Abstract
Concentration of 25-hydroxyvitamin D3 (25(OH)D3), the main circulating form of vitamin D, is inversely associated with incident, sporadic colorectal adenoma risk. We investigated whether this association differs by 2 functional variants in the vitamin D–binding protein (DBP) gene, group-specific component (GC), that encode for common protein isoforms Gc1s, Gc1f, and Gc2 linked to differences in vitamin D metabolism. We pooled data (418 patients with adenoma and 524 polyp-free control subjects) from 3 colonoscopy-based case-control studies (Minnesota, 1991–1994; North Carolina, 1994–1997; South Carolina, 2002). We estimated 25(OH)D3–adenoma associations, stratified by DBP isoforms, using multivariable logistic regression. Higher 25(OH)D3 concentrations were inversely associated with colorectal adenoma risk among those with the Gc2 isoform (per 10-ng/mL increase in 25(OH)D3, odds ratio = 0.71, 95% confidence interval: 0.56, 0.90), but not among those with only Gc1 isoforms (odds ratio = 1.07, 95% confidence interval: 0.87, 1.32; P for interaction = 0.03). Thus, the vitamin D–incident, sporadic colorectal adenoma association may differ by common DBP isoforms, and patients with the Gc2 isoform may particularly benefit from maintaining higher circulating 25(OH)D3 concentrations for adenoma prevention.
Keywords: case-control studies, colorectal neoplasms, gene-environment interaction, vitamin D, vitamin D–binding protein
Colorectal cancer is the second most common cause of cancer deaths in the United States (1). Findings from laboratory studies indicate that vitamin D may prevent colorectal neoplastic development by inducing antiproliferative gene expression, activating apoptotic pathways, and inhibiting angiogenesis (2, 3). Circulating 25-hydroxyvitamin D (25(OH)D), considered the best indicator of total vitamin D (the collective term for vitamins D2 and D3) exposure, was inversely associated with risk of colorectal neoplasms in observational studies (1, 4, 5). Two functional variants in the vitamin D–binding protein gene (formerly known as group-specific component (GC)), GC rs4588 and rs7041, encode for 3 common GC protein isoforms that are strongly associated with vitamin D–binding protein (DBP) and 25(OH)D concentrations and influence vitamin D metabolite delivery in colon cancer cell lines (6, 7). However, whether the association of 25(OH)D with colorectal neoplasms differs by these isoforms is unknown.
Vitamin D metabolism and delivery to tissues is complex. Approximately 90%–95% of vitamin D exposure is from sunlight: Ultraviolet B radiation converts 7-dehydrocholesterol to pro-vitamin D3 in the skin (8, 9). Vitamin D3 is hydroxylated in the liver into 25-hydroxyvitamin D3 (25(OH)D3), which enters the circulation and is delivered to tissues, including the colon, where it is hydroxylated to the biologically active 1,25-dihydroxyvitamin D form (9). Approximately 88% of circulating 25(OH)D and 85% of circulating 1,25-dihydroxyvitamin D are bound by the DBP (6, 10). An important role of DBP is to maintain adequate circulating stores of vitamin D, particularly when vitamin D sources are low, by protecting vitamin D from excretion and by prolonging its half-life in circulation (10, 11).
Nearly 80% of the variability in circulating DBP concentrations is determined by the combined genotype at GC rs7041 and rs4588, which encode for 3 common protein isoforms: Gc1s, Gc1f, and Gc2 (6, 12). The associations of these isoforms with 25(OH)D3 concentrations appear to be mediated by their strong association with DBP concentrations (6, 13). DBP and 25(OH)D plasma concentrations are similar among persons with Gc1s and Gc1f isoforms, determined by rs7041’s genotype (12, 14, 15). However, the Gc2 isoform, encoded by rs4588’s minor allele, is associated with lower DBP and 25(OH)D concentrations relative to the Gc1 isoform (combined Gc1s and Gc1f) (12, 15, 16). Findings from recent studies suggest higher serum 25(OH)D concentrations are more strongly inversely associated with colorectal cancer risk among persons with lower DBP concentrations (17) and more strongly inversely associated with diabetes risk among persons with the Gc2 isoform–encoding genotype (18). Given Gc2’s association with lower DBP concentrations, higher circulating 25(OH)D concentrations may be more beneficial among individuals with Gc2 isoforms because higher 25(OH)D concentrations may be needed to compensate for their lower DBP-related capacity to otherwise maintain adequate vitamin D concentrations.
Accordingly, we hypothesized that the inverse association of 25(OH)D3 with incident, sporadic, colorectal adenoma risk would be stronger among persons with the Gc2 isoform (associated with lower DBP and 25(OH)D concentrations) and weaker among persons with only Gc1 isoforms (associated with higher DBP and 25(OH)D concentrations). In one of the largest, pooled, US case-control studies of incident, sporadic colorectal adenomas (n = 616 patients with adenoma, n = 770 polyp-free control subjects), we found a statistically significant inverse association of circulating 25(OH)D3 concentrations with colorectal adenoma risk (4). Using data from this same study population, we investigated in the present study associations of GC genotypes or isoforms with 25(OH)D3 concentrations, and associations of 25(OH)D3 concentrations with risk for colorectal adenoma according to GC genotypes or isoforms.
METHODS
Study population
Study participants were part of 3 colonoscopy-based case-control studies conducted by the same principal investigator in Minnesota (Cancer Prevention Research Unit (CPRU) Study), North Carolina (Markers of Adenomatous Polyps-I (MAPI) Study), and South Carolina (Markers of Adenomatous Polyps-II (MAPII) Study). Details concerning the study populations, recruitment, and protocols for the CPRU (19), MAPI (20), and MAPII (21) studies, and a pooled analysis of the 3 populations (4) were published previously. Briefly, patients with no history of colorectal neoplasms, high-risk genetic syndromes for colon cancer (e.g., familial adenomatous polyposis), or inflammatory bowel disease being scheduled for outpatient, elective colonoscopy visits were recruited during 1991–1994 for the CPRU study, 1994–1997 for the MAPI study, and 2002 for the MAPII study. In the original CPRU study, of 1,890 potential participants who met final eligibility criteria, 1,281 (68%) signed consent. In the original MAPI and MAPII studies, of the 649 potential participants who met final eligibility criteria, 522 (80%) signed consent. Of the pooled 1,803 participants (n = 797 patients with incident, sporadic colorectal adenoma, n = 1,006 colonoscopy control subjects without hyperplastic polyps), those who were nonwhite (n = 90) or missing 25(OH)D3 concentration data (n = 372) or GC genotyping results (n = 444) were excluded, leaving 942 participants (n = 418 case patients, n = 524 control subjects) for the present analysis.
Participants with at least 1 adenomatous polyp detected during colonoscopy and confirmed by pathologic evaluation were defined as case patients, and participants with no adenomatous or hyperplastic polyps were defined as control subjects. Demographic, medical, family history, lifestyle, and dietary information was collected via questionnaire prior to colonoscopy; the dietary information was collected via a semiquantitative Willett food frequency questionnaire. One study index pathologist examined all excised polyps and reported the histologic findings using diagnostic criteria described by the National Polyp Study (22). Informed consent was obtained from each patient, and each institution’s review board approved the study protocol.
Laboratory methods
Fasting peripheral venous blood samples were taken 30–60 minutes before colonoscopy and stored at −70°C prior to performing the assay. Plasma and serum samples were separated using a standardized protocol. 25(OH)D3 and 25-hydroxyvitamin D2 (25(OH)D2) concentrations were assayed using serum samples in the MAP studies and plasma samples in the CPRU study. We analyzed paired serum and plasma samples from 20 participants to assess the comparability of serum and plasma 25(OH)D3 concentrations (plasma mean concentration = 21.3 ng/mL, standard deviation, 6.7; serum mean concentration = 23.9 ng/mL, standard deviation, 8.8; Spearman ρ ≥ 0.8, P < 0.001) (4).
25(OH)D3 and 25(OH)D2 concentrations were measured at the Molecular Epidemiology and Biomarker Research Laboratory at the University of Minnesota using liquid chromatography–tandem mass spectrometry (23). The average interassay coefficients of variation for circulating 25(OH)D3 and 25(OH)D2 were 3% and 80%, respectively. Because of the poor measurement reliability for 25(OH)D2, only 25(OH)D3 data were included in the primary analyses.
Genotyping
Single nucleotide polymorphisms (SNPs) were genotyped at the University of Minnesota Genomics Center using a Taqman platform (Applied Biosystems, Foster City, California) with standard quality control measures, as described previously (24). SNPs in vitamin D–related genes were originally selected for genotyping based on HapMap population differences in allelic distributions hypothesized to reflect evolutionary divergences in vitamin D metabolism. We selected rs3755967 as a proxy for rs4588 because the 2 SNPs were in perfect linkage disequilibrium (LD) (r2 = 1.0) in the HapMap CEU population (i.e., Utah residents of northern and western European ancestry) of US whites with European ancestry (1000 Genomes Project proxy SNPs; Broad Institute, Cambridge, Massachusetts). This population is highly comparable to our study population, which was restricted to whites of European ancestry. The SNP rs3755967 was also used as proxy for rs4588 in a recent genome-wide association study, given the strong LD (r2 > 0.99) among whites in the TwinsUK population (25); also, the r2 values for the 2 SNPs in HapMap’s Finish and British populations were 0.97 and 1.0, respectively, indicating a strong pattern of LD across various white populations. In our study population, rs7041 and rs3755967 were in Hardy-Weinberg equilibrium (χ2P > 0.05; Table 1).
Table 1.
Genotype Information on GC Single Nucleotide Polymorphisms Genotyped in the Cancer Prevention Research Unit and Markers of Adenomatous Polyps Case-Control Studies of Incident, Sporadic Colorectal Adenomas, United States, 1991–2002a
| Chr | Gene | SNP | Locationb | A > a | No. Genotyped | MAFc | MAFCEUd | HWE P Value | Amino Acid Change | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | Aa | aa | |||||||||
| 4 | GC | rs7041 | 72618334 | G > T | 297 | 446 | 199 | 0.46 | 0.43 | 0.25 | rs7041G > T: Asp416Glu |
| 4 | GC | rs3755967e | 72618323 | G > A | 466 | 389 | 87 | 0.30 | 0.24 | 0.10 | rs4588 C > A: Thr420Lys |
Abbreviations: a, minor allele; A, major allele; CEU, Utah residents of northern and western European ancestry; Chr, chromosome; GC, group-specific component (encoding vitamin D–binding protein); HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; SNP, single nucleotide polymorphism.
a Limited to white persons genotyped for GC SNPs (n = 942).
b Location determined using Ensembl GRChr37 (https://grch37.ensembl.org/index.html).
c For all participants.
d For CEU population only, using 1000 Genomes Project, phase 3, data.
e SNP rs3755967 is a proxy for rs4588 (linkage disequilibrium, 1.0, CEU population; 1000 Genomes Project, phase 3).
Statistical methods
Selected characteristics of the participants by case-control status were summarized and compared using the Fisher exact test for categorical variables and the t test for continuous variables (normalized as appropriate). 25(OH)D3 concentrations were adjusted for seasonal variation using the method described by Gail et al. (26). 25(OH)D3 concentrations were regressed on week of blood sampling using a cos/sin function accounting for seasonal variation; seasonally adjusted values were calculated by adding participant residuals from this regression model to the study-specific mean among the control subjects. These adjustments were conducted separately for the CPRU and MAP studies because seasonal variation in 25(OH)D3 concentrations may vary by latitude. The seasonally adjusted value may be interpreted as a participant’s predicted 25(OH)D3 concentration for a given year, accounting for the seasonal variation in vitamin D concentrations observed among the control subjects in the same study.
Linear regression models were used to estimate associations of rs7041 and rs3755967 genotypes with 25(OH)D3 concentrations using an additive model of inheritance in each study separately and pooled. All models were adjusted for age and sex; pooled analyses were further adjusted for study. We estimated associations separately among case patients and control subjects and then among all study participants in models further adjusted for case-control status.
The combined rs7041 and rs3755967 genotypes were used to infer the 3 common GC isoforms (Gc1s, Gc1f, and Gc2) and 6 resultant isoform combinations observed in appreciable frequencies: Gc1s-1s, Gc1s-1f, Gc1s-2, Gc1f-1f, Gc2-1s, Gc2-1f, and Gc2-2(16). Patients with rare isoforms combinations (n = 4) were excluded from subsequent analyses involving isoforms (Web Table 1, available at https://academic.oup.com/aje).
In pooled analyses, we estimated mean 25(OH)D3 concentrations and their 95% confidence intervals among those with each GC isoform combination, using multivariable general linear regression models (with the Tukey pairwise comparison test for calculating P values) adjusted for age, sex, case-control status, and study. The Gc1s-1s isoform (defined by the homozygous dominant genotype at both SNPs) was considered the reference group. After combining those with Gc1s and Gc1f isoforms, we calculated and compared mean 25(OH)D3 concentrations among those with the Gc1-2 versus Gc1-1 and Gc2-2 versus Gc1-1 isoforms in the same manner.
We investigated whether the circulating 25(OH)D3 concentration–adenoma association differed by GC isoform by stratifying the multivariable logistic regression models by the Gc1-1 versus the Gc1-2/Gc2-2 isoforms (based on their previously reported differences in DBP and 25(OH)D concentrations) (12, 15, 16). 25(OH)D3 was assessed as a continuous (per 10 ng/mL) variable, by quartiles, and by common clinical categories, consistent with other studies in which 25(OH)D–adenoma associations were reported (4, 27, 28). Potential confounders, chosen based on biological plausibility and previous literature, included age (years), sex (male or female), family history of colorectal cancer in a first-degree relative (yes or no), body mass index (measured as weight (kg)/height (m)2), regular weekly use of aspirin or other nonsteroidal anti-inflammatory drugs (yes or no), and intakes of total energy (kcal/day), total (dietary plus supplemental) calcium (mg/day), total fat (g/day), alcohol (g/day), dietary fiber (g/day), total fruits (servings per day), total vegetables (servings per day), and total red and processed meats (servings per day). Inclusion in the final model was based on consideration of biological plausibility, previous literature, and whether inclusion of the variable in the model changed the odds ratio for the 25(OH)D3–adenoma association by 10% or greater. The covariates for the final adjusted models are noted in the footnotes to the tables accompanying this article.
All statistical tests were 2-sided, and a 2-sided P < 0.05 or a 95% confidence interval that excluded 1.0 was considered statistically significant. All data were analyzed using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Selected characteristics of the study participants are shown in Table 2. Case patients, on average, were slightly older and consumed more total fat and alcohol, and they were more likely to be male or to smoke and less likely to regularly take nonsteroidal anti-inflammatory drugs or, if a woman, use hormone replacement therapy.
Table 2.
Selected Characteristics of Participants in the Cancer Prevention Research Unit and Markers of Adenomatous Polyps Case-Control Studies of Incident, Sporadic Colorectal Adenomas, United States, 1991–2002a
| Characteristics | CPRU | MAP | Pooled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case Patients (n = 318) | Control Subjects (n = 360) | Case Patient Subjects (n = 100) | Control Subjects (n = 164) | Case Patients (n = 418) | Control Subjects (n = 524) | |||||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Age, years | 58.0 (9.8) | 52.9 (11.0) | 56.8 (7.8) | 55.9 (8.9) | 57.7 (9.3) | 53.9 (10.5) | ||||||
| Male sex | 63 | 37b | 52 | 43 | 61 | 39b | ||||||
| 25(OH)D3 concentration, ng/mL | ||||||||||||
| Unadjusted | 24.1 (9.3) | 24.8 (10.6) | 26.0 (12.3) | 27.1 (11.0) | 24.5 (10.1) | 25.5 (10.7) | ||||||
| Seasonally adjusted | 24.6 (9.2) | 24.8 (10.1) | 25.7 (11.7) | 27.1 (10.7) | 24.9 (9.8) | 25.5 (10.3) | ||||||
| Season of blood sampling | ||||||||||||
| Winter | 28 | 21 | 16 | 16 | 25 | 20b | ||||||
| Spring | 30 | 30 | 35 | 41 | 31 | 33 | ||||||
| Summer | 25 | 27 | 28 | 27 | 25 | 27 | ||||||
| Fall | 17 | 22 | 21 | 16 | 18 | 20 | ||||||
| Family history of colorectal cancerc | 15 | 29b | 18 | 26 | 16 | 28 | ||||||
| Physical activity, MET-hours/week | 261 (272) | 238 (232) | 185 (141) | 179 (123) | 243 (250) | 219 (207) | ||||||
| Body mass indexd | 27.3 (4.7) | 27.1 (5.1) | 28.2 (6.7) | 27.9 (6.1) | 27.5 (5.3) | 27.3 (5.5) | ||||||
| Postmenopausal womene | 79 | 69 | 78 | 84 | 78 | 73 | ||||||
| Hormone replacement therapy usef | 41 | 66b | 74 | 73 | 50 | 68b | ||||||
| Regular NSAID or aspirin use, At least once per week | 38 | 48b | 52 | 60 | 41 | 52b | ||||||
| Current smoker | 21 | 16b | 31 | 9b | 23 | 14b | ||||||
| Dietary intake, per day | ||||||||||||
| Total energy, kcal | 1,991 (685) | 2,120 (822) | 1,939 (739) | 1,773 (936) | 2,077 (806) | 1,924 (777) | ||||||
| Vitamin D, IUg | 336 (264) | 313 (235) | 356 (271) | 347 (298) | 341 (265) | 324 (257) | ||||||
| Calcium, mgg | 982 (562) | 960 (509) | 841 (434) | 901 (506) | 949 (537) | 942 (509) | ||||||
| Total fat, g | 74.2 (35.2) | 68.0 (28.5)b | 71.6 (34.7) | 65.0 (41.6) | 73.5 (35.1) | 67.1 (33.0) | ||||||
| Alcohol, g | 10.3 (16.4) | 6.7 (14.0)b | 5.9 (11.9) | 4.9 (10.2) | 9.3 (15.6) | 6.1 (12.9) | ||||||
| Dietary fiber, g | 22.0 (10.2) | 21.6 (9.6) | 20.9 (10.2) | 19.5 (10.4) | 21.7 (10.2) | 20.6 (10.0) | ||||||
| Fruit, servings | 2.4 (1.7) | 2.5 (1.8) | 1.9 (1.6) | 1.8 (1.4) | 2.3 (1.7) | 2.3 (1.7) | ||||||
| Vegetables, servings | 3.6 (2.3) | 3.7 (2.5) | 3.7 (2.7) | 3.0 (2.1) | 3.7 (2.4) | 3.5 (2.4) | ||||||
| Red and processed meats, servings | 4.9 (3.4) | 4.6 (3.6) | 4.8 (4.2) | 5.4 (9.35) | 4.8 (3.6) | 4.9 (6.0) | ||||||
Abbreviations: 25(OH)D3, 25-hydroxyvitamin D3; CPRU, Cancer Prevention Research Unit; MAP, Markers of Adenomatous Polyps; MET, metabolic equivalents of task; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
a Limited to white persons genotyped for GC single nucleotide polymorphisms (n = 942).
bP < 0.05 for comparison with adenoma case patients by Fisher exact test for categorical variables and t test for continuous variables.
c In a first-degree relative.
d Calculated as weight (kg)/height (m)2.
en = 485.
f Calculated among postmenopausal women only (n = 358).
g From dietary and supplements intake.
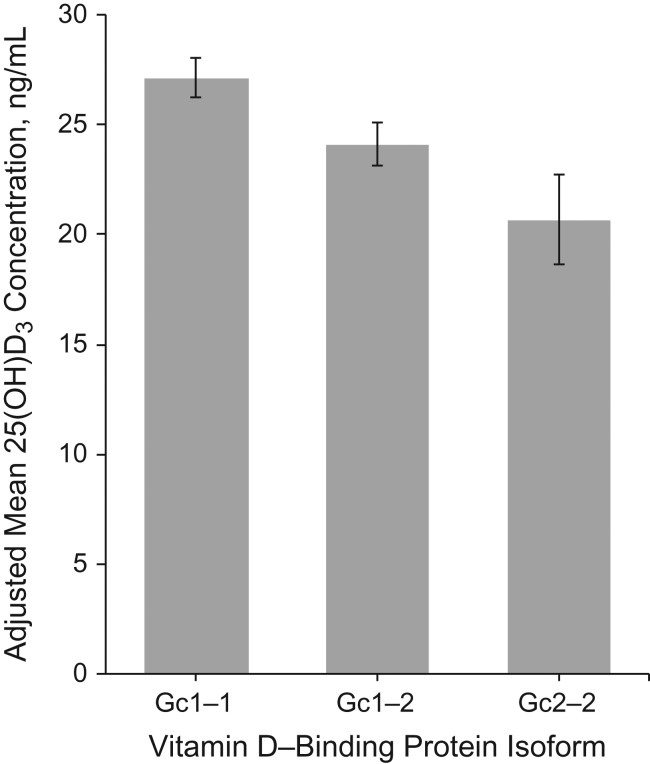
In the pooled CPRU and MAP studies, the mean 25(OH)D3 blood concentrations were statistically significantly lower by 2.05 and 3.15 ng/mL per rs7041 and rs3755967 minor allele, respectively (Web Table 2). The inverse associations of each SNPs’ minor allele with 25(OH)D3 concentrations were similar when examined separately by case-control status and by study. The adjusted mean 25(OH)D3 concentrations were 27.4 ng/mL among Gc1s-1s individuals (95% confidence interval (CI): 26.2, 28.5) and 20.7 ng/mL among Gc2-2 individuals (95% CI: 18.6, 22.7) in the pooled analysis (Web Table 3). Combining the Gc1s and Gc1f isoforms, the adjusted mean 25(OH)D3 concentrations were 27.1, 24.1, and 20.7 ng/mL for those with the Gc1-1, Gc1-2, and Gc2-2 isoforms, respectively (Figure 1). This corresponds to statistically significant 3.0 ng/mL (11.1%) and 6.4 ng/mL (23.6%) lower mean 25(OH)D3 concentrations among those with the Gc1-2 and Gc2-2 isoforms, respectively, relative to those with the Gc1-1 isoform (detailed data in Web Table 3).
Figure 1.
Adjusted mean circulating 25-hydroxyvitamin D3 (25(OH)D3) concentrations according to Gc1-1, Gc1-2, and Gc2-2 isoforms in the pooled Cancer Prevention Research Unit and Markers of Adenomatous Polyps case-control studies of incident, sporadic colorectal adenomas, United States, 1991–2002. Adjusted means, 95% confidence intervals, and P values were estimated using general linear regression models adjusted for age, sex, case-control status, and study, with P values calculated using the Tukey test for pairwise comparisons of 25(OH)D3 concentrations among study participants with the Gc1-2 versus Gc1-1 (P = 0.01) and Gc2-2 versus Gc1-1 isoforms (P < 0.0001) (see Web Table 3 for detailed data).
The associations of seasonally adjusted 25(OH)D3 concentrations with adenoma risk, stratified by GC isoform, are shown in Table 3. Concentration of 25(OH)D3 was associated with a statistically significant, approximately 29% lower risk of adenoma per 10 ng/mL higher 25(OH)D3 concentration among participants with the Gc2 isoforms (Gc1-2 and Gc2-2 combined), but the estimated association among those with the Gc1-1 isoforms was close to the null (P for interaction = 0.03). Among participants with the Gc2 isoform, those in the highest (>31.5 ng/mL) relative to those in the lowest (<17.9 ng/mL) quartile of 25(OH)D3 were statistically significantly less likely (by >50%) to have an adenoma. The estimated associations among participants with the Gc1-1 isoforms were close to the null. Using commonly used clinical cutoffs for vitamin D deficiency (20 ng/mL (29) or 30 ng/mL (30), depending on professional society), among individuals with the Gc2 isoform, a 25(OH)D3 concentration of at least 20 ng/mL relative to less than 20 ng/mL was associated with a 49% lower risk of adenoma, whereas concentrations of 20–30 ng/mL and greater than 30 ng/mL, relative to less than 20 ng/mL, were associated with statistically significant 48% and 52% lower risks of adenoma, respectively; however, the estimated associations among individuals with no inherited Gc2 isoforms (Gc1-1) were close to the null. Our findings did not substantially differ by adenoma size, multiplicity, histologic type, location, shape, or degree of dysplasia, nor according to sex (data not shown).
Table 3.
Associations of Seasonally Adjusted Circulating 25-hydroxyvitamin D3 Concentrations With Incident, Sporadic Colorectal Adenoma Risk Stratified by Vitamin D–Binding Protein (Group Component Gene) Isoforms in Pooled Cancer Prevention Research Unit and Markers of Adenomatous Polyps Case-Control Studies, United States, 1991–2002
| Variable | Among Individuals With Gc1-1 Isoforma | Among Individuals With Gc1-2/Gc2-2 Isoformsb | P Value for Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Case Patients | No. of Control Subjects | ORc | 95% CI | No. of Case Patients | No. of Control Subjects | ORc | 95% CI | ||
| 25(OH)D3 concentration (per 10 ng/mL)d | 209 | 257 | 1.07 | 0.87, 1.32 | 208 | 264 | 0.71 | 0.56, 0.90 | 0.03 |
| 25(OH)D3 quartile | |||||||||
| 1 (<17.9 ng/mL) | 39 | 46 | 1.00 | Referent | 69 | 79 | 1.00 | Referent | |
| 2 (17.9–24.3 ng/mL) | 52 | 60 | 1.10 | 0.58, 2.08 | 57 | 66 | 0.80 | 0.47, 1.38 | |
| 3 (24.4–31.5 ng/mL) | 53 | 71 | 0.98 | 0.52, 1.85 | 47 | 65 | 0.55 | 0.31, 0.97 | |
| 4 (>31.5 ng/mL) | 65 | 80 | 1.02 | 0.55, 1.91 | 35 | 54 | 0.46 | 0.24, 0.88 | 0.04 |
| P for trende | 0.94 | 0.008 | |||||||
| Clinical 25(OH)D3 cutofff | |||||||||
| Deficient (<20 ng/mL) | 51 | 62 | 1.00 | Referent | 91 | 97 | 1.00 | Referent | |
| Nondeficient (≥20 ng/mL) | 158 | 195 | 1.11 | 0.68, 1.82 | 117 | 167 | 0.51 | 0.33, 0.80 | 0.05 |
| Deficient (<20 ng/mL) | 51 | 62 | 1.00 | Referent | 91 | 97 | 1.00 | Referent | |
| Insufficient (20–30 ng/mL) | 86 | 101 | 1.13 | 0.66, 1.93 | 71 | 102 | 0.52 | 0.32, 0.85 | |
| Sufficient (>30 ng/mL) | 72 | 94 | 1.08 | 0.62, 1.89 | 46 | 65 | 0.48 | 0.27, 0.87 | 0.09 |
| P for trende | 0.83 | 0.01 | |||||||
Abbreviations: 25(OH)D3, 25-hydroxyvitamin D3; CI, confidence interval; OR, odds ratio.
a Gc1-1: combined Gc1s-1s, Gc1s-1f, and Gc1f-1f.
b Gc1-2: combined Gc2-1s and Gc2-1f.
c Adjusted for age (continuous), sex, study (Cancer Prevention Research Unit or Markers of Adenomatous Polyps), regular use of aspirin or nonsteroidal anti-inflammatory drugs, family history of colorectal cancer in a first-degree relative, smoking status (current, ever, never), alcohol intake (continuous), total calcium intake from diet and supplements (continuous), body mass index (continuous), and physical activity (continuous).
d Coded as a continuous variable in the model.
e Calculated by including the 25(OH)D3 predictor variable as a continuous variable in the model.
DISCUSSION
According to our findings, circulating 25(OH)D3 concentrations may be inversely associated with incident, sporadic colorectal adenoma among those who have inherited the Gc2 isoform-encoding genotype (which was previously associated with lower DBP and 25(OH)D concentrations). However, 25(OH)D3 concentrations greater than those considered deficient (i.e., >20 ng/mL) may not be associated with adenoma risk among those who have inherited only Gc1 isoform-encoding genotypes. To our knowledge, this is the first study to report that the increasingly supported vitamin D-colorectal neoplasm association may differ by common DBP isoforms.
Two functional variants in the highly polymorphic GC locus encode for 3 common DBP isoforms (Gc1s, Gc1f, and Gc2) (16, 31). The frequencies of Gc1s, Gc1f, and Gc2 in our study population were 0.56, 0.15, and 0.30, respectively, similar to those reported in other studies of white US adults (32, 33). The full physiological consequence of these variants has not been fully elucidated; however, their strong associations with DBP and vitamin D metabolite concentrations have been consistently reported (15, 16). Consistent with previous studies, mean 25(OH)D3 concentrations were more than 20% lower among Gc2-2 individuals than among Gc1-1 (combined Gc1s and Gc1f) individuals in our study population (12, 13). Individuals with the Gc2 isoform appear predisposed to lower 25(OH)D3 concentrations as a result of having lower DBP concentrations (6, 13, 15). One important role of the DBP is to maintain adequate circulating levels of vitamin D when vitamin D sources are scarce (10, 11). DBP can increase 25(OH)D3 reabsorption in the kidneys, prolonging its circulating half-life, and DBP is required to prevent vitamin D–deficiency phenotypes in mice when vitamin D exposure is limited (11, 34). Thus, higher 25(OH)D3 blood concentrations, the best indicator of total vitamin D exposure, may be particularly beneficial for adenoma prevention among individuals with the Gc2 isoform who may have a lower DBP-related capacity to otherwise maintain adequate vitamin D concentrations. In contrast, higher 25(OH)D3 concentrations may not be associated with adenoma risk among individuals with only Gc1 isoforms, because higher DBP concentrations associated with this isoform may be able to “compensate” and maintain adequate circulating vitamin D concentrations even when vitamin D exposure is low. Additional research is needed to investigate this hypothesis.
Supporting this hypothesis are other observational studies in which similar patterns of effect modification by DBP concentrations or GC isoforms in relation to circulating 25(OH)D’s association with vitamin D–related health outcomes were reported. In the Nurse’s Health Study of predominantly white women, the association of 25(OH)D3 concentrations with colorectal cancer risk was stronger among individuals with lower (below median) DBP concentrations (for the highest quintile vs. the lowest, odds ratio = 0.57, 95% CI: 0.32, 1.02) than among those with higher DBP concentrations (for the highest quintile vs. the lowest, odds ratio = 0.84, 95% CI: 0.49, 1.46) (17). Although this interaction was not statistically significant, given that the Gc2 isoform is strongly associated with lower DBP concentrations, the pattern for possible effect modification was similar to, and thus supports, our own (15). A potential limitation of that study, however, was the use of monoclonal enzyme-linked immunosorbent assay–based measurement of DBP, the accuracy of which may vary by GC isoform (35). In addition, 25(OH)D concentration was inversely associated with diabetes risk among white individuals with the Gc2-2 isoform genotype rs4588*AA (per a 1 standard deviation–higher baseline serum concentration of 25(OH)D, hazard ratio = 0.75, 95% CI: 0.64, 0.90), but not among persons who had inherited no A allele at rs4588 (i.e., no Gc2 isoforms) (hazard ratio = 0.99, 95% CI: 0.93, 1.05) (18). Our results, in combination with previous studies’ findings, warrant additional investigation of whether these isoforms modify the association of circulating 25(OH)D3 with other vitamin D-related outcomes.
Findings across studies that investigated associations of GC SNPs with colorectal cancer are inconsistent. Two studies in the United States in mostly white populations found no associations of GC SNPs with colorectal cancer (36, 37); however, rs4588 was associated with higher colorectal cancer risk in a Han Chinese population (38). Poynter et al. (36) found that the overall null association of GC SNPs with colorectal cancer risk persisted within strata of self-reported vitamin D intake (via food and supplements). To our knowledge, no study reported associations of GC genotypes with colorectal neoplasms stratified by circulating 25(OH)D3 concentrations, considered the most reliable marker of total vitamin D exposure (4, 36). Our results indicate associations of GC SNPs with colorectal neoplasms may differ by 25(OH)D3 concentrations.
Our study has several limitations. We did not measure DBP concentrations; however, our estimated associations of GC isoforms with 25(OH)D3 concentrations (which appear to be mediated by DBP concentrations), were consistent with findings in previous studies. Thus, we infer that the GC isoforms are also associated with DBP concentrations in our population, which underlies our hypothesis of effect modification by GC isoform. Studies are needed to investigate whether this potential 25(OH)D–GC isoform interaction may be explained by DBP concentrations. On the one hand, the solid-phase liquid chromatography–tandem mass spectrometry we used is a more accurate method for measuring 25(OH)D concentrations than are immunoassays, which are limited by DBP-concentration–dependent inaccuracies (39). On the other hand, there is concern that liquid chromatography–tandem mass spectrometry could lead to falsely low measured 25(OH)D3 concentrations in patients with high DBP concentrations due to incomplete extraction (40, 41); however, we would expect this to attenuate our results of higher 25(OH)D3 concentrations among individuals with the Gc1 isoform toward the null. In addition, control subjects were patients undergoing elective outpatient colonoscopy and thus may have been at higher risk of adenomas than was the general population, which may also have attenuated our estimated associations toward the null. We used rs3755967 as a proxy for rs4588, given their perfect LD (r2 = 1.0) in the HapMap CEU population of US whites of European ancestry. Although LD structure may vary by population, our study population of white US adults of European ancestry is highly comparable to the HapMap CEU population, supporting the appropriateness of this proxy SNP. Only 25(OH)D3 was used in our primary analyses because of the poor reliability of our 25(OH)D2 measurements. Most participants (96%) in our study had undetectable or very low (<10 ng/mL) 25(OH)D2 concentrations, consistent with findings of other studies (42), and substitution of total 25(OH)D (i.e., D2 + D3) for 25(OH)D3 did not materially affect our results. Hormone replacement therapy may influence circulating vitamin D and DBP concentrations (43) and is associated with colorectal adenoma risk (44); however, further adjusting for hormone replacement therapy use did not change our results. Last, because GC isoform frequency and the effects of the GC isoforms on vitamin D metabolism may differ by race, our findings may not be generalizable to other populations.
Strengths of our study include the use of study-specific, seasonally adjusted 25(OH)D3 concentrations to assess total vitamin D exposure, thereby reducing misclassification of vitamin D status, which may vary throughout the year and in study populations living at different latitudes. Using the approach of most previous epidemiologic studies, in which unadjusted 25(OH)D3 concentration is the predictor variable and season of blood sampling is included as a covariate in the model, did not change our results. Misclassification of adenoma outcome was minimized by verifying the adenoma and hyperplastic polyp–free status of control subjects by colonoscopy and pathologic review. We also had one of the largest case-control populations to investigate 25(OH)D–adenoma associations; the number of participants in most previous studies ranged from 200 to 700 (45), compared with 942 in our study.
In conclusion, our findings, taken together with those of previous studies, indicate the risk of colorectal adenoma associated with vitamin D exposure may differ by common, inherited DBP isoforms that affect circulating DBP concentrations. In particular, according to our findings, those with the Gc2 isoform may particularly benefit from maintaining higher circulating 25(OH)D3 concentrations for adenoma prevention. This possibility warrants investigation into whether these isoforms modify the association of circulating 25(OH)D3 with other vitamin D–related health outcomes.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (David C. Gibbs, Veronika Fedirko, Caroline Um, Roberd M. Bostick); Winship Cancer Institute, Emory University, Atlanta, Georgia (Veronika Fedirko, Roberd M. Bostick); and Department of Laboratory Medicine and Pathology, University of Minnesota Medical School, Minneapolis, Minnesota (Myron D. Gross, Bharat Thyagarajan).
This research was funded by National Institutes of Health (grants P01 CA50305, R01 CA66539, and R01 CA116795); Fullerton Foundation; Emory Winship Cancer Institute; a Georgia Cancer Coalition Distinguished Scholar award (to R.M.B.); and Franklin Foundation.
Conflict of interest: none declared.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- 25(OH)D2
25-hydroxyvitamin D2
- 25(OH)D3
25-hydroxyvitamin D3
- CI
confidence interval
- CPRU
Cancer Prevention Research Unit Study
- DBP
vitamin D–binding protein
- GC
group-specific component
- LD
linkage disequilibrium
- MAP
Markers of Adenomatous Polyps Study
- SNP
single nucleotide polymorphism
REFERENCES
- 1. Siegel RL, Miller KD, Fedewa SA, et al. . Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 2. Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952(1):73–87. [DOI] [PubMed] [Google Scholar]
- 3. Iseki K, Tatsuta M, Uehara H, et al. . Inhibition of angiogenesis as a mechanism for inhibition by 1α-hydroxyvitamin D3 and 1, 25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. 1999;81(5):730–733. [DOI] [PubMed] [Google Scholar]
- 4. Fedirko V, Bostick RM, Goodman M, et al. . Blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk: a pooled case-control study. Am J Epidemiol. 2010;172(5):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garland CF, Comstock GW, Garland FC, et al. . Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;334(8673):1176–1178. [DOI] [PubMed] [Google Scholar]
- 6. Powe CE, Evans MK, Wenger J. Vitamin D–binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hibler EA, Jacobs ET, Stone AD, et al. . Associations between vitamin D–binding protein isotypes, circulating 25(OH)D levels, and vitamin D metabolite uptake in colon cancer cells. Cancer Prev Res (Phila). 2014;7(4):426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bostick RM, Goodman M, Sidelnikov E. Calcium and vitamin D In: Potter JD, Lindor NM, eds. Genetics of Colorectal Cancer. New York, NY: Springer Science + Business Media, LLC; 2009:277–298. [Google Scholar]
- 9. Christakos S, Ajibade DV, Dhawan P, et al. . Vitamin D: metabolism. Rheum Dis Clin North Am. 2012;38(1):1–11. [DOI] [PubMed] [Google Scholar]
- 10. Speeckaert M, Huang G, Delanghe JR, et al. . Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372(1–2):33–42. [DOI] [PubMed] [Google Scholar]
- 11. Safadi FF, Thornton P, Magiera H, et al. . Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103(2):239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malik S, Fu L, Juras DJ, et al. . Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci. 2013;50(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lauridsen AL, Vestergaard P, Hermann AP, et al. . Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77(1):15–22. [DOI] [PubMed] [Google Scholar]
- 14. Braun A, Bichlmaier R, Cleve H. Molecular analysis of the gene for the human vitamin-D-binding protein (group-specific component): allelic differences of the common genetic GC types. Hum Genet. 1992;89(4):401–406. [DOI] [PubMed] [Google Scholar]
- 15. Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47(4):753–756. [PubMed] [Google Scholar]
- 16. Daiger SP, Miller M, Chakraborty R. Heritability of quantitative variation at the group-specific component (Gc) locus. Am J Hum Genet. 1984;36(3):663–676. [PMC free article] [PubMed] [Google Scholar]
- 17. Song M, Konijeti GG, Yuan C, et al. . Plasma 25-hydroxyvitamin D, vitamin D binding protein, and risk of colorectal cancer in the Nurses’ Health Study. Cancer Prev Res (Phila). 2016;9(8):664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reis JP, Michos ED, Selvin E, et al. . Race, vitamin D–binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015;101(6):1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Potter JD, Bigler J, Fosdick L, et al. . Colorectal adenomatous and hyperplastic polyps: smoking and N-acetyltransferase 2 polymorphisms. Cancer Epidemiol Biomarkers Prev. 1999;8(1):69–75. [PubMed] [Google Scholar]
- 20. Boyapati SM, Bostick RM, McGlynn KA, et al. . Calcium, vitamin D, and risk for colorectal adenoma: dependency on vitamin D receptor BsmI polymorphism and nonsteroidal anti-inflammatory drug use? Cancer Epidemiol Biomarkers Prev. 2003;12(7):631–637. [PubMed] [Google Scholar]
- 21. Daniel CR, Bostick RM, Flanders WD, et al. . TGF-α expression as a potential biomarker of risk within the normal-appearing colorectal mucosa of patients with and without incident sporadic adenoma. Cancer Epidemiol Biomarkers Prev. 2009;18(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winawer SJ, Zauber AG, O’Brien MJ, et al. . The National Polyp Study design, methods, and characteristics of patients with newly diagnosed polyps. Cancer. 1992;70(S3):1236–1245. [DOI] [PubMed] [Google Scholar]
- 23. Saenger AK, Laha TJ, Bremner DE, et al. . Quantification of serum 25-hydroxyvitamin D2 and D3 using HPLC–tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125(6):914–920. [DOI] [PubMed] [Google Scholar]
- 24. Yang B, Thyagarajan B, Gross MD, et al. . Genetic variants at chromosome 8q24, colorectal epithelial cell proliferation, and risk for incident, sporadic colorectal adenomas. Mol Carcinog. 2014;53(S1):E187–E192. [DOI] [PubMed] [Google Scholar]
- 25. Wang TJ, Zhang F, Richards JB, et al. . Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gail MH, Wu J, Wang M, et al. . Calibration and seasonal adjustment for matched case–control studies of vitamin D and cancer. Stat Med. 2016;35(13):2133–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grau MV, Baron JA, Sandler RS, et al. . Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95(23):1765–1771. [DOI] [PubMed] [Google Scholar]
- 28. Peters U, McGlynn KA, Chatterjee N, et al. . Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1267–1274. [PubMed] [Google Scholar]
- 29. Francis RM, Aspray TJ, Bowring CE, et al. . National Osteoporosis Society practical clinical guideline on vitamin D and bone health. Maturitas. 2015;80(2):119–121. [DOI] [PubMed] [Google Scholar]
- 30. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. . Evaluation, treatment, prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 31. Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox Sang. 1988;54(4):215–225. [DOI] [PubMed] [Google Scholar]
- 32. Schellenberg D, Paré PD, Weir TD, et al. . Vitamin D binding protein variants and the risk of COPD. Am J Respir Crit Care Med. 1998;157(3):957–961. [DOI] [PubMed] [Google Scholar]
- 33. Gaensslen RE, Bell SC, Lee HC. Distributions of genetic markers in United States populations: III. Serum group systems and hemoglobin variants. J Forensic Sci. 1987;32(6):1754–1774. [PubMed] [Google Scholar]
- 34. Nykjaer A, Dragun D, Walther D, et al. . An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. [DOI] [PubMed] [Google Scholar]
- 35. Denburg MR, Hoofnagle AN, Sayed S, et al. . Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res. 2016;31(6):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poynter JN, Jacobs ET, Figueiredo JC, et al. . Genetic variation in the vitamin D receptor (VDR) and the vitamin D–binding protein (GC) and risk for colorectal cancer: results from the Colon Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2010: 19(2):525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hibler EA, Hu C, Jurutka PW, et al. . Polymorphic variation in the GC and CASR genes and associations with vitamin D metabolite concentration and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2012;21(2):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu L, Sheng H, Li H, et al. . Associations between common variants in GC and DHCR7/NADSYN1 and vitamin D concentration in Chinese Hans. Hum Genet. 2012;131(3):505–512. [DOI] [PubMed] [Google Scholar]
- 39. Heijboer AC, Blankenstein MA, Kema IP, et al. . Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012;58(3):543–548. [DOI] [PubMed] [Google Scholar]
- 40. Wallace A, Gibson S, de la Hunty A, et al. . Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75(7):477–488. [DOI] [PubMed] [Google Scholar]
- 41. Vogeser M. Quantification of circulating 25-hydroxyvitamin D by liquid chromatography–tandem mass spectrometry. J Steroid Biochem Mol Biol. 2010;121(3–5):565–573. [DOI] [PubMed] [Google Scholar]
- 42. Kobayashi T, Okano T, Shida S, et al. . Variation of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 levels in human plasma obtained from 758 Japanese healthy subjects. J Nutr Sci Vitaminol (Tokyo). 1983;29(3):271–281. [DOI] [PubMed] [Google Scholar]
- 43. Rejnmark L, Lauridsen AL, Brot C, et al. . Vitamin D and its binding protein Gc: long‐term variability in peri- and postmenopausal women with and without hormone replacement therapy. Scand J Clin Lab Invest. 2006;66(3):227–238. [DOI] [PubMed] [Google Scholar]
- 44. Grodstein F, Martinez ME, Platz EA, et al. . Postmenopausal hormone use and risk for colorectal cancer and adenoma. Ann Intern Med. 1998;128(9):705–712. [DOI] [PubMed] [Google Scholar]
- 45. Gandini S, Boniol M, Haukka J, et al. . Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414–1424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.