Abstract
Background & objectives:
CD9 and CD146 are important adhesion molecules that play a role in the implantation of an embryo. This study was undertaken to correlate the expression of these markers in fertile and infertile women's endometrial stromal cells.
Methods:
Human endometrial stromal cell culture from endometrial biopsies of fertile (n=50) and infertile females (n=50) was performed and primary cell lines were established. Expression of CD9 and CD146 was studied for all the 100 cell lines with the help of flow cytometry. Gene expression of CD9 and CD146 was performed by real-time polymerase chain reaction.
Results:
There was a significant difference in endometrial stromal cells of fertile and infertile females. Flow cytometric results revealed significantly lower expression of CD9 (P=0.0126) and CD146 (P=0.0006) in the infertile endometrial stromal cells as compared to fertile endometrial stromal cells. These results were comparable with real-time data.
Interpretation & conclusions:
This study showed that endometrial stromal cells from infertile females had lower expression of adhesion molecules, CD9 and CD146. Our findings suggest that CD9 and CD146 may have a role in infertility. Infertile female's endometrial stromal cells have decreased expression of CD9 and CD146 which can be the cause of infertility related to implantation failure.
Keywords: Adhesion molecules, CD9, CD146, endometrial stromal cells, infertility
Infertility affects nearly 15 per cent of all couples in reproductive age group. Female infertility has a major role in almost 50 per cent of the infertile couple population, endometrium being one of the concerns such as endometrium hyperproliferation, including endometrial cancer, endometrial hyperplasia, endometriosis, endometrial infection such as tuberculosis, thin endometrium and the female is incapable of carrying a pregnancy to term. Implantation failure of the blastocyst is also one of the important issues1. Endometrium plays a central role in the maintenance of pregnancy with the help of many signalling molecules2.
Human endometrium has a remarkable regenerative capacity3, growing from 0.5 to 1 mm following menstruation to 5-7 mm in thickness in average menstrual cycle4, and is characterized by cyclic processes of cellular proliferation, differentiation and shedding. The endometrial thickness between 7 and 14 mm with a triple-line configuration is considered favorable for embryo implantation2.
Prianishnikov in 19785 has brought the concept that endometrial regeneration is mediated by stem cells located in the basalis endometrium, rather than the functionalis or myometrium. The characteristics of the endometrium are suggestive of the presence of an adult stem/progenitor cell population, which is responsible for the expressed regenerative capacity of this tissue6. Endometrial stem cells are the specialized cells which modulate endometrium for self-renewal, proliferation, differentiation and shedding off during the monthly menstrual cycle3. These cells also prepare the endometrium to receive the fertilized egg by hyperproliferation and angiogenesis of endometrium. Many groups have illustrated the concept of endometrial stromal/mesenchymal stem cells which reside in basalis layer of the endometrium. There are several markers expressed by the endometrial stromal cells such as platelet-derived growth factor receptor-beta, CD9, CD146, CD90, CD73, CD105, CD44 and CD297,8,9,10,11,12.
Whether these endometrial stromal/mesenchymal stem cells play any role in the establishment or maintenance of the pregnancy is not known. CD9 and CD146 are the two markers that have been studied in mice and shown to be associated with implantation13. CD9, a marker of the tetraspanin family, is a 24-27 kDa cell surface protein with four predicted transmembrane domains13. CD9 is recognized as a motility-related protein 1 that plays a key role in sperm-egg fusion. CD9-deficient eggs fail to fuse with sperm14. CD9 is associated with integrin adhesion receptors and controls integrin-dependent cell migration and invasion during blastocyst implantation12. In an experimental study, CD9-deficient endometrium in mouse failed to implant compared to the CD9-positive endometrium13. CD9 is associated with blastocyst implantation by producing matrix metalloproteinase-29.
CD146 is a cell adhesion molecule (CAM) which belongs to the immunoglobulin (Ig) superfamily. CAMs are proteins located on the cell surface involved in the process of cell adhesion through binding with other cells or with the extracellular matrix (ECM)15. There have been reports stating the importance of CD146 to be involved in trophoblast invasion during pregnancy establishment in mice15,16. CD146 is shown to be selectively expressed by invasive trophoblasts, whereas non-invasive trophoblast has no expression of CD14615. CD146 is expressed in receptive maternal uterus and invasive embryonic trophoblasts but found absent in non-pregnant uterus in mouse. Blocking the function of CD146 in vivo and in vitro inhibits blastocyst attachment and trophoblastic invasion, leading to pregnancy failure15.
In some studies luminal epithelium of the endometrium has been studied for infertility17,18, but there are no reports on role of endometrial stromal cells in infertility. Therefore, the present study was conducted to examine the human endometrial stromal cell obtained from both fertile and infertile women for the presence of adhesion molecules CD9 and CD146 to assess whether dysregulation in these molecules could be a factor for infertility.
Material & Methods
This study was carried out from 2009 to 2012 for the period of three years. Patients were recruited for this study from Jaslok Hospital and Inkus Infertility Clinic by convenient sampling. Enrolment period of the study was 19 months. The study protocol was approved by the Ethics Committee of Jaslok Hospital and Research Centre and informed written consent was obtained from each patient. The inclusion criteria were the women who had regular menstrual cycle, aged between 21 and 40 yr and had not taken any steroid hormones before three months of endometrial biopsy. The exclusion criteria were cases of pregnancy, male factor infertility and women with polycystic ovarian syndrome, endometriosis, abnormal uterine bleeding, endometrial hyperplasia and body mass index (BMI) of ≥30 kg/m2. Fertile group women had proven fertility (natural conception with full-term delivery and no history of missed abortion) and infertile group women had no conception and no history of missed abortion before the recruitment. Based on the above criteria, cases and controls were matched. A portion of each endometrial specimen obtained was examined histologically.
Human endometrial biopsy samples were collected from 100 women (n=100). Infertile group samples were collected from females undergoing diagnostic hysteroscopy (n=50). Basal characteristics of the study groups are shown in Table I. Mean age of infertile group women was 29.62±4.08 yr (range 21-40 yr), and of fertile group women was 34.12±6.6 yr (range 22-37 yr). Fertile group samples were collected from females undergoing tubal ligation (n=50). All the samples were collected in the secretory phase of menstrual cycle.
Table I.
Basal characteristics of infertile group compared to fertile group
Collection of endometrial samples: Endometrium sample was collected in bench medium containing HEPES-buffered Dulbecco modified Eagle medium/Hams F-12 (DMEM/F-12; Gibco Invitrogen, USA), 1 per cent antibiotic-antimycotic solution (Gibco Invitrogen, USA) and 5 per cent foetal bovine serum (Gibco Invitrogen).
Primary endometrial stromal cell culture: After the collection of the endometrial samples, the samples were brought to the laboratory at 4°C. Endometrial tissue was dissected and minced to 1-2 mm3 pieces using scissors. Minced tissue was digested with DMEM containing 0.1 per cent collagenase type IV (Sigma, USA) in a humidified 5 per cent CO2/95 per cent air atmosphere at 37°C for one hour. The cell suspension was filtered through a 40 μm cell strainer (BD Falcon, USA) to remove mucus and undigested tissue6. The cell filtrates were centrifuged at 250 ×g for five minutes and the cell pellet was resuspended in culture medium containing DMEM/F-12, 1 per cent antibiotic-antimycotic solutions (Gibco Invitrogen) and 1 per cent L-glutamine supplemented with 15 per cent heat-inactivated sterile-filtered foetal bovine serum.
The cells were plated in a T25 cm2 tissue culture flask (Corning, USA). The cells were cultured overnight in a humidified 5 per cent CO2/95 per cent air atmosphere at 37°C and non-adherent cells were discarded. The culture medium was replaced every 48 h. Primary cell lines were established for all the 100 patients’ endometrial samples5.
Flow cytometric analysis: Immunophenotyping of the all 100 cultured endometrial stromal cells was performed after fifth passage by means of flow cytometry. Cells were washed with Dulbecco's phosphate-buffered saline (PBS) and harvested using acutase (Invitrogen); cells were pelleted to one ml of cell suspension, concentration 1×106/ml. Staining was performed with antibodies against CD9 (PerCP) from Becton Dickinson (BD, USA), primary CD146 monoclonal antibody from Millipore (USA) and FITC-conjugated goat anti-mouse IgM secondary antibody from Millipore. Cells were incubated in primary antibody (10 μl in case of conjugated and 1:100 for unconjugated) for 30 min at room temperature after the incubation cells were washed with FACS solution (0.1% bovine serum albumin, 0.1% sodium azide in PBS) and incubated in 10 μl secondary antibody (1:1000) in the dark at 2-8°C for 30 min. At the end, the cells were fixed in 1 per cent paraformaldehyde and re-suspended in FACS solution5. The specific fluorescent labelling was analyzed at FACSCalibur flow cytometer (BD) using the built-in CellQuest Pro software (Version 5.1); 10,000 events were analyzed for each sample.
Real-time polymerase chain reaction (PCR): Total RNA was harvested from 100 cultured endometrial stromal cells, using the TRIzol™ reagent (Invitrogen) and treated with DNase I (Bangalore Genei Pvt. Ltd., Bengaluru).First-strand cDNA was synthesized using the Clontech Advantage RT Kit (Clontech BD Biosciences, USA) using of Moloney murine leukemia virus (MMLV) reverse transcriptase.
CD9 and CD146 expression levels were studied in fertile and infertile group in relation to 18S rRNA (housekeeping gene) on the R Corbett Research Real-Time PCR system (Rotor-Gene) using SYBR green chemistry (Bio-Rad, USA). The amplification conditions, primer concentration and annealing temperature were standardized to obtain single peak melt curve. The homogeneity of the PCR amplicons was verified by running the products on 1.5 per cent agarose gels and also by studying the melt curve. All PCR amplifications were carried out in duplicate. Mean cycle threshold (Ct) values generated in each experiment using the rotor-gene analysis software (Rotor-Gene Q, Qiagen, Germany) were used to calculate fold change. The relative gene expression ratios were calculated manually by the 2−ΔΔCt method19.
PCR primers were designed using Primer3 Input (v.0.4.0) http://bioinfo.ut.ee/primer3-0.4.0/. The following forward (F) and reverse (R) primers were used: for CD9 F: 5’ TTG GAC TAT GGC TCC GAT TC 3’ and R: 3’ GGC GAA TAT CAC CAA GAG GA 5’; for CD146 F: 5’ CCA AGG CAA CCT CAG CCA TGT C 3’ and R: 3’CTC GAC TCC ACA GTC TGG GAC G 5’ and for 18S F:5’GGA GAG GGA GCC TGA GAA AC 3’ and R: 3’CCT CCA ATG GAT CCT CGT TA 5’.
Statistical analysis: Infertile females and fertile females were compared for the difference in baseline characteristics using the Student's t test. Differences between fertile and infertile groups for the CD9 and CD146 markers were assessed using the non-parametric Mann-Whitney U-test. Logistic regression analysis was performed for the confounding factors such as age, BMI and menstrual pattern to explore the interaction of infertility and fertility with CD9 and CD146 expression. Univariate logistic regression was performed for all the factors; factors giving P<0.1 were further analyzed by multivariate logistic regression analysis. By modelling each parameter, the fertility prediction equation was accessed. The data were analyzed using Statistical Package for the Social Sciences (SPSS) software (version 20.0; SPSS Inc., Chicago, IL, USA).
Results
Basal characteristics of the study groups are shown in Table I. Mean age of infertile group women was 29.62±4.08 yr, age range 21-40 yr, and of the fertile group women was 34.12±6.6 yr, age range 22-37 yr (P<0.001). Mean BMI kg/m2 of the infertile group was 19.69±5.55 and of the fertile group was 5.02±9.54 (P<0.01) whereas mean of menstrual pattern in the infertile group was 28.62±1.14 and in the fertile group showed 28.9±1.26. Age and BMI were found to be significant in both the groups.
Endometrial stromal cells (primary cell lines): Endometrial stromal cell lines (primary cell lines) were established from 100 endometrial samples including fertile and fertile patients. Morphologically, there was no difference in the endometrial stromal cells of both the groups. Endometrial stromal cells were stable in culture and multiplied every 19-20 h.
After 24 h of culture both epithelial and stromal cells were observed. Passage one onwards, fibroblast-like stromal cells were the only cells growing in the culture. After 10-14 days, these cells formed a single colony at the beginning and then merged as a monolayer. These cells are also called as endometrial stromal/mesenchymal stem cells. After the third passage, the spindle-like stromal cells were the only cell type found in the culture. Morphologically, no significant difference was observed between the endometrial stromal cells of fertile samples and infertile samples.
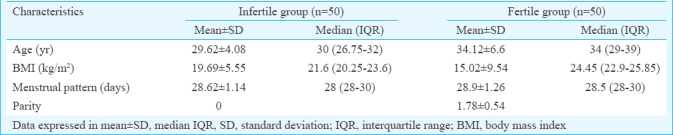
Expression of CD9 and (CD146) by flow cytometry: Differential expression of CD9 and CD146 was observed in fertile and infertile endometrial stromal cells by flow cytometry (n=100). Significantly (P<0.05) lower levels of adhesion molecule CD9 were observed in the infertile endometrial stromal cells as compared to fertile endometrial stromal cells (Figs. 1, 2).
Fig. 1.
Representative scatter plot showing flow cytometric expression of CD9 in cultured endometrial stromal cells of fertile group (A) and infertile group (B).
Fig. 2.
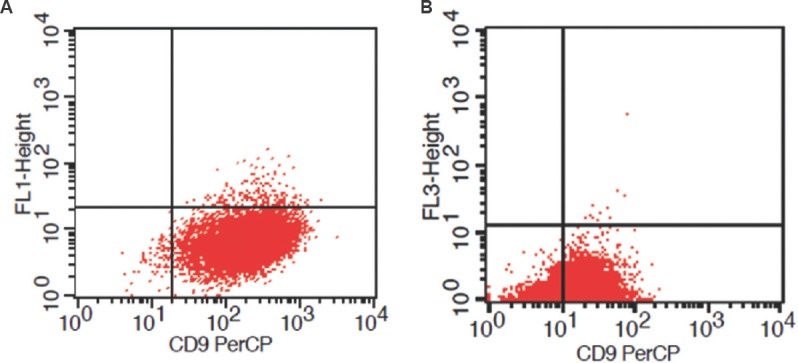
Flow cytometric expression analysis of CD9 and CD146 levels in the fertile and infertile endometrial stromal cells showing significant lower expression in infertile group having (P<0.01) for CD9 and (P<0.01) for CD146 (height of bar represents median and error bar indicates standard deviation).
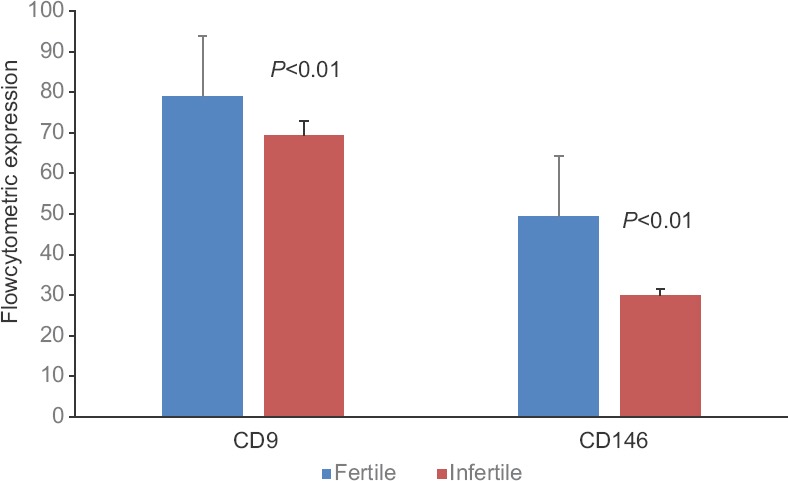
Strong expression of CD146 adhesion molecule was detected in fertile females’ endometrial stromal cells (Fig. 3A) and the infertile group showed less expression of CD146 (Fig. 3B). Results of flow cytometry showed significantly (P<0.01) lower levels of CD146 in the infertile endometrial stromal cells compared to fertile endometrial stromal cells (Fig. 2).
Fig. 3.
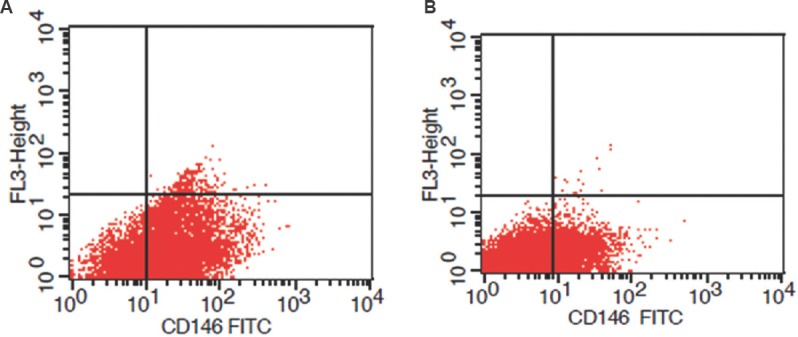
Representative scatter plot showing flow cytometric expression of CD146 in cultured endometrial stromal cells of fertile group (A) and infertile group (B).
Differential expression of CD9 and CD146 by real-time PCR: Endometrial stromal cells of the fertile and infertile females (n=100) were subjected to real-time PCR and gene expression results showed significantly (P<0.001) lower expression of both CD9 and CD146 in the infertile endometrial stromal cells compared to fertile endometrial stromal cells (Fig. 4).
Fig. 4.
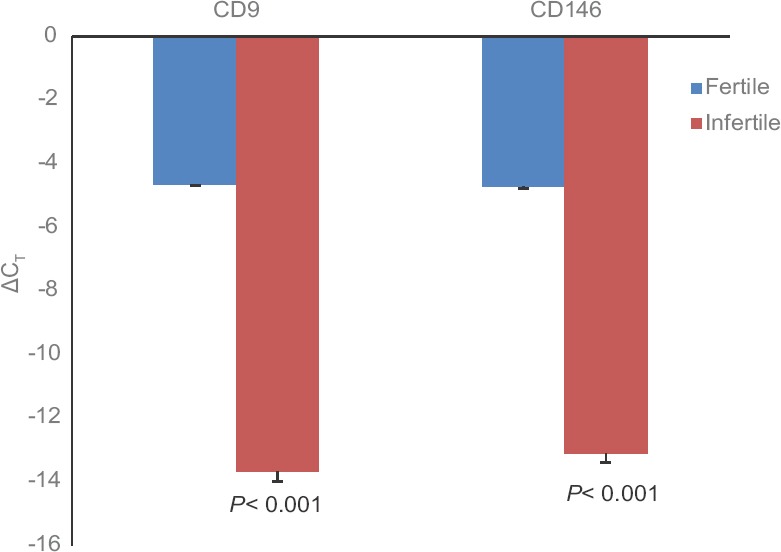
Differential gene expression of CD9 and CD146 in fertile and infertile endometrial cells (height of bar represents median and error bar indicates standard deviation).
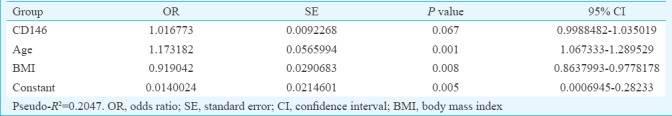
Association of CD9 and CD146 with age, BMI and menstrual pattern: Multiple logistic regression was done for the variables that gave P<0.1 in univariate regression analysis; age and BMI had a P=0.001 and P=0.0033, respectively, whereas menstrual pattern showed P=0.2424. When univariate logistic regression of CD9 was performed in the fertile and infertile group, the model showed the R2 value of 0.0444 (Table II) which was a poor predictor of fertility. When the age and BMI were included in the model, the R2 value increased to 0.2175 which was a better predictive probability of fertility in females (Table III). Similarly, in case of CD146 in univariate logistic regression, the model showed R2 value of 0.0562 (Table IV) which was a poor predictor of fertility. When age and BMI were added to the model, the R2 value increased to 0.2047 which was a better predictive probability of fertility in females (Table V).
Table II.
Logistic univariate analysis for CD9

Table III.
Multiple logistic regression analysis in group CD9 with age and body mass index
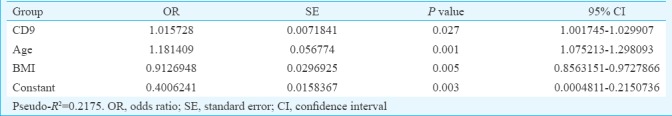
Table IV.
Logistic group univariate analysis for CD146

Table V.
Multiple logistic regression analysis in group CD146 with age and body mass index
Discussion
In the current study, the population of endometrial stromal cells isolated from human endometrium of fertile and infertile females was compared. Morphologically, there was no significant difference in the endometrial stromal cells obtained from fertile and infertile patients. The expression of CD9 and CD146 was found to be significantly associated with fertility. The endometrial stromal cell expression of CD9 and CD146 has been reported to be associated with infertility related to the endometrium12,16.
Our results showed that the CD9 and CD146 expression was significantly lower in the infertile female's endometrial stromal cells in culture compared to fertile females. CD9 and CD146 markers are found to be significant in the fertile and infertile groups, and their expression influences the outcome of the patient being fertile. Age, BMI and menstrual pattern are confounding factors that are known to affect the fertility. Infertility rate in females increases with increased BMI and obesity20. Fertility decreases with increase in age21. When taken into univariate logistic analysis, CD9 was able to explain only 0.04 per cent (pseudo-R2=0.0444) and CD146 (pseudo-R2=0.0563) 0.05 per cent in predicting the outcome. CD9 could predict the outcome of fertility by 0.04 per cent; when age and BMI were included into the equation, the probability of predicting fertility increased to 0.21 per cent.
With an age of 30 yr and BMI to be 21 kg/m2 which was mean of the study population, if CD9 expression was 100, that means it will have predictive value was 0.51.
In CD146 group it turned out to be marginally insignificant including age and BMI, hence, probably, increasing the sample size or adding more variables to the study will help to better predict the outcome of the patient being fertile.
Several reports showed that adhesion molecules play a vital role in embryo attachment and trophoblasts invasion during the process of implantation. CD9 and CD146 have been previously reported as the important adhesion molecules which take part in the adhesion and invasion of blastocyst13,15,16.
Liu et al16 showed the effect of CD146 antibody in vitro and in vivo during blastocyst attachment and trophoblast invasion. They found out that CD146 expression was absent in the uteri of non-pregnant mice and was found to be upregulated during the implantation window.
CD9 has been reported to associate with several integrins in a number of migration, adhesion and fusion systems, including a3, a5, 6 or b1 subunits21. A study in mice showed that blocking of CD9 antibody in vivo and in vitro inhibited implantation of blastocyst and invasion of trophoblast, resulting in failure of pregnancy15.
Decidualization is an important event in the establishment of pregnancy. In humans, decidualization is initiated by endometrial stromal cells which occur in the secretory phase of menstrual cycle independently of pregnancy3. Decidualizing endometrial stromal cells prepare the endometrium for embryo recognition and selection by means of biological functions such as cell adhesion, signal transduction, cell proliferation, ECM organization, cell differentiation and apoptosis22,23. Decidualization is basically a differentiation of endometrial stromal cells into secretory epithelioid-like decidual cells under the influence of progesterone24,25.
In conclusion, our study demonstrates that CD9 and CD146 adhesion molecules in female's endometrial stromal cells have the possible role in the decidualization and attachment to the embryo. In future, it would be interesting to see the expression of these molecules on blastocyst as well as on decidual cells to draw a conclusion on the role of these molecules in infertility. However, based on the findings of this study it can be suggested that, intrauterine release of adhesion promoting factors may improve implantation. More studies need to be done to see if the administration of CD9 and CD146-positive cells (autologous bone marrow) in the endometrium improves implantation in infertile females.
Footnotes
Financial support & sponsorship: Authors thank Lady Tata memorial Trust for awarding Junior Research Fellowship to the first author (MSCK) for two years and acknowledge Jaslok Hospital and Research Centre for funding the project (Research project code 503). Authors thank Dr Sripriya Natarajan (P.D. Hinduja Hospital and Research Centre, Mumbai) for guidance in statistical analysis.
Conflicts of Interest: None.
References
- 1.Cha J, Sun X, Dey SK. Mechanisms of implantation: Strategies for successful pregnancy. Nat Med. 2012;18:1754–67. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao J, Zhang Q, Li Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol. 2012;10:100. doi: 10.1186/1477-7827-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gargett CE. Uterine stem cells: What is the evidence? Hum Reprod Update. 2007;13:87–101. doi: 10.1093/humupd/dml045. [DOI] [PubMed] [Google Scholar]
- 4.McLennan CE, Rydell AH. Extent of endometrial shedding during normal menstruation. Obstet Gynecol. 1965;26:605–21. [PubMed] [Google Scholar]
- 5.Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18:213–23. doi: 10.1016/s0010-7824(78)80015-8. [DOI] [PubMed] [Google Scholar]
- 6.Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P, et al. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135:551–8. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- 7.Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–34. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 8.Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124–30. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 9.Chan RWS, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–50. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 10.Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, et al. Stem cell-like properties of the endometrial side population: Implication in endometrial regeneration. PLoS One. 2010;5:e10387. doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghobadi F, Mehrabani D, Mehrabani G. Regenerative potential of endometrial stem cells: A mini review. World J Plast Surg. 2015;4:3–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano N, Miyado K, Yoshii N, Kanai S, Saito H, Miyado M, et al. Absence of CD9 reduces endometrial VEGF secretion and impairs uterine repair after parturition. Sci Rep. 2014;4:4701. doi: 10.1038/srep04701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu WM, Cao YJ, Yang YJ, Li J, Hu Z, Duan EK, et al. Tetraspanin CD9 regulates invasion during mouse embryo implantation. J Mol Endocrinol. 2006;36:121–30. doi: 10.1677/jme.1.01910. [DOI] [PubMed] [Google Scholar]
- 14.Hemler ME. Targeting of tetraspanin proteins - Potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–58. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330:150–62. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Zhang B, Zhao X, Zhang Y, Liu Y, Yan X, et al. Blockade of adhesion molecule CD146 causes pregnancy failure in mice. J Cell Physiol. 2008;215:621–6. doi: 10.1002/jcp.21341. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Cao G, Zhang SL. Is CD146 pivotal in neoplasm invasion and blastocyst embedding? Med Hypotheses. 2011;76:378–80. doi: 10.1016/j.mehy.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Melorose J, Perroy R, Careas S. Luminal and glandular endometrial epithelium express integrins differentially throughout the menstrual cycle: Implications for implantation, contraception, and infertility. Am J Reprod Immunol. 1996;35:195–204. doi: 10.1111/j.1600-0897.1996.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Maheshwari A. Overweight and obesity in infertility: Cost and consequences. Hum Reprod Update. 2010;16:229–30. doi: 10.1093/humupd/dmp058. [DOI] [PubMed] [Google Scholar]
- 21.Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod. 2008;23:538–42. doi: 10.1093/humrep/dem431. [DOI] [PubMed] [Google Scholar]
- 22.Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–38. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- 23.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–53. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 24.Weimar CHE, Macklon NS, Post Uiterweer ED, Brosens JJ, Gellersen B. The motile and invasive capacity of human endometrial stromal cells: Implications for normal and impaired reproductive function. Hum Reprod Update. 2013;19:542–57. doi: 10.1093/humupd/dmt025. [DOI] [PubMed] [Google Scholar]
- 25.Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod. 2010;25:862–73. doi: 10.1093/humrep/dep468. [DOI] [PubMed] [Google Scholar]


