epigenetics. 2011;6(8):971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van Mil NH, Tiemeier H, Bongers-Schokking JJ, et al. . Low urinary iodine excretion during early pregnancy is associated with alterations in executive functioning in children. J Nutr. 2012;142(12):2167–2174. [DOI] [PubMed] [Google Scholar]
- 85. Yang BZ, Zhang H, Ge W, et al. . Child abuse and epigenetic mechanisms of disease risk. Am J Prev Med. 2013;44(2):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hava G, Vered L, Yael M, et al. . Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 2006;48(2):162–168. [DOI] [PubMed] [Google Scholar]
- 87. Golan HM, Lev V, Hallak M, et al. . Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48(6):903–917. [DOI] [PubMed] [Google Scholar]
- 88. Meyer U, Feldon J, Schedlowski M, et al. . Immunological stress at the maternal–foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20(4):378–388. [DOI] [PubMed] [Google Scholar]
- 89. Bergeron JD, Deslauriers J, Grignon S, et al. . White matter injury and autistic-like behavior predominantly affecting male rat offspring exposed to group B streptococcal maternal inflammation. Dev Neurosci. 2013;35(6):504–515. [DOI] [PubMed] [Google Scholar]
- 90. Le Belle JE, Sperry J, Ngo A, et al. . Maternal inflammation contributes to brain overgrowth and autism-associated behaviors through altered redox signaling in stem and progenitor cells. Stem Cell Reports. 2014;3(5):725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Malkova NV, Yu CZ, Hsiao EY, et al. . Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26(4):607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bauman MD, Iosif AM, Smith SE, et al. . Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75(4):332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dinkler L, Lundström S, Gajwani R, et al. . Maltreatment‐associated neurodevelopmental disorders: a co‐twin control analysis. J Child Psychol Psychiatry. 2017;58(6):691–701. [DOI] [PubMed] [Google Scholar]
- 94. Briscoe-Smith AM, Hinshaw SP. Linkages between child abuse and attention-deficit/hyperactivity disorder in girls: behavioral and social correlates. Child Abuse Negl. 2006;30(11):1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ouyang L, Fang X, Mercy J, et al. . Attention-deficit/hyperactivity disorder symptoms and child maltreatment: a population-based study. J Pediatr. 2008;153(6):851–856. [DOI] [PubMed] [Google Scholar]
- 96. Capusan AJ, Kuja-Halkola R, Bendtsen P, et al. . Childhood maltreatment and attention deficit hyperactivity disorder symptoms in adults: a large twin study. Psychol Med. 2016;46(12):2637–2646. [DOI] [PubMed] [Google Scholar]
- 97. Johnston C, Mash EJ, Miller N, et al. . Parenting in adults with attention-deficit/hyperactivity disorder (ADHD). Clin Psychol Rev. 2012;32(4):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Arnold EH, O’leary SG, Edwards GH. Father involvement and self-report parenting of children with attention deficit-hyperactivity disorder. J Consult Clin Psychol. 1997;65(2):337–342. [DOI] [PubMed] [Google Scholar]
- 99. Minde K, Eakin L, Hechtman L, et al. . The psychosocial functioning of children and spouses of adults with ADHD. J Child Psychol Psychiatry. 2003;44(4):637–646. [DOI] [PubMed] [Google Scholar]
- 100. Mokrova I, O’Brien M, Calkins S, et al. . Parental ADHD symptomology and ineffective parenting: the connecting link of home chaos. Parent Sci Pract. 2010;10(2):119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sonuga-Barke EJ, Daley D, Thompson M. Does maternal ADHD reduce the effectiveness of parent training for preschool children’s ADHD? J Am Acad Child Adolesc Psychiatry. 2002;41(6):696–702. [DOI] [PubMed] [Google Scholar]
- 102. Biederman J, Faraone SV, Monuteaux MC. Impact of exposure to parental attention-deficit hyperactivity disorder on clinical features and dysfunction in the offspring. Psychol Med. 2002;32(5):817–827. [DOI] [PubMed] [Google Scholar]
- 103. Chronis-Tuscano A, Raggi VL, Clarke TL, et al. . Associations between maternal attention-deficit/hyperactivity disorder symptoms and parenting. J Abnorm Child Psychol. 2008;36(8):1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Karbalaei Sabagh A, Khademi M, Noorbakhsh S, et al. . Adult attention deficit hyperactivity disorder and parenting styles. Indian J Pediatr. 2016;83(3):254–257. [DOI] [PubMed] [Google Scholar]
- 105. Roberts AL, Lyall K, Weisskopf MG. Maternal exposure to childhood abuse is associated with mate selection: implications for autism in offspring. J Autism Dev Disord. 2017;47(7):1998–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25–29. [PubMed] [Google Scholar]
- 107. Lewczyk CM, Garland AF, Hurlburt MS, et al. . Comparing DISC-IV and clinician diagnoses among youths receiving public mental health services. J Am Acad Child Adolesc Psychiatry. 2003;42(3):349–356. [DOI] [PubMed] [Google Scholar]
- 108. Sciutto MJ, Eisenberg M. Evaluating the evidence for and against the overdiagnosis of ADHD. J Atten Disord. 2007;11(2):106–113. [DOI] [PubMed] [Google Scholar]
- 109. Bruchmüller K, Margraf J, Schneider S. Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. J Consult Clin Psychol. 2012;80(1):128–138. [DOI] [PubMed] [Google Scholar]
- 110. Troy LM, Hunter DJ, Manson JE, et al. . The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570–572. [PubMed] [Google Scholar]
- 111. Wolf AM, Hunter DJ, Colditz GA, et al. . Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. [DOI] [PubMed] [Google Scholar]
- 112. Tomeo CA, Rich-Edwards JW, Michels KB, et al. . Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774–777. [PubMed] [Google Scholar]
- 113. Thapar A, Rice F, Hay D, et al. . Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66(8):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kessler RC, Adler L, Barkley R, et al. . The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lee SS, Humphreys KL, Flory K, et al. . Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31(3):328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Barbaresi WJ, Colligan RC, Weaver AL, et al. . Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dalsgaard S, Østergaard SD, Leckman JF, et al. . Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190–2196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.