Abstract
Dispersin B (DspB) from Aggregatibacter actinomycetemcomitans is a β-hexosaminidase exhibiting biofilm detachment activity. A series of β-(1→6)-linked N-acetyl-D-glucosamine thiophenyl glycosides with degree of polymerisation (DP) of 2, 3, 4 and 5 were synthesized, and substrate specificity of DspB was studied on the obtained oligosaccharides. For oligomer synthesis a 1+2, 2+2, 1+4 coupling strategy was applied, using bromo-sugars as glycosyl donors. The formation of 1,2-trans interglycosidic bond has been ensured by 2-phtalimido protecting group; chloroacetyl group was installed to mask temporarily the 6-hydroxyl and acetate esters were applied as permanent protecting groups. Enzymatic studies revealed that DP of the GlcNAc oligomers strongly affected the hydrolysis rate, and the hydrolytic activity of DspB on the tetramer and pentamer have been found to be approximately 10-fold higher than that of the dimer. This fact indicates that four units are required for a strong binding at the active centre of DspB. The role of aromatic amino acids W237, Y187 and Y278 in substrate specificity and catalysis was also examined using mutant enzymes.
Keywords: Biofilm, Dispersin B, β-(1→6)-Oligoglucosamine, Synthesis, Mutation, Substrate specificity
1. Introduction
Glucosamines are common components of many biologically important oligosaccharides and frequently encountered in glycoproteins and glycolipides.1 Isolation of glucosamine oligosaccharides from natural sources is a difficult task, therefore, the chemical synthesis would be important to provide substrates for biochemical studies. Oligo-β-(1→6)-glucosamine derivatives attract growing interest being fragments of the bacterial surface polysaccharides. However, only a few papers dealing with the preparation of β-(1→6)-linked glucosamine oligosaccharides have been published to date.2–8 Yang and coworkers reported the first synthesis of (1→6)-β-D-glucosamine hexa- and nonasaccharides2 using isopropyl thioglycosides as suitable donors in all coupling steps. Efficient one-pot syntheses of tri- and tetraglucosamines were carried out by Fridman et al. exploiting the different reactivity of N-trichloroethoxycarbonyl- and N-phthaloyl-protected glucosamine donors.3 Melean et al. applied glucosamine trichloroacetimidates and phosphates for the construction of β-(1→6)-oligoglucosamines in solution and solid-phase.4 Gening et al. synthesized a series of 3-β-acetamidopropyl oligo-β-(1→6)- glucosamines consisting of 5, 7, 9 and 11 monomers, using a convergent blockwise approach.5 Homologous cyclic oligo-(1→6)-β-D-glucosamines consisting of two to seven residues have been obtained by intramolecular glycosylation of the linear oligosaccharides. 6 Unprotected N-acetyl-D-glucosamine (GlcNAc) could be also utilized for regio- and stereoselective synthesis of β-(1→6)- linked oligomers by means of an acid reversion reaction in hydrogen fluoride.7,8
These synthetic oligosaccharides represent fragments of the bacterial surface polysaccharide produced by numerous bacterial pathogens such as Staphylococci. The most notable staphylococci, Staphylococcus aureus and Staphylococcus epidermidis bring about infections related to colonization of medical implants and formation of biofilms. Biofilms formed on different medical devices are one of the most serious problems of present-day clinical practice.9 According to the National Institute of Health (US) 80% of all infections are biofilm related.10 The treatment of biomaterial-related infections has not been fully developed, but enzymatic approaches are promising for both erasing a developed biofilm and prevention its formation.11,12 A major component of the biofilm matrix is a poly-β-D-(1→6)-N-acetyl-glucosamine (PNAG) that mediates intercellular adhesion.13,14
Kaplan et al. have reported15 that Dispersin B (DspB, EC 3.2.1.52) a soluble β-N-acetylglucosaminidase originated from periodontal pathogen Aggregatibacter actinomycetemcomitans16 was able to disperse and detach biofilms produced by S. epidermidis. On the basis of biofilm-releasing activity of DspB the authors suggested that this enzyme could be used as an antibiofilm agent to remove S. epidermidis biofilms from medical devices. Combination of Dispersin B and antiseptic triclosan showed synergistic broad-spectrum antibiofilm and antimicrobial activity against S. aureus, S epidermidis and Escherichia coli.17
DspB belongs to family 20 glycoside hydrolases. In general, these hydrolases exhibit broad substrate specificity, but DspB, according to our earlier ligand docking and hydrolysis experiments, appears to be specific to a linear polymer of β-(1→6)-linked N-acetyl-D-glucosamine (GlcNAc) and removes the terminal GlcNAc moiety from the non-reducing end.18 For these studies β-(1→6)-linked GlcNAc oligomers with DP 2, 4 and 6 were synthesized as p-methoxyphenyl glycosides. The results suggest that β-(1→6)-linked GlcNAc oligomer bound to the active site better than β-(1→4)-linked GlcNAc oligosaccharide and DspB is an exo-acting enzyme. Unfortunately, the beta-linked p-methoxyphenyl aglycone of the hydrolysis products can be further hydrolysed by DspB disturbing the precise HPLC measurements. In addition, due to the applied blockwise approach only the even numbered oligomers were available.
Here we present the synthesis of a novel thiophenyl glycoside series of β-(1→6)-linked oligosaccharides and its application to further delineate the substrate specificity of DspB. Since the thio-glycosidic bond is stable to enzymatic hydrolysis the reducing end products bear always a chromophore group allowing the detection of the substrate and the reducing end hydrolysis products with UV detection after HPLC separation.
The importance of aromatic amino acids at the active centre of DspB has been studied by Manuel and coworkers using mutant enzymes. 19 Mutation of W237 completely abolished the hydrolytic activity on 4-nitrophenyl N-acetyl-β-D-glucosaminide (PNP-β-Glc-NAc) substrate but showed a low biofilm detachment activity. Mutation of Y187 and Y278 amino acids decreased the hydrolytic activity measured on both PNP-β-Glc-NAc and PNAG substrate. Activity of mutant enzymes on the new oligomer substrate series was also studied.
2. Results and discussion
2.1. Synthesis of substrate series
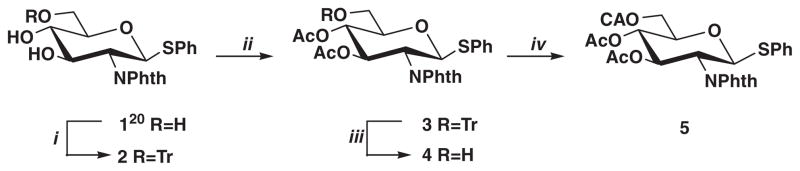
Fully protected 2-phthalimido-containing thioglucosides (5, 6 and 10) equipped with monochloroacetate ester as temporary protecting group and acetate esters as permanent protecting groups were utilized for the synthesis of the desired β-(1→6)-linked Glc-NAc oligosaccharides. These key-intermediates were transformed either to a donor by bromination or to an acceptor by selective liberation of the terminal primary hydroxyl group. The 6-O-chloroacetate proved to be stable to both bromination and the glycosylation reaction but could be readily removed with thiourea in an assisted cleavage.
The known phenyl 2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (1)20 was resorted as starting compound. Tritylation of 1 gave compound 2, whose acetylation followed by removal of trityl group by acidic hydrolysis (2→3→4) afforded acceptor 4 in an overall 65% yield. Probably, acyl migration from O-4 to O-6 under the used acidic conditions for detritylation could cause this moderate yield.21 Then chloroacetyl group, as a temporary protecting group, was introduced with chloroacetyl chloride in dichloromethane in the presence of pyridine at −10 °C to give derivative 5 in good yield (75%) (Scheme 1).
Scheme 1.
Reagents and conditions: (i) triphenylmethyl chloride, DMAP, Py, rt, 24 h; (ii) Ac2O, Py, rt, 24 h; (iii) 80% aq acetic acid, 50 °C, 3 h, 65% for 4 for three steps; (iv) chloroacetyl chloride, Py, DCM, −20 °C→rt, 75%.
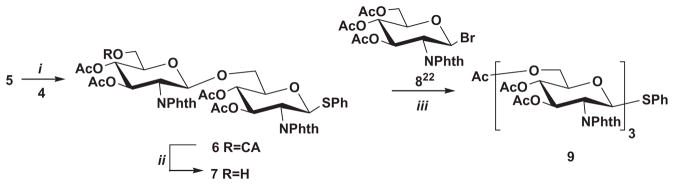
After bromination of 5 with bromine at room temperature the obtained bromo sugar without purification was coupled with acceptor 4 using AgOTf promotion to give the disaccharide 6 in 77% yield. Treatment of 6 with thiourea gave disaccharide 7 which was used as an acceptor for preparation of trisaccharide 9 by its coupling with 3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl bromide (8)22 (Scheme 2).
Scheme 2.
Reagents and conditions: (i) (a) Br2, DCM, rt, 1 h; (b) AgOTf in toluene; collidine, DCM, −74 °C→rt, 24 h, 77%; (ii) thiourea, DCM–MeOH, 50 °C, 24 h, 76%; (iii) AgOTf in toluene; collidine, DCM, −74 °C→rt, 24 h, 53%.
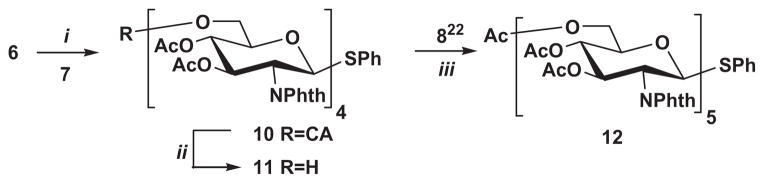
Disaccharide 6 was also converted to glycosyl bromide whose coupling with disaccharide acceptor 7 afforded tetrasaccharide 10 in 75% yield.
Since the yield of the formation of the tetrasaccharide 10 was higher than in the case of trisaccharide 9, we planned to use 1+4 block synthesis for the preparation of pentasaccharide 12 instead of 2+3. Thus, synthesis of pentasaccharide 12 was achieved starting from compound 10 in similar manner (10→11→12) as the preparation of trisaccharide 9 (Scheme 3).
Scheme 3.
Reagents and conditions: (i) (a) Br2, DCM, rt, 1 h; (b) AgOTf in toluene; collidine, DCM, −74 °C→rt, 24 h, 72%; (ii) thiourea, DCM–MeOH, 50 °C, 24 h, 76%; (iii) AgOTf in toluene; collidine, DCM, −74 °C→rt, 24 h, 72%.
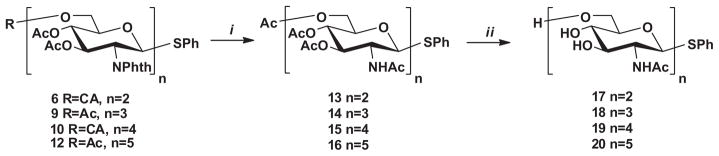
Deprotection of oligosaccharides 6, 9, 10 and 12 by ethylenediamine in ethanol, followed by acetylation afforded 13, 14, 15 and 16 in overall 83%, 81%, 73%, and 71%, yields, respectively (Scheme 4). We performed peracetylation after the removal of the phthaloyl groups since dephthaloylation resulted in a rather complex reaction mixture and the isolation of the fully acetylated derivatives was easier from it by silica gel column chromatography than the selectively N-acetylated derivatives.
Scheme 4.
Reagents and conditions: (i) (a) ethylenediamine, EtOH, 70 °C, 24 h; (b) Ac2O, Py, rt, 24 h, 83% for 13 for, 81% for 14, 73% for 15, 71% for 16; (ii) NaOMe, MeOH, 86% for 17, 86% for 18, 88% for 19, 85% for 20.
Finally, de-O-acetylation of 13–16 by Zemplén transesterification gave the intended β-(1→6)-linked GlcNAc oligosaccharides 17, 18, 19 and 20 (Scheme 4) which were tested as substrates for DspB as follows.
2.2. Hydrolysis of thioglycosides catalysed by Dispersin B
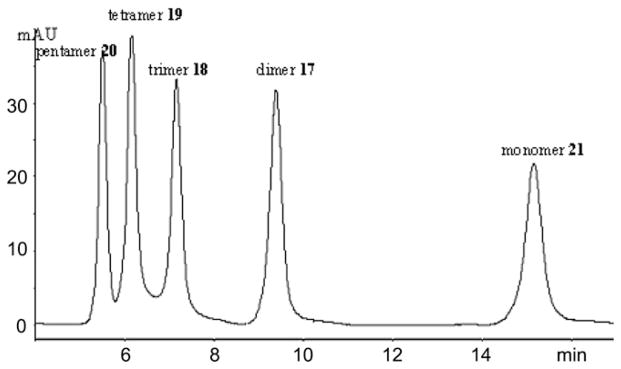
Substrate specificity of DspB was studied separately on each thiophenyl glycoside 17– 20. Product analysis was carried out by HPLC resulted in base-line separation of 17–20 and phenyl 2-deoxy-2-acetamido-1-thio-β-D-glucopyranoside (21)23 as shown Fig. 4 in Section 3.
Figure 4.
HPLC separation of 1 mM standard mixture of GlcNAc oligomers 17–20 and monomer 21.
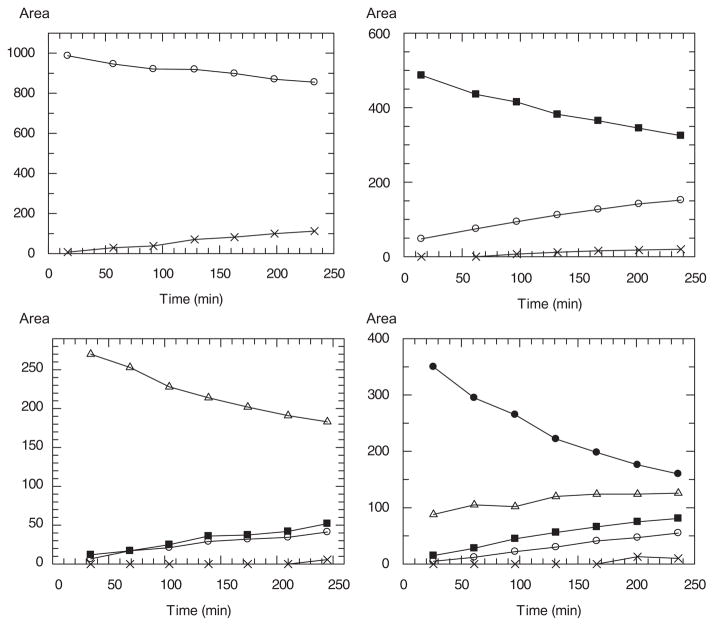
Figure 1 shows the change of product distribution of thiophenyl glycoside substrates during 4 h incubation with DspB. These diagrams demonstrate that hydrolysis occurs step-by-step from the non-reducing end of the substrates leading to the formation of shorter chromophore-containing reducing end products. Dimer substrate gives monomer product, dimer and monomer formed from trimer substrate, while tetramer hydrolysis results in trimer, dimer and monomer thioglycoside products. Product distribution of pentamer exhibits the most interesting profile; while the amount of the tetrasaccharide 19, after a short increasing, is practically constant, the quantity of trimer 18 and dimer 17 increase linearly and the monomer appears after a longer incubation time.
Figure 1.
HPLC product distribution of DspB catalysed hydrolysis of thiophenyl glycosides. Measured area for: x monomer 21, ○ dimer 17, ■ trimer 18, △ tetramer 19, ● pentamer 20.
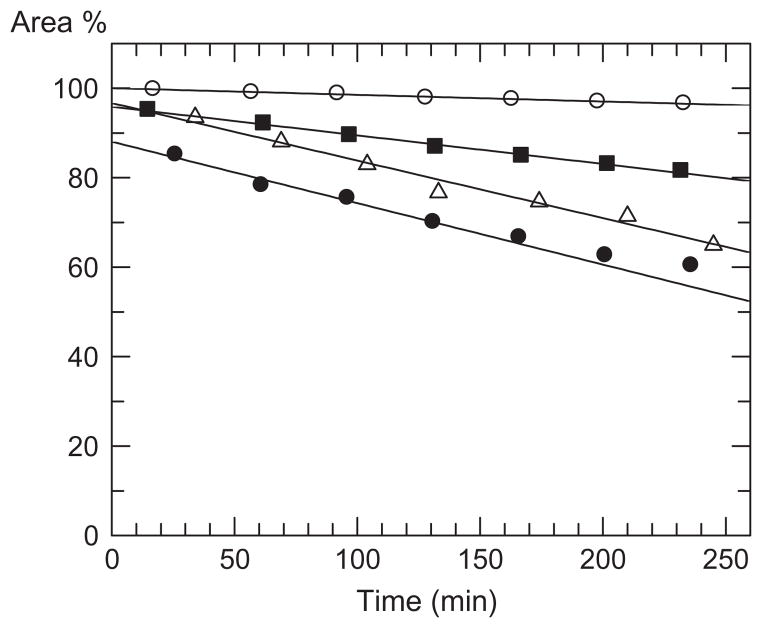
Effect of the DP on hydrolysis rate shows that β-(1→6)-linked GlcNAc thioglycosides longer than the dimer 17 are good substrates and linear decrease in peak area% of substrate versus time can be observed using HPLC product distribution measurements for the oligomers (Fig. 2). After 4 h incubation time the conversion of dimer 17 was very low (3.6%), while hydrolysis of trimer 18, tetramer 19 and pentamer 20 resulted in higher conversion 19.3%, 33.2%, and 39.6%, respectively. However, it is interesting that the reaction rates (slopes of the curves) for the hydrolysis of tetramer 19 and pentamer 20 were similar. Reaction rate increased with degree of polymerisation until tetramer, but the fifth GlcNAc unit in case of pentamer did not result in further increase of velocity. These results indicate that effective binding requires at least four glucosamine residues in substrate molecule. The points of pentamer hydrolysis deviate from the straight line above 30% conversion due to the decrease of substrate concentration.
Figure 2.
Time course of hydrolysis reaction on thiophenyl glycosides 17, 18, 19 and 20 catalysed by DspB. [S] = 0.4 mM; [E] = 2 μM; pH 5.9; T = 25 °C; ○ 17, ■ 18, △ 19, ● 20.
2.3. Effect of mutation of aromatic amino acids at the active centre of DspB
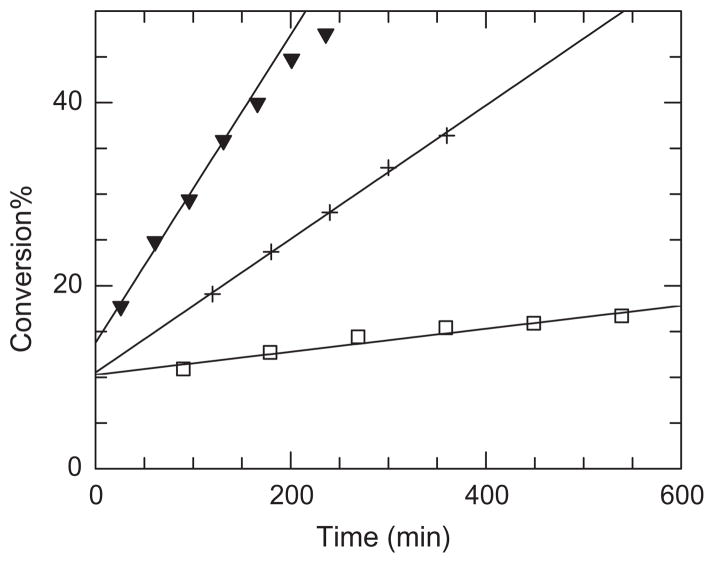
Hydrolytic activity of Y187A, Y278A and W237A mutants was also studied on thiophenyl glycosides 17–20 as substrates and compared to hydrolytic activity of wild type enzyme. To compare the reaction rates of enzymes on pentasaccharide substrate 20 the conversions were plotted versus reaction time (Fig. 3).
Figure 3.
Conversion of pentamer substrate 20 hydrolysis catalysed by: ▼ DspB, + Y187A and □ Y278A mutants. Data of fitted curves: DspB m = 0.17 b = 13.76 r = 0.997; Y187A m = 0.073 b = 10.5 r = 0.999; Y278A m = 0.013 b = 10.3 r = 0.973.
We have found that wild type enzyme is a much more active on pentamer substrate 20 than Y187A mutant and Y278A mutant shows a low activity. W237A mutant was completely inactive on pentamer substrate, therefore, it was not represented in the graph. Our results confirm the conclusion of Manuel and coworkers,19 who find the mutant to be inactive on monomer and polymer substrate, and suggested that W237 is an important participant of hydrophobic substrate binding pocket of DspB.
The conversions determined on substrates with different DP are summarised in Table 1.
Table 1.
Conversion% after 6 h incubation of substrates in hydrolysis reaction catalysed by DspB and mutants
| Substrate | Enzyme
|
||
|---|---|---|---|
| Wild type | Y187A | Y278A | |
| Dimer 17 | 4.3 | 2.8 | 0 |
| Trimer 18 | 23.1 | 14.4 | 2.6 |
| Tetramer 19 | 39.8 | 15.4 | 4.36 |
| Pentamer 20 | 49.8 | 18.0 | 7.7 |
Results show that Y187A mutation decreased hydrolytic activity and changed substrate specificity. Highest conversion was measured on pentamer substrate, hydrolysis rate of tetramer was lower (~80% of 20), followed by trimer (46% of 20) and dimer showed the lowest conversion (<10% of 20) in case of wild type enzyme. In contrast, the conversions were similar on trimer and dimer in Y187A catalysed reaction, 85% and 80% of 20, respectively. Our results verify the presence of multiple subsites, since mutation of Tyr187 (an amino acid farther away form the cleavage site) influenced rather the hydrolysis of tetramer and pentamer than that of trimer or dimer.
In conclusion, our measurements confirm the applicability of new synthetic compounds 17–20 for investigation of Dispersin B and its mutants. Substrate series are suitable for activity measurement, studying the effect of mutation and exploring the active site composition/structure. These experiments will be extended to study new mutants for detailed understanding of enzyme function.
3. Experimental
3.1. Chemistry
Optical rotations were measured at room temperature with a Perkin–Elmer 241 automatic polarimeter in CHCl3. TLC was performed on Kieselgel 60 F254 (Merck) with detection by charring with 50% aqueous sulfuric acid. Column chromatography was performed on Silica Gel 60 (Merck 63–200 mesh). The 1H (360 and 400 MHz) and 13C NMR (90.54 and 128 MHz) spectra were recorded with Bruker AM-360 and Bruker DRX-400 spectrometers. Internal references: TMS (0.000 ppm for 1H), CDCl3 (77.00 ppm for 13C for organic solutions). The 1H and 13C NMR assignments have been established from 1D NMR spectra and the proton-signal assignments were supported by analysis of two-dimensional 1H–1H correlation spectra (COSY), as well as the carbon-signal assignments by two-dimensional 13C-1H correlation maps (HET-COR). MALDI TOF-MS analyses of compounds were carried out in the positive reflectron mode using a BIFLEX III mass spectrometer. Elemental analyses were performed at the analytical laboratories in Debrecen.
3.1.1. Typical procedure A for glycosylation reaction I
To a solution of starting material (2.66 mmol) in dry CH2Cl2 (10 mL) bromine (150 μL, 3.07 mmol) was added at room temperature. After stirring for 1 h the reaction mixture was concentrated and then was co-evaporated with toluene. To a solution of crude bromo sugar (2.66 mmol) and acceptor (1.77 mmol) in dry CH2Cl2 (10 mL) were added collidine (500 μL, 2.79 mmol) and 4 Ǻ molecular sieves and cooled to −74 °C. After stirring for 30 min at −74 °C, silver triflate (927 mg, 3.48 mmol) dissolved in toluene was added. Then the reaction mixture was left to attain room temperature over night. The mixture was diluted with CH2Cl2 and filtered through Celite. The filtrate was washed with 10% aq Na2S2O3 and water, dried and concentrated. The crude product was purified by silica column chromatography.
3.1.2. Typical procedure B for dechloroacetylation
To a solution of starting material (1.03 mmol) in dry CH2Cl2 (10 mL) pyridine (100 μL, 1.24 mmol) and chloroacetyl chloride (99 μL, 1.24 mmol) were added at −10 °C. After stirring for 30 min the reaction was quenched with methanol (0.5 mL) diluted with CH2Cl2, extracted with aq 1 M HCl and saturated NaHCO3 solution, dried and concentrated. The crude product was purified by silica column chromatography.
3.1.3. Typical procedure C for glycosylation reaction II
To a solution of 3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl bromide (8)22 (174 mg, 0.35 mmol) and acceptor (0.23 mmol) in dry CH2Cl2 (5 mL) collidine (66 μL, 0.35 mmol) and 4 Å molecular sieves were added and cooled to −74 °C. After stirring for 30 min at −74 °C, silver triflate (120 mg, 0.46 mmol) dissolved in toluene was added. Then the reaction mixture was left to attain room temperature over night. The mixture was diluted with CH2Cl2 and filtered through Celite. The filtrate was washed with 10% aq Na2S2O3 and water, dried and concentrated. The crude product was purified by silica column chromatography.
3.1.4. Typical procedure D for dephthaloylation and peracetylation of the obtained amine
To a solution of starting material (0.18 mmol) in ethanol (15 mL) was added ethylene diamine (5 equiv per phthaloyl group of starting material). After stirring for 1 day at reflux temperature the mixture was concentrated. The residue was dissolved in pyridine (10 mL) and acetic anhydride (5 mL) was added. After stirring for 1 day at room temperature the mixture was concentrated and then was co-evaporated with toluene. The residue was diluted with CH2Cl2, washed with water, dried and concentrated. The crude product was purified by silica column chromatography.
3.1.5. Typical procedure E for de-O-acetylation
To a solution of starting material (0.13 mmol) in methanol (15 mL) catalytic amount of NaOMe (pH ~8) was added. After stirring for 1 day at room temperature the mixture was diluted with water, neutralized with Amberlite IR-120 H+ ion-exchange resin, filtered and concentrated. The residue was lyophilized.
3.1.6. Phenyl 3,4-di-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (4)
To a solution of phenyl 2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside 120 (3 g, 7.48 mmol) in pyridine (50 mL) triphenylmethyl chloride (5.22 g, 18.76 mmol) and 4-dimethylaminopyridine (62 mg) were added. After stirring for 1 day at room temperature the mixture was concentrated and then was co-evaporated with toluene. To a solution of crude product 2 (7.48 mmol) in pyridine (30 mL) was added Ac2O (15 mL). After stirring for 1 day at room temperature the mixture was concentrated. The residue was diluted with CH2Cl2, extracted with aq 1 M HCl and saturated NaHCO3 solution, dried and concentrated. Compound 3 thus obtained was used in the next step without further purification. A solution of compound 3 (7.48 mmol) in 80% aq acetic acid (100 mL) was stirred at 50 °C for 3 h. Then the mixture was concentrated and then was co-evaporated with toluene. The crude product was purified by silica column chromatography (CH2Cl2–acetone 95:5) to yield 4 (2.4 g, 65%) as a colourless syrup. [α]D +65.8 (c 0.12 in CHCl3); 1H NMR (CDCl3): δ 7.92–7.73 (m, 4H, Ar), 7.45–7.24 (m, 5H, Ar), 5.85 (dd, 1H, J3,4 9.5 Hz, H-3), 5.77 (d, 1H, J1,2 10.5 Hz, H-1), 5.11 (t, 1H, J4,5 9.5 Hz, H-4), 4.36 (t, 1H, J2,3 10.5 Hz, H-2), 3.84–3.61 (m, 3H, H-5, H-6, H-6′), 2.41 (s, 1H, OH), 2.05 and 1.87 (2s, 6H, 2COCH3); 13C NMR (CDCl3): δ 170.06 (2COCH3), 82.90 (C-1), 78.22, 71.41 and 68.94 (C-3, C-4, C-5), 61.49 (C-6), 53.64 (C-2), 20.59 and 20.35 (2COCH3). Anal. Calcd for C24H23NO8S: C, 59.37; H, 4.77. Found: C, 59.42; H, 4.69.
3.1.7. Phenyl 3,4-di-O-acetyl-6-O-chloroacetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (5)
To a solution of compound 4 (500 mg, 1.03 mmol) in dry CH2Cl2 (10 mL) pyridine (100 μL, 1.24 mmol) and chloroacetyl chloride (99 μL, 1.24 mmol) were added at −10 °C. After stirring for 30 min the reaction was quenched with methanol (0.5 mL) diluted with CH2Cl2, extracted with aq 1 M HCl and saturated NaHCO3 solution, dried and concentrated. The crude product was purified by silica column chromatography (hexane–acetone 3:2) to yield 5 (435 mg, 75%) as a colourless syrup. [α]D +52.23 (c 0.43 in CHCl3); 1H NMR (CDCl3): δ 7.91–7.71 (m, 4H, Ar), 7.43–7.25 (m, 5H, Ar), 5.81 (dd, 1H, J3,4 9.5 Hz, H-3), 5.75 (d, 1H, J1,2 10.5 Hz, H-1), 5.13 (t, 1H, J4,5 9.5 Hz, H-4), 4.42–4.30 (m, 3H, H-2, H-6, H-6′), 4.10 (s, 2H, COCH2Cl), 3.97–3.90 (m, 1H, H-5), 2.03 and 1.85 (2s, 6H, 2COCH3); 13C NMR (CDCl3): δ 169.95, 169.41 and 166.93 (CO), 82.95 (C-1), 75.54, 71.38 and 68.47 (C-3, C-4, C-5), 63.59 (C-6), 53.42 (C-2), 40.58 (COCH2Cl), 20.50 and 20.28 (2COCH3). Anal. Calcd for C26H24ClNO9S: C, 55.57; H, 4.30. Found: C, 55.44; H, 4.42.
3.1.8. Phenyl 3,4-di-O-acetyl-6-O-chloroacetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (6)
Compound 5 (1.5 g, 2.66 mmol) was reacted with bromine and then the obtained bromo sugar was coupled with acceptor 4 according to typical procedure A. The crude product was purified by silica column chromatography (hexane–acetone 1:1) to yield 6 (1.28 g, 77%) as a colourless syrup. [α]D +46.76 (c 0.20 in CHCl3); 1H NMR (CDCl3): δ 7.86–7.61 (m, 8H, Ar), 7.32–7.23 (m, 5H, Ar), 5.75 (dd, 1H, J2′,3′ 10.5 Hz, H-3′), 5.70 (dd, 1H, J3,4 9.5 Hz, H-3), 5.58 (d, 1H, J1,2 10.5 Hz, H-1), 5.54 (d, 1H, J1′,2′ 8.5 Hz, H-1′), 5.15 (t, 1H, J4′,5′ 9.5 Hz, H-4′), 4.86 (t, 1H, J4,5 9.5 Hz, H-4), 4.41–4.28 (m, 3H, H-2′, H-6a′, H-6b′), 4.20 (t, 1H, J2,3 10.5 Hz, H-2), 4.20 (s, 2H, COCH2Cl), 3.92–3.80 (m, 3H, H-5, H-5′, H-6a), 3.69 (dd, 1H, J5,6b 6.5 Hz, J6a,6b 10.5 Hz, H-6b), 2.06, 1.94, 1.87 and 1.77 (4s, 12H, 4COCH3); 13C NMR (CDCl3): δ 170.01, 169.94, 169.45, 169.39, 167.63, 167.14 and 166.75 (CO), 98.24 (C-1′), 82.23 (C-1), 76.84 (C-5), 71.66 (C-5′), 71.34 (C-3), 70.59 (C-3′), 69.25 (C-4), 68.58 (C-4′), 69.13 (C-6), 63.32 (C-6′), 54.27 (C-2′), 53.32 (C-2), 40.70 (COCH2Cl), 20.57, 20.49, 20.36 and 20.29 (4COCH3). Anal. Calcd for C44H41ClN2O17S: C, 56.38; H, 4.41. Found: C, 56.47; H, 4.50.
3.1.9. Phenyl 3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (7)
Compound 6 (0.9 g, 0.96 mmol) was treated with thiourea according to typical procedure B. The crude product was purified by silica column chromatography (CH2Cl2–acetone 95:5) to yield 7 (625 mg, 76%) as a colourless syrup. [α]D +42.0 (c 0.37 in CHCl3); 1H NMR (CDCl3): δ 7.93–7.56 (m, 8H, Ar), 7.44–7.16 (m, 5H, Ar), 5.78 (t, 1H, J3′,4′ 10 Hz, H-3′), 5.68 (t, 1H, J3,4 10 Hz, H-3), 5.58 (d, 1H, J1,2 10 Hz, H-1), 5.53 (d, 1H, J1′,2′ 8.5 Hz, H-1′), 5.12 (t, 1H, J4′,5′ 10 Hz, H-4′), 4.94 (t, 1H, J4,5 10 Hz, H-4), 4,30 (dd, 1H, J2,3 9 Hz, H-2′), 4.19 (t, 1H, J 10 Hz, H-2), 3.95 (dd, 1H, J6a,6b 10 Hz, J5,6a 3 Hz H-6a), 3.86–3.76 (m, 2H, H-5, H-6a′), 3.86–3.76 (m, 3H, H-5′, H-6b, H-6b′), 2.78 (br s, 1H, OH), 2.07, 1.97, 1.87 and 1.78 (4s, 12H, 4COCH3); 13C NMR (CDCl3): δ 170.14, 169.91, 167.70 and 166.81 (CO), 97.83 (C-1′), 82.38 (C-1), 76.52 (H-5), 74.16 (H-5′), 71.39 (C-3), 70.68 (C-3′), 69.80 (C-4), 69.17 (C-4′), 69.01 (C-6), 61.21 (C-6′), 54.39 (C-2′), 53.39 (C-2), 20.70 and 20.36 (4COCH3). Anal. Calcd for C42H40N2O16S: C, 58.60; H, 4.68. Found: C, 58.71; H, 4.76.
3.1.10. Phenyl 3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (9)
Bromo sugar 822 (174 mg, 0.35 mmol) was coupled with acceptor 7 according to typical procedure C. The crude product was purified by silica column chromatography (CH2Cl2–acetone 95:5) to yield 9 (155 mg, 53%) as a colourless syrup. [α]D +40.07 (c 0.28 in CHCl3); 1H NMR (CDCl3): δ 7.95–7.55 (m, 12H, Ar), 7.32–7.19 (m, 5H, Ar), 5.79 (dd, 1H, J2″,3″ 10.5 Hz, H-3″), 5.63 (t, 1H, J3,4 10 Hz, H-3), 5.59 (t, 1H, J2′,3′ 10.5 Hz, H-3′), 5.56 (d, 1H, J1″,2″ 10 Hz, H-1″), 5.51 (d, 1H, J1,2 10 Hz, H-1), 5.33 (d, 1H, J1′,2′ 8.5 Hz, H-1′), 5.19 (t, 1H, J3″,4″ 9.5 Hz, H-4″), 4.92 (t, 1H, J3′,4′ 9.5 Hz, H-4′), 4.80 (t, 1H, J4,5 9.5 Hz, H-4), 4.41–4.32 (m, 2H, H-6a″, H-2″), 4.25–4.17 (m, 2H, H-2′, H-6b″), 4.14 (t, 1H, J2,3 10 Hz, H-2), 3.97–3.84 (m, 3H, H-5″, H-6a, H-6a′), 3.77–3.55 (m, 3H, H-6b′, H-5′, H-5), 3.45 (dd, 1H, J5,6b 6 Hz, J6a,6b 10 Hz, H-6b), 2.14, 2.04, 1.95, 1.92, 1.86, 1.79 and 1.75 (7s, 21H, 7COCH3); 13C NMR (CDCl3): δ 170.68, 170.02, 169.98, 169.44, 169.31, 167.65 and 166.75 (CO), 97.81 (C-1′), 97.78 (C-1″), 82.29 (C-1), 77.42 (C-5), 73.21 (C-5′), 71.87 (C-5″), 71.45 (C-3), 70.64 (C-3″, C-3′), 69.42 (C-4′), 69.01 (C-4), 68.78 (C-4″), 68.52 (C-6), 67.63 (C-6′), 61.83 (C-6″), 54.37 (C-2″), 54.26 (C-2′), 53.28 (C-2), 20.74, 20.58, 20.44, 20.37 and 20.31 (7COCH3). MALDI TOF-MS calcd for C62H59N3O25S: 1277.31 [M]. Found: 1300.15 [M+Na]+.
3.1.11. Phenyl 3,4-di-O-acetyl-6-O-chloroacetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (10)
Compound 6 (340 mg, 0.36 mmol) was reacted with bromine and then the obtained bromo sugar was coupled with acceptor 7 according to typical procedure A. The crude product was purified by silica column chromatography (hexane–acetone 1:1) to yield 10 (280 mg, 72%) as a colourless syrup. [α]D +44.04 (c 0.13 in CHCl3); 1H NMR (CDCl3): δ 7.95–7.54 (m, 16H, Ar), 7.30–7.19 (m, 5H, Ar), 5.80 (dd, 1H, J2‴,3‴ 10.5 Hz, H-3‴), 5.67 (dd, 1H, J2′,3′ 10.5 Hz, H-3′), 5.63 (t, 1H, J3,4 10 Hz, H-3), 5.57 (dd, 1H, J2″,3″ 10.5 Hz, H-3″), 5.51 (d, 1H, J1‴,2‴ 8.5 Hz, H-1‴), 5.50 (d, 1H, J1,2 10 Hz, H-1), 5.34 (d, 1H, J1′,2′ 8.5 Hz, H-1′), 5.29 (d, 1H, J1″,2″ 8.5 Hz, H-1″), 5.18 (t, 1H, J3‴,4‴ 9.5 Hz, H-4‴), 4.92 (t, 1H, J3′,4′ 9.5 Hz, H-4′), 4.81 (dd, 1H, J3″,4″ 8 Hz, H-4″), 4.79 (t, 1H, J4,5 10 Hz, H-4), 4.45 (dd, 1H, J6a‴,6b‴ 12 Hz, J5‴,6a‴ 5 Hz, H-6a‴), 4.39–4.33 (m, 2H, H-2‴, H-6b‴), 4.26–4.16 (m, 2H, H-2′, H-2″), 4.22 (s, 2H, COCH2Cl), 4.13 (t, 1H, J2,3 10 Hz, H-2), 3.99–3.65 (m, 6H, H-5‴, H-6a′, H-6a, H-5′, H-6a″, H-6b′), 3.62–3.56 (m, 1H, H-5), 3.52–3.39 (m, 3H, H-5″, H-6b″, H-6b), 2.05, 1.95, 1.93, 1.86, 1.79, 1.75 and 1.60 (8s, 24H, 8COCH3); 13C NMR (CDCl3): δ 169.82, 169.39, 169.22, 167.52, 167.04 and 166.62 (CO), 97.80 (C-1‴), 97.69 (C-1″), 97.37 (C-1′), 82.23 (C-1), 76.75 (C-5), 72.73 (C-5′, C-5″), 71.60 (C-5‴), 71.35 (C-3), 70.47 (C-3‴), 70.39 (C-3′, C-3″), 69.33 (C-4′, C-4″), 68.78 (C-4), 68.60 (C-4‴), 68.22 (C-6), 67.98 (C-6′), 67.39 (C-6″), 63.29 (C-6‴), 54.26 (C-2‴), 54.15 (C-2′), 54.11 (C-2″), 53.17 (C-2), 40.62 (COCH2Cl), 20.46, 20.34 and 20.21 (8COCH3). MALDI TOF-MS calcd for C80H75ClN4O33S: 1686.37 [M]. Found: 1709.11 [M+Na]+.
3.1.12. Phenyl 3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (11)
Compound 10 (240 mg, 0.14 mmol) was treated with thiourea according to typical procedure B. The crude product was purified by silica column chromatography (CH2Cl2–acetone 9:1) to yield 11 (173 mg, 76%) as a colourless syrup. [α]D +46.45 (c 0.16 in CHCl3); 1H NMR (CDCl3): δ 8.03–7.54 (m, 16H, Ar), 7.33–7.18 (m, 5H, Ar), 5.83 (dd, 1H, J3‴,4‴ 9.5 Hz, H-3‴), 5.68 (dd, 1H, J3′,4′ 9.5 Hz, H-3′), 5.64 (t, 1H, J3,4 10 Hz, H-3), 5.58 (dd, 1H, J3″,4″ 9.5 Hz, H-3″), 5.52 (d, 1H, J1‴,2‴ 8.5 Hz, H-1‴), 5.51 (d, 1H, J1,2 10.5 Hz, H-1), 5.39 (d, 1H, J1′,2′ 8.5 Hz, H-1′), 5.32 (d, 1H, J1″,2″ 8.5 Hz, H-1″), 5.16 (t, 1H, J4‴,5‴ 9.5 Hz, H-4‴), 5.04 (t, 1H, J4′,5′ 9.5 Hz, H-4′), 4.87 (t, 1H, J4″,5″ 9.5 Hz, H-4″), 4.81 (t, 1H, J4,5 10 Hz, H-4), 4.33 (dd, 1H, J2‴,3‴ 10.5 Hz, H-2‴), 4.24 (dd, 1H, J2′,3′ 10.5 Hz, H-2′), 4.21 (dd, 1H, J2″,3″ 10.5 Hz, H-2″), 4.15 (t, 1H, J2,3 10 Hz, H-2), 4.01–3.95 (m, 1H, H-6a‴), 3.90–3.51 (m, 10H, H-5, H-5′, H-5″, H-5‴, H-6a, H-6b, H-6a′, H-6b′, H-6a″, H-6b‴), 3.47 (dd, 1H, J5″,6b″ 6 Hz, J6a″,6b″ 11 Hz H-6b″),, 3.05 (br s, 1H), 2.08, 1.97, 1.94, 1.88, 1.80 and 1.77 (8s, 24H, 8COCH3); 13C NMR (CDCl3): δ 170.05, 169.95, 169.66, 169.56, 169.36, 167.62, 167.54 and 166.74 (CO), 97.82 (C-1″), 97.56 (C-1′), 97.40 (C-1‴), 82.37 (C-1), 76.96 (C-5), 74.18 (C-5‴), 72.82 (C-5″), 72.39 (C-5′), 71.46 (C-3), 70.72 (C-3‴), 70.57 (C-3″, C-3′), 69.89 (C-4′), 69.69 (C-4″), 69.13 (C-4‴), 68.96 (C-4), 68.44 (C-6″), 67.99 (C-6′), 67.76 (C-6‴), 61.11 (C-6), 54.38 (C-2‴), 54.25 (C-2′, C-2″), 53.30 (C-2), 20.62, 20.60, 20.51, 20.45, 20.37 and 20.32 (8COCH3). MALDI TOF-MS calcd for C78H74N4O32S: 1610.40 [M]. Found: 1633.24 [M+Na]+.
3.1.13. Phenyl 3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-glucopyranoside (12)
Bromo sugar 822 (75 mg, 0.15 mmol) was coupled with acceptor 11 according to typical procedure C. The crude product was purified by silica column chromatography (CH2Cl2–acetone 95:5) to yield 12 (146 mg, 72%) as a colourless syrup. [α]D +38.44 (c 0.16 in CHCl3); 1H NMR (CDCl3): δ 7.96–7.55 (m, 20H, Ar), 7.32–7.17 (m, 5H, Ar), 5.80 (dd, 1H, J 9 Hz, J 10.5 Hz, H-3″″), 5.72–5.44 (m, 6H, H-3, H-3′, H-3″, H-3‴, H-1″″, H-1), 5.34–5.26 (m, 3H, H-1′, H-1″, H-1‴), 5.21 (t, 1H, J 9.5 Hz, H-4″″), 4.93 (t, 1H, J 9.5 Hz, H-4‴), 4.88–4.74 (m, 3H, H-4, H-4′, H-4″), 4.43–4.34 (m, 2H, H-2″″, H-6a″″), 4.28–4.08 (m, 5H, H-6b″″, H-2, H-2′, H-2″, H-2‴), 3.99–3.34 (m, 13H, H-5″″, H-6a, H-6a′, H-6a″, H-6a‴, H-5, H-5′, H-5″, H-5‴, H-6b, H-6b′, H-6b″, H-6b‴), 2.26, 2.17, 2.05, 1.97, 1.94, 1.87, 1.79 and 1.75 (11s, 33H, 11COCH3); 13C NMR (CDCl3): δ 170.60, 169.86, 169.39, 169.29, 167.58 and 166.68 (CO), 97.89 (C-1″″), 97.79, 97.54 and 97.40 (C-1′, C-1″, C-1‴), 82.29 (C-1), 77.61 (C-5), 72.86, 72.76 and 72.51 (C-5′, C-5″, C-5‴), 71.88 (C-5″″), 71.44, 70.61 and 70.43 (C-3, C-3′, C-3″, C-3‴, C-3″″), 69.45, 69.33 and 68.86 (C-4, C-4′, C-4″, C-4‴), 68.79 (C-4″″), 68.29, 67.99, 67.66 and 67.15 (C-6, C-6′, C-6″, C-6‴), 61.84 (C-6″″), 54.29 and 54.20 (C-2′, C-2″, C-2‴, C-2″″), 53.24 (C-2), 20.69, 20.53, 20.40 and 20.27 (11COCH3). MALDI TOF-MS calcd for C98H93N5O41S: 2027.51 [M]. Found: 2050.66 [M+Na]+.
3.1.14. Phenyl 3,4,6-tri-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside (13)
Compound 6 (170 mg, 0.18 mmol) was treated with ethylene diamine and the obtained amine was peracetylated according to typical procedure D. The crude product was purified by silica column chromatography (CH2Cl2–acetone 1:1) to yield 13 (110 mg, 83%) as a colourless syrup. [α]D −32.36 (c 0.15 in CHCl3); 1H NMR (CDCl3): δ 7.53–7.30 (m, 5H, Ar), 5.59 (d, 1H, J 9 Hz, NH), 5.48 (d, 1H, J 9 Hz, NH), 5.17 (t, 1H, J3,4 10 Hz, H-3), 5.16 (d, 1H, J2′,3′ 10 Hz, H-3′), 5.03 (t, 1H, J3′,4′ 9.5 Hz, H-4′), 4.98 (t, 1H, J4,5 10 Hz, H-4), 4.86 (d, 1H, J1,2 10 Hz, H-1), 4.51 (d, 1H, J1′,2′ 8.5 Hz, H-1′), 4.25 (dd, 1H, J6a′,6b′ 12, J5,6a′ 4.5 Hz, H-6a′), 4.11 (dd, 1H, J5,6b′ 10.5 Hz, H-6b′), 3.96 (t, 1H, J2,3 10 Hz, H-2), 3.93–3.86 (m, 2H, H-6a, H-2′), 3.71–3.59 (m, 2H, H-5, H-5′), 3.53 (dd, 1H, J6a,6b 12, J5,6b 6 Hz, H-6b), 2.07, 2.02, 2.00, 1.96, 1.80 and 1.63 (7s, 21H, 7COCH3); 13C NMR (CDCl3): δ 170.98, 170.82, 170.75, 170.54, 169.78 and 169.43 (CO), 100.74 (C-1′), 86.14 (C-1), 76.92 (C-5), 73.64 (C-3), 72.66 (C-3′), 71.66 (C-5′), 68.73 (C-4), 68.41 (C-4′), 68.10 (C-6), 61.80 (C-6′), 54.00 (C-2′), 52.69 (C-2), 22.67, 22.61, 20.48 and 20.37 (COCH3). MALDI TOF-MS calcd for C32H42N2O15S: 726.23 [M]. Found: 749.01 [M+Na]+.
3.1.15. Phenyl 3,4,6-tri-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside (14)
Compound 9 (81 mg, 0.06 mmol) was treated with ethylene diamine and then the obtained amine was peracetylated according to typical procedure D. The crude product was purified by silica column chromatography (CH2Cl2–methanol 95:5) to yield 14 (52 mg, 81%) as a colourless syrup. [α]D −16.98 (c 0.17 in CHCl3); 1H NMR (CDCl3): δ 7.58–7.29 (m, 5H, Ar), 6.45 (d, 1H, J 9 Hz, NH), 6.40 (d, 1H, J 9 Hz, NH), 5.98 (d, 1H, J 8.5 Hz, NH), 5.24 (t, 1H, J3,4 10 Hz, H-3), 5.18 (t, 2H, J 9.5 Hz, H-3′, H-3″), 5.04 (t, 1H, J3″,4″ 9.5 Hz, H-4″), 4.93 (t, 2H, J 10 Hz, H-4, H-4′), 4.91 (d, 1H, J1,2 10 Hz, H-1), 4.58 (d, 1H, J1′,2′ 8 Hz, H-1′), 4.48 (d, 1H, J1″,2″ 8.5 Hz, H-1″), 4.26 (dd, 1H, J6a″,6b″ 12 Hz, J5,6a″ 5 Hz, H-6a″), 4.12 (dd, 1H, J6a″,6b″ 12 Hz, J5,6b″ 2 Hz, H-6b″), 4.01 (t, 1H, J2″,3″ 8.5 Hz, H-2″), 3.98 (t, 1H, J2,3 10 Hz, H-2), 3.92–3.77 (m, 4H, H-2′, H-6a′, H-6b′, H-5), 3.74–3.57 (m, 3H, H-5″, H-5′, H-6a), 3.45 (dd, 1H, J6a,6b 11.5 Hz, J5,6b 6 Hz, H-6b), 2.12, 2.08, 2.05, 2.04, 2.02, 1.97, 1.95 and 1.83 (10s, 30H, 10COCH3); 13C NMR (CDCl3): δ 171.14, 171.09, 170.94, 170.89, 170.83, 170.69, 170.66, 170.20, 169.97 and 169.54 (CO), 101.50 (C-1″), 100.85 (C-1′), 86.10 (C-1), 76.48 (C-5), 73.72 (C-3), 72.87 (C-3″), 72.71 (C-3′), 72.58 (C-5′), 71.74 (C-5″), 69.21 (C-4), 69.06 (C-4′), 68.65 (C-4″), 68.58 (C-6), 68.48 (C-6′), 62.11 (C-6″), 54.01 (C-2), 53.80 (C-2″), 52.78 (C-2′), 20.74, 20.58, 20.44, 20.37 and 20.31 (7COCH3). MALDI TOF-MS calcd for C44H59N3O22S: 1013.33 [M]. Found: 1036.36 [M+Na]+.
3.1.16. Phenyl 3,4,6-tri-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside (15)
Compound 10 (180 mg, 0.107 mmol) was treated with ethylene diamine and then the obtained amine was peracetylated according to typical procedure D. The crude product was purified by silica column chromatography (CH2Cl2–methanol 95:5) to yield 15 (102 mg, 73%) as a colourless syrup. [α]D −1.90 (c 0.16 in CHCl3); 1H NMR (CDCl3): δ 7.91 (d, 1H, J 9 Hz, NH), 7.58–7.51 (m, 2H, Ar), 7.46 (d, 1H, J 9 Hz, NH), 7.40–7.26 (m, 4H, Ar, NH), 7.18 (d, 1H, J 9 Hz, NH), 5.38 (t, 1H, J2′,3′ 10 Hz, H-3′), (t, 1H, J2,3 10 Hz, H-3), 5.08 (t, 1H, J 10 Hz, H-3‴), 5.03 (t, 1H, J3″,4″ 10 Hz, H-4″), 4.94 (t, 1H, J3‴,4‴ 9 Hz, H-4‴), 4.91 (d, 1H, J1,2 10.5 Hz, H-1), 4.89 (t, 1H, J3,4 9.5 Hz, H-4), 4.74 (t, 1H, J3′,4′ 9.5 Hz, H-4′), 4.73 (d, 1H, J1″,2″ 9 Hz, H-1″), 4.70 (d, 1H, J1′,2′ 10 Hz, H-1′), 4.44 (dd, 1H, J6a‴,6b‴ 12 Hz, J5‴,6a‴ 6 Hz, H-6a‴), 4.38–4.18 (m, 4H, H-1‴, H-5, H-2‴, H-2), 4.11–4.00 (m, 3H, H-2″, H-5′, H-6b‴), 3.92–3.65 (m, 6H, H-5‴, H-2′, H-5″, H-6a″, H-6b″, H-6a), 3.60–3.44 (m, 2H, H-6b, H-6a′), 3.32 (dd, 1H, J6a′,6b′ 12, J5′,6b′ 8 Hz, H-6b′), 2.16, 2.10, 2.08, 2.06, 2.05, 2.03, 2.01, 2.00 1.97, 1.94 and 1.88 (10s, 39H, 13COCH3); 13C NMR (CDCl3): δ 171.50, 170.92, 170.73, 170.07, 170.02, 169.93 and 169.56 (CO), 103.56 (C-1‴), 102.70 (C-1″), 101.26 (C-1′), 86.75 (C-1), 75.39 (C-5), 74.20 (C-3), 73.48 (C-5‴), 72.59 (C-3′), 72.39 (C-5″, C-3‴), 71.96 (C-3″), 71.63 (C-5′), 70.92 (C-6′), 70.77 (C-6, C-6″), 70.41 (C-4), 70.36 (C-4′), 69.59 (C-4‴), 69.08 (C-4″), 62.69 (C-6‴), 55.27 (C-2′), 54.67 (C-2″), 54.12 (C-2), 52.92 (C-2‴), 23.25, 23.17, 23.05, 20.91, 20.68 and 20.61 (COCH3). MALDI TOF-MS calcd for C56H76N4O29S: 1300.43 [M]. Found: 1323.44 [M+Na]+.
3.1.17. Phenyl 3,4,6-tri-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-3,4-di-O-acetyl-2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside (16)
Compound 12 (136 mg, 0.067 mmol) was treated with ethylene diamine and then the obtained amine was peracetylated according to typical procedure D. The crude product was purified by silica column chromatography (CH2Cl2–methanol 95:5) to yield 16 (76 mg, 71%) as a colourless syrup. [α]D −8.24 (c 0.12 in CHCl3); 1H NMR (CDCl3): δ 8.18 (d, 1H, J 9.5 Hz, NH), 7.72 (d, 2H, J 9 Hz, NH), 7.69–7.61 (m, 2H, Ar), 7.40–7.33 (m, 3H, Ar), 7.13 (d, 1H, J 9 Hz, NH), 6.93 (br s, 1H, NH), 5.35–5.23 (m, 2H, H-3‴, H-3″″), 5.21–4.81 (m, 8H, H-3, H-3′, H-3″, H-4′, H-4″, H-4‴, H-4″″, H-1), 4.66 (t, 1H, J 9.5 Hz, H-4), 4.63–4.46 (m, 5H, H-1′, H-1″, H-1‴, H-1″″, H-6a″″), 4.41–4.13 (m, 6H, H-2, H-2′, H-2″, H-2‴, H-2″″, H-5), 4.10–3.06 (m, 13H, H-6b″″, H-6a, H-6b, H-5′, H-5″, H-5‴, H-5″″, H-6a′, H-6b′, H-6a″, H-6b″, H-6a‴, H-6b‴), 2.23, 2.15, 2.11, 2.08, 2.07, 2.05, 2.03, 2.02, 2.00 1.99 and 1.95 (16s, 48H, 16COCH3); 13C NMR (CDCl3): δ 171.74, 171.29, 171.11, 171.01, 170.96, 170.38, 170.27, 170.12, 170.05, 169.86, 169.66 and 169.49 (CO), 104.43, 104.16, 103.35 and 101.81 (C-1″″, C-1‴, C-1″, C-1′), 86.96 (C-1), 74.64, 74.41, 73.66, 73.52, 72.78, 72.51 and 72.22 (C-3, C-3′, C-3″, C-3‴, C-3″″, C-5, C-5′, C-5″, C-5‴, C-5″″), 71.71, 71.53 and 71.44 (C-6, C-6′, C-6″, C-6‴), 70.91, 70.86, 70.60, 69.65 and 69.06 (C-4, C-4′, C-4″, C-4‴, C-4″″), 63.02 (C-6″″), 54.38, 54.32, 54.19 and 53.01 (C-2, C-2′, C-2″, C-2‴, C-2″″), 23.33, 23.30, 23.19, 23.04, 21.13, 20.83, 20.70 and 20.63 (COCH3). MALDI TOF-MS calcd for C68H93N5O36S: 1587.53 [M]. Found: 1610.29 [M+Na]+.
3.1.18. Phenyl 2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside (17)
Compound 13 (95 mg, 0.13 mmol) was treated with NaOMe according to typical procedure E. The crude product was lyophilized to give 17 (58 mg, 86%) as a white solid. [α]D −53.23 (c 0.14 in H2O); 1H NMR (D2O): δ 7.58–7.40 (m, 5H, Ar), 4.94 (d, 1H, J1,2 10 Hz, H-1), 4.58 (d, 1H, J1′,2′ 8.5 Hz, H-1′), 4.22 (dd, 1H, J5,6a 11 Hz, J6a,6b 1 Hz, H-6a), 3.97 (dd, 1H, J5,6a′ 11 Hz, J6a′,6b′ 1 Hz, H-6a′), 3.83 (t, 1H, J2,3 10 Hz, H-2), 3.85–3.72 (m, 3H, H-6b, H-6b′, H-2′), 3.70–3.43 (m, 6H, H-5, H-3, H-3′, H-4′, H-4, H-5), 2.04 and 1.96 (2s, 6H, 2COCH3); 13C NMR (D2O): δ 175.38, 175.17 (CO), 102.40 (C-1′), 87.56 (C-1), 79.78 (C-5), 76.92 (C-5′), 76.22 (C-3), 75.00 (C-3′), 70.97 (C-4), 70.75 (C-4′), 69.59 (C-6), 61.76 (C-6′), 56.50 (C-2), 55.47 (C-2′), 23.18 and 23.14 (2COCH3). MALDI TOF-MS calcd for C22H32N2O10S: 516.18 [M]. Found: 539.18 [M+Na]+.
3.1.19. Phenyl 2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside (18)
Compound 14 (46 mg, 0.045 mmol) was treated with NaOMe according to typical procedure E. The crude product was lyophilized to give 18 (28 mg, 86%) as a white solid. [α]D −70.37 (c 0.07 in H2O); 1H NMR (DMSO): δ 7.58–7.31 (m, 5H, Ar), 4.87 (d, 1H, J1,2 10.5 Hz, H-1), 4.45 (d, 2H, J 8.5 Hz, H-1′, H-1″), 4.09 (dd, 1H, J6a″,6b″ 10 Hz, J5″,6a″ 2 Hz, H-6a″), 3.82 (dd, 1H, J6a,6b 10 Hz, J5,6a 1 Hz, H-6a), 3.74 (t, 1H, J2,3 10 Hz, H-2), 3.70–3.55 (m, 7H, H-5, H-6b, H-2′, H-2″, H-6b″, H-6a′, H-6b′), 3.51 (t, 1H, J3,4 10 Hz, H-3), 3.47–3.35 (m, 3H, H-3′, H-3″, H-5″), 3.31–3.15 (m, 4H, H-4, H-5′, H-4′, H-4″), 1.98, 1.90 and 1.89 (3s, 9H, 3COCH3); 13C NMR (DMSO): δ 170.48, 170.40 and 170.24 (CO), 102.23 and 102.05 (C-1′, C-1″), 86.31 (C-1), 79.03 (C-5), 76.97 (C-5′), 75.81 (C-5″), 75.35 (C-3), 74.56 (C-3″), 74.35 (C-3′), 70.88 (C-4), 70.65 (C-4′), 70.43 (C-4″), 69.70 (C-6), 69.54 (C-6′), 61.22 (C-6″), 55.72 (C-2′, C-2″), 54.42 (C-2), 23.23 and 23.15 (3COCH3). MALDI TOF-MS calcd for C30H45N3O15S: 719.26 [M]. Found: 742.36 [M+Na]+.
3.1.20. Phenyl 2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-1-thio-β-D-glucopyranoside (19)
Compound 15 (92 mg, 0.07 mmol) was treated with NaOMe according to typical procedure E. The crude product was lyophilized to give 19 (58 mg, 88%) as a white solid. [α]D −27.34 (c 0.08 in H2O); 1H NMR (DMSO): δ 7.53–7.31 (m, 5H, Ar), 4.88 (d, 1H, J1,2 10.5 Hz, H-1), 4.49–4.39 (m, 3H, H-1′, H-1″, H-1‴), 4.11–4.01 (m, 3H, H-6a, H-6a′, H-6a″), 3.86–3.34 (m, 15H, H-6a‴, H-2, H-6b, H-6b′, H-6b″, H-6b‴, H-2′, H-2″, H-2‴, H-5, H-5‴, H-3, H-3′, H-3″, H-3‴), 3.31–3.16 (m, 6H, H-5′, H-5″, H-4, H-4′, H-4″, H-4‴), 1.99, 1.98, 1.91 and 1.90 (4s, 12H, 4COCH3); 13C NMR (DMSO): δ 170.84, 170.73 and 170.51 (CO), 102.30, 102.24 and 102.08 (C-1′, C-1″, C-1‴), 86.31 (C-1), 78.98 (C-5), 77.00, 75.71, 75.50, 75.43, 74.74 and 74.33 (C-5′, C-5″, C-5‴, C-3, C-3′, C-3″, C-3‴), 71.10, 70.73 and 70.60 (C-4, C-4′, C-4″, C-4‴), 69.82, 69.77 and 69.40 (C-6, C-6′, C-6″), 61.30 (C-6‴), 55.84 (C-2′, C-2″, C-2‴), 54.52 (C-2), 23.25 and 23.18 (4COCH3). MALDI TOF-MS calcd for C38H58N4O20S: 922.34 [M]. Found: 945.44 [M+Na]+.
3.1.21. Phenyl 2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-β-D-glucopyranosyl-(1→6)-2-acetamido-2-deoxy-β-Dglucopyranosyl-(1→6)-2-acetamido-2-deoxy-1-thio-β-Dglucopyranoside (20)
Compound 16 (72 mg, 0.045 mmol) was treated with NaOMe according to typical procedure E. The crude product was lyophilized to give 20 (43 mg, 85%) as a white solid. [α]D −37.23 (c 0.10 in H2O); 1H NMR (DMSO): δ 7.59–7.27 (m, 5H, Ar), 4.87 (d, 1H, J 10.5 Hz, H-1), 4.50–4.39 (m, 4H, H-1′, H-1″, H-1‴, H-1″″), 4.16–3.97 (m, 4H, H-6a, H-6a′, H-6a″, H-6a‴), 3.83–3.14 (m, 26H, H-6a″″, H-2, H-5, H-6b, H-6b′, H-6b″, H-6b‴, H-6b″″, H-2′, H-2″, H-2‴, H-2″″, H-3, H-3′, H-3″, H-3‴, H-3″″, H-5′, H-5″, H-5‴, H-5″″, H-4, H-4′, H-4″, H-4‴, H-4″″), 2.09, 2.06, 2.01 and 1.98 (5s, 15H, 5COCH3); 13C NMR (DMSO): δ 170.34, 170.31, 170.28, 170.19 and 170.02 (CO), 102.25, 102.16 and 102.03 (C-1′, C-1″, C-1‴, C-1″″), 86.13 (C-1), 78.77 (C-5), 76.96, 75.66, 75.40, 75.28, 75.15, 74.79, 74.28, 74.19, 74.11, 71.25, 70.70 and 70.63 (C-5′, C-5″, C-5‴, C-5″″, C-3, C-3′, C-3″, C-3‴, C-3″″, C-4, C-4′, C-4″, C-4‴, C-4″″), 70.01, 69.94, 69.84 and 69.52 (C-6, C-6′, C-6″, C-6‴), 61.18 (C-6″″), 55.68 (C-2′, C-2″, C-2‴, C-2″″), 54.37 (C-2), 23.22, 23.17 and 23.13 (5COCH3). MALDI TOF-MS calcd for C46H71N5O25S: 1125.42 [M]. Found: 1148.44 [M+Na]+.
3.2. Enzymatic studies
3.2.1. Expression and purification of DspB and mutants
The enzyme DspB was expressed and purified using the plasmid pRC3 that carried the dspB gene encoding amino acids 21–381 fused directly to a hexahistidine metal-binding C-terminal tail located downstream from an IPTG-inducible tac promoter as previously described.18 Y187A, Y278A and W237A mutants of DspB were generated using the primers and method published earlier.19
3.2.2. HPLC separation of substrates and products of the enzymatic reaction
Compounds were separated on a reversed phase column (Zorbax Eclipse XDB-C18 150 × 4.6 mm, 5 μm) with acetonitrile/water (10:90) as the mobile phase at a flow rate of 1 mL/min at 25 °C using a Hewlett–Packard 1090 Series II liquid chromatograph equipped with diode array detector and automatic sampler. Five microlitres of the standard mixture or the reaction mixture was injected into the chromatographic column. Effluent was monitored for the products containing thiophenyl group at 254 nm. The HPLC profile of the standard mixture (Fig. 4) shows that substrates (DP 2–5, 1 mM) and products (DP 1–4) are separated very well with reasonable retention time. Retention order is reversed on C18 column, first eluted peak is the substrate, followed by the shorter hydrolysis products. The products of the hydrolysis were identified and measured by using relevant standards with application of CHEMSTATION software. The quality of acetonitrile was HPLC gradient grade. Purified water was obtained from a laboratory purification system equipped with both ion exchange and carbon filters (Millipore, Bedford, MA, USA).
3.2.3. Hydrolysis on oligomer substrates catalysed by DspB enzyme and its mutants
Hydrolytic reactions were carried out on β-(1→6)-linked Glc-NAc oligosaccharides and the reaction products were analysed by HPLC. Incubations of oligosaccharides DP 2–5 at substrate concentration of 0.4–2 mM were carried out in 50 mM phosphate buffer (pH 5.9) containing 100 mM NaCl at room temperature (25 °C). The reactions were initiated by the addition of enzyme (2 μM). To follow the reaction 5 μL reaction mixture was injected into the chromatographic column at different time intervals. Reaction products were separated as described above. The hydrolysis conversion is defined as the percentage of substrate converted to shorter products. The conversions were calculated from HPLC peak area of substrate and products. The sum of peak areas of products was divided by the total area of substrate and products. This ratio was multiplied with 100 to get conversion% of the enzyme reactions.
Acknowledgments
This work was supported by the Hungarian Scientific Research Fund (Project No. PD73064) and by the TÁMOP 4.2.1./ B-09/1/KONV-2010-0007 project. The project is implemented through the New Hungary Development Plan, co-financed by the European Social Fund and the European Regional Development Fund.
References
- 1.Hakomori S, Zhang Y. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 2.(a) Yang F, He H, Du Y. Tetrahedron Lett. 2002;43:7561–7563. [Google Scholar]; (b) Yang F, He H, Du Y. Carbohydr Res. 2003;338:495–502. doi: 10.1016/s0008-6215(02)00494-9. [DOI] [PubMed] [Google Scholar]
- 3.Fridman M, Solomon D, Yogev S, Baasov T. Org Lett. 2002;4:281–283. doi: 10.1021/ol017054d. [DOI] [PubMed] [Google Scholar]
- 4.Melean LG, Love KR, Seeberger PH. Carbohydr Res. 2002;337:1893–1916. doi: 10.1016/s0008-6215(02)00299-9. [DOI] [PubMed] [Google Scholar]
- 5.Gening ML, Tsvetkov YE, Pier GB, Nifantiev NE. Carbohydr Res. 2007;342:567–575. doi: 10.1016/j.carres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Gening ML, Titov DV, Grachev AA, Gerbst AG, Yudina ON, Shashkov AS, Chizhov AO, Tsvetkov YE, Nifantiev NE. Eur J Org Chem. 2010:2465–2475. [Google Scholar]
- 7.Defaye J, Gadelle A, Pedersen C. Carbohydr Res. 1989;186:177–188. [Google Scholar]
- 8.Leung C, Chibba A, Gómez-Biagi RF, Nitz M. Carbohydr Res. 2009;344:570–575. doi: 10.1016/j.carres.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Rohde H, Frankenberger S, Zähringer U, Mack D. Eur J Cell Biol. 2010;89:103–111. doi: 10.1016/j.ejcb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Haro JM, Peters BM, O’May GA, Archer N, Kerns P, Prabhakara R, Shirtiff ME. FEMS Immunol Med Microbiol. 2010;59:306–323. doi: 10.1111/j.1574-695X.2010.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fey PD. Curr Opin Microbiol. 2010;13:610–615. doi: 10.1016/j.mib.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan JB. Int J Artif Organs. 2009;32:545–554. doi: 10.1177/039139880903200903. [DOI] [PubMed] [Google Scholar]
- 13.Izano EA, Sadovskaya I, Wang H, Vinogradov E, Ragunath C, Ramasubbu N, Jabbouri S, Perry MB, Kaplan JB. Microb Pathog. 2008;44:52–60. doi: 10.1016/j.micpath.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Mark D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Joyce JG, Abeygunawardana C, Xu Q, Cook R, Hepler JC, Przysiecki CT, Grimm KM, Roper K, Ip CC, Cope L. Carbohydr Res. 2003;338:903–922. doi: 10.1016/s0008-6215(03)00045-4. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N. Antimicrob Agents Chemother. 2004;48:2633–2636. doi: 10.1128/AAC.48.7.2633-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. J Bacteriol. 2003;185:4693–4698. doi: 10.1128/JB.185.16.4693-4698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. J Antimicrob Chemother. 2009;64:88–93. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 18.Kerrigan JE, Ragunath C, Kandra L, Gyémánt G, Lipták A, Jánossy L, Kaplan JB, Ramasubbu N. Acta Biol Hung. 2008;59:439–451. doi: 10.1556/ABiol.59.2008.4.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manuel SGA, Ragunath C, Sait HBR, Izano EA, Kaplan JB, Ramasubbu N. FEBS J. 2007;274:5987–5999. doi: 10.1111/j.1742-4658.2007.06121.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa T, Nakabayashi S, Sasajima K. Carbohydr Res. 1981;95:308–312. [Google Scholar]
- 21.Peters T, Weimar T. Liebigs Ann Chem. 1991:237–242. [Google Scholar]
- 22.Akiya S, Ogawa T. Chem Pharm Bull. 1960;8:583–587. [Google Scholar]
- 23.Buskas T, Garegg P, Konradsson P, Maloisel JL. Tetrahedron: Asymmetry. 1994;5:2187–2194. [Google Scholar]