Abstract
This study examined the mechanisms underlying pudendal and tibial neuromodulation of bladder function at the single neuron level in the spinal cord. A microelectrode was inserted into the S2 spinal cord of anesthetized cats to record single neuron activity induced by bladder distention over a range of constant intravesical pressures (10–40 cmH2O). Pudendal nerve stimulation (PNS) or tibial nerve stimulation (TNS) was applied at 5 Hz frequency and 0.2 ms pulse width and at multiples of the threshold (T) intensities for inducing anal or toe twitches. A total of 14 spinal neurons from 11 cats were investigated. Both PNS and TNS at 2 T intensity significantly (p < .05) reduced by 40–50% the frequency of firing induced by bladder distention at 20–40 cmH2O in the same spinal neurons. This reduction was not changed by blocking opioid receptors with naloxone (1 mg/kg, i.v.). Activation of pudendal afferents by repeatedly stroking (3–5 times per second) the genital skin using a cotton swab also inhibited the neuron activity induced by bladder distention. Prolonged (30 min) TNS at 4 T intensity produced a short lasting (10–18 min) post-stimulation inhibition that reduced by 40–50% bladder-related neuron activity at different bladder pressures. These results indicate that PNS and TNS inhibition of reflex bladder activity may be mediated in part by convergence of inhibitory inputs onto the same population of bladder-related interneurons in laminae V-VII of the S2 spinal cord and that an opioid receptor mechanism is not involved in the inhibition.
Keywords: Bladder, Neuromodulation, Spinal Neuron, Cat
1. Introduction
Pudendal and tibial neuromodulation are effective therapies to treat overactive bladder (Peters et al. 2009, 2010) – a disorder that is defined by the International Continence Society as a syndrome characterized by urinary urgency with or without urge incontinence, usually with urinary frequency and nocturia (Abrams et al. 2002). However, the mechanisms underlying these neuromodulation therapies are not fully understood. It is commonly assumed that pudendal or tibial neuromodulation stimulates afferent axons that in turn activate inhibitory mechanisms either in the spinal cord or in the brain to suppress the neuron activity in the micturition reflex pathway (de Groat and Tai 2017). Our previous studies showed that: (1) in acute spinal cord transected cats spinal reflex bladder activity is inhibited by pudendal nerve stimulation (PNS) but not by tibial nerve stimulation (TNS) (Xiao et al. 2014b); (2) in spinal intact cats electrical stimulation of the pontine micturition center to activate the descending limb of the micturition reflex pathway elicits bladder activity that is inhibited by PNS but not by TNS (Lyon et al. 2016); (3) intrathecal administration of a GABAA receptor antagonist in cats suppresses PNS inhibition of bladder overactivity (Xiao et al. 2014a); and (4) application of an opioid receptor antagonist to the pons suppresses TNS inhibition of bladder overactivity (Ferroni et al. 2015). These results indicate that PNS inhibition of bladder reflexes can occur at the spinal level but TNS inhibition may not.
To directly test the hypothesis that pudendal but not tibial neuro-modulation acts in the spinal cord to suppress reflex bladder activity, this study was conducted in anesthetized cats to record the effects of PNS or TNS on the firing of bladder-related neurons in the S2 spinal cord. The neurons were identified by their responses to bladder distention. The results of this study indicate that PNS as well as TNS acts in the spinal cord to modulate bladder function.
2. Materials and methods
The Animal Care and Use Committee at the University of Pittsburgh approved the protocol and animal use in this study.
2.1. Surgical procedures
A total of 11 cats (4 male and 7 female, 2.9–4.1 kg; Liberty Research, Waverly, NY) were used. The animals were anesthetized initially with isoflurane (2–5% in oxygen) during surgery and then switched to α-chloralose anesthesia (initial dose 65 mg/kg i.v. followed by supplemental doses as needed) during data collection. The left cephalic vein was catheterized for intravenous administration of fluid and drug. A midline anterior cervical incision was used to access the airway, which was kept patent via tracheostomy. The right carotid artery was catheterized for monitoring arterial blood pressure. Oxygen saturation and heart rate were measured via a pulse oximeter (9847VNONIN Medical, Plymouth, MN) attached to the tongue. A midline laparotomy was performed and the ureters were transected, ligated, and then drained externally. A single-lumen urethral catheter (2.3 mm inner diameter) was inserted into the bladder via a small anterior urethrotomy and fixed in place by a suture around the urethra. The single-lumen catheter was attached to a T-adapter to allow for simultaneous bladder pressure recording via a pressure transducer and bladder distention via a saline reservoir. Bladder pressure was maintained at a constant level by adjusting the height of the saline reservoir connected to the urethral catheter. Small incisions were made in the right sciatic notch lateral to the tail and on the medial side of the left hindlimb above the ankle to expose the right pudendal nerve and left tibial nerve, respectively. A tripolar cuff electrode (NC223pt, Microprobes, Gaithersburg, MD) was placed around each intact nerve and connected to a stimulator (S88; Grass Medical Instruments, Quincy, MA). All incisions were then closed by sutures.
The spinal cord and cauda equina were then exposed between the L5 and S3 vertebrae via a dorsal laminectomy. The spinal dura was cut and the S2 spinal cord was exposed. The animal was mounted in a modified Narishige spinal frame in which the hip was supported by metal pins, and the spinous process at the rostral end of the laminectomy was supported by a clamp. The skin, which had been cut posteriorly from L4 to S3 was tied along the margin to form a pool that was filled with warm (35–37 °C) mineral oil.
2.2. Searching for bladder-related neurons in S2 spinal cord
This study focused on the identification of bladder-related neurons in S2 spinal cord because previous tracing studies showed that S2 spinal cord is a major spinal segment for bladder afferent projections (Morgan et al. 1981). A tungsten microelectrode (WE50030.5A10, 0.5 MΩ, Microprobes, Gaithersburg, MD) attached to a microelectrode driver was lowered to the dorsal surface of the spinal cord on the right side in the middle of the S2 spinal cord segment along the dorsal root entry zone. With bladder pressure maintained at a constant pressure of 40 cmH2O by setting the height of the saline reservoir, the microelectrode was slowly advanced in 5 μm steps into the spinal cord. At each electrode location, neuron activity amplified 20,000 times by an amplifier (P511, Grass Instruments, MA) was displayed on an oscilloscope and sent to an audio monitor. When single neuron firing was detected, the bladder pressure was lowered to 0 cmH2O to assess the contribution of bladder afferent input to the neuron activity. If the neuron stopped firing at 0 cmH2O bladder pressure, the microelectrode was kept in place to further investigate PNS or TNS effects on the activity. Otherwise, the microelectrode was advanced further into the cord. The microelectrode was withdrawn once it reached 2 mm depth before entering the ventral horn of the spinal cord. Then, the microelectrode was moved 0.5–1 mm rostral/caudal or medio/lateral to a new location on the spinal cord surface to repeat the search procedure until a bladder-related neuron was identified.
2.3. Stimulation protocol and drug administration
Uniphasic rectangular pulses (5-Hz frequency, 0.2-ms pulse width), which have previously been shown to be effective in inhibiting bladder activity (Larson et al. 2011; Tai et al. 2012), were delivered to the tibial or pudendal nerve. The intensity threshold (T) for inducing toe or anal sphincter twitch was determined at the beginning of the experiment. Based on our prior studies, stimulation intensity of 2 T was used for PNS or TNS of short duration (about 3 min) to acutely suppress bladder-related neuron activity during the stimulation (Fuller et al. 2017; Uy et al. 2017), while 4 T was used for prolonged (30-min duration) TNS to produce a post-stimulation inhibitory effect (Tai et al. 2011b).
Table 1 shows the sequence of different tests as described below in detail. When a bladder neuron was identified, the single unit activity was sampled at 20 kHz by an analog-to-digital converter (PCI-6024E, National Instruments, TX) and saved in a computer running the Lab-VIEW program (National Instruments, TX). Initially, the pressure response was determined by recording the activity at a range of bladder pressures (10, 20, 30, or 40 cmH2O) for 20–30 s with a 20–30 s interval of 0 cmH2O pressure between each pressure increase. To evaluate the effect of PNS on neuron activity (N = 13 neurons), the pressure response test was repeated under the following conditions: 1) control without PNS; 2) during 2 T PNS; 3) control after PNS. The same test was then performed using TNS (N = 10 neurons). Following the PNS and TNS tests, the effect of peri-genital stimulation (PGS) on neuron activity was determined at 10 and 30 cmH2O bladder pressures first without PGS (control) then during PGS (N = 6 neurons). PGS was applied by repeatedly stroking (3–5 times per second) the genital area with a cotton swab to determine if physiological stimulation of a population of mechanosensitive cutaneous afferents in the pudendal nerve can mimic the effect of electrical stimulation of the whole pudendal nerve.
Table 1.
The sequence of different tests.
| Order | Test |
|---|---|
| 1 | Bladder neuron identified |
| 2 | Pudendal nerve stimulation |
| 3 | Tibial nerve stimulation (TNS) |
| 4 | Peri-genital stimulation |
| 5 | Post-TNS effect |
| 6 | Naloxone treatment |
After testing the acute effects of PNS/TNS/PGS during the stimulation, the post-stimulation inhibitory effect induced by prolonged (30-min duration) TNS as revealed in our previous studies (Tai et al. 2011b) was investigated. The bladder pressure response (10–40 cmH2O) was first determined without any stimulation to serve as the control condition, and then followed by a period of 4 T TNS for 30 min with the bladder pressure at 0 cmH2O. Within 0.5–1.5 min after cessation of 30-min TNS, the bladder pressure response (10–40 cmH2O) was tested again to determine the post-stimulation inhibition. Then, at an interval of 1–11 min the bladder pressure was increased from 0 cmH2O to 40 cmH2O for 20 s to assess the recovery from the post-stimulation inhibition. Once the neuron activity at 40 cmH2O recovered to control level, the full pressure response test (10–40 cmH2O) was performed again.
At the end of the experiment, naloxone (1 mg/kg) was administered intravenously with the bladder pressure at 0 cmH2O. Five minutes after naloxone treatment, the acute effect of PNS (N = 6 neurons) or TNS (N = 7 neurons) on neuron activity was evaluated again using the bladder pressure response test.
2.4. Histological identification of the microelectrode locations
After conclusion of every experiment, the S2 segment of the spinal cord was excised and fixed in formalin (10%) for 3 days. The fixed tissue was then processed with 30% sucrose buffer, embedded in OTC compound, and frozen at −80 °C for 24 h. Then, 30–40 μm thick sections were cut serially by a cryostat, stained with cresyl violet, and examined using light microscopy. In each cat there were only 1–5 microelectrode tracks distributed in 3 sections (rostral, middle, and/or caudal) of the S2 spinal segment, i.e. only 1–2 microelectrode tracks were identified in a single section. Most of the microelectrode tracks were identified in the histological sections by lesions made by the microelectrode insertion. Using an estimated 15% tissue shrinkage (Tai et al. 2001), the recording location was determined based on the depth of the microelectrode tip where the single neuron activity was investigated. Then, the microelectrode locations in different animals were marked on a standard template of S2 spinal cord based on their relative distances to the landmarks of the spinal cord (dorsal surface, central canal, and lateral edge of the gray matter, etc.). Estimated microelectrode locations were then compared to the laminar organization of the cat sacral S2 spinal cord described by Rexed (1954).
2.5. Data analysis
During PNS or TNS, the stimulation artifacts of amplitude larger than the action potentials of the bladder neuron were identified by thresholding and then removed by a LabVIEW program together with the first 10–30 ms of recording following each stimulus which in some cases contained large amplitude field potentials and/or neuron activity from other neurons that were inactive prior to electrical stimulation. Then, the number of bladder-related neuron action potentials during the 20-s bladder distention at different pressures (10, 20, 30, or 40 cmH2O) was counted using the LabVIEW program by setting a new threshold level to distinguish the bladder neuron spikes from any low amplitude neuron spikes or baseline activity. To calculate the firing rate of bladder neuron spikes, a 1-s window was used to count the number of spikes and the results were displayed as pulses per second (pps) over time. The number of action potentials measured during each 20-s bladder distention at different pressures (10, 20, 30, or 40 cmH2O) and under different conditions (control, PNS, TNS, or PGS) were all normalized to the initial control measurement with the bladder distended at 40 cmH2O. The normalized data from different animals under the same conditions are presented as means ± SE. Statistical significance (p < .05) was determined by linear regression analysis or ANOVA followed by Bonferroni multiple comparisons.
3. Results
3.1. Single neuron activity in S2 spinal cord induced by bladder distention
Action potentials from single neurons were identified based on their similar amplitude and shape (Fig.1B). In addition, the single neuron potentials were very sensitive to the location of the microelectrode tip. A 5–10 μm displacement of the microelectrode could result in the loss of activity. A total of 15 neurons were identified from the 11 cats as bladder-related neurons. Fourteen neurons responded positively to an increase in bladder pressure (Fig.1A). One neuron responded negatively by decreasing activity during an increase in bladder pressure. Among the 14 bladder-related positive neurons that were studied during PNS and TNS, 6 exhibited stable activity for a period (4–5 h) that was long enough to complete all the tests described in method section. There is no statistically significant difference between the neurons from male (N = 5 neurons) and female (N = 9 neurons) cats in their response (firing rate) to the PNS or TNS.
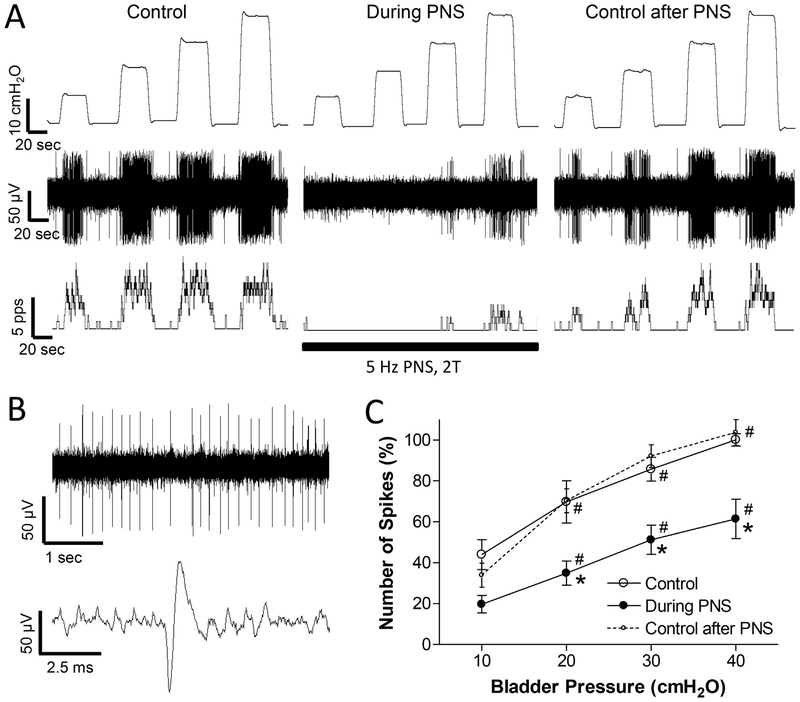
Fig. 1.
Pudendal nerve stimulation (PNS) inhibits spinal interneuronal activity induced by bladder distention. A: Single neuron activity induced by bladder distention at 10, 20, 30, and 40 cmH2O before PNS (Control), during PNS, and 30 s after PNS. Top trace: bladder pressure; Middle trace: single neuron spikes; Bottom trace: neuron firing rate. The black bar indicates PNS duration. PNS: 5 Hz, 0.2 ms, 2 T = 0.7 V. T – threshold intensity for inducing anal twitch. Microelectrode tip location: 1.1 mm below the spinal cord surface. B: Single neuron spikes and a single action potential are shown in an expanded time scale from the same neuron as shown in A. C: Number of single neuron spikes at different bladder pressures with or without PNS normalized to the initial control value measured at 40 cmH2O. * significantly (p < .05) different from control (two-way ANOVA). # significantly (p < .05) different from 10 cmH2O for different conditions (one-way ANOVA). N = 13 neurons from 10 cats. PNS: 5 Hz, 0.2 ms, 2 T = 0.3–11.2 V. Microelectrode tip locations: 0.5–1.64 mm below the spinal cord surface.
Since the neuron activity was detected during bladder distention at 40 cmH2O constant pressure, only those neurons that maintained tonic firing during prolonged bladder distention were identified (Fig.1A). In addition, only those neurons that stopped firing when the bladder pressure was lowered to 0 cmH2O were investigated in this study. Some of these neurons (N = 4) stopped firing within a few seconds at 0 cmH2O (Figs. 1 and 2), while others (N = 8) gradually reduced the firing rate during a 10–38 s period (average 19 ± 3 s) before a complete cessation of firing or maintained a low level of firing between distentions (N = 2) (Fig.3A). On average, the number of action potentials during the 20-s bladder distention increased linearly (slope = 1.8 ± 0.3) as the bladder pressure increased from 10 to 40 cmH2O (Fig.1C). The average maximal firing rate was 18.6 ± 2.6 Hz at 40 cmH2O bladder pressure.
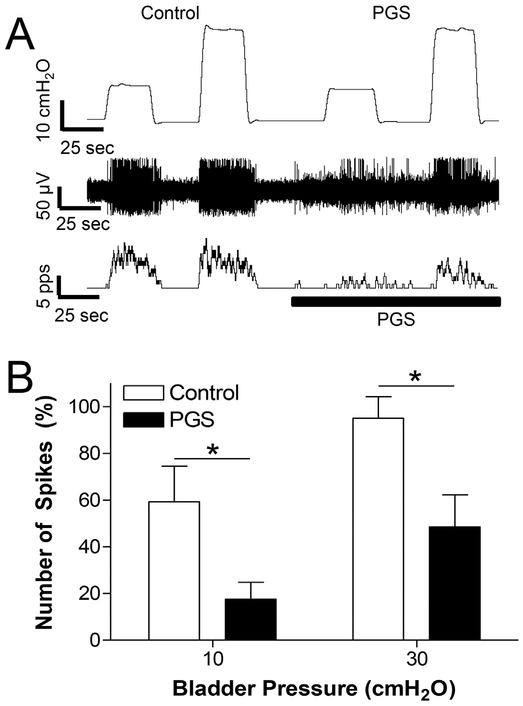
Fig. 2.
Perigenital stimulation (PGS) inhibits spinal neuron activity induced by bladder distention. A: Single neuron activity induced by bladder distention at 10 and 30 cmH2O before PGS (control) or during PGS. PGS: repeatedly stroking (3–5 times/s) the perigenital skin area using a cotton swab. Top trace: bladder pressure; Middle trace: single neuron spikes; Bottom trace: neuron firing rate. The black bar indicates the duration of PGS. Microelectrode tip location: 1.1 mm below the spinal cord surface. B: Number of single neuron spikes at different bladder pressures with or without PGS normalized to the initial control value measured at 40 cmH2O. * significantly (p < .05) different between control and PGS (two-way ANOVA). N = 6 neurons from 5 cats. Microelectrode tip locations: 0.75–1.2 mm below the spinal cord surface.
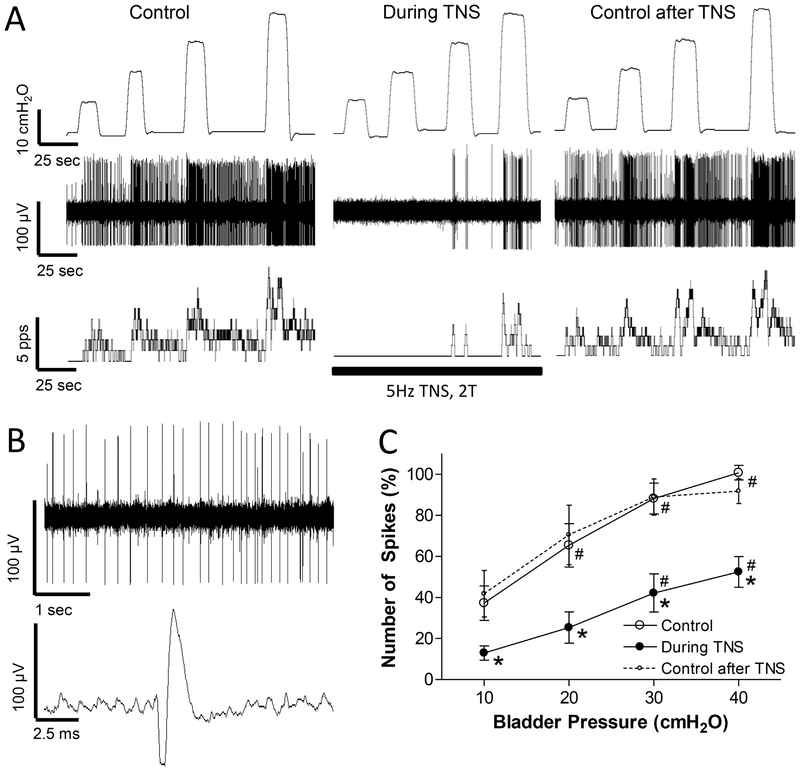
Fig. 3.
Tibial nerve stimulation (TNS) inhibits spinal interneuronal activity induced by bladder distention. A: Single neuron activity induced by bladder distention at 10, 20, 30, and 40 cmH2O before TNS (Control), during TNS, and 30 s after TNS. Top trace: bladder pressure; Middle trace: single neuron spikes; Bottom trace: neuron firing rate. The black bar indicates the TNS duration. TNS: 5 Hz, 0.2 ms, 2 T = 4.8 V. T – threshold intensity for inducing toe twitch. Microelectrode tip location: 0.5 mm below the spinal cord surface. B: Single neuron spikes and a single action potential are shown in an expanded time scale from the same neuron as shown in A. C: Number of single neuron spikes at different bladder pressures with or without TNS normalized to the initial control value measured at 40 cmH2O. * significantly (p < .05) different from control (two-way ANOVA). # significantly (p < .05) different from 10 cmH2O for different conditions (one-way ANOVA). N = 10 neurons from 9 cats. TNS: 5 Hz, 0.2 ms, 2 T = 1.0–4.8 V. Microelectrode tip locations: 0.5–1.64 mm below the spinal cord surface.
3.2. Inhibitory effect of PNS or PGS on bladder neuron activity
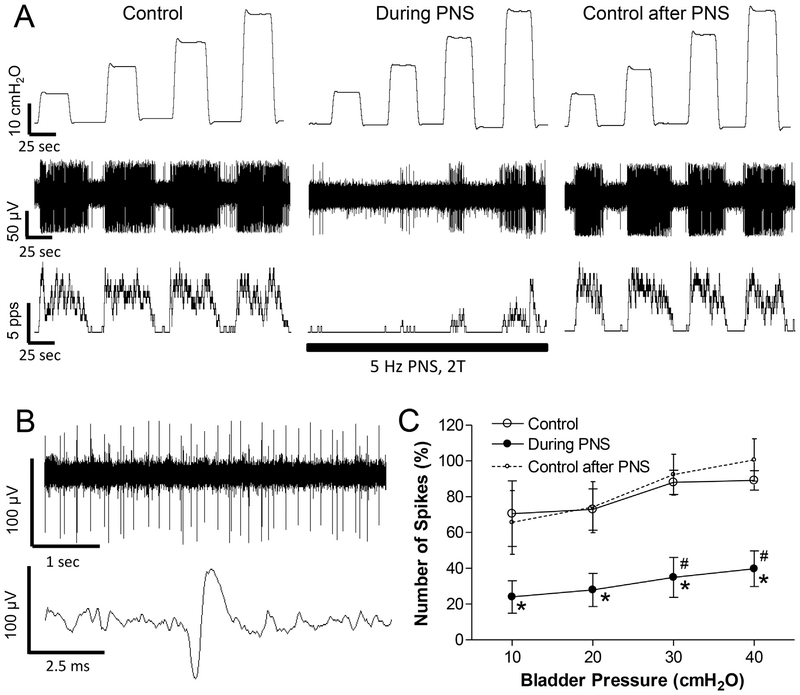
PNS (5 Hz, 0.2 ms, 2 T) inhibited the spinal neuron activity induced by bladder distention in all neurons tested (N = 13, Fig.1). On average the activity during the 20-s bladder distention was significantly (p < .05) reduced on average by 40–50% at 20, 30, and 40 cmH2O bladder pressures (Fig.1C). In 3 neurons, a complete inhibition occurred at low bladder pressure (10–20 cmH2O, Fig.1A). During PNS inhibition the neuron activity increased linearly (slope = 1.4 ± 0.3) with increasing bladder pressure and the slope of the curve was not significantly different from that before PNS. The inhibited neuron activity recovered quickly (30–90 s) after terminating PNS (Fig.1C). Neuron activity before and after PNS was not significantly different (Fig.1C).
To determine if physiological activation of cutaneous afferents in the pudendal nerve could mimic the effect of electrical stimulation of the nerve, PGS was applied by repeatedly stroking (3–5 times per second) the peri-genital skin area using a cotton swab. When tested on nine quiescent neurons with an empty bladder at 0 cmH2O pressure, PGS elicited firing in three neurons and did not elicit firing in the other six neurons. PGS was not tested further on the three neurons that exhibited excitatory responses. However, when tested in the other six neurons during firing induced by increases in bladder pressure to 10 and 30 cmH2O, PGS suppressed the firing (Fig.2).
3.3. Inhibitory effect of TNS on bladder neuron activity
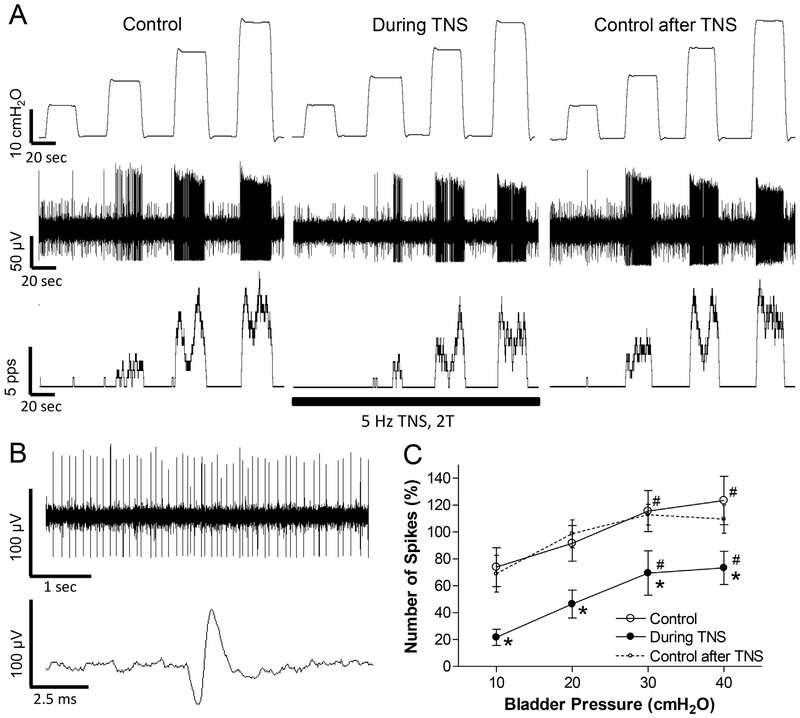
TNS (5 Hz, 0.2 ms, 2 T) of a short duration (approximately 3 min) inhibited the activity induced by bladder distention in all neurons tested (N = 10, Fig.3). These 10 neurons were also inhibited by PNS as shown in Fig.1, indicating a convergence of pudendal and tibial inhibition at the spinal cord level. In 3 neurons, a complete inhibition occurred at low bladder pressure (10–20 cmH2O, Fig.3A). On average the neuron activity increased linearly (slope = 1.3 ± 0.3) with increasing bladder pressure during TNS inhibition. The slope of the increase was not significantly different from the slope (2.1 ± 0.3) before TNS (Fig.3C). The bladder neuron activity also recovered quickly (30–90 s) after terminating TNS (Fig.3 A and C). Neuronal activity before and after TNS was not significantly different (Fig.3C). The magnitude of the inhibition of neuron activity during TNS (Fig.3C) was not significantly different (p > .05, two-way ANOVA) from that elicited by PNS (Fig.1C).
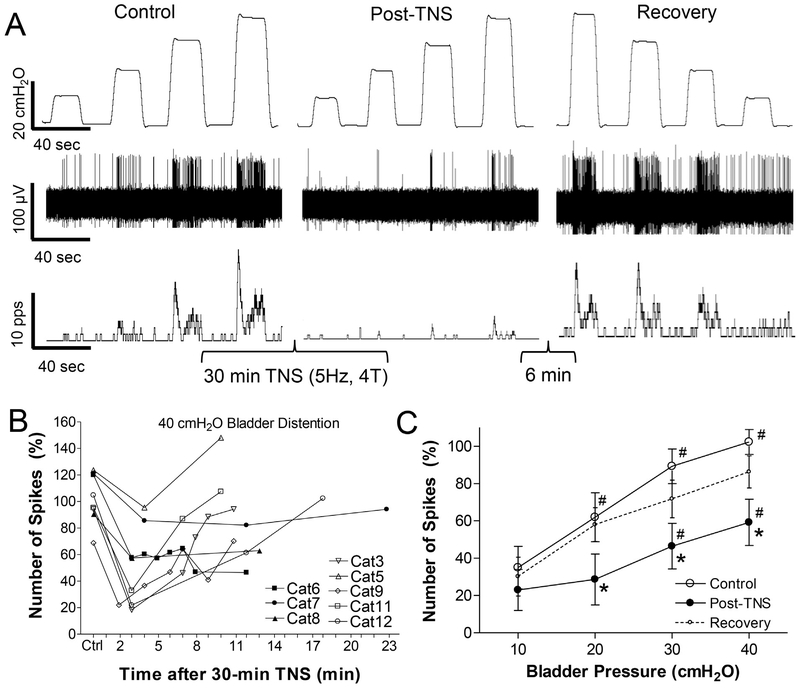
Prolonged (30-min duration) TNS (5 Hz, 0.2 ms) at a higher intensity (4 T) produced post-stimulation inhibition in all neurons tested (N = 8, Fig.4). In 5 neurons the activity induced by 40 cmH2O bladder distention fully recovered within 10–18 min after the cessation of the 30-min TNS, but in the other 3 neurons the activity did not recover during an observation period of 12–23 min (Fig.4B). On average, 0.5–1.5 min after cessation of the 30-min TNS the neuron activity was reduced by 40–50% at bladder pressures 20–40 cmH2O (Fig.4C). After a recovery period of 10–23 min, the average activity was not significantly different from that in the control condition before the 30-min TNS (Fig.4C).
Fig. 4.
Tibial nerve stimulation (TNS) of 30-min duration produces a post-stimulation inhibition of spinal neuron activity induced by bladder distention. A: Single neuron activity induced by bladder distention at 10, 20, 30, and 40 cmH2O before TNS (Control), 30 s after the 30-min TNS (Post-TNS), and 6 min after the stimulation (Recovery). Top trace: bladder pressure; Middle trace: single neuron spikes; Bottom trace: neuron firing rate. TNS: 30 min, 5 Hz, 0.2 ms, 4 T = 3.6 V. T – threshold intensity for inducing toe twitch. Microelectrode tip location: 0.75 mm below the spinal cord surface. B: Number of single neuron spikes induced by 40 cmH2O bladder distention at different time points after terminating the 30-min TNS showing that neuron activity recovered in 5 cats (open symbols) but did not recover in 3 cats (black symbols). C: Number of single neuron spikes at different bladder pressures before (Control), 0.5–1.5 min after the 30-min TNS (Post-TNS), and 10–23 min after the stimulation (Recovery). The number of single neuron spikes in B and C is normalized to the initial control value measured at 40 cmH2O. * significantly (P < .05) different from control (two-way ANOVA). # significantly (P < .05) different from 10 cmH2O for different conditions (one-way ANOVA). N = 8 neurons from 8 cats. TNS: 5 Hz, 0.2 ms, 4 T = 2.0–9.6 V. Microelectrode tip locations: 0.5–1.64 mm below the spinal cord surface.
3.4. Effects of naloxone on PNS or TNS inhibition of bladder neuron activity
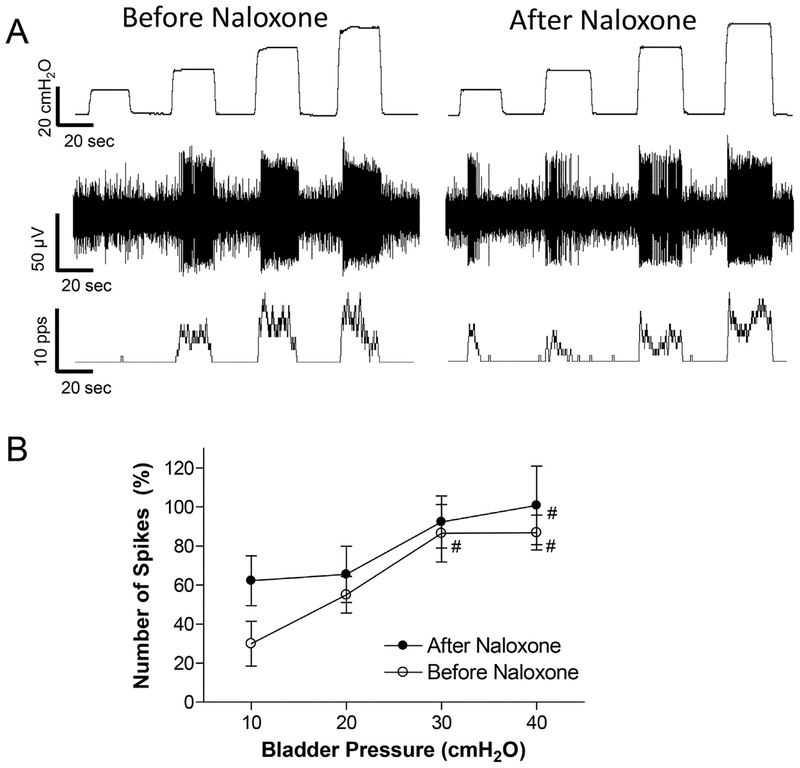
Naloxone (1 mg/kg, i.v.) did not significantly change the neuron activity induced by bladder distention (Fig.5). Although there was a trend toward an increase in activity after naloxone at the low bladder pressure (10 cmH2O in Fig.5B), this change was not statistically significant. The slopes of the linear relationship between neuron firing and bladder pressure are also not significantly different (p > .05) before (2.0 ± 0.5) and after (1.4 ± 0.7) naloxone treatment. After naloxone treatment, the neuron activity was still significantly (p < .05) reduced about 50% by PNS or TNS (Fig.6C and Fig.7C), and recovered quickly (30–90 s) after a short period of PNS or TNS (Fig.6A and Fig.7A).
Fig. 5.
Naloxone (1 mg/kg, i.v.) did not significantly change spinal neuron activity induced by bladder distention. A: Typical example showing spinal neuron activity before and 5 min after naloxone treatment. Top trace: bladder pressure; Middle trace: single neuron spikes; Bottom trace: neuron firing rate. Microelectrode tip location: 0.7 mm below the spinal cord surface. B: Number of single neuron spikes at different bladder pressure before and after naloxone treatment. The number of spikes is normalized to the initial control value measured at 40 cmH2O. # significantly (p < .05) different from 10 cmH2O for the two conditions (one-way ANOVA). N = 7 neurons from 7 cats. Microelectrode tip locations: 0.5–1.64 mm below the spinal cord surface.
Fig. 6.
After naloxone treatment pudendal nerve stimulation (PNS) still inhibits spinal neuron activity induced by bladder distention. A: Single neuron activity induced by bladder distention at 10, 20, 30, and 40 cmH2O before PNS (Control), during PNS, and 30 s after PNS. Top trace: bladder pressure; Middle trace: single neuron spikes; Bottom trace: neuron firing rate. The black bar indicates PNS duration. PNS: 5 Hz, 0.2 ms, 2 T = 0.7 V. T - threshold intensity for inducing anal twitch. Microelectrode tip location: 1.1 mm below the spinal cord surface. B: Single neuron spikes and a single action potential are shown in an expanded time scale from the same neuron as shown in A. C: Number of single neuron spikes at different bladder pressures with or without PNS normalized to the initial control value measured at 40 cmH2O. * significantly (p < .05) different from control (two-way ANOVA). # significantly (p < .05) different from 10 cmH2O (one-way ANOVA). N = 6 neurons from 6 cats. PNS: 5 Hz, 0.2 ms, 2 T = 0.3–11.2 V. Microelectrode tip locations: 0.7–1.64 mm below the spinal cord surface.
Fig. 7.
After naloxone treatment tibial nerve stimulation (TNS) still inhibits spinal neuron activity induced by bladder distention. A: Single neuron activity induced by bladder distention at 10, 20, 30, and 40 cmH2O before TNS (Control), during TNS, and 30 s after TNS. Top trace: bladder pressure; Middle trace: single neuron spikes; Bottom trace: neuron firing rate. The black bar indicates the TNS duration. TNS: 5 Hz, 0.2 ms, 2 T = 1.0 V. T – threshold intensity for inducing toe twitch. Microelectrode tip location: 1.2 mm below the spinal cord surface. B: Single neuron spikes and a single action potential are shown in an expanded time scale from the same neuron as shown in A. C: Number of single neuron spikes at different bladder pressures with or without TNS normalized to the initial control value measured at 40 cmH2O. * significantly (p < .05) different from control (two-way ANOVA). # significantly (p < .05) different from 10 cmH2O for different conditions (one-way ANOVA). N = 7 neurons from 7 cats. TNS: 5 Hz, 0.2 ms, 2 T = 1.0–4.8 V. Microelectrode tip locations: 0.5–1.64 mm below the spinal cord surface.
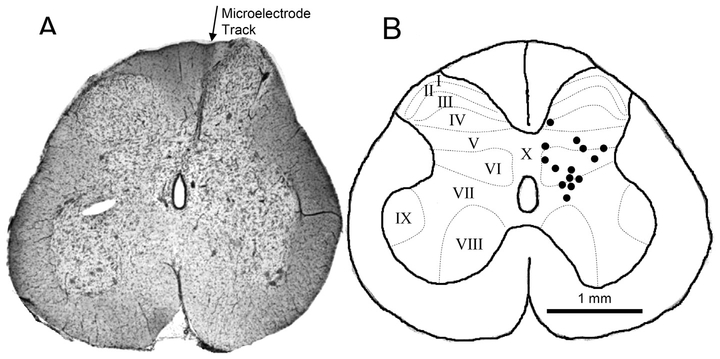
3.5. Locations of bladder neurons identified in the S2 spinal cord
The locations of the 14 neurons investigated in this study were identified histologically by measurements of the electrode tracks in the S2 spinal cord (Fig.8). Most of the neurons were located within the lamina V-VII. The average depth of these neurons was 1.08 ± 0.08 mm (range 0.5–1.64 mm) below the spinal cord surface. An estimation of 15% tissue shrinkage (Tai et al. 2001) was used to determine the recording locations in the histological sections. Therefore, the average error of the identified locations should be < 5% (0.05 mm) for an average 10–20% shrinkage of a 1-mm length of microelectrode track.
Fig. 8.
Histologically identified locations in the S2 spinal cord where single neuron activity induced by bladder distention was recorded by microelectrode. A. Histological section of S2 spinal cord showing a microelectrode track. B. Locations of microelectrode tips where neuron activity were recorded. N = 14 neurons from 11 cats. Rexed laminae (I-X) are indicated by dotted lines that were redrawn based on Rexed’s study (1954).
4. Discussion
Activity of single neurons in the S2 spinal cord of the cat evoked by bladder distention was significantly inhibited during short periods of PNS (Fig.1), PGS (Fig.2) or TNS (Fig.3) and rapidly recovered following termination of stimulation. However, prolonged (30-min duration) TNS induced post-stimulation inhibition of this activity (Fig.4). Intravenous administration of a large dose of naloxone did not significantly change the bladder distention-induced activity (Fig.5) or the PNS/TNS inhibition of this activity (Fig.6 and Fig.7). These results indicate that inter-neurons in the intermediate region of the sacral spinal cord (Fig.8) might be the site of convergence of visceral afferent excitatory and somatic afferent-induced inhibitory inputs that regulate micturition and that an opioid receptor mechanism is not involved in the inhibition.
It is well known that micturition is mediated by a spinobulbospinal reflex pathway that is relayed through circuitry in the rostral pons (de Groat et al. 2015; Fowler et al. 2008). Thus, neuromodulation of reflex bladder activity could occur at various sites along this reflex pathway. Previous studies (Ferroni et al. 2015; Reese et al. 2015; Rogers et al. 2015; Xiao et al. 2014b) indicated that PNS and TNS inhibits acetic acid induced bladder overactivity in cats by actions at different sites in the central nervous system, i.e., PNS acting in the spinal cord and TNS acting in the brain stem. However, the present experiments provide evidence that both PNS and TNS suppress normal reflex bladder activity at least in part by targeting the same population of interneurons in the spinal cord. The S2 spinal cord of the cat is the major spinal segment for bladder afferent (Morgan et al. 1981) as well as pudendal afferent projections (Thor et al. 1989). Thus, PNS inhibition of bladder-related neuron activity could be mediated by intra-segmental circuitry. However, tibial afferents project to L5-S1 spinal segments with a major projection to L6–L7 spinal segments (Gustafson et al. 2006). Therefore, TNS inhibition of bladder-related neuron activity in S2 spinal cord must be mediated by an inter-segmental pathway or due to TNS activation of a supraspinal center that in turn generates a descending inhibitory input to the S2 spinal cord.
Although the type of spinal neuron exhibiting PNS and TNS inhibition was not identified in the present experiments, the distribution of these neurons mediolaterally across laminae V-VII (Fig.8) suggests that they are spinal tract neurons that form the sensory or ascending limb of the spinobulbospinal micturition reflex pathway. Previous anatomical tracing studies revealed that these spinal tract neurons projecting to the caudal thoracic – rostral lumbar spinal cord (de Groat et al. 1981) or to the periaqueductal gray (Klop et al. 2005) are located in a band extending from lamina I on the lateral edge of the dorsal horn ventromedially through lamina V-VII in the same location as the neurons studied in our experiments. This location (Fig.8) matches very well with the distribution of bladder primary afferent projections in lamina V-VII of the S2 spinal cord in cats (Morgan et al. 1981) and suggests that the neurons are second order neurons in the bladder sensory pathway receiving direct primary afferent from the bladder. The sensory function of the neurons is consistent with the absence of neuron firing with the bladder empty (Fig.1A) and the linear increase in firing with graded increases in bladder pressure (Fig.1C). The neurons in our experiments are clearly not parasympathetic preganglionic neurons, which are located primarily on the lateral edge of the gray matter (Nadelhaft et al. 1980) and which exhibit a bursting pattern of firing with a mean firing rate of 2–8 Hz in response to graded increases in bladder pressure (de Groat et al. 1982). The neurons identified in this study exhibited a tonic firing pattern of a higher firing rate (18.6 Hz), which agrees with our previous study in cats showing that interneurons in sacral spinal cord respond to bladder distention with a tonic firing at a rate of 10–20 Hz (deGroat et al. 1979). The effect of PNS or TNS to produce a parallel downward shift in the relationship between neuron activity and intravesical pressure (Figs. 1C and 3C) is consistent with the effect of PNS or TNS during cystometry to increase the volume threshold (i.e., bladder capacity) for evoking a micturition reflex without changing the amplitude of the reflex bladder contractions (Chen et al. 2010; Tai et al. 2012). This selective effect on bladder capacity is most reasonably explained by either a reduction in the afferent input to the switching circuit in the pontine micturition center (de Groat and Wickens 2013) or a direct effect on the switching circuit to shift the set point for triggering the micturition reflex (Mallory et al. 1991; Noto et al. 1991). The present experiments indicate that the former is at least partially responsible for the neuromodulatory actions of PNS and TNS to enhance bladder storage function.
The spinal neurons inhibited by PNS and TNS respond to low intravesical pressures (10–20 cmH2O) and therefore must be activated by non-nociceptive Aδ bladder afferent fibers which comprise the afferent limb of the spinobulbospinal micturition reflex pathway (de Groat et al. 1981). The bladder is also innervated by nociceptive C-fiber afferents which are activated by bladder irritation with noxious agents (Häbler et al. 1990) or following chronic spinal cord injury (Cheng et al. 1999; de Groat et al. 1990). After acute transection of the spinal cord at the thoracic level, which eliminates the spinobulbospinal micturition re-flex, stimulation of C-fiber bladder afferents by intravesical infusion of dilute acetic acid can elicit reflex bladder contractions via spinal circuitry (Xiao et al. 2014b). These reflex bladder contractions are suppressed by PNS but not by TNS (Rogers et al. 2015; Xiao et al. 2014b). Thus, it is likely that spinal interneurons receiving nociceptive afferent inputs from the bladder do not receive convergent inhibitory neuro-modulation from the pudendal and tibial nerves but rather only receive an inhibitory input activated by pudendal afferent pathways. However, TNS is effective in reducing bladder overactivity elicited by intravesical infusion of acetic acid in spinal cord intact cats and this effect is suppressed by application of naloxone to the brain stem indicating that it occurs at a supraspinal site by activation of opioid receptors (Ferroni et al. 2015).
When used clinically to treat overactive bladder symptoms, tibial neuromodulation is applied for 30 min once per week for an initial 12 weeks of treatment followed by once per month application (Peters et al. 2009), while pudendal neuromodulation is applied continuously (Peters et al. 2010). The different treatment regimens for pudendal and tibial neuromodulation indicate that tibial neuromodulation activates a long-lasting post-stimulation inhibition to suppress bladder activity, but pudendal neuromodulation does not. Our previous studies in cats also showed that prolonged TNS but not PNS produced long-lasting (at least 2 h) post-stimulation inhibition of bladder reflex activity (Larson et al. 2011; Tai et al. 2011b). Therefore, in this study TNS of 30-min duration was tested to determine if it could produce a long-lasting post-stimulation inhibition of spinal neuron activity. Although post-TNS inhibition was observable in every tested neuron (N = 8 neurons, Fig.4), the inhibition in most of these neurons (N = 5) only lasted for 10–18 min (Fig.4B) and the average activity of all tested neurons also fully recovered at this time (Fig.4C). This result indicates that the long-lasting (hours or weeks) post-TNS inhibition in clinical application of tibial neuromodulation might occur at a supraspinal site.
PNS at 5 Hz inhibited every neuron tested in this study (N = 13 neurons, Fig.1). However, PGS, which activates a population of cutaneous afferents passing through the pudendal nerve, inhibited 6 neurons (Fig.2) but excited 3 neurons (data not shown). Thus, it is likely that the cutaneous afferents have mixed excitatory and inhibitory effects on bladder-related neurons and that the excitatory effects are obscured when the entire pudendal nerve which also contains muscle afferents is stimulated at 5 Hz. These results agree with previous observations that pudendal afferent input can inhibit or excite the bladder depending on the frequency of afferent firing, i.e. 5 Hz inhibits the bladder, but 20 Hz excites the bladder (Tai et al. 2011a). PGS probably produced pudendal afferent firing over a wide range of frequencies for different afferent fibers. Therefore, it can inhibit some neurons but excite other neurons. It is known that PGS inhibits the bladder in spinal intact cats, but excites the bladder in chronic spinal cord injured cats (Tai et al. 2011a). Therefore, it is possible that the PGS inhibitory effect dominates the excitatory effect in spinal intact cats, explaining our observation that more neurons were inhibited than excited. In chronic spinal cord injured cats, we might observe more neurons excited than inhibited by PGS.
After naloxone treatment, PNS/TNS still inhibited every bladder related neuron tested in this study (Figs.6–7). This result agrees with our previous studies showing that after naloxone treatment PNS or TNS still inhibits the micturition reflex and increases bladder capacity during saline cystometry (Chen et al. 2010; Tai et al. 2012). However, our previous studies in cats also showed that naloxone applied intravenously or directly to the pontine area in the brain completely removes TNS inhibition of bladder overactivity induced by acetic acid irritation of the bladder (Ferroni et al. 2015; Tai et al. 2012), indicating a supraspinal site of action for one type of TNS inhibition. Whether TNS inhibits spinal neuron firing induced by acetic acid irritation of the bladder and whether naloxone treatment eliminates this inhibition still needs to be determined.
It is known that the micturition reflex is tonically inhibited by an opioid receptor mechanism and that naloxone treatment can reduce bladder capacity and facilitate the micturition reflex (Roppolo et al. 1983). However, in this study naloxone did not significantly change bladder-related neuron activity. This result suggests that the tonic opioid inhibition in the sensory limb of the spinobulbospinal micturition reflex pathway might occur at a supraspinal site instead of at a spinal site. Previous studies in cats (Hisamitsu and de Groat 1984; Noto et al. 1991) also indicated that the opioid receptor mechanism in the spinal cord controls the magnitude of bladder contractions mediated by the descending limb of the spinobulbospinal micturition reflex pathway, while the frequency of bladder contractions which reflects bladder capacity and is controlled by the sensory limb of the spinobulbospinal micturition reflex pathway is modulated by an opioid receptor mechanism in the brain stem.
In summary, this study revealed that both pudendal and tibial neuromodulation of bladder activity is associated with a reduction in the firing of the same population of neurons in the spinal cord, indicating either that neuromodulation by PNS or TNS directly targets these spinal neurons or that the modulation occurs upstream of these neurons to affect their excitatory input. This question could be addressed in future experiments by electrically stimulating bladder afferents in the pelvic nerve to determine if the interneurons exhibiting PNS and TNS inhibition are second-order neurons in the bladder sensory pathway (i.e. that they respond at short latency and thus must receive direct afferent input from the bladder). If this is true, then it would establish that PNS and TNS inhibition occur at the first synapse on the afferent limb of the micturition reflex pathway.
Acknowledgment
This study is supported by the National Institutes of Diabetes, Digestive and Kidney Diseases under grants DK-102427, DK-091253, and DK-111382.
Footnotes
Conflict of Interests
No conflicts of interests are declared by the authors.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A, 2002. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am. J. Obstet. Gynecol 187, 116–126. [DOI] [PubMed] [Google Scholar]
- Chen ML, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, Tai C, 2010. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp. Neurol 224, 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC, 1999. Effect of capsaicin on the micturition reflex in normal and chronic spinal cats. Am. J. Phys 277, R786–R794. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Tai C, 2017. Mechanisms of action of sacral nerve and peripheral nerve stimulation for disorders of bladder and bowel In: Krames ES, Peckham PH, Rezai AR (Eds.), Neuromodulation, Second Ed. Vol. 1 Academic Elsevier Press, New York, N.Y, pp. 221–236 (Chapter 19). [Google Scholar]
- de Groat WC, Wickens C, 2013. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol. 207, 66–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K, 1981. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J. Auto Nerv. Syst 3, 135–160. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Milne RJ, Roppolo JR, 1982. Parasympathetic preganglionic neurons in the sacral spinal cord. J. Auto Nerv. Syst 5, 23–43. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR, 1990. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auto Nerv Syst 30, S71–S78. [DOI] [PubMed] [Google Scholar]
- Ferroni MC, Slater RC, Shen B, Xiao Z, Wang J, Lee A, Roppolo JR, de Groat WC, Tai C, 2015. Role of the brain stem in tibial inhibition of the micturition reflex in cats. Am. J. Physiol. Renal. Physiol 309, F242–F250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC, 2008. The neural control of micturition. Nat. Rev. Neurosci 9, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller TW, Jiang X, Bansal U, Lamm V, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C, 2017. Sex difference in the contribution of GABAB receptors to tibial neuromodulation of bladder overactivity in cats. Am J Physiol Regul Integr Comp Physiol 312, R292–R300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Krier J, Milne RJ, Morgan C, Nadelhaft I (1979) Neural control of the urinary bladder and large intestine IN: Integrative Functions of the Autonomic Nervous System, Eds: Brooks CM, Koizumi K and Sato A, University of Tokyo Press, pp. 50–67. [Google Scholar]
- de Groat WC, Griffiths D, Yoshimura N, 2015. Neural control of the lower urinary tract In: Terjung RL (Ed.), Comprehensive Physiology, Handbook of Physiology John Wiley & Sons, Inc, New York, pp. 327–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson KJ, Moffitt MA, Wang X, Sun J, Snyder S, Grill WM, 2006. Topography of spinal neurons active during hindlimb withdrawal reflexes in the decerebrate cat. Neurosci 141, 1983–1994. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M, 1990. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J. Physiol 425, 545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamitsu T, de Groat WC, 1984. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res 298, 51–65. [DOI] [PubMed] [Google Scholar]
- Klop EM, Mouton LJ, Kuipers R, Holstege G, 2005. Neurons in the lateral sacral cord of the cat project to periaqueductal grey, but not to thalamus. Eur. J. Neurosci 21, 2159–2166. [DOI] [PubMed] [Google Scholar]
- Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C, 2011. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J. Physiol 589, 5833–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon TD, Ferroni MC, Kadow BT, Slater RC, Zhang Z, Chang V, Lamm V, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C, 2016. Pudendal but not tibial nerve stimulation inhibits bladder contractions induced by stimulation of pontine micturition center in cats. Am J Physiol Regul Integr Comp Physiol 310, R366–R374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory BS, Roppolo JR, de Groat WC, 1991. Pharmacological modulation of the pontine micturition center. Brain Res 546, 310–320. [DOI] [PubMed] [Google Scholar]
- Morgan C, Nadelhaft I, de Groat WC, 1981. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J. Comp. Neurol 201, 415–440. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, de Groat WC, Morgan C, 1980. Location and morphology of para-sympathetic preganglionic neurons in the sacral spinal cord of the cat revealed by retrograde axonal transport of horseradish peroxidase. J. Comp. Neurol 193, 265–281. [DOI] [PubMed] [Google Scholar]
- Noto H, Roppolo JR, de Groat WC, Nishizawa O, Sugaya K, Tsuchida S, 1991. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol. Int 47 (Suppl. 1), 19–22. [DOI] [PubMed] [Google Scholar]
- Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA, 2009. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J. Urol 182, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Peters KM, Killinger KA, Boguslawski BM, Boura JA, 2010. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol. Urodyn 29, 1267–1271. [DOI] [PubMed] [Google Scholar]
- Reese JN, Rogers MJ, Xiao Z, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C, 2015. Role of spinal metabotropic glutamate receptor 5 in pudendal inhibition of the nociceptive bladder reflex in cats. Am. J. Physiol. Renal Physiol 308, F832–F838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexed B, 1954. A cytoarchitectonic atlas of the spinal cord in the cat. J. Comp. Neurol 100, 297–379. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Xiao Z, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C, 2015. Propranolol, but not naloxone, enhances spinal reflex bladder activity and reduces pudendal inhibition in cats. Am. J. Physiol. Regul. Integr. Comp. Physiol 308, R42–R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo JR, Booth AM, de Groat WC, 1983. The effects of naloxone on the neural control of the urinary bladder of the cat. Brain Res 264, 355–358. [DOI] [PubMed] [Google Scholar]
- Tai C, Booth AM, de Groat WC, Roppolo JR, 2001. Colon and anal sphincter contractions evoked by microstimulation of the sacral spinal cord in cats. Brain Res 889, 38–48. [DOI] [PubMed] [Google Scholar]
- Tai C, Chen M, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, 2011a. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Exp. Neurol 228, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC, 2011b. Prolonged poststimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am. J. Physiol. Renal Physiol 300, F385–F392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, 2012. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am. J. Physiol. Renal Physiol 302, F1090–F1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Morgan C, Nadelhaft I, Houston M, De Groat WC, 1989. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J. Comp. Neurol 288, 263–279. [DOI] [PubMed] [Google Scholar]
- Uy J, Yu M, Jiang X, Jones C, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C, 2017. Glutamatergic mechanisms involved in bladder overactivity and pudendal neuromodulation in cats. J. Pharmacol. Exp. Ther 362, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Reese J, Schwen Z, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C, 2014a. Role of spinal GABAA receptors in pudendal inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am. J. Physiol. Renal Physiol 306, F781–F789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Rogers MJ, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C, 2014b. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. Am J Physiol Renal Physiol 307, F673–F6769. [DOI] [PMC free article] [PubMed] [Google Scholar]