Abstract
Introduction
Advancing research and treatment for Alzheimer's disease (AD) and the search for effective treatments depend on a complex financial ecosystem involving federal, state, industry, advocacy, venture capital, and philanthropy funding approaches.
Methods
We conducted an expert review of the literature pertaining to funding and financing of translational research and drug development for AD.
Results
The federal government is the largest public funder of research in AD. The National Institute on Aging, National Institute of Mental Health, National Institute of General Medical Sciences, and National Center for Advancing Translational Science all fund aspects of research in AD drug development. Non-National Institutes of Health federal funding comes from the National Science Foundation, Veterans Administration, Food and Drug Administration, and the Center for Medicare and Medicaid Services. Academic Medical Centers host much of the federally funded basic science research and are increasingly involved in drug development. Funding of the “Valley of Death” involves philanthropy and federal funding through small business programs and private equity from seed capital, angel investors, and venture capital companies. Advocacy groups fund both basic science and clinical trials. The Alzheimer Association is the advocacy organization with the largest research support portfolio relevant to AD drug development. Pharmaceutical companies are the largest supporters of biomedical research worldwide; companies are most interested in late stage de-risked drugs. Drugs progressing into phase II and III are candidates for pharmaceutical industry support through licensing, mergers and acquisitions, and co-development collaborations.
Discussion
Together, the funding and financing entities involved in supporting AD drug development comprise a complex, interactive, dynamic financial ecosystem. Funding source interaction is largely unstructured and available funding is insufficient to meet all demands for new therapies. Novel approaches to funding such as mega-funds have been proposed and more integration of component parts would assist in accelerating drug development.
Keywords: NIH, NIGMS, NCATS, NIMH, NINDS, Venture capital, Advocacy, Philanthropy, Alzheimer's disease, Clinical trials, Pharmaceutical industry, Biotechnology companies, SBIR, STTR
Alzheimer's disease (AD) is increasing in frequency as the world's population ages and poses a major threat to the public health. AD doubles in frequency every 5 years after the age 65, and the number of individuals in the United States with AD dementia is projected to grow from a current 5.5 million to an estimated 14 million by the year 2050 [1], [2]. The world's population of AD dementia will increase from 35 million to an astonishing 135 million by 2050 [3]. The corresponding toll in human suffering and socioeconomic costs will be enormous. The identification of milder forms of cognitive impairment and preclinical AD further enlarges considerations regarding the impact of AD on society [2], [4], [5].
Prevention and treatment of AD by 2025 has been articulated as a goal of the US government and has been endorsed by other countries [6], [7]. Prevention and treatment require the development of new treatments that prevent or delay the onset, slow the progression, or improve the symptoms (cognitive, functional, and behavioral) of AD. The failure rate of AD drug development is 99% [8]; the failure rate of the development of disease-modifying therapies for AD is 100%. Despite these discouraging outcomes in drug development programs, the urgent need to address the socioeconomic crisis posed by AD requires that we continue to advance understanding of AD drug development. Lessons learned from AD are likely to generalize to other neurodegenerative disorders (NDDs), given the many similarities in protein aggregation and cell injury across NDD [9]. To advance the research agenda in AD, financial resources are required including funding from government, industry, venture capital, foundations, and philanthropy. Federal research funding programs include the National Institutes of Health (NIH), National Science Foundation (NSF), Food and Drug Administration (FDA), Department of Defense, and Veterans Administration (VA). Private sector funding includes sources in the biopharma industry, venture capital investments, foundations, advocacy organizations, and support from philanthropists. Public-private partnerships have formed to help ameliorate the financial burden to individual entities, and industry collaborations have evolved to de-risk investments [10], [11]. Funding and financing resources form a complex financial ecosystem, which is a key to advancing research in AD. Here, we describe major elements of this network of support especially as it pertains to development of new drug treatments for AD.
1. Cost of AD drug development
Total costs of an AD drug development program are estimated at $5.6 billion, and the process takes 13 years from preclinical studies to approval by the FDA [12]. This compares to an estimated cost of cancer treatment development of $793.6 million per agent (assuming 9% cost of capital) [13]. Considering the pharmaceutical industry as a whole bringing a new agent to approval has an estimated cost of $2.8 billion [14]. AD drug development costs substantially exceed most estimates for drugs in other therapeutic areas.
Table 1 shows the average cost and duration of each phase of AD drug development. These figures include the cost of capital and the cost of failures that companies must sustain if they work in the AD drug development arena. The high rate of failure of AD drug development is partly responsible for the high costs of advancing AD drug development [8], but out-of-pocket costs for development of a single AD agent approach $500 million (Table 1). Phase III trials are the most costly part of AD drug development, and pharmaceutical companies are among the few enterprises that can sustain such costs.
Table 1.
Cost and duration of each aspect of AD drug development
| Stage of process | Duration (months) | Cost (billions)∗ ($) | Cumulative out-of- pocket costs (at end of each stage) (millions) ($) |
|---|---|---|---|
| Preclinical | 50.1 | 1.65 | |
| Phase I | 12.8 | 1.19 | 71 |
| Phase II | 27.7 | 1.04 | 126 |
| Phase III | 50.9 | 1.79 | 413 |
| FDA | 18 | 0.02 | |
| Total | 13.3 years | 5.69 |
Abbreviations: AD, Alzheimer's disease; FDA, Food and Drug Administration.
Capitalized and including cost of failures of drug development (from Scott et al, 2014) [12].
2. National Institutes of Health
The principle public funder of research is the US NIH, investing more in health research than any other public enterprise in the world with an annual budget of approximately $34 billion U.S. dollars. The federal budget devoted to NIH has had support from both Republican and Democratic parties. There is a mismatch between the cost of disease to society and the amount of research devoted to it. AD, for example, costs the US society more than $216 billion annually, and it has an NIH budget of $1.8 billion; for every $1 spent on AD, less than 1% of that amount is devoted to research [15], [16]. AD has a greater impact on the US economy than cancer or cardiovascular disease [15]; it has a smaller NIH research budget than either of these disorders (cancer – $6.0 billion, cardiovascular disease - $2.2 billion; www.nih.gov).
Neuroscience research at NIH is guided by the Neuroscience Blueprint and within that the NIH Neurotherapeutics Blueprint was launched to create a virtual pharmaceutical company aimed at advancing discovery and development of small molecules to treat Central Nervous System disease including NDDs [17]. The goal was to foster the development of potential therapies in Academic Medical Centers (AMCs) and biotechnology companies and to advance new therapies to clinical trials and potential industry partnership. Once funding is approved, lead discovery teams from the National Institute of Neurological Disease and Stroke work collaboratively and guide the grantee's development program. The lead team assists in bioactivity/efficacy hit-to-lead studies, medicinal chemistry and lead optimization, pharmacokinetics and toxicity, data management, manufacturing and formulation, and phase 1 clinical trials [17].
Within the NIH, the major funding agency for AD research is the National Institute on Aging (NIA). To support the development of new therapies for AD and related dementias, the NIA funds a trial coordinating center—the Alzheimer Clinical Trial Consortium—that conducts clinical trials on AD and related disorders and advances tools and methods relevant to trials in this population. The NIA provides grant support for promising therapies to be tested with the Alzheimer Clinical Trial Consortium and its trial network. The Alzheimer Clinical Trial Consortium continues the themes of AD clinical trials initiated with the Alzheimer's Disease Cooperative Study [18]. The NIA participates in a public-private partnership—the Alzheimer's Disease Neuroimaging Initiative (ADNI)—funded partially by pharmaceutical companies and NIH whose goal is to simulate a clinical trial and collect data relevant to trial planning. The ADNI studies brain imaging and biomarker changes in longitudinal cohorts of cognitively normal individuals, participants with mild cognitive impairment, and mild AD dementia patients [19]. The ADNI has been very scientifically productive and has produced publically available data relevant to calculating sample sizes needed to power clinical trials, the predictive value of biomarkers and biomarker combinations, and the relationship of biomarkers to clinical measures [20], [21]. The ADNI is seen as a model of research acceleration by a public-private partnership [19], [22].
The NIA has funded a project to create a Trial-Ready Cohort for Preclinical and Prodromal AD to develop means of enhancing recruitment of participants to clinical trials using electronic means, following them with serial on-line assessments, and creating algorithms that help to predict which among the registrants have positive amyloid scans required for participation in AD clinical trials [23]. Other NIA programs relevant to AD drug development are shown in Table 2.
Table 2.
NIA-supported resources relevant to AD drug development
| NIA-supported program | Relevance to AD drug development |
|---|---|
| Alzheimer Clinical Trial Consortium | Conducts clinical trials of AD treatments with an organized network of academic clinical trial sites |
| Alzheimer's Disease Neuroimaging Initiative | Longitudinal multisite study of biomarkers in preclinical AD, prodromal AD, and mild AD dementia in a simulated trial structure [19], [20], [21], [22] |
| Trial-Ready Cohort for Preclinical and Prodromal AD | Study to identify how best to use innovative technologies to engage participants in clinical trials and predict their biomarker status important for clinical trials [23]; conducted in partnership with GAP |
| AD Genetics Consortium | Identify genes related to AD risk and progression and indicative of pathways amenable to treatment [24] |
| National Cell Repository for AD | Repository of biological material derived from AD and other NDD available for study to find disease mechanisms that can be modified by treatment [25] |
| Dominantly Inherited AD Network | Characterize the natural history of patients with autosomal dominant AD |
| DIAN-Treatment Unit (DIAN-TU) | Conduct clinical trials in populations of participants with autosomal dominant AD mutations (funded as a partnership with the Alzheimer's Association) [26] |
| Alzheimer Prevention Initiative | Conducts clinical trials in patients at high genetic risk of developing AD (funded as a public-private partnership with pharmaceutical companies) [26] |
| Alzheimer's Disease Centers | Network of Centers that collect longitudinal data on AD and conduct AD research |
| National Alzheimer's Coordinating Center | Monitors, collects, archives, and provides access to data collected by the ADCs [27], [28] |
| Alzheimer's Drug Development Program | Supports therapy development activities including medicinal chemistry, pharmacokinetics, absorption, distribution, metabolism, excretion, toxicology efficacy in animal models, formulation development, chemical synthesis under Good Manufacturing Practices, Investigational New Drug enabling studies and initial phase I clinical testing. |
| Pilot Clinical Trials for the Spectrum of Alzheimer's Disease and Age-related Cognitive Decline (PAR-18-175) | Funds development and implementation of phase I or II clinical trials of promising pharmacological and nonpharmacological interventions in individuals with age-related cognitive decline and in individuals with AD across the spectrum from pre-symptomatic to more severe stages of disease, as well as to stimulate studies to enhance trial design and methods. |
| Phase III Clinical Trials for the Spectrum of Alzheimer's Disease and Age-related Cognitive Decline (PAR-18-028) | Funds R01 grant applications that propose to develop and implement phase III clinical trials of promising pharmacological and nonpharmacological interventions in individuals with age-related cognitive decline and across the AD spectrum from presymptomatic to more severe stages of disease. |
| AD Sequencing Project | Whole genome and whole exome sequencing of genes relevant to AD (discovery and follow-up study) |
| Molecular Mechanisms of the Vascular Etiology of Alzheimer's Disease Consortium | Supports research to better understand how the vascular system may be involved in the onset and progression of AD and related dementias. |
| Alzheimer's Preclinical Efficacy Database (AlzPED) | AlzPED provides tg model data across relevant translational criteria data sets such as therapeutic agents and targets. AlzPED is designed to help identify the critical data, design elements, and methodology missing from studies; making them susceptible to misinterpretation, less likely to be reproduced, and reducing their translational value. Through this function, AlzPED is intended to influence the development and implementation of reproducibility strategies, including guidelines for standardized best practices for the rigorous preclinical testing of AD candidate therapeutics. |
| Accelerating Medicines Partnership-Alzheimer's Disease Target Discovery and Preclinical Validation Project | The goal is to shorten the time between the discovery of potential drug targets and the development of new drugs for AD treatment and prevention by integrating the analyses of large-scale molecular data from human brain samples with network modeling approaches [29]. |
Abbreviations: AD, Alzheimer's disease; NIA, National Institute on Aging; NDD, neurodegenerative disorders; GAP, Global Alzheimer Platform.
The National Center for Advancing Translational Science (NCATS) approaches disease states agnostically and emphasizes the development of methods, infrastructure, and collaborations applicable to all human diseases including AD. The NCATS supports both preclinical and clinical aspects of drug development [30]. Resources useful in preclinical drug development are shown in Table 3 (www.ncats.nih.gov).
Table 3.
NCATS resources for preclinical drug development
|
Abbreviations: FDA, Food and Drug Administration; NIH, National Institutes of Health; NCATS, National Center for Advancing Translational Science.
The NCATS Bridging Interventional Development Gaps program enables research collaborations between individual researchers and NCATS experts to generate preclinical and clinical data through government contracts for use in Investigational New Drug applications to regulatory authorities such as the FDA (www.ncats.nih.gov). Using the Bridging Interventional Development Gap approach, the NIH outsources preclinical studies to contract research organizations (CROs) under the direction of NCATS intramural researchers with expertise in the relevant drug development areas (Table 4).
Table 4.
Components of the NCATS BrIDGs program
|
Abbreviations: NCATS, National Center for Advancing Translational Science; BrIDGs, Bridging Interventional Development Gaps; ADME, absorption, distribution, metabolism, and excretion; IND, investigational new drug application.
The NCATS supports clinical translational research and preclinical drug development (Table 5). The NCATS Clinical and Translational Science Awards (CTSAs) form a nationwide collaborative network of clinical trial sites that advance clinical trial training and conduct trials on many disease states [31], [32], [33]. The development of a single institutional review board for trials is an example of an initiative led by the NCATS and applied across the NIH to facilitate trials [34].
Table 5.
Clinical drug development resources of NCATS
|
Abbreviations: NCATS, National Center for Advancing Translational Science; BEST, Biomarkers, Endpoint, and Other Tools Resource.
The NCATS supports federal-pharmaceutical partnerships in the Accelerating Medicines Partnership to develop agents within companies that have repositioning potential [29]. These agents were originally intended for one indication but development was halted. Their mechanism of action suggests that they may be useful in another condition, and the NCATS supports these repositioning efforts in conjunction with the pharmaceutical company and AMC investigators. AD therapies are included in the Accelerating Medicines Partnership [29].
The National Institute of General Medical Sciences (NIGMS) supports research in AD and NDD as well as many other disease states and normal physiology [35]. The NIGMS comprises three scientific divisions including Biophysics, Biomedical Technology, and Computational Biosciences; Genetics and Molecular, Cellular, and Developmental Biology; and Pharmacology, Physiology, and Biological Chemistry and the Center for Research Capacity Building. The NIGMS is responsible for basic science research grants that explore new cellular pathways and new laboratory methods, research training, and diversification of the scientific workforce. The latter includes recruitment and training of an ethnically diversified workforce as well as leadership in funding programs and projects in states that historically have received low levels of NIH funding and have not had an opportunity to develop mature scientific programs, training, and resources [36]. Work force development in states with limited NIH funding is supported by the Institutional Development Award (IDeA) program. The IDeA program includes Clinical Translational Research awards, Center of Biomedical Research Excellence grants, and IDeA Networks of Biomedical Research Excellence [37].
The Center for Neurodegeneration and Translational Neuroscience, a collaboration between the Cleveland Clinic Lou Ruvo Center for Brain Health (LRCBH) [38] and the University of Nevada, Las Vegas, is supported by a Center of Biomedical Research Excellence award and exemplifies the support by the NIGMS of research in AD and NDD. The Center for Neurodegeneration and Translational Neuroscience comprises administrative, data management and statistics, and clinical and translational research cores, as well as projects studying brain imaging and cognitive deficits in AD and Parkinson's disease and animal models of AD (see accompanying articles in this e-book).
Research in AD may be part of the portfolio of other NIH institutes. Research in behavioral issues in AD may be supported by the National Institute of Mental Health. An example of National Institute of Mental Health-funded AD research is the Clinical Antipsychotic Trials of Intervention Effectiveness—Alzheimer's Disease [39], [40]. Similarly, a study of ginkgo biloba for prevention of cognitive decline in older adults was supported by the National Center for Complementary and Alternative Medicines [41].
Research funds are accessed through competitive grants that support various types of research (Table 6). The NIH grants include “direct costs” that cover the expenses of the proposed research and “indirect costs” that are provided to the institutions hosting the research to account for research-related expenses not covered by the direct costs including facilities, personnel management, and administration. These indirect costs can comprise up to 60% or more of the total award and have become a major source of revenue for research-intensive institutions [42]. This indirect support is an essential part of the research ecosystem.
Table 6.
Major NIH grant types (https://grants.nih.gov/grants/funding/ac_search_results.htm)
| Grant title | Grant number | Grant description |
|---|---|---|
| Research Construction Programs | C06 | Research Facilities Construction Grant |
| Institutional Training and Director Program Projects | DP1 | NIH Director's Pioneer Award |
| Institutional Training and Director Program Projects | DP2 | NIH Director's New Innovator Awards |
| Institutional Training and Director Program Projects | DP4 | NIH Director's Pathfinder Award - Multi-Year Funding |
| Research Career Programs | K12 | Physician Scientist Award (Program) |
| Research Career Programs | K21 | Scientist Development Award |
| Research Career Programs | K23 | Mentored Patient-Oriented Research Career Development Award |
| Research Program Projects and Centers | P20 | Exploratory Grants |
| Research Program Projects and Centers | P30 | Center Core Grants |
| Research Program Projects and Centers | P50 | Specialized Center |
| Research Projects | R01 | Research Project |
| Research Projects | R13 | Conference |
| Research Projects | R21 | Exploratory/Developmental Grants |
| Research Projects | R33 | Exploratory/Developmental Grants phase II |
| Research Projects | R34 | Planning Grant |
| Research Projects | R41/42 | Small Business Technology Transfer Grants—phase I and phase II |
| Research Projects | R43/44 | Small Business Innovation Research Grants—phase I and phase II |
| Research-Related Programs | S06 | Minority Biomedical Research Support-MBRS |
| Research-Related Programs | S11 | Minority Biomedical Research Support Thematic Project Grants |
| Research-Related Programs | S21 | Research and Institutional Resources Health Disparities Endowment Grants -Capacity Building |
| Training Programs | T32 | Institutional National Research Service Award |
| Training Programs | T37 | Minority International Research Training Grants (FIC) |
| Cooperative Agreements | U01 | Research Project--Cooperative Agreements |
Abbreviation: NIH, National Institutes of Health.
In addition to grants, the NIH supports small business initiations through Small Business Innovation Research and Small Business Technology Transfer grants. These grants are a key channel through which discoveries in academic laboratories can be commercialized through small start-up companies that begin the process of product development with the aim of eventually partnering the agent, device, or process for regulatory approval and commercialization. The IDeA program sponsors four Regional Technology Transfer Accelerator Hubs to facilitate development of Small Business Innovation Research and Small Business Technology Transfer applications from IDeA state investigators. The grants show the value of science in stimulating the economy and creating jobs.
The NIH sponsors some large-scale trans-institute programs that address problems applicable to many institutes and populations. The Brain Research through Advancing Innovative Neurotechnologies initiative is one such activity. The Brain Research through Advancing Innovative Neurotechnologies is supported by a partnership of the NIH, NSF, Defense Advanced Research Projects Agency, private foundations, and researchers [43]. The goal of the Brain Research through Advancing Innovative Neurotechnologies is “to accelerate the development and application of innovative technologies to construct a dynamic picture of brain function that integrates neuronal and circuit activity over time and space” [44], [45]. Understanding of brain networks in AD will be among the many benefits of this project.
3. Non-NIH federal funding
Non-NIH federal agencies have smaller research budgets and grant portfolios related to AD. These agencies include the NSF, VA, Department of Defense, FDA, Department of Energy Office of Science, National Library of Medicine, and Centers for Medicare and Medicaid Services.
The VA funds Geriatric Research, Education, and Clinical Centers that support research projects in AD. The VA projects that approximately 218,000 veterans will be diagnosed with dementia in 2017, an increase of more than 40,000 since 2008 and an urgent cause of concern for how to best meet the needs of aging veterans.
The NSF has grants in Integrative Organismal Systems, Molecular and Cellular Biosciences, and Computational Neurosciences among many areas of investment (www.nsf.gov). Some of these address issues important to understanding AD.
The FDA created the Critical Path Institute (C-Path) which sponsors the Clinical Data Interchange Standards Consortium and the Coalition Against Major Diseases (CAMD). These enterprises develop strategies for data interoperability and for qualification by the FDA of clinical trial assessments and biomarkers. The CAMD led the successful effort to qualify a simulation method of AD clinical trials useful for trial planning [46]. The CAMD also created the CAMD Online Data Repository for AD consisting of standardized placebo group data from 24 AD trials numbering 6500 subjects. The CAMD Online Data Repository for AD represents a unique integrated standardized clinical trial database whose size facilitates a comprehensive understanding of disease heterogeneity and progression [47].
The Centers for Medicare and Medicaid Services funds demonstration projects such as the Imaging Dementia: Evaluating Amyloid Scanning study that is assessing the impact of amyloid imaging on short- and long-term mild cognitive impairment and AD patient outcomes. These data are critical to decide whether amyloid imaging should be reimbursed by the Centers for Medicare and Medicaid Services as part of clinical care. Amyloid imaging is routinely used in clinical trials and Imaging Dementia: Evaluating Amyloid Scanning will help in the translation of trial observations to clinical care.
The Department of Defense has funded imaging research involving positron emission tomography and funds research in traumatic brain injury and chronic traumatic encephalopathy relevant to AD.
4. State funding
Some states provide funds for AD centers or AD-related research projects. For example, California funds California Alzheimer's Disease Centers and provides grant support for research projects. Texas funds a Consortium of Alzheimer's Disease Centers, New York supports Centers for Excellence for Alzheimer's Disease, and the Nevada legislature has supported the Cleveland Clinic LRCBH that provides AD and Parkinson's disease care and research in conjunction with the Center for Neurodegeneration and Translational Neuroscience.
5. Academic Medical Centers
AMCs are key to innovation in understanding disease biology, discovery of potential treatment interventions, and initiation of projects that can lead to product commercialization including new drugs for prevention and treatment of AD. AMCs have two main goals: teaching of the next generation of clinicians and biomedical scientists and discovery of new knowledge by their clinical and scientific faculty. In the course of achieving their goals, AMCs deliver care to patients and are part of the health-care system.
Most basic science research conducted at AMCs is funded by the NIH augmented by philanthropists, state funding, and biopharma partnerships. The pharmaceutical industry has downsized its internal research capacities and focused on late stage drug development and commercialization. To insure a steady flow of candidate compounds into their pipelines, many pharmaceutical companies have forged alliances with AMCs [48], [49], [50], [51], [52], [53], [54]. They fund AMC investigator research in areas of mutual interest in return for access to information, technology transfer, and commercialization opportunities. AMCs protect the intellectual property of the institution and the investigator through contractual arrangements implemented by Technology Transfer Offices [55], [56]. A recent survey identified 78 AMC-based drug discovery centers in the United States with 45 addressing neuropsychiatric and NDD targets [57]. The majority of funding for these centers came from federal sources, but some centers had substantive relationships with for-profit enterprises, mostly pharmaceutical companies.
Investigators in AMCs “spin off” biotechnology start-ups that typically focus on one promising compound, device, or discovery that has commercial potential. The Small Business Innovation Research and Small Business Technology Transfer grants facilitate this process of initiating new biotech start-ups. Angel funds, seed capital, and philanthropy assist AMC faculty in advancing the commercialization process. The spin-off companies are important sources of innovative new drugs. Approximately half of recently approved agents came from small biotechnology companies and AMC laboratories [58], [59], [60]. An entrepreneurial spirit is required to bridge the gap between academic culture and attracting private funding in the quest to commercialize a product. Products can be new drugs and treatments but might also be biomarkers with commercial potential or patentable processes that save time or money. Recently, venture capital companies have begun to form relationships directly with AMCs to encourage innovation, support start-ups, and access new products moving toward commercialization.
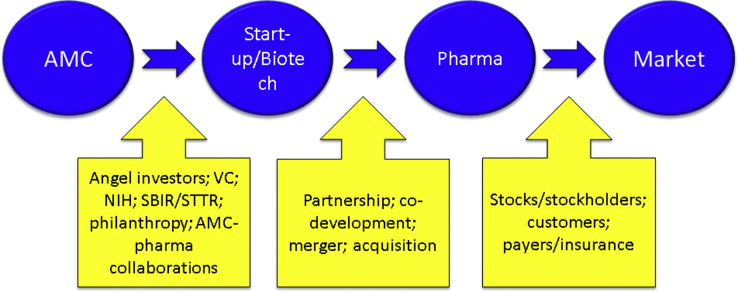
Fig. 1 provides an overview of how ideas for products originating in AMCs generate financial support, leading to eventual commercialization.
Fig. 1.
Financial ecosystem beginning with discovery in an academic medical center (AMC) and progressing through biotechnology to the pharmaceutical industry and eventually to market. Each stage of the process is supported by specific types of capital.
Adjustments are required by AMCs to facilitate drug development by faculty. The AMC conflict of interest policies often impose stringent limitations on academic-industry relationships and have the unintended consequence of hindering the participation of academic investigators in the drug discovery and development process [61], [62]. As industries increasingly turn to academic laboratories for target identification and early-stage treatment candidate development and to academic clinics for clinical trial leadership and execution, conflict of interest rules must evolve and be sufficiently flexible to allow AMC investigators to take advantage of the opportunities offered through industry collaboration while limiting influences that may be perceived as inappropriate [49]. Similarly, recognition of the important role of faculty involved in drug development including industry-sponsored research through academic promotion and award of tenure is critical to establishing a culture of drug development in AMCs.
To enhance their role in AD drug discovery and development AMCs need to provide students, residents, fellows, doctoral candidates, and others interested in AD therapeutics with courses, learning experiences, programs, and leadership that will acquaint them with the drug development processes and opportunities. The Stanford SPARK program offers a model of how this can be achieved [63]. SPARK is a hands-on training program in translational research providing guidance and seed funds to teach how to develop and commercialize drugs and diagnostics.
A major threat to AMC-industry collaboration is the lack of reproducibility of many findings reported from academic laboratories. Protocol errors, lack of statistical rigor in data analysis, and inadequate reporting have resulted in poor reproducibility and lack of confidence in research executed in AMC laboratories [64]. Rigorous adherence to conduct and reporting of basic and animal research is necessary to restore confidence in academic laboratory reports and facilitate academic-industry collaborations [65].
6. Biotechnology companies and private equity investment in AD drug development
Biotechnology companies can be defined as venture-backed drug development firms using technological applications centered on biological systems, living organisms, or their derivatives [66]. “Biotech” includes the disciplines of genetics, molecular biology, biochemistry, embryology, and cell biology and is linked to biomaterials, cell therapy, gene therapy, immunotherapy/vaccines, protein therapeutics, and some specialty pharmaceuticals and small-molecule therapeutics [66]. Success in AD drug development will produce a very high return on investment. This possibility attracts venture capital investment to AD research, but the high rate of failure has kept this funding stream small [67]. Venture capital investment in Central Nervous System disease declined 40% in the 2009–2013 period compared with the 2004–2008 period [68]. Angel investors or seed capital providers have high risk tolerance and supply small amounts of money to encourage novel ideas. If the concepts begin to mature and promise to lead to a successful program, venture capital may be attracted to allow more advanced drug development. Venture capital funds are usually raised in “rounds” of stock option sales (rounds A, B, and C) as milestones are reached in the drug development process. Venture capital investors typically want relatively fast turn-around on their investment; exit strategies for venture capital investors include transition of the biotech to partnerships, licensing agreements, co-development or co-marketing agreements, and progression to stock sales and initial public offerings. Venture capital investments available specifically to support AD drug development include Dolby Family Ventures and the United Kingdom-based Dementia Discovery Fund. Bill Gates of the Gates Foundation recently contributed $50,000,000 to the Dementia Discovery Fund and is providing $50,000,000 of additional venture capital to encourage AD drug development in the biotechnology sector [69].
Candidate therapies may pass from smaller to larger biotech companies as biotechs seek to strengthen their pipelines, progress toward vertically integrated Central Nervous System companies, or attract investors interested in a broader portfolio. This can be a healthy process allowing drugs to progress in testing before major pharmaceutical companies invest; however, the process also may lead to abuse by passing flawed agents from company to company and attracting capital from enthusiastic but under-informed investors.
7. Advocacy organizations
The Alzheimer Association is the largest private noncorporate funder of AD research. In 2016, the association invested $90 million in research, including $25 million in new project investments and the rest in support of on-going multi-year commitments [70]. The new project support included $7 million for clinical trials targeting brain inflammation and $4.3 million for the Dominantly Inherited AD Network-Treatment Unit [71] (Table 2).
The Alzheimer Foundation of America and UsAgainstAlzheimer's support advocacy for AD funding and have helped advance the national AD research agenda including maintaining and increasing funding for AD research. UsAgainstAlzheimer's helped advance the Global Alzheimer Platform whose goal is to enhance recruitment and trial conduct to accelerate AD drug development [23].
Advocacy plays a critical role in raising consciousness about AD, referring patients to trials, supporting families, providing research grants, and advocating for increased funding. In some cases, advocacy collaborates directly with laboratories or biotech companies to raise funds for drug development [72], [73].
8. Philanthropy
Philanthropists make contributions to advocacy organizations or directly to universities and scientists to support research projects. Many philanthropists are motivated by the experience of AD afflicting a family member, and many family philanthropies have originated with the intent of honoring a family member. Philanthropy plays a critically important role in the AD research ecosystem. Philanthropy often provides seed money for small projects that do not yet have preliminary data that would support a federal grant application. Philanthropy can fund high-risk/high-reward projects that might be too risky to receive funding from other sources such as the NIH.
The Alzheimer's Drug Discovery Foundation (ADDF) is a venture-philanthropy organization that is a key player and innovator in the AD drug discovery and development landscape. The ADDF funds studies in animal models, provides grants to fund animal toxicity testing of promising therapies, and supports early-stage proof-of-concept clinical trials. The venture philanthropy model allows the ADDF to take an ownership position in early-stage companies they fund and re-invest any revenues generated. Venture philanthropy is being more commonly applied as a vehicle for collaboration of foundation and advocacy groups with biotechnology companies [74].
The Cleveland Clinic LRCBH is an AD care and research organization in Las Vegas, Nevada [38]. It is a leader in drug development and clinical trials. The LRCBH was created and continues to be supported by philanthropists. The LRCBH demonstrates how philanthropy can influence a community to develop resources for AD research, creating a new AD research and drug development enterprise where none existed previously. Once established, philanthropy-based projects can attract federal funding and build clinical trials programs to garner support from other sources. The LRCBH now hosts a Center of Biomedical Research Excellence award from NIGMS as well as other federal funding and biopharma industry support. Multiple funding sources are critical to the sustainability of an AD research organization.
The Cure Alzheimer's Fund and Bright Focus Foundation are two philanthropies that provide grants to AD researchers doing innovative research and have had a substantial influence on research progress.
FasterCures is a disease-agnostic organization promoting information about drug development, convening meetings of drug development stakeholders, and doing analyses of and publishing novel means of advancing drug development (e.g., patient engagement strategies). FasterCures has a Philanthropy Advisory Service that studies disease areas and advises philanthropists on where investments will have maximum impact. The Philanthropy Advisory Service conducted such as analysis for AD [75].
9. Pharmaceutical industry
The pharmaceutical industry is the largest funder of drug discovery and development research in the world, exceeding that of NIH or any other funding organization. Biopharma funds approximately 60% of all annual US research and development activities. The total annual research and development budget for biopharma (biotechnology and pharmaceutical industry) in 2016 was $75 billion [76]. Over 70% of all AD clinical trials are sponsored or co-sponsored by the pharmaceutical industry [77].
Payments from biopharma support much of the AD drug development ecosystem. New agents may be accessed through AMC collaborations, in-house discovery teams, acquisitions of biotechnology companies, mergers with other pharmaceutical companies, in-licensing of promising compounds, and partnering and co-development arrangements. Each of these has corresponding financial support by the pharmaceutical company. Extensive in-house resources and out-sourcing to CROs are needed for each aspect of drug development—toxicity testing, manufacturing, supply line management, site management, recruitment of participants to trials, regulatory affairs, and so on. Outsourcing to CRO's accounted for approximately $20 billion of the 2016 biopharma research and development budget. For global drug development much of the infrastructure must exist in each country in which the company supports research activities [78].
Clinical trial sites are reimbursed for all activities provided to conduct biopharmaceutical trials, including trial site start up, gaining the institutional review board permission, managing the drug supply, advertising for participants to enter the trial, conducting all assessments (imaging, clinical interviews, rating scales, lumbar puncture, and so on), providing all data to the sponsor, and eventually closing the trial and maintaining records for 5 years after trial completion. Indirect payments (usually in the range of 30%–35% of total costs) are provided to the institutions hosting the research program. These payments comprise an important part of the financial infrastructure of many research organizations conducting clinical and translational research. All research must be free of charge to participants.
10. Drug development ecosystem
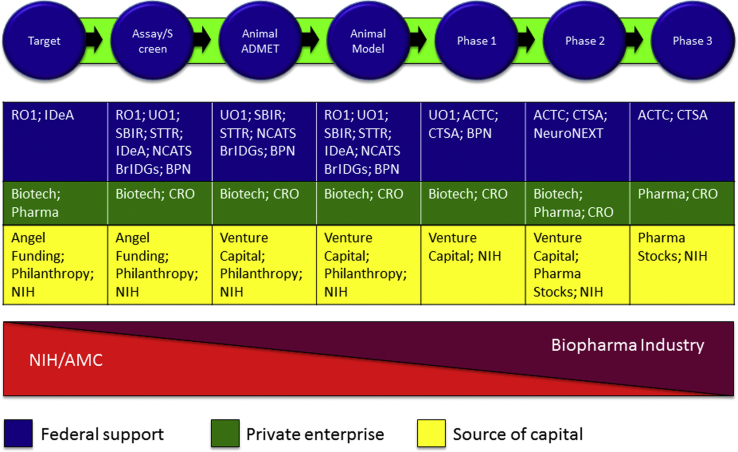
Fig. 2 summarizes the interactions of the organizations described previously to compose an ecosystem that supports drug development for AD. Advancing new treatments is not the only outcome on which the scientific enterprise is brought to bear, but it is among the most important to citizen-taxpayers who fund aspects of this work, and it serves as an important example of the interaction of the public and private sectors to improve public health.
Fig. 2.
Drug development ecosystem: phases of drug development and sources of support for each phase. Abbreviations: ACTC, Alzheimer Clinical Trial Consortium; BrIDGs, Bridging Interventional Development Gaps; BPN, Blueprint Neurotherapeutics Network; CRO, Contract Research Organization; CTSA, Clinical and Translational Science Award; IDeA, Institutional Development Award from National Institute of General Medical Sciences (NIGMS); NCATS, National Center for Advancing Translational Science; NIH, National Institutes of Health; SBIR, Small Business Innovation Research; STTR, Small Business Technology Transfer grants.
The NIH is the principle supporter of investigator-initiated research that leads to new targets and potential new interventions. Pharmaceutical companies partner with AMCs to support basic science research as they increasingly divest themselves of in-house research laboratories. Following optimization, the lead agent is tested for efficacy in animal models to determine if effects in an animal model system are supportive of the goals for the molecule. Animal models have not predicted efficacy in humans, but advancing an agent without knowledge of its effects in models would be unwise [79]. Animal model assessments might be financed through NIH funding to AMC investigators, by biotechnology companies, or by pharmaceutical companies.
Once there is sufficient confidence in efficacy at the animal model or test system level, the agent must be assessed for toxicity and the range of doses safe in animals established. Rodent and dog species are commonly used for toxicity assessments. Financing this aspect of drug development can be very difficult and comprises part of the “valley of death,” where promising drugs stall because no funding is available for this critically important step in new drug development [22], [80], [81]. CROs exist to conduct these studies, and other potential sources of support include the NIH NCATS program. Biotechnology companies supported by venture capital can fund this step if the investors are convinced of the return on investment, and venture philanthropy such as the ADDF has supported these studies.
Once safety and efficacy have been shown at the animal level, the drug can be advanced to phase I first-in-human trials. Phase I typically involves healthy volunteers to determine dose, tolerability, and pharmacokinetics of an agent in humans. This phase also faces substantial funding challenges and is part of the valley of death. The NIH may support phase I trials through Clinical and Translational Science Award programs. Biotechnology and pharmaceutical companies may subcontract to CROs to perform the phase I assessments using venture capital or internal budgets generated by sales of other products. Pharmaceutical companies prefer to engage in drug development in late phase II or phase III but sometimes use partnership, in-licensing, acquisition, or co-development strategies earlier in the drug development process if the agent seems very likely to succeed and has a good strategic fit with company objectives. Phase II (learning trials to establish proof-of-concept in patients with AD) is usually financed through biotechnology and pharmaceutical companies, and phase III (confirmatory trials required to advance an agent to regulatory review) is dominated by large pharmaceutical companies although large- and medium-sized biotechnology companies may sometimes advance agents through phase III and to regulatory approval. CROs are typically used to conduct phase II and III trials; some pharmaceutical companies have in-house trial execution capacity. Regulatory review preparation is typically led by in-house regulatory affairs teams, but CROs with regulatory expertise are available to support all or part of this process. Marketing of approved agents to make the new treatment widely accessible to patients is performed by pharmaceutical companies or the large- and mid-sized biotechnology companies that have escorted the drug through phase III trials and regulatory approval.
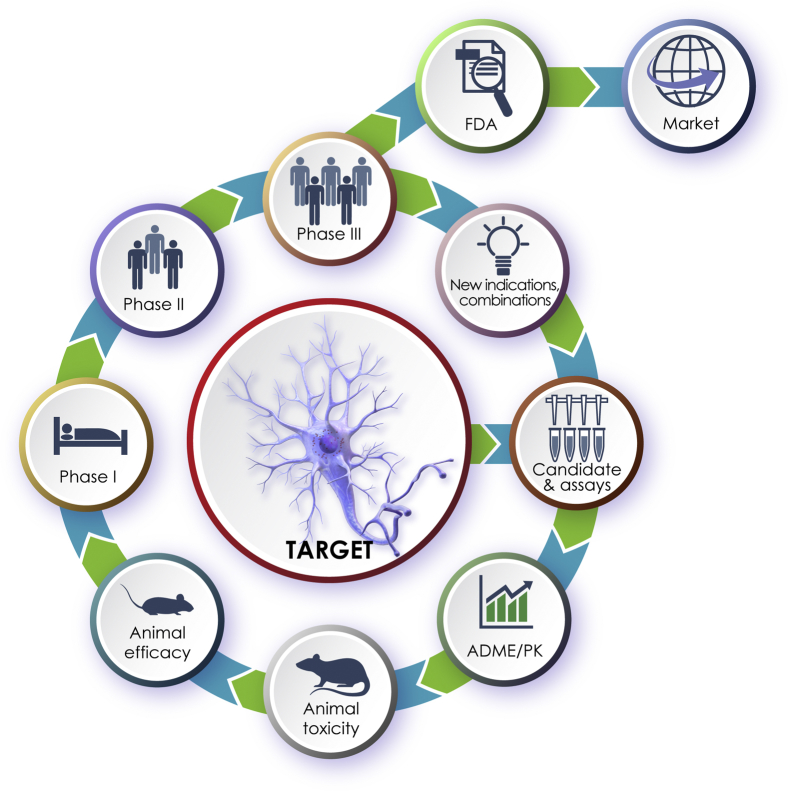
Ideally, the drug development process will produce products for FDA review that will eventually come to market while also serving as a learning experience to generate new agents as understanding of AD biology progresses. Effective life cycle management of approved agents will extend their use to new populations and new indications (Fig. 3).
Fig. 3.
The drug development system envisioned as a cycle that develops new products for FDA review and feeds back to the cycle for improved product development. Abbreviation: FDA, Food and Drug Administration.
11. Innovations in financing translational research
The extreme expense of current drug development for AD is not sustainable (Table 1), discourages companies from working in the AD research arena, dissuades venture capital from investing in AD drug development, and diminishes the opportunity to advance new therapies for patients with AD. Innovation is needed to improve the financial underpinnings of AD drug development and translational research.
Modeling suggests that it will take an estimated $38.4 billion over a decade to deliver a robust pipeline of AD therapeutics [82]. No single investment entity can undertake such a financial burden; a combination of federal and private equity would allow the development of a mega-fund structure to cover the costs and underwrite AD therapeutic development [83]. This would accommodate a high failure rate and decrease the risk of the investment by distributing the opportunity for success among multiple agents and allowing parallel development of multiple treatment approaches.
Public-private partnerships are an effective means of advancing research by distributing the cost among federal and private sources [10], [11], [84], [85]. This can be especially effective in precompetitive arenas such as biomarker development, disease modeling, and advancing analytics [86]. As noted, the ADNI is an example of a very productive research program jointly funded by the NIH and several pharmaceutical companies.
A novel approach that has emerged involves venture funding approaches adopted by some advocacy groups to directly fund drug development [72].
Crowd funding is another innovation using web-based means of raising funds. This has succeeded in generating small amounts of funding to inaugurate new drug development programs [87], [88]. Crowd-sourcing of drug development problems is another innovation using motivational prizes to harness the creativity of web-connected individuals.
Collaboration of two or more pharmaceutical companies is a means of distributing financial risk of AD drug development. Co-development and risk sharing is an increasingly popular strategy. Current examples include collaborative development of a β-site amyloid precursor protein cleaving enzyme inhibitor by Eli Lilly and AstraZeneca and co-development of a β-site amyloid precursor protein cleaving enzyme inhibitor and an anti-amyloid antibody by Eisai and Biogen. The Alzheimer Prevention Initiative is an example of collaboration among NIH, a private institute (Banner Alzheimer Institute), and two pharmaceutical companies [26].
Research centers poised at the interface of health-care systems and academic universities and committed to advancing treatment innovations represent another evolving development that can advance drug development. The Oxford Biomedical Research Center is an example [89].
Funding from the NIH, the Alzheimer's Association, and many other organizations is awarded on a competitive basis with each application scored by scientific peers with funds given primarily on the basis of the rank of the score. An alternative model is used by the Adelson Medical Research Foundation. In this approach, a field-limiting problem is identified by a group of experts, means of solving the problem are posed, and the quality of the proposed solutions reviewed. Skills and resources from several laboratories are usually required to address the identified problem. All participants must agree to collaborate and share data. Once these requirements are fulfilled, all collaborators are funded.
More innovation in financial structures is needed to sustain and accelerate AD drug development. In addition, the ecosystem is relatively unstructured, lacking a comprehensive roadmap for how to optimize and accelerate the process of moving promising treatments through the pipeline. In some cases, promising compounds are not supported while flawed agents find funding and are advanced. The current funding and financing ecosystem is too limited to advance new therapies quickly enough to meet the needs of the burgeoning patient population.
12. Summary
AD research and treatment development requires extensive capital. Funding from federal agencies, state appropriations, private equity, philanthropy, and advocacy is needed to achieve the goal of developing treatments to prevent, delay, slow the progress, or improve the symptoms of AD. Given the high cost of caring for these disorders and the projected increase in the population of those affected, the investment will more than repay itself in decreased costs, market revenue, and improved quality of life for patients.
AD drug development must be accelerated to address the unmet needs of the growing AD population. Greater collaboration among stakeholders, more precompetitive cooperation among industry members, more flexible AMC-industry partnerships, greater investment in basic research to identify viable targets and biomarkers, improved preparation of students for careers in drug discovery and development, more open forums for exchange of ideas about promising compounds, greater risk sharing in the expensive later stages of drug development, and more innovation in drug discovery/development financing can all contribute to finding effective treatments urgently needed by those with or at immanent risk of manifesting AD. The efficiency of drug development must also be improved. Faster assessment of drugs in nonclinical settings, improved biomarkers to detect effects with smaller sample sizes, and improved conduct of trials can all contribute to decreasing costs of drug development [90], [91].
Research in Context.
-
1.
Systematic review: Drug development for Alzheimer's disease (AD) and neurodegenerative disorders (NDD) has a high failure rate and the costs of drug development are very high. These factors combine to reduce interest in AD drug development and discourage investment from venture capital, biotechnology, philanthropy, and pharmaceutical companies in AD therapeutic development. Understanding the financial ecosystem underpinning AD drug development provides insights into this complex process and suggests opportunities for improvement.
-
2.
Interpretation: Drug development typically begins with National Institutes of Health (NIH)-supported basic science research. These investigations might be supported by National Institute on Aging, National Institute of Neurological Disease and Stroke (NINDS), or National Institute of General Medical Sciences (NIGMS). Spinoffs and startups from academic laboratories are financed through small business awards from the NIH, angel funding, or seed monies from philanthropists and donors. Increasing confidence in a drug through toxicity studies and animal efficacy is supported by biotechnology companies and venture capital. As compounds mature into the clinical phase of testing, support from pharmaceutical companies is typical although biotechnology companies and federal agencies can also support advanced drug development.
-
3.
Future directions: AD drug development depends on a complex funding and financing ecosystem. Novel mechanisms for funding drug development are evolving and improvement in the efficacy of drug development funding can accelerate the development of new therapy for patients with AD and other NDD.
Acknowledgments
This work was supported by a COBRE grant from the NIH/MIGMS (P20GM109025) and Keep Memory Alive. Neither funding source was involved in the report preparation or interpretation of data.
Footnotes
Disclosures: J.C. has provided consultation to Axovant, BiOasis Technologies, Biogen, Boehringer-Ingelheim, Bracket, Dart, Eisai, Genentech, Grifols, Hisun, Intracellular Therapies, Kyowa, Lilly, Lundbeck, Medavante, Merck, Neurotrope, Novartis, Nutricia, Orion, Otsuka, Pfizer, Probiodrug, QR, Resverlogix, Samus, Servier, Suven, Takeda, Toyama, and United Neuroscience companies. C.R. has no disclosures; he is a full-time employee of University of Nevada, Las Vegas. P.K. has no disclosures; he is a full-time employee of University of Nevada, Las Vegas.
References
- 1.Alzheimer's Association 2017 Alzheimer's disease facts and figures. Alzheimer's Dement. 2017;13:325–373. [Google Scholar]
- 2.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer's Disease International . Alzheimer Disease International; London, England: 2015. World Alzheimer's Report 2015: The Global Impact of Dementia. [Google Scholar]
- 4.Brodaty H., Heffernan M., Kochan N.A., Draper B., Trollor J.N., Reppermund S. Mild cognitive impairment in a community sample: the Sydney Memory and Ageing Study. Alzheimers Dement. 2013;9:310–317.e1. doi: 10.1016/j.jalz.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Roberts R., Knopman D.S. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aisen P.S., Cummings J., Jack C.R., Jr., Morris J.C., Sperling R., Frolich L. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. 2017;9:60–69. doi: 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vradenburg G. A pivotal moment in Alzheimer's disease and dementia: how global unity of purpose and action can beat the disease by 2025. Expert Rev Neurother. 2015;15:73–82. doi: 10.1586/14737175.2015.995638. [DOI] [PubMed] [Google Scholar]
- 8.Cummings J.L., Morstorf T., Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37–43. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings J., Pillai J. Oxford University Press; United Kingdom: 2016. Neurodegenerative Diseases: Unifying Principles. [Google Scholar]
- 10.Gottwald M., Becker A., Bahr I., Mueller-Fahrnow A. Public-private partnerships in lead discovery: overview and case studies. Arch Pharm (Weinheim) 2016;349:692–697. doi: 10.1002/ardp.201600078. [DOI] [PubMed] [Google Scholar]
- 11.Muller S., Weigelt J. Open-access public-private partnerships to enable drug discovery–new approaches. IDrugs. 2010;13:175–180. [PubMed] [Google Scholar]
- 12.Scott T.J., O'Connor A.C., Link A.N., Beaulieu T.J. Economic analysis of opportunities to accelerate Alzheimer's disease research and development. Ann N Y Acad Sci. 2014;1313:17–34. doi: 10.1111/nyas.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad V., Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med. 2017;177:1569–1575. doi: 10.1001/jamainternmed.2017.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiMasi J.A., Grabowski H.G., Hansen R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Hurd M.D., Martorell P., Langa K.M. Monetary costs of dementia in the United States. N Engl J Med. 2013;369:489–490. doi: 10.1056/NEJMc1305541. [DOI] [PubMed] [Google Scholar]
- 16.Deb A., Thornton J.D., Sambamoorthi U., Innes K. Direct and indirect cost of managing alzheimer's disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res. 2017;17:189–202. doi: 10.1080/14737167.2017.1313118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cywin C.L., Tamiz A.P. National Institutes of Health Blueprint Neurotherapeutics Network: results to date and path forward. Neurotherapeutics. 2017;14:1066–1069. doi: 10.1007/s13311-017-0530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thal L.J. The Alzheimer's disease cooperative study in 2004. Alzheimer Dis Assoc Disord. 2004;18:183–185. [PubMed] [Google Scholar]
- 19.Jones-Davis D.M., Buckholtz N. The impact of the Alzheimer's Disease Neuroimaging Initiative 2: what role do public-private partnerships have in pushing the boundaries of clinical and basic science research on Alzheimer's disease? Alzheimers Dement. 2015;11:860–864. doi: 10.1016/j.jalz.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiner M.W., Veitch D.P. Introduction to special issue: overview of Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement. 2015;11:730–733. doi: 10.1016/j.jalz.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Cedarbaum J. 2014 Update of the Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2015;11:e1–e120. doi: 10.1016/j.jalz.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkbeiner S. Bridging the Valley of Death of therapeutics for neurodegeneration. Nat Med. 2010;16:1227–1232. doi: 10.1038/nm.2222. [DOI] [PubMed] [Google Scholar]
- 23.Cummings J.L., Aisen P., Barton R., Bork J., Doody R., Dwyer J. Re-engineering Alzheimer clinical trials: Global Alzheimer Platform Network. J Prevent Alz Dis. 2016;3:114–120. doi: 10.14283/jpad.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridge P.G., Hoyt K.B., Boehme K., Mukherjee S., Crane P.K., Haines J.L. Assessment of the genetic variance of late-onset Alzheimer's disease. Neurobiol Aging. 2016;41:200.e13. doi: 10.1016/j.neurobiolaging.2016.02.024. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijsman E.M., Pankratz N.D., Choi Y., Rothstein J.H., Faber K.M., Cheng R. Genome-wide association of familial late-onset Alzheimer's disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011;7:e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiman E.M., Langbaum J.B., Fleisher A.S., Caselli R.J., Chen K., Ayutyanont N. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26 Suppl 3:321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beekly D.L., Ramos E.M., van Belle G., Deitrich W., Clark A.D., Jacka M.E. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 28.Edland S.D., Emond J.A., Aisen P.S., Petersen R.C. NIA-funded Alzheimer centers are more efficient than commercial clinical recruitment sites for conducting secondary prevention trials of dementia. Alzheimer Dis Assoc Disord. 2010;24:159–164. doi: 10.1097/WAD.0b013e3181c9983f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodes R.J., Buckholtz N. Accelerating Medicines Partnership: Alzheimer's Disease (AMP-AD) Knowledge Portal aids Alzheimer's drug discovery through open data sharing. Expert Opin Ther Targets. 2016;20:389–391. doi: 10.1517/14728222.2016.1135132. [DOI] [PubMed] [Google Scholar]
- 30.Huang R., Southall N., Wang Y., Yasgar A., Shinn P., Jadhav A. The NCGC pharmaceutical collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci Transl Med. 2011;3:80ps16. doi: 10.1126/scitranslmed.3001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute of Medicine . The National Academies Press; Washington, DC: 2013. The CTSA Program at NIH: Opportunities for Advancing Clinical and Translational Research. [PubMed] [Google Scholar]
- 32.Colvis C.M., Austin C.P. Innovation in therapeutics development at the NCATS. Neuropsychopharmacology. 2014;39:230–232. doi: 10.1038/npp.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurwitz D., Lunshof J.E. A deserving role for the National Center for Advancing Translational Sciences. Lancet. 2011;377:1745–1746. doi: 10.1016/S0140-6736(11)60729-0. [DOI] [PubMed] [Google Scholar]
- 34.Splinter K., Hull S.C., Holm I.A., McDonough T.L., Wise A.L., Ramoni R.B. Implementing the single institutional review board model: lessons from the Undiagnosed Diseases Network. Clin Transl Sci. 2018;11:28–31. doi: 10.1111/cts.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker G.J. The National Institute of General Medical Sciences. J Am Coll Radiol. 2005;2:790–792. doi: 10.1016/j.jacr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Jeffe D.B., Andriole D.A. A national cohort study of MD-PhD graduates of medical schools with and without funding from the National Institute of General Medical Sciences' Medical Scientist Training Program. Acad Med. 2011;86:953–961. doi: 10.1097/ACM.0b013e31822225c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapes S.K., Velasquez S.E. Assessment of the Impact of the Kansas IDeA Network of Biomedical Research Excellence Program on Undergraduate Participation in Research. J Microbiol Biol Educ. 2013;14:47–57. doi: 10.1128/jmbe.v14i1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings J., Zhong K., Bernick C. The Cleveland Clinic Lou Ruvo Center for Brain Health: keeping memory alive. J Alzheimers Dis. 2014;38:103–109. doi: 10.3233/JAD-130791. [DOI] [PubMed] [Google Scholar]
- 39.Ismail M.S., Dagerman K., Tariot P.N., Abbott S., Kavanagh S., Schneider L.S. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness- Alzheimer's Disease (CATIE-AD): baseline characteristics. Curr Alzheimer Res. 2007;4:325–335. doi: 10.2174/156720507781077214. [DOI] [PubMed] [Google Scholar]
- 40.Schneider L.S., Tariot P.N., Dagerman K.S., Davis S.M., Hsiao J.K., Ismail M.S. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 41.Snitz B.E., O'Meara E.S., Carlson M.C., Arnold A.M., Ives D.G., Rapp S.R. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston S.C., Desmond-Hellmann S., Hauser S., Vermillion E., Mia N. Predictors of negotiated NIH indirect rates at US institutions. PLoS One. 2015;10:e0121273. doi: 10.1371/journal.pone.0121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bargmann C.I., Newsome W.T. The Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) initiative and neurology. JAMA Neurol. 2014;71:675–676. doi: 10.1001/jamaneurol.2014.411. [DOI] [PubMed] [Google Scholar]
- 44.Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Working Group . Current Alzheimer Research; Washington, DC: 2013. Advisory Committee to the NIH Director Interim Report. [Google Scholar]
- 45.Insel T.R., Landis S.C., Collins F.S. Research priorities. The NIH BRAIN Initiative. Science. 2013;340:687–688. doi: 10.1126/science.1239276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero K., Ito K., Rogers J.A., Polhamus D., Qiu R., Stephenson D. The future is now: model-based clinical trial design for Alzheimer's disease. Clin Pharmacol Ther. 2015;97:210–214. doi: 10.1002/cpt.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neville J., Kopko S., Broadbent S., Aviles E., Stafford R., Solinsky C.M. Development of a unified clinical trial database for Alzheimer's disease. Alzheimers Dement. 2015;11:1212–1221. doi: 10.1016/j.jalz.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Loregian A., Palu G. How academic labs can approach the drug discovery process as a way to synergize with big pharma. Trends Microbiol. 2013;21:261–264. doi: 10.1016/j.tim.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Tralau-Stewart C.J., Wyatt C.A., Kleyn D.E., Ayad A. Drug discovery: new models for industry-academic partnerships. Drug Discov Today. 2009;14:95–101. doi: 10.1016/j.drudis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Germann P.G., Schuhmacher A., Harrison J., Law R., Haug K., Wong G. How to create innovation by building the translation bridge from basic research into medicinal drugs: an industrial perspective. Hum Genomics. 2013;7:5. doi: 10.1186/1479-7364-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asadullah K., Busch A., Gottwald M., Reinke P., Landeck L. Industry-academia collaborations for biomarkers. Nat Rev Drug Discov. 2015;14:805–806. doi: 10.1038/nrd4727. [DOI] [PubMed] [Google Scholar]
- 52.Pizzo P.A., Lawley T.J., Rubenstein A.H. Role of leaders in fostering meaningful collaborations between academic medical centers and industry while also managing individual and institutional conflicts of interest. JAMA. 2017;317:1729–1730. doi: 10.1001/jama.2017.2573. [DOI] [PubMed] [Google Scholar]
- 53.Hammonds T. Academic-Pharma drug discovery alliances: seeking ways to eliminate the valley of death. Future Med Chem. 2015;7:1891–1899. doi: 10.4155/fmc.15.111. [DOI] [PubMed] [Google Scholar]
- 54.Yokley B.H., Hartman M., Slusher B.S. Role of academic drug discovery in the quest for new CNS therapeutics. ACS Chem Neurosci. 2017;8:429–431. doi: 10.1021/acschemneuro.7b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cockburn I., Long G. The importance of patents to innovation: updated cross-industry comparisons with biopharmaceuticals. Expert Opin Ther Pat. 2015;25:739–742. doi: 10.1517/13543776.2015.1040762. [DOI] [PubMed] [Google Scholar]
- 56.Glorikian H., Warburg R.J., Moore K., Malinowski J. Intellectual property considerations for molecular diagnostic development with emphasis on companion diagnostics. Expert Opin Ther Pat. 2018;28:123–128. doi: 10.1080/13543776.2018.1409209. [DOI] [PubMed] [Google Scholar]
- 57.Frye S., Crosby M., Edwards T., Juliano R. US academic drug discovery. Nat Rev Drug Discov. 2011;10:409–410. doi: 10.1038/nrd3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kneller R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nat Rev Drug Discov. 2010;9:867–882. doi: 10.1038/nrd3251. [DOI] [PubMed] [Google Scholar]
- 59.Moscicki R.A., Tandon P.K. Drug-development challenges for small biopharmaceutical companies. N Engl J Med. 2017;376:469–474. doi: 10.1056/NEJMra1510070. [DOI] [PubMed] [Google Scholar]
- 60.Stevens A.J., Jensen J.J., Wyller K., Kilgore P.C., Chatterjee S., Rohrbaugh M.L. The role of public-sector research in the discovery of drugs and vaccines. N Engl J Med. 2011;364:535–541. doi: 10.1056/NEJMsa1008268. [DOI] [PubMed] [Google Scholar]
- 61.Stossel T.P. Overregulation of conflicts hinders medical progress. Cleve Clin J Med. 2007;74 Suppl 2:S14–S15. doi: 10.3949/ccjm.74.suppl_2.s14. discussion S6–22. [DOI] [PubMed] [Google Scholar]
- 62.Vallance P., Williams P., Dollery C. The future is much closer collaboration between the pharmaceutical industry and academic medical centers. Clin Pharmacol Ther. 2010;87:525–527. doi: 10.1038/clpt.2010.29. [DOI] [PubMed] [Google Scholar]
- 63.Kim E.S., Omura P.M.C., Lo A.W. Accelerating biomedical innovation: a case study of the SPARK program at Stanford University, School of Medicine. Drug Discov Today. 2017;22:1064–1068. doi: 10.1016/j.drudis.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Bustin S., Nolan T. Talking the talk, but not walking the walk: RT-qPCR as a paradigm for the lack of reproducibility in molecular research. Eur J Clin Invest. 2017;47:756–774. doi: 10.1111/eci.12801. [DOI] [PubMed] [Google Scholar]
- 65.Jilka R.L. The road to reproducibility in animal research. J Bone Miner Res. 2016;31:1317–1319. doi: 10.1002/jbmr.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrence S. Biotech's wellspring-a survey of the health of the private sector in 2016. Nat Biotechnol. 2017;35:413–420. doi: 10.1038/nbt.3867. [DOI] [PubMed] [Google Scholar]
- 67.Fleming J.J. The decline of venture capital investment in early-stage life sciences poses a challenge to continued innovation. Health Aff (Millwood) 2015;34:271–276. doi: 10.1377/hlthaff.2014.1051. [DOI] [PubMed] [Google Scholar]
- 68.Thomas D., Wessel C. Biotechnology Industry Organization; Washington, DC: 2015. Venture Funding of Therapeutic Innovation: A Comprehensive Look at a Decade of Venture Funding of Drug R&D. [Google Scholar]
- 69.Gates B. The Gates Notes LLC; Seattle, Washington, USA: 2017. Why I'm digging deep into Alzheimer's. [Google Scholar]
- 70.Alzheimer's Association Annual Report: Fiscal Year 2016. Alzheimer's Association; Chicago, Ill: 2017. [Google Scholar]
- 71.Mills S.M., Mallmann J., Santacruz A.M., Fuqua A., Carril M., Aisen P.S. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris) 2013;169:737–743. doi: 10.1016/j.neurol.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramsey B.W., Nepom G.T., Lonial S. Academic, foundation, and industry collaboration in finding new therapies. N Engl J Med. 2017;376:1762–1769. doi: 10.1056/NEJMra1612575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joyce C. Transforming our approach to translational neuroscience: the role and impact of charitable nonprofits in research. Neuron. 2014;84:526–532. doi: 10.1016/j.neuron.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 74.Bartek R.J. Foundation-industry relationships–a new business model joint-venture philanthropy in therapy development. Curr Top Med Chem. 2014;14:313–318. doi: 10.2174/1568026613666131127154903. [DOI] [PubMed] [Google Scholar]
- 75.Milken Institute Philanthropy Advisory Service . 2015. Alzheimer's Disease: A Giving Smarter Guide to Accelerate Development of New Therapies. [Google Scholar]
- 76.Pharmaceutical Research and Manufacturers of America . 2017. Biopharmaceutical Research Industry Profile. Washington, DC. [Google Scholar]
- 77.Cummings J., Lee G., Mortsdorf T., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2017. Alzheimer's Dement. 2017;3:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cummings J., Reynders R., Zhong K. Globalization of Alzheimer's disease clinical trials. Alzheimers Res Ther. 2011;3:24–32. doi: 10.1186/alzrt86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sabbagh J.J., Kinney J.W., Cummings J.L. Animal systems in the development of treatments for Alzheimer's disease: challenges, methods, and implications. Neurobiol Aging. 2013;34:169–183. doi: 10.1016/j.neurobiolaging.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 80.Gamo N.J., Birknow M.R., Sullivan D., Kondo M.A., Horiuchi Y., Sakurai T. Valley of death: a proposal to build a “translational bridge” for the next generation. Neurosci Res. 2017;115:1–4. doi: 10.1016/j.neures.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hudson J., Khazragui H.F. Into the valley of death: research to innovation. Drug Discov Today. 2013;18:610–613. doi: 10.1016/j.drudis.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Lo A.W., Ho C., Cummings J., Kosik K.S. Parallel discovery of Alzheimer's therapeutics. Sci Transl Med. 2014;6:241cm5. doi: 10.1126/scitranslmed.3008228. [DOI] [PubMed] [Google Scholar]
- 83.Montazerhodjat V., Frishkopf J.J., Lo A.W. Financing drug discovery via dynamic leverage. Drug Discov Today. 2016;21:410–414. doi: 10.1016/j.drudis.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Portilla L.M., Rohrbaugh M.L. Leveraging public private partnerships to innovate under challenging budget times. Curr Top Med Chem. 2014;14:326–329. doi: 10.2174/1568026613666131127155703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy D.G., Goldman M., Loth E., Spooren W. Public-private partnership: a new engine for translational research in neurosciences. Neuron. 2014;84:533–536. doi: 10.1016/j.neuron.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Sidders B., Brockel C., Gutteridge A., Harland L., Jansen P.G., McEwen R. Precompetitive activity to address the biological data needs of drug discovery. Nat Rev Drug Discov. 2014;13:83–84. doi: 10.1038/nrd4230. [DOI] [PubMed] [Google Scholar]
- 87.Dragojlovic N., Lynd L.D. Crowdfunding drug development: the state of play in oncology and rare diseases. Drug Discov Today. 2014;19:1775–1780. doi: 10.1016/j.drudis.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 88.Carter A.J., Donner A., Lee W.H., Bountra C. Establishing a reliable framework for harnessing the creative power of the scientific crowd. PLoS Biol. 2017;15:e2001387. doi: 10.1371/journal.pbio.2001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenhalgh T., Ovseiko P.V., Fahy N., Shaw S., Kerr P., Rushforth A.D. Maximising value from a United Kingdom Biomedical Research Centre: study protocol. Health Res Policy Syst. 2017;15:70. doi: 10.1186/s12961-017-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Low L.A., Tagle D.A. Microphysiological systems (“Organs-on-Chips”) for drug efficacy and toxicity testing. Clin Transl Sci. 2017;10:237–239. doi: 10.1111/cts.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu J., Yang B., Ke J., Li W., Suen W.C. Antibody-based drugs and approaches against amyloid-beta species for Alzheimer's disease immunotherapy. Drugs Aging. 2016;33:685–697. doi: 10.1007/s40266-016-0406-x. [DOI] [PubMed] [Google Scholar]