Abstract
The transpiration stream that passes through a plant may follow an apoplastic route, with low resistance to flow, or a cell-to-cell route, in which cellular membranes impede water flow. However, passage of water through membranes can be facilitated by aquaporins thereby decreasing resistance. We investigated the relationship between transpiration, which can be down-regulated by abscisic acid (ABA) or by high humidity, and the osmotic water permeability (Pos) of protoplasts. By using leaf protoplasts of wild-type (wt) Arabidopsis thaliana plants and of mutants that are low in ABA (aba1) or insensitive to ABA (abi1 and abi2), we found that protoplasts from aba1 and abi mutants have very low Pos values compared with those from wt plants when the plants are grown at 45% relative humidity. High values of Pos were found 3 h after the addition of ABA to the culture medium of aba1 plants; addition of ABA to abi plants did not restore the Pos to wt levels. There was no such increase in Pos when excised leaves of aba1 plants were treated with ABA. When the transpiration stream was attenuated by growing the plants at 85% relative humidity, the Pos of protoplasts from all plants (wt and mutants) was higher. We suggest that attenuation of the transpiration stream in whole plants is required for the up-regulation of the Pos of the membranes, and that this up-regulation, which does not require ABA, is mediated by the activation of aquaporins in the plasma membrane.
Plant growth requires a tradeoff between the need to acquire CO2 for photosynthesis and the need to minimize water loss from the leaves. Water lost through the transpiration stream is replaced by water that is taken up from the soil. The force that drives this water uptake is the tension created by the evaporation of water from the leaf cells. Water that moves through living tissues can follow an apoplastic or cell-to-cell path. Careful measurements of the hydraulic properties of plant tissues and organs show that the relative contribution of each pathway to overall hydraulic conductivity may change substantially depending on the intensity of water flow and other factors (1). When the rate of water flow is high (open stomates, low relative humidity), water flows along the apoplast and around the protoplasts because the apoplastic path has the lower hydraulic resistance. Conversely, when the rate of water flow is small, a larger proportion of water follows the cell-to-cell path and needs to pass through cellular membranes (and possibly through plasmodesmata). Water flow along this path has a much higher hydraulic resistance and can be modulated by aquaporins, proteins that facilitate transmembrane water transport (2–4). An important unanswered question is whether the magnitude of flow in the apoplastic path affects the resistance in the cell-to-cell path.
In plants, aquaporins form a family of 35–37 sequence-related proteins (5, 6) that transport water and neutral solutes, such as glycerol, across membranes. Aquaporins have been found in the tonoplast (vacuolar membrane) and the plasma membrane, and many among them can increase the Pos of the Xenopus laevis oocyte plasma membrane by 10- to 20-fold. Sequence comparisons show that they fall into four different clades, and the plasma membrane intrinsic protein (PIP) clade has about a dozen members. The most abundantly and ubiquitously expressed PIPs are in the PIP1 and PIP2 subfamilies. These subgroups also have a conserved phosphorylation site that may be a target for regulation of aquaporin activity and overall plasma membrane hydraulic conductivity (7, 8).
In the study presented here we questioned how the magnitude of the transpiration stream affects the hydraulic conductivity of the membranes. The transpiration stream can be modulated by changing the relative humidity (RH) of the atmosphere or by the application of the hormone abscisic acid (ABA), which causes stomatal closure. For plants growing at 20°C, the transpiration stream will decrease more than 80% when the RH is raised from 45% to 85%. Plants sense this change in humidity and open their stomates further (9). Although not all of the plants react in the same way, the most common response consists of sensing of the stomatal transpiration rate through the guard cells (10). Other mechanisms, such as cuticular transpiration (11, 12) or leaf water potential (12, 13), could also be involved in sensing RH. All of these mechanisms find experimental support in the literature. However, ABA is not involved in this sensing mechanism (14). This is in contrast to its role in bringing about stomatal closure in response to a decrease in soil water potential.
The synthesis of ABA is enhanced in response to soil water deficit, causing the rapid closure of stomates and thereby decreasing the intensity of the transpiration stream. In addition, ABA, which is involved in long-distance signaling between root and shoot (15), may directly affect the hydraulic conductivity of both root and shoot tissues. A number of studies carried out with decapitated root systems show that ABA applied to the roots increases the exudation volume from the cut stump of the stem by increasing the hydraulic conductivity (Lr) of the root system about 2-fold. ABA thus functions to allow faster uptake of water by the roots and better transport of water within the plant (16–18). This effect of ABA appears to be rapid (within 30 min) and long lasting (17).
ABA-deficient mutants are very useful for investigating the role of this hormone in plant water relations. The cut stump of a plant of the wilty tomato mutant flacca has an exudation rate that is only half that of a control plant (19). By using this same mutant, Bradford (20) showed that spraying the shoot with ABA not only caused stomatal closure, but also raised the hydraulic conductivity of the root system.
Application of the cell pressure probe allows a direct approach to studying the effect of ABA on transmembrane water transport. With this technique, Hose et al. (21) investigated the effect of ABA on the hydraulic conductivity of maize roots and cortex cells. They found that ABA facilitates the cell-to-cell component of water transport across the root cylinder. The effects on cellular hydraulic conductivity were large (7- to 27-fold) but highly transient and postulated to be mediated by aquaporins.
To understand how the transpiration stream affects the hydraulic conductivity of cellular membranes, we used the technique of Ramahaleo et al. (22) to measure the Pos of leaf protoplasts derived from wild-type (wt) Arabidopsis ecotype Landsberg erecta (Ler) plants and from mutants that are impaired in ABA synthesis (aba1) or are insensitive to ABA (abi1 and abi2) (23, 24). In these mutants, the stomates are wide open. As a result, the apoplastic water flow, with its low hydraulic resistance, dominates the water transport path. What happens to the permeability of the membranes in these plants? Our results show that attenuating the transpiration stream by closing the stomates with ABA or increasing the RH causes an increase in the Pos of the protoplasts. Aba1 and abi mutant plants have very low Pos values compared with wt. When ABA was added to the growth medium of the aba1 plants, stomatal closure preceded a dramatic increase in Pos of the leaf protoplasts up to the levels found in wt plants. ABA had no effect on the Pos of protoplasts isolated from excised leaves that were incubated in ABA solution. We conclude that leaves regulate the Pos of the plasma membrane in response to the magnitude of the transpiration stream.
Materials and Methods
Plant Material and Growing Conditions.
Throughout this study, Arabidopsis thaliana ecotype Ler was used as the wt. Plants were grown either on a soil–peat mixture (J.M. McConkey and Co., Sumner, WA) during 4–5 weeks in a growth room (20°C ± 1°C) with a continuous light and a RH of 45 ± 5% or in hydroponic system, as described (25). The Magenta boxes were covered with a vented lid allowing gas exchange and were autoclaved before use. The plants were grown in the same room as the plants grown in soil. The effect of RH was studied as follows. Ten days after the seeds germinated, the lids of the boxes were removed. During the next 4 weeks, the plants grown without lids at the RH of the growth room (45%), and some water was added every week to maintain a constant level. To study plant growth at high RH, the lids were replaced on the boxes (after 4 weeks) to maintain the RH at 85%. The RH was measured with a recording thermo–hygro pen (Fisher Scientific) placed inside the box. The leaf temperature and box temperature were recorded with a thermometer with two thermocouples (Doric Scientific, San Diego). The temperature was recorded several times during a week.
ABA Treatment of Whole Plants and Excised Leaves.
The control and ABA treatments were performed by transferring the pots for 3 or 24 h to a basin containing water or 1 μM ABA (Sigma) solution buffered with 10 mM Mes/Tris at pH 5.5 (21).
Excised leaves were incubated under vacuum in 1 μM ABA solution, 10 mM Mes/Tris, pH 5.5, for 1 or 3 h in a Petri dish before measurement of the size of the stomatal pore or the preparation of protoplasts.
Isolation of Protein Fractions and Immunodetection.
For each gel lane, the same amount of protein (about 30 μg) was loaded. The proteins were denatured with 1% SDS and 100 mM ethanedithiol at 40°C. The immunodetection of PIP1 and PIP2 proteins was performed as described in Daniels et al. (26) by using, respectively, a serum against the amino acid sequence (KSLGSFRSAANV) found in PIP2 aquaporins or a serum against the N terminus of PIP1a aquaporins (27).
Measurements of Stomatal Aperture.
Two leaves of each line were cut and the lower surface was stuck to a microscope slide with a medical adhesive (Hollister, Libertyville, IL). Three repeats from three different plants were done in each case. After 1 min, the leaf was peeled away under water, leaving the lower epidermis stuck to the glass. Then, under the microscope, stomatal apertures were recorded with a video recorder for 10 min, allowing the image capture of more than 100 stomata. Measurements of the stomatal apertures were performed subsequently on the filmed images. In each case more than 100 measurements were performed.
Preparation of Protoplasts and Pos Measurements.
Leaf protoplast preparation and osmotic water permeability measurements on isolated protoplasts were performed as described (22).
To determine whether ABA affects the permeability of protoplasts, Pos was measured on leaf protoplasts from aba1 plants. The protoplasts were incubated for 10 min with 1 or 10 μM ABA in the measuring solutions.
Statistical Analysis.
The Pos values are given as the mean ± SD or as frequency histograms. We used SIGMASTAT from SPSS (Chicago; www.spss.com/software/science) to analyze the data. The t test and ANOVA test were used to detect differences between the lines and the growing conditions at the usual probability level of P = 0.05.
Results
aba and abi Mutants of Arabidopsis Have Plasma Membranes with a Low Pos.
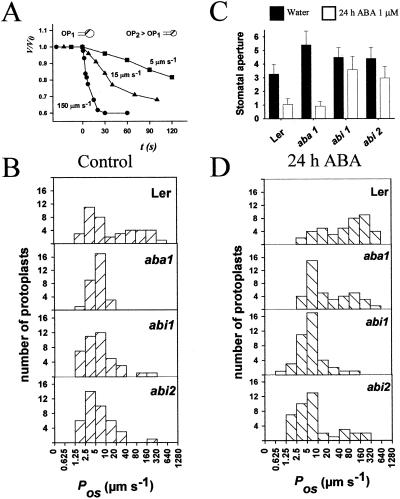
To find out whether mutant plants that are defective in ABA synthesis or ABA perception have an altered Pos of the plasma membrane, we applied the method of Ramahaleo et al. (22) to leaf protoplasts obtained from wt, aba1, abi1, and abi2 plants. aba1 mutants have very low levels of ABA, and the wilty phenotype of the mutant can be restored by ABA treatment (23, 24), whereas abi mutants have defects in ABA perception and stomatal closure (24, 28). We generally determined the Pos of 40 protoplasts obtained from 4–5 different protoplast preparations, with each preparation being made from the fully expanded leaves of 3–4 plants. Fig. 1A shows the change in volume (shrinkage) for three different leaf protoplasts transferred from a solution of 0.4 mol⋅kg−1 to 0.6 mol⋅kg−1 sorbitol. Each preparation usually has protoplasts with a range of Pos values, and this range of values is reproducible from one experiment to the next. The range of Pos values obtained for protoplasts derived from wt plants is shown in Fig. 1B Upper. The histogram shows two subpopulations with half the protoplasts in the 1.25–10 μm⋅s−1 range and the other half in the 20–540 μm⋅s−1 range. Experiments in which the Arrhenius energy of activation for cell swelling was measured (22, 29, 30) indicate that values around 5–15 μm⋅s−1 for plant membranes correspond to an energy of activation of 50–70 kJ. Such a high value for the energy of activation of water transport corresponds to a situation where there are no water channels, or the water channels are not in operation.
Figure 1.
The effect of 24-h ABA treatment on stomatal aperture and Pos values of leaf protoplasts from Arabidopsis wt and mutant plants. (A) Time courses showing rates of shrinkage and calculated Pos values for three different Arabidopsis leaf protoplasts. The initial diameters of the protoplasts were about 30 μm in each case (OP1 = 0.4 mol⋅kg−1; OP2 = 0.6 mol⋅kg−1). (B) Histograms of Pos values. Measurements were performed on leaf protoplasts from the wt and the mutants, aba1, abi1, and abi2. Leaves of 4-week-old plants were harvested and 4–5 different preparations of protoplasts were used for each condition. The Pos of 40 protoplasts was measured for each line and in each condition. (C) Mean stomatal aperture of well-watered plants and plants treated during 24 h with 1 μM ABA. Bars represent the mean ± SD. (D) Pos values of protoplasts from plants treated with 1 μM ABA for 24 h.
In the aba1 and abi mutants, the distribution of Pos values was much narrower with essentially all of the plants in the 1.25–40 μm⋅s−1 range. The mean Pos value of protoplasts of the aba1 plants (6 μm⋅s−1) is only one-tenth of that of the protoplasts from the wt plants (69 μm⋅s−1), whereas the abi mutant protoplasts have a mean value of about one-fifth (15 μm⋅s−1) of wt. Statistical analysis using Dunn's test for ANOVA shows that the Pos values of the protoplasts from the mutant plants are significantly different (P = 0.05) from the wt values (Table 1). The low Pos values in the mutants indicate that plasma membrane aquaporins may be inactive in most protoplasts.
Table 1.
Statistical analysis of Pos values (μm⋅s−1) of leaf protoplasts from the wt and aba mutants
| ABA treatment | Mutant lines | Mean | SD | Median | Dunn's test |
|---|---|---|---|---|---|
| − | Wt | 69 | 116 | 9.5 | A |
| − | aba1–1 | 6 | 4 | 4.6 | B |
| − | abi1 | 14 | 32 | 5.0 | B |
| − | abi2 | 14 | 39 | 4.9 | B |
| + | Wt | 126 | 132 | 96.0 | C |
| + | aba1–1 | 67 | 129 | 11.0 | C |
| + | abi1 | 12 | 23 | 6.0 | D |
| + | abi2 | 26 | 56 | 6.6 | D |
The data are extracted from the histograms in Fig. 1. For each condition, a Dunn's test ANOVA was carried out separately and used to detect significant variation: the same letter indicates no significant difference (P = 0.05). Forty protoplasts were measured for each line in each condition.
ABA Treatment of Intact aba1 Plants Increases the Pos of Protoplasts.
To understand how increasing the level of ABA affects the Pos values, we placed the pots for 24 h in a basin containing a solution of 1 μM ABA. First, we determined the effect of ABA on stomatal aperture (Fig. 1C). Under well-watered conditions, stomatal pore size was about 3 μm for wt Ler plants. The stomates of the mutants were more widely open (4–5 μm). This conforms to expectations for plants that have low levels of ABA (aba1) or are deficient in ABA sensing (abi). Addition of ABA caused closure of the stomates in the wt and aba1 plants, but the abi plants were, as expected, much less sensitive to ABA and the stomates remained open (28).
The 24-h treatment of the wt and aba1 mutant plants with ABA pushed the histogram of protoplast Pos values to the right (Fig. 1D), with more protoplasts in the 20 to 640 μm⋅s−1 range. The mean value of the wt plants went from 69 μm⋅s−1 to 126 μm⋅s−1 as a result of the addition of ABA, and the value for aba1 plants went from 6 μm⋅s−1 to 67 μm⋅s−1. A statistical analysis of the effect of ABA on wt or aba1 plants in a pairwise comparison shows that the effect of ABA is significant (t test; P < 0.05). In the abi1 and abi2 plants there was no shift in the histogram (compare Fig. 1 B and D) and differences in the mean values were not significant (t test; P > 0.05). We interpret these findings to mean that ABA presence or perception is necessary to maintain protoplasts in a high Pos state, and that this effect is most likely mediated by aquaporins.
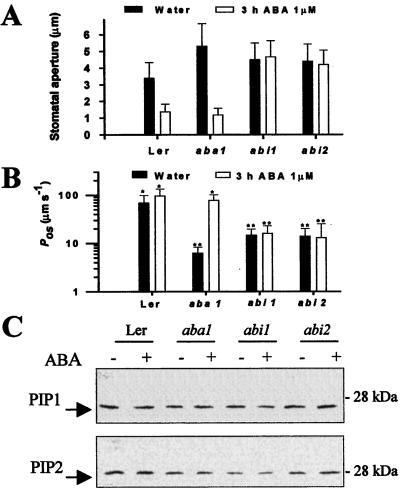
A 3-h ABA Treatment Is Sufficient to Raise the Pos.
ABA could exert its effect on plasma membrane Pos at the level of aquaporin activity, aquaporin abundance, and/or gene expression. We repeated the entire experiment 3 h after the addition of ABA to the growth medium and the results were almost identical. The effect of ABA on stomatal opening is shown in Fig. 2A. Once again, ABA induced rapid stomatal closure in wt and aba1 plants, but not in abi plants. Fig. 2B shows mean Pos values rather than histograms as in Fig. 1. It is clear that ABA addition 3 h before protoplast preparation was sufficient to raise the Pos of the aba1 protoplasts to wt levels. Fig. 2C shows the effect of ABA on the abundance of two major classes of plasma membrane aquaporins (PIP1 and PIP2) by using an immunoblot and two different and specific antisera. Equal amounts (40 μg) of microsomal protein were loaded in each lane. The results show that the mutant plants may have somewhat lower levels of the aquaporins (the bands appear less intense), but that 3 h of ABA treatment did not alter those levels. These results strongly suggest that the effect of ABA must be at the level of aquaporin activity.
Figure 2.
The effect of 3 h of ABA treatment on stomatal aperture and Pos values of leaf protoplasts from wt and mutant Arabidopsis plants. (A) Mean stomatal aperture of well-watered plants and plants treated for 3 h with 1 μM ABA. The aperture is given as the mean ± SD. (B) Mean Pos values of leaf protoplasts of Arabidopsis wt and mutants. In each case, the Pos value of 20 protoplasts was measured from three preparations. Bars with the same sign are not significantly different (Dunn's test) and the results are presented as the mean ± SD. (C) Immunoblot analysis of microsomal extracts from Arabidopsis leaves of the wt and mutants using anti-PIP1 serum (Upper) and anti-PIP2 serum (Lower). The minus signs correspond to a water treatment and the plus sign to a 3-h ABA treatment. Equal amounts of leaf microsomal protein (40 μg) were loaded in each lane. The arrow indicates the position of the protein. The numbers on the right indicate the molecular mass standards.
The Effect of ABA Is Seen Only in Whole Plants, Not in Excised Leaves.
An experiment was also conducted to determine the time course of the effect of ABA on stomatal closure and protoplast Pos. The apperture of the stomatal pores was examined 1, 2, and 3 h after ABA treatment of the plants. At these times we also prepared protoplasts from the leaves and determined their Pos. The results in Fig. 3A show that after 2 h of ABA treatment the stomates began to close, but the change in Pos had not yet taken place. Thus, attenuating the transpiration stream comes before the Pos change. Nevertheless, the effect of ABA may be direct and require more time.
Figure 3.
ABA and membrane permeability. (A) The effect of treating aba1 plants for 1, 2, and 3 h with ABA on stomatal aperture and Pos values of leaf protoplasts. Details are as in Fig. 2. (B) The effect of 1 or 3 h of ABA treatment of excised leaves from the wt and aba1 mutant on stomatal aperture and Pos values of leaf protoplasts.
To find out whether ABA can have a direct effect on leaf cells, as it does on cells of excised root systems, we tested its effect on excised leaves. Leaves from control and aba1 plants were incubated with ABA for 1 or 3 h after vacuum infiltration to facilitate entrance of the hormone into the leaf, before protoplast preparation. An examination of the stomatal apertures showed that 1 h of ABA treatment was sufficient to close the stomates. However, there was no increase in the Pos of the protoplasts prepared from the leaves either after 1 or 3 h of ABA treatment (t test, P > 0.05). These results are not consistent with a direct effect of ABA on the cells, though we cannot rule out that the ABA had a direct effect on the guard cells but not on the mesophyll, which yields most of the protoplasts. We suggest that attenuation of the transpiration stream is an important component of the up-regulation of Pos by ABA. We also tested the effect of ABA directly on protoplasts of aba1 mutant plants. Incubation for 10 min with ABA did not increase the Pos of the protoplasts (data not shown). Although 10 min is a brief exposure, it should be sufficient for the hormone to enter the cells and trigger an activation pathway. For example, a 5-min exposure of guard cells to ABA is sufficient to dramatically enhance potassium-channel currents (31).
The Role of RH in Changing the Pos of the Protoplasts.
To find out whether a change in the transpiration stream might affect the permeability of the cells, we switched to a hydroponic system in which single plants were floated on rafts in Magenta boxes either without a lid (45% humidity) or with a loose lid (85% humidity). The aperture of the stomates of the plants grown at the higher humidity was significantly greater for the Landsberg ecotype (usually around 4.5 μm) when compared with the aperture of the plants grown at 45% RH (usually around 3.5 μm), indicating that the plants responded to this change in humidity. No significant difference in stomatal aperture was observed when the aba1 or abi mutants or the wt plants were grown at different RH values (data not shown). Measurements of leaf temperature with a thermocouple of plants growing in a box without a lid or with a lid showed the temperatures to be the same: 20°C ± 1°C. Determination of the Pos values revealed striking differences in the Pos of the protoplasts, depending on the RH. The data are presented in Fig. 4. The histograms clearly show that, in all plants examined, raising the RH to 85% increased the proportion of protoplasts with a high Pos value significantly for the aba and abi mutants. Thus, attenuation of the transpiration stream by raising the RH increased the Pos of the protoplasts of the mutant plants.
Figure 4.
Histograms of Pos values from leaf protoplasts of plants grown in hydroponics respectively at 45% and 85% of RH. Leaves of 5-week-old plants were harvested, and 2–3 different preparations of protoplasts were used. The Pos of 20 protoplasts was measured for each line and in each condition. Bars with the same sign are not significantly different (Dunn's test), and the results are presented as the mean ± SD.
Discussion
According to the composite model of water transport, developed by Steudle and his collaborators over the last 10 years (1), water transport through the plant can follow either an apoplastic path or a cell-tocell path. How much water travels along each pathway depends on the magnitude of the total water flow and especially on the nature of the driving forces. When the stomatal pores are open and there are strong pressure gradients because of evaporative demand, water flows largely around the protoplasts because this is the path of least hydraulic resistance. In the absence of transpiration, water moves cell-to-cell by osmotic forces through semipermeable membranes; membranes provide a variable resistance to water flow depending on the abundance and activity of aquaporins or water channel proteins. Steudle (1) postulated that the two pathways interact with each other and that such interactions are responsible for regulating the circulation of water in the plant. The results presented here support such an interaction: when the stomates were wide open, as in the aba1 and abi plants, the Pos of the protoplasts was found to be very low, much lower than in control plants that had partially opened stomates; when the stomates were closed because ABA had been added to the growth medium, the Pos increased more than 10-fold. The relationship between apoplastic flow, cell-to-cell flow, stomatal aperture, and ABA is illustrated in Fig. 5A. The effect of ABA is to dramatically reduce the apoplastic flow and to increase the cell-to-cell flow. Does ABA play a direct role in this modification of the Pos or is its effect indirect and mediated by stomatal closure and the attenuation of the transcription stream?
Figure 5.
A model of water flux in a leaf depending of the intensity of the transpiration stream. (A) In the well-watered condition without ABA, the water flux is large in the wt and even larger in the mutant plants; the Pos of the protoplasts from the mesophyll of the mutant plants is low. ABA treatment of wt and the aba1 mutant causes closure of the stomates, which leads to a decrease of the transpiration stream and an activation of the aquaporins in the plasma membranes of the mesophyll cells. (B) In hydroponics when the RH is low (45%), the water flux is large in the wt and even larger in the mutant plants; the Pos of the protoplasts from the mesophyll of the mutant plants is low (same as above). An increase of the RH to 85% leads to a decrease of the transpiration stream and strong increase of the water permeability of the mesophyll cells for all of the lines.
Is the Effect of ABA Direct or Indirect?
Most of the literature of the role of ABA in the water economy of plants deals with the role of ABA in stomatal closure. When roots experience water deficit, but the leaves are still fully turgid, ABA moving up the xylem causes the stomates to close through a collapse of the osmotic pressure of the guard cells. This collapse is caused by the efflux of potassium ions and anions as well as the conversion of malate into starch (32) and by the efflux of water from the cells. Whether ABA directly regulates the Pos of guard cell plasma membranes is not known.
Both indirect and direct evidence indicates that ABA can have an effect on the aqueous permeability of the membranes of root cells. Thirty years ago, Tal and Imber (19) and Glinka and Reinhold (33) reported that the application of ABA to the root medium of decapitated plants increases the exudation rate from the cut stump. Since that time, a number of studies have been published on this subject (16, 17, 20, 34). Stress conditions such as flooding, chilling, and salt cause a decrease in the hydraulic conductivity of the root system that may be ameliorated by preadaptation or ABA treatment. For example, Perez de Juan et al. (35) found that drought hardening of maize, which induces ABA synthesis, or ABA application, improved the water status of chilling sensitive lines. A direct effect of ABA on aquaporins in these maize plants was postulated on the basis of experiments in which HgCl2 was used to shut down plasma membrane aquaporins (36). An elegant recent study by Hose et al. (21) that used cell and root pressure probes on maize roots led to the conclusion that ABA added to excised root systems increased the root hydraulic conductivity by 3- to 4-fold and the cell conductivity by 7- to 27-fold. The effect on cellular hydraulic conductivity was transient, lasting only an hour. The authors concluded that ABA acts at the plasma membrane presumably by activating aquaporins. This would facilitate the uptake of water by the roots under nontranspiring conditions when the apoplastic pathway of water transport is minimal and the cell-to-cell pathway needs to be maximized. In all of these experiments, ABA was applied to excised root systems, eliminating the possibility that the effect is mediated by stomatal closure and a reduction in the transpiration stream. Surprisingly, mutants of maize, or Arabidopsis that contain very low levels of ABA or are insensitive to ABA, have not yet been used for hydraulic conductivity measurements on excised root systems.
We were able to dissociate the effect of ABA on Pos of leaf cells from its effect on stomatal closure, by examining these parameters 1, 2, and 3 h after the addition of ABA to the medium. We found that after 2 h the stomates began to close, but the Pos of the membranes had not yet changed. This means that closing of the stomates may be a necessary condition, though it may not be sufficient to change the Pos; ABA may also have a function in Pos regulation. By applying ABA to excised immersed leaves, we found that ABA caused stomatal closure, as expected, but did not result in a change in the Pos of the membranes. We presume that a 3 h of ABA treatment would be sufficient, as ABA brings about stomatal closure in 1 h. ABA, in the absence of a transpiration stream, does not appear to be sufficient to promote the increase in Pos of leaf cells. This surprising result contrasts with the already mentioned direct increase of the hydraulic conductivity induced by ABA on the roots of decapitated plants, independently of the transpiration stream. We suggest that both a change in the transpiration stream and ABA are needed to alter the Pos of the membranes of the leaf cells.
High humidity also attenuates the transpiration stream by decreasing the vapor pressure deficit. We performed Pos measurement on protoplasts from plants grown in hydroponics at different humidities. Leaf protoplasts from wt plants grown at 85% RH had similar Pos values compared with those of plants grown at 45% RH. However, in the aba1 and abi mutants, raising the humidity to 85% also raised the Pos to well above control values. Because the relative humidity directly depends on the temperature, we measured the leaf temperature and the temperature in the box with thermocouples. In both conditions, the temperature was 20 ± 1°C. Because the temperature was constant during the experiment, we may assume that the saturation value of water vapor was also the same in both conditions. We can therefore assume that the transpiration water flux is determined mainly by the degree of stomatal aperture and by the difference in vapor pressure between the stomatal chamber and the air outside the leaf. If we assume that the RH at 20°C in the stomatal chamber is close to 100% (37) at both outside RH values, the increase of RH corresponds to a 4-fold decrease in the transpiration stream. Thus, there are two conditions that increase the Pos of the cells: decreasing the transpiration stream by closing the stomates with ABA or decreasing the transpiration stream by growing mutant plants that have low Pos at high RH.
Why Should There Be an Increase in Pos of the Mesophyll Cells When the Transpiration Stream Is Weak?
Stomatal closure, whether it occurs at night or during the day as a result of water deficit, is accompanied by the continued uptake of water from the soil and the redistribution of water within the plant. As a result of these processes, the entire plant is fully rehydrated if sufficient water is available in the soil. Furthermore, even when photosysnthesis is diminished because the stomates are closed, water needs to circulate between the phloem and the xylem to transport solutes. We postulate that this cell-to-cell movement of water will be accelerated by an increase in Pos when there is no transpiration stream, as illustrated in Fig. 5A. The lower osmotic pressure differences, which could be the result of a lowered rate of photosynthesis, would require higher Pos values to move water cell-to-cell and support the high rate of xylem to phloem recycling that occurs in plants (38). Rehydratating a partially dehydrated plant may be speeded up if the Pos of the cell membranes is higher.
When the vapor pressure difference is high and the stomates are open, the transpiration flux is maximum. In this condition, the Pos values of the leaf protoplasts are low, suggesting, as proposed by Steudle and Peterson (39) for the root, that the water flux in the plant is mainly apoplastic and does not involve a substantial symplastic or trans-cellular water flux. When the vapor pressure difference is low, the transpiration flux is dramatically decreased and the transcellular water flux becomes much more significant (39). The significant increase of the Pos values at high RH we measured is in agreement with this hypothesis (Fig. 5B). In the case of the leaf, the increase of the water permeability of the cells would not allow to speed up the rate of transpiration, but it could facilitate the recycling between xylem and phloem and other internal processes that require transcellular water transport.
How Could ABA and Relative Humidity Exert Their Effects?
The lack of effect of ABA on the Pos of the protoplasts from the abi mutants indicates that the two signal-transduction pathways—one involving ABA and one involving the sensing of the transpiration stream—may share common elements. The signal-transduction pathway for stomatal closure has been analyzed in considerable detail (40, 41). Phosphorylation events are important mediators of signaling in all cells and play a major role in ABA signaling in guard cells. Genes encoding protein phosphatases that regulate stomatal movement were identified several years ago (42). Guard cells contain an ABA-activated protein kinase (43). An ABA-activated serine–threonine kinase of guard cells that is essential for stomatal closure was recently identified by Li et al. (44). Activation of anion channels by phosporylation mediated by this kinase is thought to result in stomatal closure. Interestingly, phosphorylation of PIPs is regulated by apoplastic water potential (7), and phosphorylation at a conserved site increased the activity of PM28A, a PIP2 aquaporin (8). This conserved phosphorylation site is in the cytoplasmic domain between the second and third transmembrane α-helices and is situated close to the aqueous pore. Our results show that ABA did not alter the abundance of PIP1 and PIP2 aquaporins—we define PIP2 aquaporins more narrowly than they are defined in the most recent article of Johanson et al. (6)—but cannot, of course, exclude that ABA causes an increase in PIPs not recognized by our antibodies (like PIP 2.6, PIP 2.7, and PIP 2.8 in ref. 6). Genomics and proteomics approaches need to be applied to these and other mutants to determine how gene expression and protein abundance change in this important protein family and how this relates to the hydraulic permeability of cells and tissues.
Acknowledgments
We thank Dr. Tony Schaeffner who generously provided the PIP1-specific antiserum. This work was supported in part by the U.S. Department of Agriculture Research Initiative Competitive Grants Program.
Abbreviations
- ABA
abscisic acid
- PIP
plasma membrane intrinsic protein
- Pos
osmotic water permeability
- RH
relative humidity
- wt
wild type
References
- 1.Steudle E. Plant Soil. 2000;226:45–56. [Google Scholar]
- 2.Tyerman S D, Bohnert H, Maurel C, Steudle E, Smith J A C. J Exp Bot. 1999;50:1055–1071. [Google Scholar]
- 3.Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P. Biochim Biophys Acta. 2000;1465:324–342. doi: 10.1016/s0005-2736(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 4.Maurel C, Chrispeels M J. Plant Physiol. 2001;125:135–138. doi: 10.1104/pp.125.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaumont F, Barrieu F, Wojcik E, Chrispeels M J, Jung R. Plant Physiol. 2001;25:1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig A R, Kjellbom P. Plant Physiol. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson I, Larsson C, Ek B, Kjellbom P. Plant Cell. 1996;8:1181–1191. doi: 10.1105/tpc.8.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson I, Karlsson M, Shukla V K, Chrispeels M J, Larsson C, Kjellbom P. Plant Cell. 1998;10:451–460. doi: 10.1105/tpc.10.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mott K A, Parkhurst D F. Plant Cell Environ. 1991;14:509–515. [Google Scholar]
- 10.Monteith J L. Plant Cell Environ. 1995;18:357–364. [Google Scholar]
- 11.Farquhar G D. Plant Physiol. 1978;5:787–800. doi: 10.1104/pp.61.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shultze E D. Annu Rev Plant Physiol. 1986;37:247–274. [Google Scholar]
- 13.Tardieu F. Plant Growth Reg. 1996;20:93–104. [Google Scholar]
- 14.Assmann S M, Snyder J A, Julie Lee Y-R. Plant Cell Environ. 2000;23:387–395. [Google Scholar]
- 15.Davies W J, Tardieu F, Trejo C L. Plant Physiol. 1994;104:309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glinka Z. Plant Physiol. 1973;51:217–219. doi: 10.1104/pp.51.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludewig M, Dorfflinger K, Seifert H. Planta. 1988;175:325–333. doi: 10.1007/BF00396337. [DOI] [PubMed] [Google Scholar]
- 18.Quintero J M, Fournier J M, Ramos J, Benlloch M. Physiol Plant. 1998;102:279–284. [Google Scholar]
- 19.Tal M, Imber D. Plant Physiol. 1971;47:849–850. doi: 10.1104/pp.47.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford K J. Plant Physiol. 1983;72:251–255. doi: 10.1104/pp.72.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hose E, Steudle E, Hartung W. Planta. 2000;211:874–882. doi: 10.1007/s004250000412. [DOI] [PubMed] [Google Scholar]
- 22.Ramahaleo T, Morillon R, Alexandre J, Lassalles J-P. Plant Physiol. 1999;119:885–896. doi: 10.1104/pp.119.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koornneef M, Dellaert L W, van der Veen J H. Mutat Res. 1982;93:109–123. doi: 10.1016/0027-5107(82)90129-4. [DOI] [PubMed] [Google Scholar]
- 24.Koornneef M, Reuling G, Karssen C M. Physiol Plant. 1984;61:377–384. [Google Scholar]
- 25.Arteca R N, Arteca J M. Physiol Plant. 2000;108:188–193. [Google Scholar]
- 26.Daniels M J, Mirkov T E, Schroeder J I, Chrispeels M J. Plant Physiol. 1994;106:1325–1333. doi: 10.1104/pp.106.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frangne N, Maeshima M, Schäffner A R, Mandel T, Martinoia E, Bonnemain J-L. Planta. 2001;212:270–278. doi: 10.1007/s004250000390. [DOI] [PubMed] [Google Scholar]
- 28.Roelfsema M R G, Prins H B A. Physiol Plant. 1995;95:373–378. [Google Scholar]
- 29.Maurel C, Tacnet F, Guclu J, Guern J, Ripoche P. Proc Natl Acad Sci USA. 1997;94:7103–7108. doi: 10.1073/pnas.94.13.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niemietz C M, Tyerman S D. Plant Physiol. 1997;115:561–567. doi: 10.1104/pp.115.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blatt M R. Planta. 1990;180:445–455. doi: 10.1007/BF00198799. [DOI] [PubMed] [Google Scholar]
- 32.MacRobbie E A. Philos Trans R Soc London B. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glinka Z, Reinhold L. Plant Physiol. 1971;48:103–105. doi: 10.1104/pp.48.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glinka Z. Plant Physiol. 1977;59:933–935. doi: 10.1104/pp.59.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez de Juan J, Irgoyen J J, Sanchez-Diaz M. Plant Sci. 1997;122:71–79. [Google Scholar]
- 36.Quintero J M, Fournier J M, Bemlloch M. J Exp Bot. 1999;50:1607–1612. [Google Scholar]
- 37.Nobel P S. Physicochemical and Environmental Plant Physiology. San Diego: Academic; 1999. [Google Scholar]
- 38.Kockenberger W, Pope J M, Xia Y, Jeffrey K R, Komor E, Callaghan P T. Planta. 1997;201:325–333. [Google Scholar]
- 39.Steudle E, Peterson C A. J Exp Bot. 1998;49:775–788. [Google Scholar]
- 40.Leung J, Giraudat J. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder J I, Allen G J, Hugouvieux V, Kwak J M, Waner D. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 42.Merlot S, Giraudat J. Plant Physiol. 1997;114:751–757. doi: 10.1104/pp.114.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori I C, Muto S. Plant Physiol. 1997;113:833–839. doi: 10.1104/pp.113.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J X, Wang X Q, Watson M B, Assmann S M. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]