Abstract
Objective(s):
Renal ischemia-reperfusion injury (IRI) as a severe condition of acute kidney injury (AKI) is the most common clinical problem with high mortality rates of 35-60% deaths in hospital. Mesenchymal stem cells (MSC) due to unique regenerative characteristics are ideal candidates for the treatment of the ischemic injuries. This work is focused on the administration of MSC to IRI-induced AKI Wistar rats and evaluating their significance in AKI treatment.
Material and Methods:
Animals underwent surgical procedure and AKI was induced by 40 min bilateral renal pedicle clamping. Immediately after reperfusion, 2×106 rat bone marrow derived MSCs were injected via intra-parenchymal or intra-aortic route.
Results:
Animals subjected to AKI after days 1 and 3 showed significant increase in the serum creatinine and blood urea nitrogen (BUN) concentration along with a declined glomerular filtration rate (GFR) when compared with non-ischemic animals. On the other hand, treated animals showed a significant enhanced regeneration as compared to ischemic animals in both administration route groups.
Conclusion:
According to the results concluded from the renoprotective effects of MSC in IRI/AKI, MSCs could be considered as promising therapeutic approach for AKI in clinical applications.
Key Words: Acute kidney injury, Acute renal failure Bone marrow-derived mesenchymal stem cell, Cell transplantation Ischemic kidney injury, Rat
Introduction
Acute kidney injury (AKI), a heterogeneous clinical syndrome, refers to the progressively kidney failure and declined glomerular filtration rate (GFR) resulting in the accumulation of urea and creatinine in the body. This accumulation of nitrogen wastes results in the impairment of body fluid, electrolytes, and acid-base homeostasis, which is accompanied by significant morbidity and mortality i.e. 23.9% among adults and 13.8% among children. The incidence of AKI among individuals, who have been hospitalized, is significantly greater than the general population (1, 2) and ischemia-reperfusion injury (IRI) has been known as the most common cause of AKI (3). Early diagnosis of AKI is difficult causing irreversible kidney damages in patients, which is being treated with current applied approaches such as dialysis or transplantation (4, 5). Several cell culturing and pre-clinical approaches in animal models are being used to understand the pathophysiology of AKI and finding novel and effective therapeutic strategies (2). Renal IRI is a common experimental model to induce AKI in rat and mice for experimental purposes (2). As per literature, following the induction of renal IRI, the inflammation process is activated and numerous inflammatory mediators such as cytokines, chemokines, and reactive oxygen species (ROS) are secreted and activated, causing recruitment of inflammatory cells for enhanced inflammation leading towards apoptosis and necrosis (4, 6).
For these reasons, fundamental strategies and cures are needed for the management and treatment of AKI.
Stem cells (SCs) especially mesenchymal stem cells (MSCs) are undifferentiated cells having self-renewal and multi-lineage differentiation capabilities (5-8). These cells have been obtained mainly from bone marrow whereas other sources such as umbilical cord, adipose tissue and other less or non-invasive sources and have potential to treat several diseases, including acute and chronic graft versus host disease, Crohn disease, Sjgren’s syndrome (SS), ulcerative colitis, multiple sclerosis, transplant rejection, systemic sclerosis and systemic lupus erythematous (7). These cells have also been applied in clinics to treat myocardial infarction (10-12), cardiomyopathy, heart failure (13), diabetes (14), osteoarthritis (15), osteogenesis imperfecta, foot and ankle fusion, liver cirrhosis, burn injuries, neurologic disease (16, 17), encephalomyelitis (18), urologic disorders (19), and experimental AKI, as well as, tumor therapy (9).
In pre-clinical and clinical practices, potential of MSCs to ameliorate renal IRI (20), glycerol model of AKI, cisplatinum model of AKI, glomerulonephritis model (17), Alport’s syndrome, diabetic nephropathy (21), and rhabdomyolysis-associated acute kidney injury (4, 9, 22–25) has been studied comprehensively and it has been concluded that MSCs after their injection, move into ischemic injured kidney tissues and influence parenchymal reconstruction and tissue regeneration, repair injured tubules and improve renal function (4).
The significant damage of ischemic injury is evidenced in the corticomedullary zone, in particular, S3 segment of the proximal tubule, located in the deep inner cortex and the outer stripe of the outer medulla (2). In spite of the paracrine effects of MSCs, their efficiency to establish a natural cell niche and to regenerate the injury is directly related to the closeness of injury site and administration of the cells. The aim of the present study was to investigate the regenerative potential of MSCs administered immediately after reflow and to compare systemic and locally injection route, in a rat model of IRI/AKI.
Materials and Methods
Animals, induction of IRI/AKI, and cell infusions
All procedures involving animals were approved by the respective Institutional Animal Care of the Mashhad University of Medical Sciences (Mashhad, Iran) and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Animals were housed at a constant temperature and humidity, with a 12:12-hr light-dark cycle, and had unrestricted access to a standard diet and tap water. Adult male Wistar rats weighing 250–300 g were used for this study (MUMS, Iran).
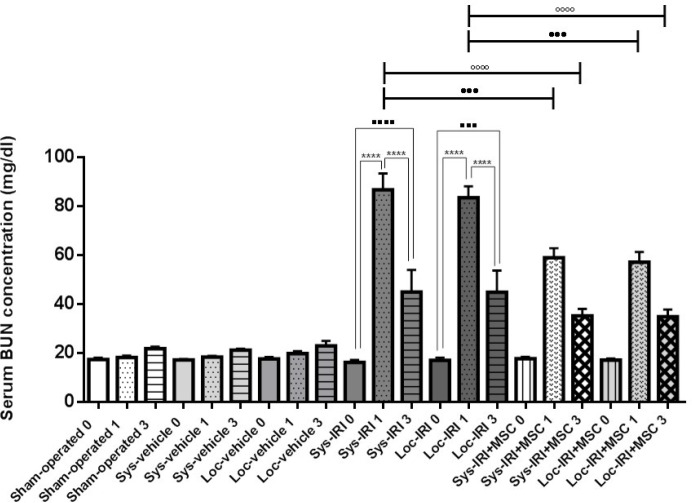
Table 2.
Effect of renal ischemia and mesenchymal stem cells (MSC) administration on serum blood urea nitrogen (BUN) level
| Groups | Day 0 | Day 1 | Day 3 |
|---|---|---|---|
| Sham-operated | 17.4±1.67 | 18.2±1.64 | 21.8±1.79 |
| Systemic vehicle | 17.2±0.84 | 18.34±1.22 | 21.24±1.26 |
| Local vehicle | 17.6±1.82 | 19.82±2.16 | 22.94±4.52 |
| Systemic IRI | 16.2±2.28 | 86.8±14.89 ≠ • | 44.98±20.02 °… § |
| Local IRI | 17±2.55 | 83.54±10.49 ≠ • | 44.88±19.72 °°— “ |
| Systemic IRI+MSC | 17.74±1.67 | 58.92±8.86 * ≠ • | 35.2±6.44 ** |
| Local IRI+MSC | 17.1±1.6 | 57.16±9.3 # ≠ • | 34.9±6.47 ## |
Serum BUN values (mg/dl) from cell-treated and non-treated groups. Values are mean±SEM.
P<0.0001, and
P=0.0002 compared to day 0 in same group,
P=0.002, and
P<0.0001 compared to Sys-IRI group, day 1 and,
P=0.0005, and
P<0.0001 compared to Loc-IRI group, day 1,
P<0.0001,
P=0.0056, and
P=0.006 compared to Sham-operated group in same day, and
P<0.0001,
P=0.0037 and,
P=0.0135 compared to vehicle-treated group in same route injection and same day
The corticomedullary zone is the best site for locally intraparenchymal injection, so in the absence of the ultrasonic guide, the injection at the proper location would be blind. We studied right and left kidneys of the 40 healthy (control) adult male Wistar rats that were killed in our labs for other experiments. Kidneys were removed and fixed in 10% formalin and dehydrated with different degrees of alcohol and paraffin-embedded blocks were prepared. Two sections (5-micrometer) were prepared from each block and were stained with hematoxylin and eosin (H&E) and the slides were evaluated by morphometric method. To confirm the location of injection site, methylene blue solution was injected in the corticomedullary zone, and then injection site was examined. Thirty gauge needles with length of 1 cm were used, while a micropipette tip of 7 mm was cut from the top and the needle was entered to it, so that 3 mm from the tip of the needle was available for injection.
The rats were randomly assigned to the following groups, each containing of five animals: sham-operated group, systemic/local vehicle treated groups (Sys/Loc-vehicle), IRI with systemic/local vehicle treated groups (Sys/Loc-IRI), and IRI with systemic/local cell treated groups (Sys/Loc-IRI+MSC).
IRI/AKI was induced in pentobarbital-anesthetized animals, and rectal temperature was maintained at 37°C, as described elsewhere (23). After a midabdominal laparotomy, kidneys were exposed and renal pedicles were clamped with atraumatic vascular clamps for 40 min. While clamps were applied, in systemic cell/vehicle treated groups, the abdominal aorta was cannulated with IV cannula (gauge 24, O.D. 0.7 mm) for intra-aortic injection. In locally cell/vehicle-treated groups, intraparenchymal injection was performed, as described above. The vehicle in control rats with IRI was infused by the same route. The sham-operated animals underwent similar operative procedures but without any other procedure.
Administration of cells or vehicle was performed immediately after reflow. After visual confirmation of reflow, ~2×106 MSC/animal were given via the supra-renal aorta or intra-parenchymal of kidney (~1×106 MSC/kidney). Control animals for IRI were infused with medium. Aorta puncture site was sutured with 10-0 nylon, covered by fibrin glue and abdominal incision was closed with 4-0 silk, and animals were allowed to recover.
Cells and culture
MSCs used for this experiment were generated by standard procedures. Briefly, under sterile conditions 6-weeks old male Wistar rats were killed by chloroform. Then, the femur and tibia were excised and all connective tissues attached to bones were removed immediately (24). Bone marrow plugs were extracted by flushing the bone marrow cavity in sterile PBS, and then the suspension was filtered through 100-µm mesh. The cells were centrifuged, resuspended in complete culture medium containing low-glucose Dulbecco’s modified Eagle’s medium (LG-DMEM; Gipco, USA) supplemented with 10% fetal bovine serum (FBS; Gipco, USA), plated in 75-cm2 primary culture flasks (Spl, Life science; Korea), and incubated at 37°C in humidified atmosphere with 5% CO2. After 3 days, the medium was changed and nonadherent cells were removed. The MSCs were isolated on the basis of its ability to adhere to the culture flask. At 90% confluence, the cells were trypsinized (0.25% trypsin-EDTA; Gipco, USA) and passaged at 1:3 ratios. The culture medium was changed every 2–3 days. Cells had a typical spindle-shaped appearance, and the MSCs phenotype was confirmed by differentiation into osteocytes and adipocytes with specific differentiation media (25). In addition, superficial markers were negative for CD45, CD11b, CD34 and positive for CD44, CD166, CD73 and CD90 expression, determined by Fluorescence-activated cell sorting (FACS) analysis (28). Passage 3-4 was used in all experiments. The viability of MSC by using trypan blue staining was verified to be >85% before use.
Biochemical analysis
Blood samples were collected at baseline and days 1 and 3 post-IRI. Urine samples were obtained from metabolic cage for 24 hr at baseline and days 1 and 3 post-IRI. Creatinine and blood urea nitrogen (BUN) concentration were determined by autoanalyzer (BT3000 Plus, Italy).
Histology and injury scores
Kidney tissues were embedded in paraffin and 5 µm tissue sections obtained for H&E staining. Histological damage was scored by the EGTI scoring system. The scoring system consists of histological damage in 4 individual components: Endothelial, Glomerular, Tubular, and Interstitial (score: 0-14). Ten non overlapping fields were randomly chosen from the cortical and corticomedullary junction areas of each section (400 ×magnification) (29).
Statistical analysis
Data are expressed as means±SEM. Primary data collection and statistical analyses were carried out using Prism (GraphPad, San Diego, CA). ANOVA and Bonferoni’s post hoc tests were used to assess differences between data. A P-value of <0.05 was considered significant.
Results
Animals were used in this study had a normal blood and urine tests at pre-surgery stage. All animals in control, sham-operated groups and pre-injury day in the treated groups showed a normal kidney function evaluated by serum creatinine and BUN and GFR measurement as well as normal renal histology.
Bone marrow -MSCs isolation, culture and characterization
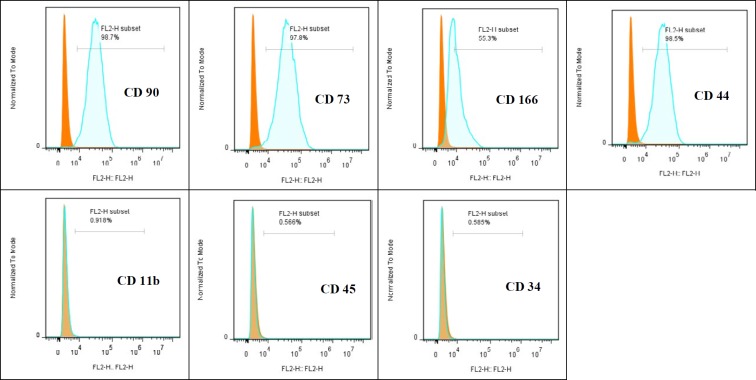
MSCs, used in the current study, were generated by standard procedures and grown for at least three passages in culture and then morphologically defined by a fibroblast-like appearance (Figure 1). Before in vivo usage of MSCs, they were characterized by confirming their ability to undergo osteogenic and adipogenic differentiation (Figure 2) as well as flowcytometry analysis. The cells were negative for CD45, CD11b, and CD34 but positive for the CD44, CD166, CD73 and CD90 superficial markers (Figure 3).
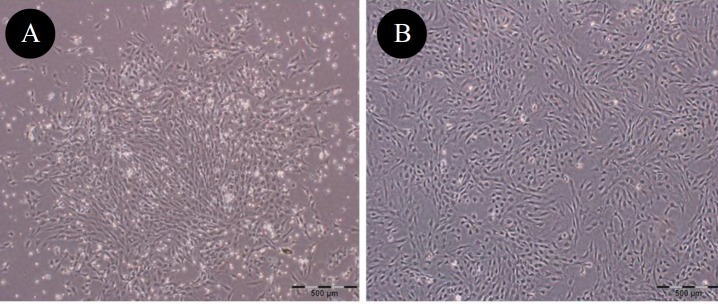
Figure 1.
Phase contrast photomicrographs showing morphological characteristics of rat bone marrow-mesenchymal stem cells (BM-MSCs). (A) passage 1, and (B) passage 3. Cells showed spindle -like fibroblastic morphology (×40)
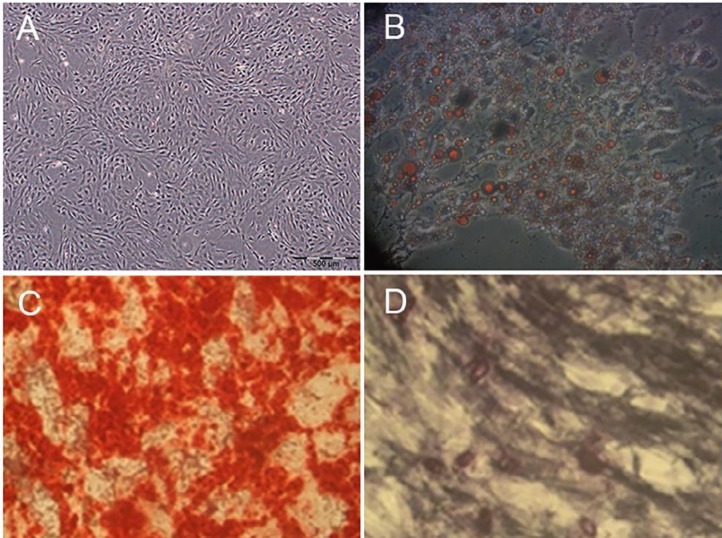
Figure 2.
Differentiation characteristics of rat bone marrow-mesenchymal stem cells (BM-MSCs). A: The control sample was cultured in routine medium (×40). B: Adipogenic differentiation was visualized by Oil Red O staining of the lipid vesicles (×200), and C and D: Osteogenic differentiation was detected by Alizarin red staining, and Alkaline phosphatase, respectively (×100)
Figure 3.
Flowcytometry analysis of rat bone marrow-mesenchymal stem cells (BM-MSCs). Rat BM-MSCs were positive for CD90, CD73, CD166, and CD44, but negative for CD11b, CD45, and CD34 surface markers
Intraparenchymal injection
Histology study of 40 healthy animals revealed that average diameter of kidney cortex in adult male Wistar rat was 2.8 mm (Mean±SEM (2.81±0.7)) as the intraparenchymal injection was performed in 3 mm deep. Due to the limited access to ultrasound guide, injection at the proper location would be blind and we tried our best for a convenient alternative way. While studying the right and left kidneys of 40 healthy (control groups) adult male Wistar rats, we observed
Biochemical analysis and functional study
There were no significant differences between sham-operated group, systemic and local vehicle treated groups in serum creatinine, BUN, and GFR values at any time (Table 1-3).
Table 1.
Effect of renal ischemia and mesenchymal stem cells (MSC) administration on serum creatinine level
| Groups | Day 0 | Day 1 | Day 3 |
|---|---|---|---|
| Sham-operated | 0.58±0.04 | 0.62±0.04 | 0.7±0.03 |
| Systemic vehicle | 0.68±0.03 | 0.71±0.02 | 0.73±0.01 |
| Local vehicle | 0.67±0.03 | 0.69±0.01 | 0.7±0.02 |
| Systemic IRI | 0.66±0.04 | 3.41±0.23 ≠ • | 1.53±0.14 ≠ • |
| Local IRI | 0.6±0.04 | 3±0.14 ≠ • | 1.32±0.15 … § |
| Systemic IRI+MSC | 0.64±0.02 | 1.05±0.09 * | 0.9±0.08 * |
| Local IRI+MSC | 0.67±0.03 | 1±0.07 # | 0.88±0.06 # |
Serum creatinine values (mg/dl) from cell-treated and non-treated groups. There is no significant difference between systemic and local MSC treated animals. Values are mean±SEM.
P<0.0001 compared to day 0 in same group,
P<0.0001 compared to Sys-IRI group, day 1 and,
P<0.0001 compared to Loc-IRI group, day 1,
P<0.0001, and
P=0.0002 compared to Sham-operated group in same day, and
P<0.0001, and
P=0.0003 compared to vehicle-treated group in same route injection and same day
Table 3.
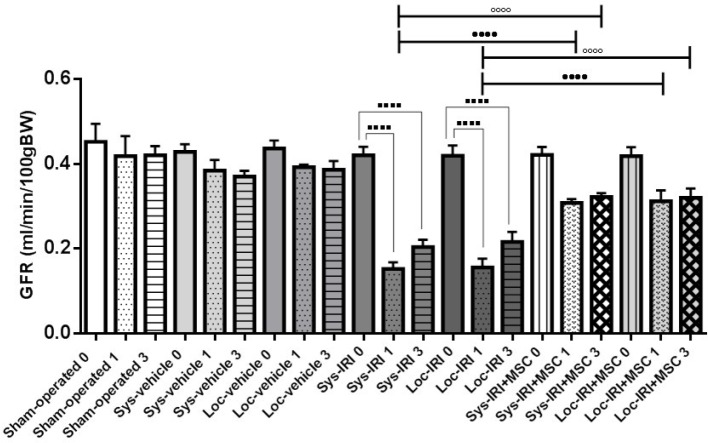
Effect of renal ischemia and mesenchymal stem cells (MSC) administration on glomerular filtration rate (GFR) level
| Groups | Day 0 | Day 1 | Day 3 |
|---|---|---|---|
| Sham-operated | 0.45±0.09 | 0.42±0.11 | 0.42±0.05 |
| Systemic vehicle | 0.43±0.04 | 0.38±0.06 | 0.37±0.03 |
| Local vehicle | 0.44±0.04 | 0.39±0.01 | 0.39±0.05 |
| Systemic IRI | 0.42±0.04 | 0.15±0.04 ≠ • | 0.20±0.04 ≠ § |
| Local IRI | 0.42±0.05 | 0.16±0.05 ≠ • | 0.22±0.05 ≠ “ |
| Systemic IRI+MSC | 0.42±0.04 | 0.31±0.02 * | 0.32±0.02 ** |
| Local IRI+MSC | 0.42±0.05 | 0.31±0.06 # | 0.32±0.05 ## |
GFR values (ml/min/100 gBW) from cell-treated and non-treated groups. There is no significant difference between systemic and local MSC treated animals. Values are mean±SEM.
P<0.0001 compared to day 0 in same group,
P=0.0012, and
P=0.0002 compared to Sys-IRI group, day 1 and,
P=0.0012, and
P=0.0004 compared to Loc-IRI group, day 1,
P<0.0001 compared to Sham-operated group in same day, and
P<0.0001,
P=0.0003, and
P=0.0002 compared to vehicle-treated group in same route injection and same day
Ischemia for 40 min in animals led to severe renal insufficiency, as evidenced by a rise in creatinine, BUN, and decline in GFR values when compared with non-ischemic animals at days 1 and 3.
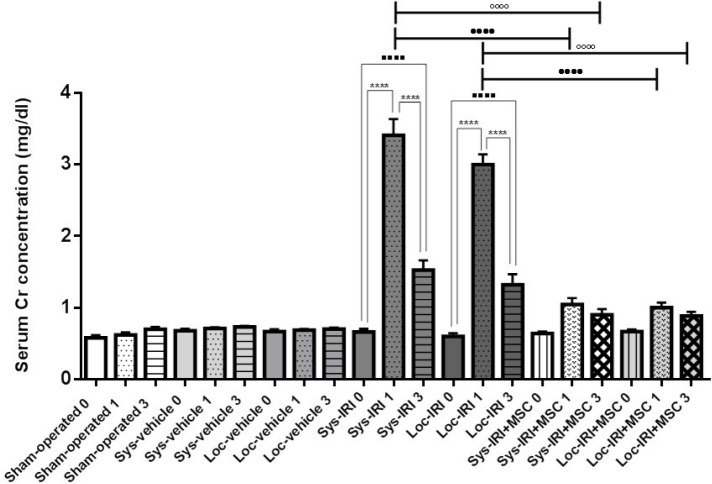
As shown in Figures 4-6, animals subjected to AKI at 24 hr post-ischemia had significant increase in the levels of serum creatinine to 3.41±0.23 mg/dl (Sys-IRI), 3±0.14 mg/dl (Loc-IRI), and serum BUN to 86.8±6.7 mg/dl (Sys-IRI) and 83.5±4.7 mg/dl (Loc-IRI) when compared with non-ischemic animals (P<0.05). The results showed that there are no significant differences between systemic and local ischemic groups at any time (Table 1-3).
Figure 4.
Effect of rat bone marrow-mesenchymal stem cells (BM-MSCs) on serum creatinine following renal ischemia-reperfusion injury (IRI). Serum creatinine was measured at pre-injury (day 0) and 1 and 3 days after surgery. Rats treated with MSCs immediately after reperfusion. **** P<0.0001 compared to day 1, ....P<0.0001 compared to day 0, ••••P<0.0001 compared to day 1 in cell treated group (same route injection), and °°°°P<0.0001 compared to day 3 in cell treated group (same route injection), (n=5 per group), Cr: creatinine
Figure 6.
Effect of rat bone marrow-mesenchymal stem cells (BM-MSCs) on glomerular filtration rate (GFR) following renal ischemia-reperfusion injury (IRI). GFR was measured at pre-injury (day 0) and 1 and 3 days after surgery. Rats treated with MSCs immediately after reperfusion. ****P<0.0001 compared to day 0, ••••P<0.0001 compared to day 1 in cell treated group (same route injection), and °°°°P<0.0001 compared to day 3 in cell treated group (same route injection), (n=5 per group)
Animals infused with MSC had significantly lower serum creatinine, 1.05±0.09 mg/dl (Sys-IRI+MSC), 1±0.07 mg/dl (Loc-IRI+MSC) (P<0.0001, Figure 4), BUN levels 58.92±3.96 mg/dl (P=0.0002, Sys-IRI+MSC) and 57.16±4.16 mg/dl (P=0.0005, Loc-IRI+MSC) at 24 hr after cell injection compared with same route vehicle-treated IRI animals (Figure 5).
Figure 5.
Effect of rat bone marrow-mesenchymal stem cells (BM-MSCs) on serum blood urea nitrogen (BUN) following renal ischemia-reperfusion injury (IRI). Serum BUN was measured at pre-injury (day 0) and 1 and 3 days after surgery. Rats treated with MSCs immediately after reperfusion. **** P<0.0001 compared to day 1, .... P<0.0001 compared to day 0, ••••P<0.0001 compared to day 1 in cell treated group (same route injection), and °°°°P<0.0001 compared to day 3 in cell treated group (same route injection), (n=5 per group)
Figure 6 represented the GFR from different treatment groups. A significant increase in the GFR was observed in MSC-treated groups, as compared to those of vehicle-treated IRI animals, Sys- IRI+MSC (0.31±0.01 ml/min/100 gBW) vs. Sys-IRI (0.15±0.02 ml/min/100 gBW) and Loc-IRI+MSC (0.31±0.03 ml/min/100 gBW) vs. Loc-IRI (0.16±0.02 ml/min/100 gBW), (P=0.0012, Figure 6) at 24 hr post-ischemia.
Histological analysis
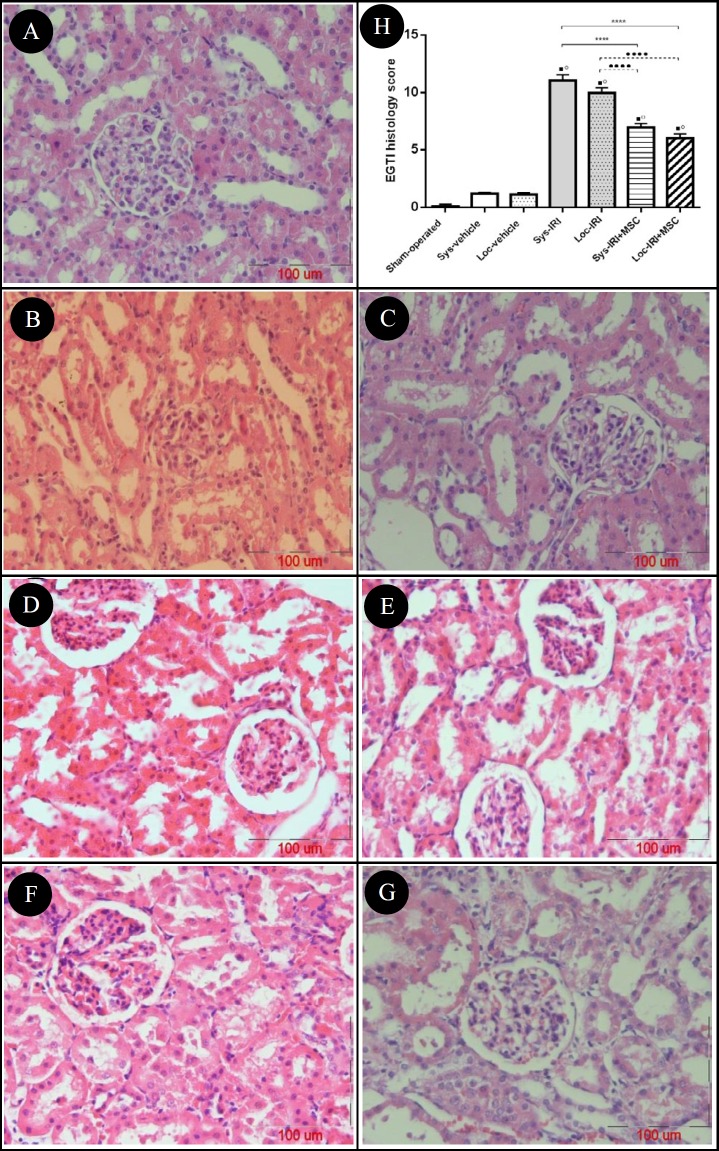
We examined the histological characteristics of kidney tissues from each group. Compared with the sham-operated and systemic/local vehicle-treated groups injected with media, there was notable damage in IRI kidneys (Figure 7). When compared with IRI rats, 11±0.5 (Sys-IRI) and 10±0.5 (Loc-IRI), kidneys from MSC-treated rats showed significantly lower degree of EGTI score, 6.98±0.31 and 6.04±0.35 in systemic and local IRI+MSC groups, respectively (Figure 7).
Figure 7.
Photomicrograph of pathological changes induced by renal ischemia-reperfusion injury (IRI). The kidney sections were stained by hematoxylin and eosin (H&E) and examined using a light microscope (x400) and scored using the EGTI histology scoring system to assess Endothelial, Tubular, Glomerular and Interstitial cell damage (A-G). Score 0 indicates normal histology, while score 14 displays maximal injury. A: Control (sham operated) group with normal renal morphology, B & C: Sys-vehicle and Loc-vehicle groups with non-significant changes compared with control group. D & E: Sys-IRI and Loc-IRI groups with the distinctive pattern IRI (dilation of renal tubules, cast formation, endothelial loss, as well as tubular epithelial cells necrosis). F & G: Sys-IRI+MSC and Loc-IRI+MSC groups with relatively well-preserved architecture and focal tubular necrosis with lower degree of vacuolization and cast formation. H: Effect of rat bone marrow-mesenchymal stem cells (BM-MSCs) on renal histology following renal IRI. .P<0.0001 compared to sham-operated group, °P< 0.0001 compared to same route vehicle injection, ****P<0.0001 compared to Sys-IRI group and ••••P<0.0001 compared to Loc-IRI group
Discussion
The present study provides evidence to support that the MSC therapy contributes significant renoprotection in rats with IRI-AKI. Animals infused with MSC immediately after reperfusion had significantly better renal function and lower renal injury scores than untreated animals. This was demonstrated in two different injection route.
The pathophysiological process after ischemic injury causes several functional and structural changes in renal tissue involving cellular metabolism, cytoskeleton, extra cellular matrix generation and activation of inflammatory factors such as cytokines, chemokines, and ROS molecules (30).
Despite these changes, surviving renal tubule cells have a remarkable ability to regenerate and proliferate after ischemic AKI. As our results showed, untreated animals had a relative recovery in renal function 72 hr after ischemia.
Cell-based regenerative therapeutic applications are being considered as the most promising approaches to treat several diseases including IRI/AKI (11, 31–34). Cellular mechanisms such as transdifferentiation, de-differentiation or homing of SC, paracrine or endocrine effects of administered SC and expansion/proliferation of resident stem/progenitor cell play critical role in the regenerative capabilities of injured tissues (30). The protective effects via differentiation-independent mechanisms, is due to cytokines secretion and increased expression of growth factors such as HGF, VEGF, and IGF-I, or their anti-apoptotic and mitogenic properties (3, 4, 22, 35–38). Significance of these paracrine effects of MSCs in regenerative medicine has gained much attention of scientists for IV injection of cells. In spite of such paracrine effects, the best administration route for the most effectiveness of stem cells is to be close to the site of injury and natural cell niche. It has been shown that the effect of cell injection at the site of injury in some tissues is much better than systemic administration. Several studies have focused on the local injection of the cells close to the injury site in ischemic heart and brain i.e. in many myocardial infarction models, direct injection of stem cells at the center of infarct and border zone areas of the myocardium is more effective than systemic injection (11, 31–34).
It is well known that renal ischemia usually damages the corticomedullary zone. The S3 segment represents the remainder of the proximal tubule, located in the deep inner cortex and the outer stripe of the outer medulla (2). Because of that, the corticomedullary zone is the best site for locally intraparenchymal injection. In one study, cells were injected by hydrogel to increase homing in the tissue (39). In other two studies, the cells were injected into the corticomedullary zone under ultrasound guide (40, 41).
As mentioned, the corticomedullary zone is the best site for intraparenchymal injection, so in the absence of the ultrasonic guide, the injection at the proper location was blind. We studied the 80 healthy kidneys and achieved cortical diameter.
The integration percentage of administrated stem cells is usually below 1% in any given organ. On the other hand, MSCs are known to have immunomodulatory properties and secrete various growth factors and cytokines, which may explain further possible mechanisms including inhibition of fibrosis and apoptosis, improvement of angiogenesis, stimulation of mitosis, proliferation and differentiation of intrinsic stem cells (42-45). The kidney can recover after damage following repolarization, cell de-differentiation, migration, and proliferation and the release of growth factors, chemokines and cytokines can facilitate repair process (30).
The administration of MSC, hematopoietic stem cell (HSC), or a bone marrow transplant, following glycerol-, cis-platinum, or IRI/AKI, protected kidney tissue and improved renal function in rodents (3, 44). As with other studies (3, 44), animals infused with MSC, either systemic or local injection, compared with untreated MSC animals, had significantly better renal function as early as 24 hr following reflow (3, 4), which was determined by decreasing the Cr level approximately 33%.
In accordance with comparative analysis of literature, it was observed that administration of MSC, independently to the administration route, significantly improved renal function at days 1 and 3 after induction of IRI/AKI among animals treated by MSCs.
The mechanism behind the regeneration could be transdifferentiation of MSCs and differentiation-independent protective effects such as cytokines secretion and increased expression of growth factors such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor 1 (IGF-I), or their anti-apoptotic and mitogenic properties. Pathological data was enough to justify the paracrine effects in MSC-based kidney regeneration (3, 4, 22, 35–38). As discussed by several other studies, the infusion of MSC into the suprarenal aorta in IRI/AKI model has no adverse effects like respiratory and renal problems or death (4, 44). Moreover, the advantage of local injection is directly trapping into the injury site. Results of the current work demonstrate that local injection has slightly better effect than intra-aortic injection but these differences are not statistically significant.
Conclusion
Our data showed the promising kidney-protective effect of MSC in the rat IRI/AKI experimental model employing both intra-aorta and intra-parenchymal injection routes. Administration of MSCs significantly improved renal function and histopathologic indices at days 1 and 3 after induction of IRI/AKI. These effects at least in part, mediated by complex paracrine actions that are able to significantly protect and regenerate the damaged kidneys. More investigations are required to determine the mechanism responsible for this recovery.
Acknowledgment
This work was supported by a grant from Vice Chancellor for Research and Technology of Mashhad University of Medical Sciences (MUMS), as a part of PhD thesis presented by the first author.
References
- 1.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 2.Taal M, Brenner B, Rector F. Brenner & Rector’s the kidney. 9th edition. Philadelphia, PA: Elsevier/Saunders; 2012. [Google Scholar]
- 3.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. AJP Ren Physiol. 2005;289:31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 4.Lee P-Y, Chien Y, Chiou G-Y, Lin C-H, Chiou C-H, Tarng D-C. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via down regulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant. 2012;21:2569–2585. doi: 10.3727/096368912X636902. [DOI] [PubMed] [Google Scholar]
- 5.Reule S, Gupta S. Kidney regeneration and resident stem cells. Organogenesis. 2011;7:135–139. doi: 10.4161/org.7.2.16285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian H, Lu Y, Shah SP, Wang Q, Hong S. 14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury. Stem Cells Dev. 2012;21:1187–1199. doi: 10.1089/scd.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neshati V, Mollazadeh S, Fazly Bazzaz BS, Iranshahi M, Mojarrad M, Naderi-Meshkine H, et al. Cardiogenic effects of characterized Geum urbanum extracts on adipose-derived human mesenchymal stem cells. Biochem Cell Biol. 2018:24. doi: 10.1139/bcb-2017-0313. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Mollazadeh S, Neshati V, Fazly Bazzaz BS, Iranshahi M, Mojarrad M, Naderi-Meshkine H, et al. Standardized Sophora pachycarpa root extract enhances osteogenic differentiation in adipose-derived human mesenchymal stem cells. Phytother Res. 2017;31:792–800. doi: 10.1002/ptr.5803. [DOI] [PubMed] [Google Scholar]
- 9.Togel F, Westenfelder C. The role of multipotent marrow stromal cells (MSCs) in tissue regeneration. Organogenesis. 2011;7:96–100. doi: 10.4161/org.7.2.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahzad U, Li G, Zhang Y, Yau TM. Transmyocardial revascularization induces mesenchymal stem cell engraftment in infarcted hearts. Ann Thorac Surg. 2012;94:556–5562. doi: 10.1016/j.athoracsur.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 11.Nayan M, Paul A, Chen G, Chiu RCJ, Prakash S, Shum-Tim D. Superior therapeutic potential of young bone marrow mesenchymal stem cells by direct intramyocardial delivery in aged recipients with acute myocardial infarction: in vitro and in vivo investigation. J Tissue Eng. 2011 doi: 10.4061/2011/741213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neshati V, Mollazadeh S, Fazly Bazzaz BS, de Vries AAF, Mojarrad M, Naderi-Meshkine H, et al. MicroRNA-499a-5p promotes differentiation of human bone marrow-derived mesenchymal stem cells to cardiomyocytes. Appl Biochem Biotechnol. 2018;24 doi: 10.1007/s12010-018-2734-2. doi: 10.1007/s12010-018-2734-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Mathiasen AB, Jorgensen E, Qayyum AA, Haack-Sorensen M, Ekblond A, Kastrup J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial) Am Heart J. 2012;164:285–291. doi: 10.1016/j.ahj.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:3. doi: 10.1186/1741-7015-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003 Dec;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 16.Perasso L, Cogo CE, Giunti D, Gandolfo C, Ruggeri P, Uccelli A, et al. Systemic administration of mesenchymal stem cells increases neuron survival after global cerebral ischemia in vivo ( 2VO ) Neural Plast . 2010 doi: 10.1155/2010/534925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edalatmanesh MA, Bahrami AR, Hosseini E, Hosseini M, Khatamsaz S. Bone marrow derived mesenchymal stem cell transplantation in cerebellar degeneration: A behavioral study. Behav Brain Res. 2011;225:63–70. doi: 10.1016/j.bbr.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Morando S, Vigo T, Esposito M, Casazza S, Novi G, Principato MC, et al. The therapeutic eff ect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res Ther. 2012;3:1–7. doi: 10.1186/scrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aboushwareb T, Atala A. Stem cells in urology. Nat Clin Pract Urol. 2008;5:621–631. doi: 10.1038/ncpuro1228. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda H, Yamahara K, Otani K, Okumi M, Yazawa K, Kaimori JY, et al. Transplantation of allogenic fetal membrane-derived mesenchymal stem cells protects against ischemia/reperfusion-induced acute kidney injury. Cell Transplant. 2014;23:889–899. doi: 10.3727/096368913X665594. [DOI] [PubMed] [Google Scholar]
- 21.Ende N, Chen R, Reddi AS. Transplantation of human umbilical cord blood cells improves glycemia and glomerular hypertrophy in type 2 diabetic mice. Biochem Biophys Res Commun. 2004;321:168–171. doi: 10.1016/j.bbrc.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 22.Morigi M, Rota C, Montemurro T, Montelatici E, Lo Cicero V, Imberti B, et al. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513–522. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 23.Tögel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–485. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–2212. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 25.Geng Y, Zhang L, Fu B, Zhang J, Hong Q, Hu J, et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther. 2014;5:80. doi: 10.1186/scrt469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havakhah S, Sadeghnia HR, Hajzadeh MR, Roshan NM, Shafiee S, Hosseinzadeh H, et al. Effect of Nigella sativa on ischemia-reperfusion induced rat kidney damage. Iran J Basic Med Sci. 2014;17:986–992. [PMC free article] [PubMed] [Google Scholar]
- 27.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 28.Naderi-meshkin Hojjat. "Improving the homing capacity of mesenchymal stem cells (MSCs) through pretreatment and use of an injectable scaffold percutaneously in rat animal model." PhD thesis. Ferdowsi University of Mashhad; 2013. [Google Scholar]
- 29.Khalid U, Pino-Chavez G, Nesargikar P, Jenkins RH, Bowen T, Fraser DJ, et al. Kidney ischaemia reperfusion injury in the rat: the EGTI scoring system as a valid and reliable tool for histological assessment. J Histol Histopathol. 2016 Jan;3:1. [Google Scholar]
- 30.Bagul A, Frost JH, Drage M. Stem cells and their role in renal ischaemia reperfusion injury. Am J Nephrol. 2013;37:16–29. doi: 10.1159/000345731. [DOI] [PubMed] [Google Scholar]
- 31.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Lai WH, Liao SY, Siu CW, Yang YZ, Tse HF. Lack of cardiac nerve sprouting after intramyocardial transplantation of bone marrow-derived stem cells in a swine model of chronic ischemic myocardium. J Cardiovasc Transl Res. 2012;5:359–364. doi: 10.1007/s12265-012-9350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko SF, Yip HK, Lee CC, Sheu JJ, Sun CK, Ng SH, et al. Immediate intramyocardial bone marrow-derived mononuclear cells implantation in minipig myocardium after permanent coronary artery ligation: Magnetic resonance imaging with histopathologic and immunochemical correlation. Invest Radiol. 2011;46:495–503. doi: 10.1097/RLI.0b013e318214a63f. [DOI] [PubMed] [Google Scholar]
- 34.Wang CC, Chen CH, Lin WW, Hwang SM, Hsieh PCH, Lai PH, et al. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res. 2008;77:515–524. doi: 10.1093/cvr/cvm046. [DOI] [PubMed] [Google Scholar]
- 35.Furuichi K, Shintani H, Sakai Y, Ochiya T, Matsushima K, Kaneko S, et al. Effects of adipose-derived mesenchymal cells on ischemia-reperfusion injury in kidney. Clin Exp Nephrol. 2012;16:679–689. doi: 10.1007/s10157-012-0614-6. [DOI] [PubMed] [Google Scholar]
- 36.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 37.Cao H, Qian H, Xu W, Zhu W, Zhang X, Chen Y, et al. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32:725–732. doi: 10.1007/s10529-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 38.Altun B, Yilmaz R, Aki T, Akoglu H, Zeybek D, Piskinpasa S, et al. Use of mesenchymal stem cells and darbepoetin Improve Ischemia-Induced Acute Kidney Injury Outcomes. Am J Nephrol. 2012;35:531–539. doi: 10.1159/000339167. [DOI] [PubMed] [Google Scholar]
- 39.Gao J, Liu R, Wu J, Liu Z, Li J, Zhou J, et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials. 2012;33:3673–3681. doi: 10.1016/j.biomaterials.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 40.Quimby JM, Webb TL, Gibbons DS, Dow SW. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. J Feline Med Surg. 2011;13:418–426. doi: 10.1016/j.jfms.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pino CJ, Humes HD. Stem cell technology for the treatment of acute and chronic renal failure. Transl Res. 2010;156:161–168. doi: 10.1016/j.trsl.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perin L, Giuliani S, Sedrakyan S, Da Sacco S, De Filippo RE. Stem cell and regenerative science applications in the development of bioengineering of renal tissue. Pediatr Res. 2008;63:467–471. doi: 10.1203/PDR.0b013e3181660653. [DOI] [PubMed] [Google Scholar]
- 43.Tögel FE, Westenfelder C. Treatment of acute kidney injury with allogeneic mesenchymal stem cells: preclinical and initial clinical data. In Regenerative Nephrology. Elsevier Inc; 2011. pp. 315–339. [Google Scholar]
- 44.Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl. 2011;1:103–106. doi: 10.1038/kisup.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wise AF, Ricardo SD. Mesenchymal stem cells in kidney inflammation and repair. Nephrology. 2012;17:1–10. doi: 10.1111/j.1440-1797.2011.01501.x. [DOI] [PubMed] [Google Scholar]