Abstract
Inhaled corticosteroids (ICSs) are a mainstay of COPD treatment for patients with a history of exacerbations. Initial studies evaluating their use as monotherapy failed to show an effect on rate of pulmonary function decline in COPD, despite improvements in symptoms and reductions in exacerbations. Subsequently, ICS use in combination with long-acting β2-agonists (LABAs) was shown to provide improved reductions in exacerbations, lung function, and health status. ICS-LABA combination therapy is currently recommended for patients with a history of exacerbations despite treatment with long-acting bronchodilators alone. The presence of eosinophilic bronchial inflammation, detected by high blood eosinophil levels or a history of asthma or asthma–COPD overlap, may define a population of patients in whom ICSs may be of particular benefit. Prospective clinical studies to determine an appropriate threshold of eosinophil levels for predicting the beneficial effects of ICSs are needed. Further study is also required in COPD patients who continue to smoke to assess the impact of cell- and tissue-specific changes on ICS responsiveness. The safety profile of ICSs in COPD patients is confounded by comorbidities, age, and prior use of systemic corticosteroids. The risk of pneumonia in patients with COPD is increased, particularly with more advanced age and worse disease severity. ICS-containing therapy also has been shown to increase pneumonia risk; however, differences in study design and the definition of pneumonia events have led to substantial variability in risk estimates, and some data indicate that pneumonia risk may differ by the specific ICS used. In summary, treatment with ICSs has a role in dual and triple therapy for COPD to reduce exacerbations and improve symptoms. Careful assessment of COPD phenotypes related to risk factors, triggers, and comorbidities may assist in individualizing treatment while maximizing the benefit-to-risk ratio of ICS-containing COPD treatment.
Keywords: COPD, inhaled corticosteroids, bronchodilators, dual/triple therapy, safety, pneumonia
Plain-language summary
Increasing treatment options for COPD add to the complexity of treatment and require review of clinical data to inform treatment decisions. Inhaled corticosteroids (ICSs) in combination with long-acting β2-agonists (LABAs) reduce the risk of exacerbations and improve lung function and health status in patients with COPD compared with ICS or LABA therapy alone. Certain patients may particularly benefit from ICS therapy, including those with frequent exacerbations despite long-acting bronchodilator therapy and those with evidence of eosinophilic bronchial inflammation, which can be determined by high levels of blood eosinophils and/or a history of asthma or asthma–COPD overlap. Although relatively uncommon, an increased risk of pneumonia is associated with ICS use and appears to be dependent on the specific ICS used. Recent studies of triple therapy combining an ICS, LABA, and a long-acting muscarinic antagonist demonstrated significant benefits compared with dual therapy and support their widespread use in COPD patients with frequent exacerbations, but longer-term data and comparisons of specific triple-therapy regimens are needed to optimize therapy.
History of inhaled corticosteroid use in COPD
COPD is characterized by persistent respiratory symptoms and progressive airflow limitation.1 Goals of COPD management are to minimize the impact of symptoms, improve levels of physical activity, and decrease the future risk of exacerbations responsible for disease progression.1 Achieving these goals is challenging due to the heterogeneous nature of COPD and an incomplete understanding of the pathophysiology of the disease. Inflammatory changes have been observed in the lungs as a result of inhaling cigarette smoke, as well as noxious particles and gases from other sources.1 The presence of increased numbers of inflammatory cells in airway biopsies and bronchoalveolar lavage, including neutrophils, alveolar macrophages, and T lymphocytes, in the lungs of smokers susceptible to the development of COPD may act directly on airway and alveolar tissue, promoting airway narrowing and airflow limitation.2,3 These data, in conjunction with the effectiveness of inhaled corticosteroids (ICSs) in the treatment of asthma, encouraged routine use of ICSs in patients with COPD. Over the past three decades, extensive research has been conducted evaluating the use of ICSs in such patients. This article reviews the history of the use of ICSs in COPD, with a focus on pivotal clinical studies, systematic reviews, and meta-analyses. The effect of smoking on ICS response in COPD is also discussed, and safety considerations for ICS use in COPD are examined, particularly regarding long-term safety and pneumonia risk. The applicability of these data is considered in light of current treatment practices, as well as their relevance to future therapy for COPD, including triple-therapy regimens.
Use of ICS monotherapy in COPD
In the 1990s, short-term studies of ICS monotherapy in patients with COPD and chronic bronchitis found that anti-inflammatory therapy reduced bronchial inflammation, but had varying effect on lung-function measures of forced expiratory volume in 1 second (FEV1) and peak expiratory flow (PEF).4–6 In a 6-month study, fewer exacerbations, particularly the most severe exacerbations, occurred in patients treated with ICSs compared with placebo.6 Subsequently, four long-term (3-year) randomized, placebo-controlled studies of ICS in patients with COPD were conducted to determine the effect of therapy on the rate of decline in pulmonary function, the results of which are discussed further herein.7–10 These studies identified varying effects of ICSs on outcomes of interest in COPD, but failed to show benefit of ICS monotherapy on pulmonary function (Table 1). A meta-analysis of ICS studies investigating lung function in patients with COPD showed that ICS use did not slow the rate of FEV1 decline in 3,571 patients over 24–54 months.11 In addition, a subsequent pooled analysis of 3,911 patients showed that after 6 months, ICS therapy did not modify FEV1 decline in patients with moderate-severe COPD.12
Table 1.
Long-term studies of ICSs as monotherapy for patients with COPD
| Study | Inclusion criteria/patients, n | Treatment | Primary efficacy outcome | Secondary efficacy outcomes | Safety |
|---|---|---|---|---|---|
| Wise et al7 | Participants from LHS (smoking-cessation trial) who were current smokers or had quit within 2 years; 40–69 years of age; FEV1:FVC <0.7; FEV1 30%–90% predicted; N=1,116 (ICS n=559, Pbo n=557); baseline postbronchodilator FEV1% predicted, ICS 69%, Pbo 67% | Triamcinolone acetonide MDI 100 µg/inhalation; Pbo identical vehicle MDI; six inhalations BID; total daily ICS dose 1,200 µg | Rate of decline in FEV1 postbronchodilator NS; mean decline, ICS 44.2 mL/year, Pbo 47.0 mL/year (95% CI for difference −11.0 to 5.4) | Respiratory symptoms (ATS questionnaire) at 36 months, NS for cough, phlegm, wheezing; dyspnea, P=0.02 (no dyspnea, ICS 68.2%, Pbo 61.5%); new or worsening respiratory symptoms per 100 PY, ICS 21.1, Pbo 28.2 (P=0.005); respiratory hospitalizations per 100 PY, ICS 0.99, Pbo 2.1 (P=0.07); respiratory ED visits per 100 PY, ICS 1.3, Pbo 1.0 (P=0.36); respiratory outpatient visits, ICS 1.2, Pbo 2.1 (P=0.03); airway reactivity, P=0.02 at 9 and 33 months in favor of ICS; HRQoL (SF36), mental health subscale score (change from baseline to 36 months), ICS −2.3; Pbo −0.1 (P=0.03) | All-cause mortality, ICS n=15; Pbo n=19 (P=0.49); thrush, ICS n=5; Pbo n=2; moderate-severe throat irritation, ICS 2.3%, Pbo 1.1% (P=0.02); moderate-severe bruising, ICS 0.8%, Pbo 0.4%; cataracts, ICS n=122, Pbo n=114; BMD (% change from baseline to 36 months), lumbar spine – ICS -0.35, Pbo 0.98 (P=0.007); femoral neck – ICS −2, Pbo −0.22 (P<0.001) |
| Pauwels et al8 | Current smokers ≥5 cigarettes/day or smoking history ≥10 years or ≥5 pack-years; FEV1:slow vital capacity <0.7; FEV1 50%–100% predicted, N=1,277 (ICS n=634, Pbo n=643); baseline prebronchodilator FEV1, ICS 77%, Pbo 77% | Budesonide DPI 400 µg; Pbo DPI; BID for 3 years | Change over time in FEV1: over the first 6 months, ICS improved FEV1 at rate of 17 mL/year vs Pbo decline of 81 mL/year (P<0.001) From 9 months to end of treatment, NS difference: ICS −57 mL/year, Pbo −69 mL/year >3 years in study completers: ICS −140 mL, Pbo −180 mL (P=0.05) | ICS had more beneficial effect in patients who smoked less; age, sex, baseline FEV1, presence or absence of serum IgE antibodies, and reversibility of airflow limitation had no effect on outcome | Skin bruising, ICS n=63 (10%), Pbo n=27 (4%) (P<0.001); oral candidiasis, ICS n=31, Pbo n=10 (P<0.001); BMD (measured in 194 patients), femoral trochanter decline – ICS 0.04%, Pbo 0.38% (P=0.02); cataracts, bone fractures, diabetes similarly distributed between groups |
| Vestbo et al9 | 30–70 years of age; FEV1:FVC <0.7; no self-reported asthma; N=290 (ICS n=145, Pbo n=145); baseline postbronchodilator FEV1, ICS 86%, Pbo 87% | Budesonide DPI; Pbo DPI; 800 µg in morning and 400 µg in evening for 6 months followed by 400 µg BID for 30 months | Rate of FEV1 decline: ICS 46.0 mL/year, Pbo 49.6 mL/year (P=0.70), with no effect of sex, smoking status, or baseline FEV1 | Symptoms decreased in both treatment groups with no differences; exacerbations, ICS 155, Pbo 161; reversibility to β2-agonist from visit one to end of study, ICS 8.1% to 6.7%, Pbo 7.2% to 8.2% | Pneumonia: ICS n=16, Pbo n=24 |
| Burge et al10 | Current or former smokers; 40–75 years of age; nonasthmatic COPD; FEV1 postbronchodilator ≥0.8 L, but <85% predicted normal; FEV1:FVC <0.7; N=751 (ICS n=376, Pbo n=375); baseline postbronchodilator FEV1% predicted, ICS 50%, Pbo 50% | Fluticasone propionate MDI with spacer 500 µg; Pbo MDI with spacer; BID for 3 years | Decline in FEV1 postbronchodilator: ICS 50 mL/year, Pbo 59 mL/year (P=0.16), with no effect of smoking status, age, sex, or FEV1 response to oral corticosteroids At 3 and 36 months, predicted mean FEV1 76 and 100 mL higher with ICS vs Pbo, respectively (P<0.001) |
Exacerbation rate (median), ICS 0.99/year, Pbo 1.32/year (P=0.026); SGRQ similar at baseline and 6 months, thereafter increased over time and worsened at a faster rate with Pbo (3.2 units/year) vs ICS (2.0 units/year) (P=0.004) | Hoarseness/dysphonia, ICS n=35, Pbo n=16; throat irritation, ICS n=43, Pbo n=27; oral candidiasis, ICS n=41, Pbo n=24; bruising, ICS n=27, Pbo n=15; fractures, ICS n=9, Pbo n=17; cataracts, ICS n=5, Pbo n=7 |
Abbreviations: ATS, American Thoracic Society; BID, bis in die (twice daily); BMD, bone-mineral density; DPI, dry-powder inhaler; ED, emergency department; ERS, European Respiratory Society; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HRQoL, health-related quality of life; ICS, inhaled corticosteroid; LHS, lung health study; MDI, metered-dose inhaler; NS, not significant; Pbo, placebo; PY, person-years; SGRQ, St George’s Respiratory Questionnaire.
Lung Health Study II
Participants from the Lung Health Study smoking-cessation trial were recruited for a second study to assess the effect of triamcinolone acetonide in delaying decline in lung function in participants with COPD.7 A total of 1,116 smokers (or those who had quit smoking within the prior 2 years) with airflow obstruction, defined as an FEV1 to forced vital capacity (FVC) ratio <0.70 and FEV1 30%–90% predicted, were enrolled. Although asthma diagnosis was not technically an exclusion criterion, patients who used bronchodilators or ICSs regularly were excluded, effectively excluding those with symptomatic asthma. The primary outcome measure, rate of decline in postbronchodilator FEV1, showed no significant effect of ICS treatment vs placebo (44.2 vs 47.0 mL per year, respectively). Most respiratory symptoms, including cough, phlegm, wheezing, and breathlessness, over the preceding year did not differ significantly between treatment groups at 36 months. Fewer new or worsening respiratory symptoms were found in the ICS group. The rate of unscheduled physicians’ visits and hospitalization for respiratory conditions was lower in the ICS group, but visits to the emergency department (for respiratory and nonrespiratory conditions) and all health-care visits (for nonrespiratory conditions) were similar between treatment groups. At baseline, airway reactivity was similar between groups. At 9 and 33 months, the ICS group had significantly less reactivity to methacholine challenge than placebo (P=0.02). Overall, health-related quality of life (measured by the SF36) showed no changes associated with treatment, except for a slightly worse mental health subscale score at 36 months in the ICS group compared with placebo.
European Respiratory Society study on COPD
Across nine European countries, 1,277 smokers aged 30–65 years with postbronchodilator FEV1 50%–100% predicted and prebronchodilator FEV1:slow vital capacity ratio <70% were randomized to receive budesonide dry-powder inhaler or placebo for 3 years.8 Patients with a history of asthma were excluded. Changes in postbronchodilator FEV1 over the first 6 months were significantly different between ICSs (improved at a rate of 17 mL/year) and placebo (declined by a rate of 81 mL/year, P<0.001); however, by 9 months the slopes of FEV1 decline were similar between treatment groups (P=0.39). Among those who completed the 3-year study (n=912), the median decline in FEV1 over 3 years was 140 mL in the ICS group and 180 mL in the placebo group (P=0.05). ICS use was more effective in patients who smoked less, but there was no association of the slope of FEV1 decline with age, sex, baseline FEV1, presence/absence of serum IgE antibodies, or reversibility of airflow limitation.
Copenhagen City Lung Study
Participants in the Copenhagen City Heart Study were eligible for the lung study if they were aged 30–70 years, had an FEV1:FVC ratio ≤0.7, and had no self-reported asthma.9 Smoking history was not an inclusion criterion, and long-term CS treatment (more than two episodes of >4 weeks duration) was the main exclusion criterion. A total of 290 patients were randomized to budesonide or placebo. There was no significant effect of ICSs on rate of FEV1 decline, and stratification by sex, smoking status, and baseline FEV1 did not affect these results. No significant differences were observed between treatment groups for occurrence of symptoms, exacerbations, or reversibility to β2-agonist treatment.
Inhaled Steroids in Obstructive Lung Disease in Europe (ISOLDE) study
Current or former smokers aged 40–75 years with non-asthmatic COPD, FEV1:FVC ratio <70%, baseline post-bronchodilator FEV1 ≥0.8 L but <85% predicted were eligible for the ISOLDE study.10 A total of 751 patients were randomized to fluticasone propionate or placebo. No significant difference in annual rate of FEV1 decline was observed between ICSs and placebo (50 vs 59 mL/year, P=0.16), and slopes of decline were not influenced by smoking status, age, sex, or FEV1 response to oral CSs. The predicted mean FEV1 at 3 and 36 months was significantly higher with ICSs by 76 and 100 mL, respectively, vs placebo (P<0.001). The median yearly exacerbation rate was 25% lower for ICS vs placebo (0.99 vs 1.32 per year, P=0.026). In both treatment groups, health status measured by the disease-specific St George’s Respiratory Questionnaire (SGRQ) improved after the first 6 months of treatment (slight decrease in SGRQ total score), but thereafter it worsened (increase in SGRQ score), and the rate of worsening was slower with ICSs than placebo (increase of 2.0 vs 3.2 units/year, respectively, P=0.004).
The results of these four studies consistently showed that ICS monotherapy did not reduce the accelerated rate of decline in pulmonary function that is characteristic of COPD, and thus ICS monotherapy is not disease-modifying. The only therapy proven to slow FEV1 decline in COPD is smoking cessation.13 However, ICSs improved some key secondary outcomes, including COPD symptoms, health-care utilization, airway reactivity, and notably the frequency of exacerbations. Systematic reviews of randomized controlled trials with at least 6 months of follow-up comparing ICS monotherapy vs placebo found a statistically significant reduction (18%–24%) in risk of exacerbations with ICSs.14,15 Moreover, when the reduction in exacerbation risk by ICSs was regressed against initial FEV1 percentage predicted, it was found that the more severe the airflow obstruction, the greater the risk reduction.14 In addition, ICS therapy was also shown to decelerate the rate of worsening of health status measured by the SGRQ, with a 1.4-unit improvement (decrease in SGRQ score) relative to placebo.14 A Cochrane database review of randomized controlled trials comparing ICSs and placebo also showed the effect of ICSs on reducing exacerbations and slowing the rate of decline in health-related quality of life.16 The ICS effect was not predicted by oral CS response, bronchodilator reversibility, or bronchial hyperresponsiveness.
The net impact of these studies in today’s world of combination therapy for COPD is that effects of CSs, at least when administered alone, are often slow to develop, and responses in the first 6 months of therapy may not remain the same in the long term. Far fewer studies have been done on the effect of CS withdrawal in COPD, and few current combination-therapy studies require a 6-month washout prior to initiation of the trial.
Barnes et al reviewed cellular and molecular mechanisms of COPD pathogenesis and proposed several factors that may explain the limited response to ICS monotherapy in this disease.17,18 ICSs or oral CSs do not suppress inflammation in COPD, even at high dosages. In COPD, the numbers of airway neutrophils are increased, but they are not fully suppressed by CSs. In addition, resistance to CSs may be related to decreased activity and expression of HDAC2 in inflammatory cells of patients with COPD as a result of increased oxidative and nitrative stress from cigarette smoking. ICSs may have a small bronchodilator effect that is not disease-modifying, but additional investigation is warranted. The lack of significant efficacy on the rate of decline of FEV1 may have prevented exploration of the dose response for ICSs in COPD.1 In 2014, a randomized, prospective study compared two dosages of fluticasone (500 and 1,000 µg/day) in patients with COPD and an FEV1:FVC ratio <70%, FEV1 <80% predicted, and smoking history >10 pack-years.19 The higher dosage was associated with improved lung function and symptoms, decreased exacerbations, and better quality of life compared to the lower dosage. In asthma, there is a tendency to administer higher dosages of ICS than needed for symptom control; however, this may increase the risk of adverse events, such as adrenal suppression, osteoporosis, and growth inhibition in children.20,21 Therefore, for US Food and Drug Administration (FDA) regulatory approval, efficacy differences between dosage groups must be demonstrated for COPD.22
Use of ICSs in combination with LABAs
Combining drugs with different modes of action may improve outcomes. Two-way synergistic activity between ICSs and LABAs has been demonstrated.23,24 One of the cellular actions of ICSs is to translocate glucocorticoid receptors from the cytoplasm to the nucleus.24 This action is enhanced in the presence of β-agonists and causes an anti-inflammatory effect greater than either drug alone, without the need to increase the ICS dosage.23 In addition, ICSs activate β-receptor genes to produce more β-receptors, thereby enhancing the bronchodilator effect of LABAs.25 Numerous clinical studies have been conducted evaluating ICS-LABA combinations in patients with COPD, and systematic reviews and meta-analyses have pooled their results to inform treatment decisions. Relevant studies are discussed in the following paragraphs.
The Towards a Revolution in COPD Health (TORCH) trial was a pivotal, double-blind, placebo-controlled, randomized study comparing salmeterol plus fluticasone propionate (50 and 500 µg, respectively, taken twice daily) with each component alone and placebo over 3 years.26 Patients with COPD were enrolled if they had at least a 10-pack-year smoking history, FEV1 <60% predicted, and an FEV1:FVC ratio ≤0.70.26 Among 6,184 randomized patients, the risk of death was reduced by 17.5% with the ICS-LABA combination vs placebo (P=0.052). ICS-LABA significantly reduced the rate of exacerbations by 25% compared with placebo (P<0.001) and improved health status and FEV1 compared with either component alone or placebo. A subsequent double-blind, randomized, parallel-group study included patients aged ≥40 years with COPD who had at least a 10-pack-year smoking history, FEV1:FVC ratio ≤0.70, FEV1 ≤50% predicted, and at least one exacerbation in the past year requiring oral CSs, antibiotics, or hospitalization.27 Among 782 randomized patients, a 30.5% reduction in mean annual rate of moderate-severe exacerbations was observed with salmeterol plus fluticasone propionate (50 and 250 µg, respectively) compared with salmeterol alone (P<0.001) at half the dose of fluticasone propionate used in the TORCH study.
In a 2003 review of three ICS-LABA combination-therapy studies in COPD patients, a 30% reduction in exacerbations was observed vs placebo and trough FEV1 improved vs placebo (101 mL/year, 95% CI 76–126) or either therapeutic agent alone (ICS 50 mL/year, 95% CI 26–74; LABA 34 mL/year, 95% CI 11–57).14 In two Cochrane database systematic reviews, ICS-LABA combination therapy administered in a single inhaler was compared to LABA or ICS monotherapy.28,29 Across nine eligible studies comparing ICS-LABA to LABA alone, the exacerbation rate was reduced by 24% (95% CI 0.68–0.84) with combination therapy, but there was no difference in mortality (OR 0.92, 95% CI 0.76–1.11).28
In six studies that compared ICS-LABA vs ICS monotherapy, a significant 13% reduction in the rate of exacerbations was noted (RR 0.87, 95% CI 0.80–0.94) and the odds of death were significantly lower with combination therapy (OR 0.78, 95% CI 0.64–0.94).29 In addition, a 2014 Bayesian network meta-analysis evaluated randomized controlled trials of at least 12 weeks duration comparing fixed-dose ICS-LABA combinations with active control or placebo, and found that ICS-LABA reduced moderate-severe exacerbations, with the exception of beclomethasone dipropionate–formoterol, which had the lowest sample size of all groups (Figure 1).30 HRs ranged from 0.59–0.92 for ICS-LABA vs placebo and 0.63–0.98 for ICS-LABA vs LABA monotherapy. Medium-and high-dose ICS-LABA combinations were similarly effective in reducing the rate of moderate-severe exacerbations.
Figure 1.
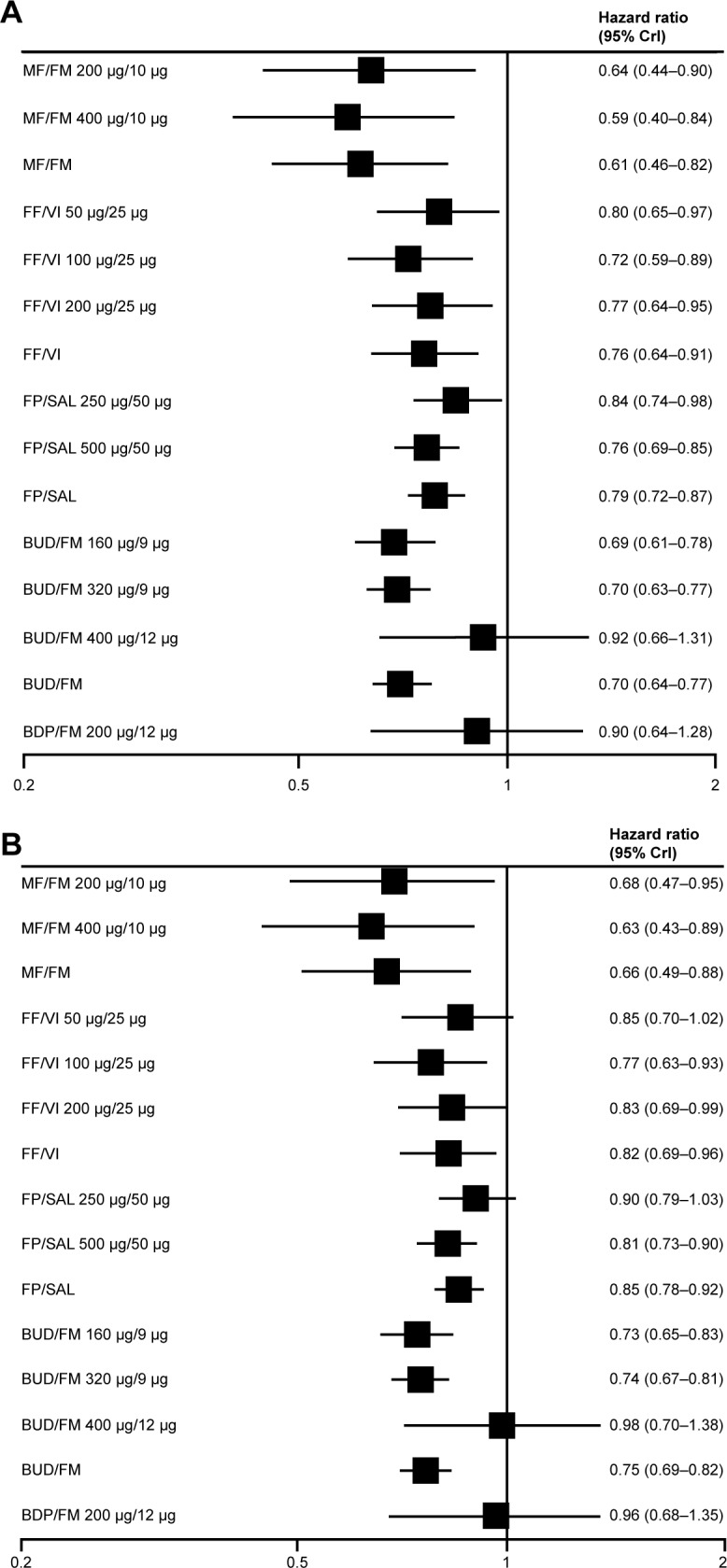
Effectiveness of ICS-LABA inhalers.
Notes: Pooled effect estimates of combined ICS-LABA inhalers vs (A) placebo and (B) LABA on moderate-severe exacerbations. Copyright ©2014. Dove Medical Press. Reproduced with permission from Oba Y and Lone NA. Comparative efficacy of inhaled corticosteroid and long-acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:469–479.30
Abbreviations: BDP, beclomethasone dipropionate; BUD, budesonide; CrI, credibility interval; FF, fluticasone furoate; FM, formoterol; FP, fluticasone propionate; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; MF, mometasone furoate; SAL, salmeterol; VI, vilanterol.
Estimates of treatment effect across these systematic reviews may have differed as a result of clinical trial heterogeneity, particularly with regard to baseline exacerbation history and lung function and how a COPD exacerbation was defined within a study.29,31 Despite these differences, a clear effect of ICS-LABA combination therapy on exacerbations was demonstrated, and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018 report concluded that ICS-LABA combination therapy was more effective than either agent alone in reducing exacerbations, as well as improving lung function and health status.1
Recent post hoc analyses of published trials have examined the early response to ICS-LABA combination therapy.32,33 Lower exacerbation rates and improved lung function (FEV1 and PEF) were evident as early as 3 months after starting treatment with budesonide–formoterol vs placebo. Early improvements in FEV1 and total score on the SGRQ were associated with future response, and early FEV1 improvements (but not SGRQ-score improvements) predicted lower risk of future COPD exacerbations.
Long-acting muscarinic antagonists (LAMAs), such as tiotropium, have a prolonged bronchodilator effect that has been shown to reduce exacerbations to a greater degree than LABA monotherapy.1,34,35 Despite being the most commonly prescribed first-line treatments, few clinical studies have compared LAMA monotherapy to ICS-LABA combination therapy.36 Using a large administrative claims database in the US, the real-world effectiveness of tiotropium was compared to budesonide–formoterol. The ICS-LABA combination reduced the risk of COPD exacerbation by 22% compared with LAMA monotherapy; however, the results could have been skewed by a high number of patients having possible comorbid asthma. The Investigating New Standards for Prophylaxis in Reducing Exacerbations (INSPIRE) study compared the efficacy of salmeterol–fluticasone with tiotropium monotherapy in preventing exacerbations in patients with severe and very severe COPD.37 The exacerbation rate was not significantly different between the groups treated with ICS-LABA therapy and LAMA monotherapy, but the ICS-LABA group had better health status and was less likely to withdraw. The fact that LABAs and LAMAs work on different receptors to induce bronchodilation provides a rationale for their use in combination to optimize bronchodilation.38 The comparative efficacy of LAMA-LABA (dual bronchodilators) vs ICS-LABA therapy in reducing exacerbations is currently a matter of keen interest. The Effect of Indacaterol Glycopyrronium vs Fluticasone Salmeterol on COPD Exacerbations (FLAME) study compared the efficacy of a LAMA-LABA combination (glycopyrronium 50 µg–indacaterol 110 µg) with ICS-LABA (fluticasone 500 µg–salmeterol 50 µg) therapy in reducing exacerbation risk for patients with COPD.39 For patients with a history of exacerbation in the previous year, the annual rate of moderate or severe exacerbation was significantly lower for the LAMA-LABA group (0.98) than for the ICS-LABA group (1.19, P<0.001).
Predictors of response
Post hoc analyses suggest eosinophil counts in blood and sputum may be used as predictive biomarkers for the efficacy of ICSs in reducing exacerbations.40–43 Dose–response effectiveness of ICSs has been demonstrated in patients with elevated eosinophils,44 and eosinophil levels may be able to direct treatment during COPD exacerbations. Eosinophilic inflammation in COPD, defined as sputum eosinophils ≥3%, has been reported in up to 28% of cases during an acute exacerbation and up to 38% of patients with stable disease.44 However, measurement of sputum eosinophils is unsuitable for point-of-care testing and requires experience to differentiate inflammatory cell counts. Measurement of blood eosinophils may be a more useful biomarker for routine practice. The threshold for eosinophilic inflammation continues to be debated, with some reports suggesting a threshold of >2%, 3%, or 4% of the total white-blood-cell count, whereas others propose a total eosinophil count of 150, 220, or 300 cells/µL.45,46
The INCONTROL study showed that for patients with blood-eosinophil counts ≥100 cells/µL, exacerbations were reduced significantly more with budesonide–formoterol compared with formoterol alone (P=0.015).42 The higher the blood-eosinophil count, the greater the exacerbation rate without ICSs and the greater the reduction in exacerbations with ICSs, which tends to reach a plateau around eosinophil counts of 400 cells/µL. The impact of blood-eosinophil count on response to ICSs was also found to be greater as the eosinophil count increased by Pascoe et al in their analysis of data from two parallel randomized controlled trials on the addition of fluticasone furoate to vilanterol.47
Disparate findings for blood eosinophils were reported in the Subpopulations and Intermediate Outcome Measures in COPD (SPIROMICS) cohort, in which blood eosinophils alone were not a reliable predictor of COPD exacerbations in contrast to sputum eosinophils, and the association between blood and sputum eosinophils was weak.48 Differences in study populations could account for the disparate findings, as the INCONTROL study had a larger number of smokers with more exacerbations compared with the SPIROMICS cohort.42,48
Elevated eosinophil counts in the blood and airway walls are also observed in patients with COPD who have other signs of inflammation.49 Tamada et al evaluated 331 COPD patients for asthma-like airway inflammation or atopic factors using fractional exhaled nitric oxide and serum IgE, respectively.50 High fractional exhaled nitric oxide (≥35 parts per billion) was present in 16% of patients, high IgE (≥173 IU/mL) occurred in 36% of patients, and both factors were present in 8% of patients. Furthermore, there is another type of COPD that occurs with asthma, termed asthma–COPD overlap (ACO). A clear definition of ACO has yet to be determined,50,51 but a systematic review and meta-analysis of 17 studies including COPD and asthma used a definition of any COPD patient with at least one of the following asthma characteristics: diagnosis of asthma, FEV1 reversibility ≥12% and ≥200 mL of change from baseline, PEF variability ≥20%, and airway hyperresponsiveness to methacholine or histamine.51 Across the 17 studies, the pooled prevalence of ACO was 27% and 28% in population- and hospital-based studies, respectively. In five studies, ACO was associated with worse outcomes, including more frequent exacerbations, hospitalizations, and emergency-department visits, and two studies reported significantly higher use of ICS-LABA combinations in patients with ACO than those with COPD. Objective measures of airway inflammation and/or atopy (eg, IgE levels) indicate a smaller proportion of patients with ACO defined by symptoms, lung function, or physician diagnosis; however, these measures may be important for identifying patients who would benefit from ICS-LABA therapy.1 The presence of eosinophilic inflammation has not been studied extensively in this population.
Effect of smoking status on ICS response
Despite a causal link between cigarette smoking and COPD, a substantial percentage of patients with moderate-severe COPD continue to smoke (30%–43%).52 Sustained smoking cessation reduces the rate of decline in FEV1 and decreases respiratory symptoms among smokers with early COPD.53 However, reductions in the amount of smoking up to 50% have no observable effect on the decline in FEV1, suggesting that total or near-total abstinence from smoking is required to reduce the effect of cigarette smoke on lung function.53
The adverse effects of smoking in patients with COPD or asthma have been attributed to increased airway inflammation and reduced CS responsiveness,54 but the majority of studies examining the effect of smoking on CS responsiveness have been conducted in patients with asthma.55 Although it has been proposed that the oxidative and nitrative stress associated with cigarette smoking may inactivate HDAC2 in COPD patients and contribute to ICS resistance,17 studies of smokers and ex-smokers generally have failed to identify a significant difference of ICSs on clinical and inflammatory parameters, including HDAC2.55,56 Reductions in bronchial mast cells have been observed with both short- and long-term ICS treatment in smokers and ex-smokers with COPD, whereas reductions in CD3+, CD4+, and CD8+ cells were observed with short-term ICSs in current smokers only.55 These differential effects of ICSs on specific cell types may be related to epigenetic regulation occurring with DNA methylation and histone modification.55 In a comparison of ICS therapy in smokers and ex-smokers with COPD, IL8 and neutrophil-related elastase activity increased in smokers and were unchanged or decreased in ex-smokers.57 Sputum eosinophils may be reduced in smokers as a result of the nitric oxide and carbon monoxide present in cigarette smoke.54 A recent post hoc analysis of budesonide–formoterol studies in patients with COPD (INCONTROL) found that among current smokers, a greater reduction in exacerbation rate was observed with ICS-LABA therapy vs LABA alone at higher peripheral blood-eosinophil counts.42 These cell- and tissue-specific responses may be related to the heterogeneous nature of COPD, and suggest that reduced steroid responsiveness is not a general characteristic of the disease.42,55 These findings highlight the need for further evaluation of appropriate biomarkers of disease severity and treatment selection and their relationship to smoking status.
Safety considerations
A review of long-term studies of ICS monotherapy in patients with COPD revealed important information about their safety (Table 1). Skin bruising and oral candidiasis were increased with ICS therapy in most studies.7,8,10,43,58 In a subset of patients from the Lung Health Study, no effect of ICSs on adrenal function was observed over the 3-year study duration.59 These pivotal long-term studies of ICS mono-therapy in COPD patients did not identify a difference in occurrence of cataracts compared with placebo.7,8,10 However, an increased risk of cataracts was observed in a population-based, cross-sectional study of more than 3,000 patients, in which ICS use was associated with significantly increased prevalence of nuclear and posterior subcapsular cataracts, and higher cumulative lifetime doses were associated with higher risks.60 In a follow-up analysis 10 years later, the risk of incident cataracts was significant only for patients who had ever used both ICSs and oral CSs.61 Moreover, using a large electronic medical record database in the UK, ICSs or ICS-LABA (fluticasone propionate–salmeterol) combination therapy was not associated with increased risks of cataracts or glaucoma.62 The 1-year Efficacy and Tolerability of Budes-onide/Formoterol in One Hydrofluoroalkane Pressurized Metered-Dose Inhaler in Patients with Chronic Obstructive Pulmonary Disease (SUN) study of budesonide–formoterol in COPD patients identified numerically more frequent adverse events typically associated with ICSs, including oral candidiasis, ocular effects, skin effects, and bone effects, in the ICS-LABA group than the LABA-alone or placebo groups.63 However, there were no differences in objectively measured changes in lenticular opacity or intraocular pressure, nor clinically relevant changes in bone-mineral density (BMD), across the three treatment groups.
Osteoporosis is a systemic feature of COPD, with prevalence that is two to five times higher than that in age-matched subjects without airflow obstruction.64 In a cross-sectional study of COPD patients, the dosage or duration of ICSs or oral CSs used was not different between those with osteoporosis and those with normal bone mass.64 Over the 3-year TORCH study, changes in BMD at the hip and lumbar spine were small, and there were no significant differences between any of the active treatment groups (ICS-LABA, ICS alone, LABA alone) and placebo.65 Long-term studies analyzed in the Cochrane database review did not show any major effect of ICS therapy on fractures or BMD over 3 years.16 In contrast, a meta-analysis of 16 randomized controlled trials (of which 14 evaluated fluticasone and two budesonide) showed an increased risk of fractures (>20%) with more than 24 weeks of ICS therapy vs control.66 Significant reduction in BMD at the lumbar spine (1.33%, P=0.007) and femoral neck (1.78%, P<0.001) was observed with triamcinolone in the Lung Health Study,7,67 but not with budesonide in the European Respiratory Society study (a small but significant decline in femoral trochanter BMD was observed with placebo vs budesonide, P=0.02).8 Current recommendations suggest measuring BMD in patients with COPD intermittently to assess fracture risk and treating those with significantly reduced BMD.68
Effect of ICSs on pneumonia risk in patients with COPD
The risk of pneumonia is increased in patients with COPD and further increased in those with a history of exacerbations and more severe disease.69,70 ICS-containing therapy for COPD has generally been associated with an increased risk of nonfatal pneumonia.70 A 2009 meta-analysis of 18 studies of ICS therapy in COPD estimated an approximately 60% increased risk of pneumonia without a significant increase in pneumonia-related death or overall mortality.71 In a new-user cohort study using a medical record database, ICS use was associated with a 49% increased risk of pneumonia (vs long-acting bronchodilator) that was attenuated to 19% with at least 6 months of exposure.70
Differences in study design and duration, population studied, and definition of pneumonia events may contribute to variability in pneumonia rates across studies.69,70 Pneumonia risk may also differ by the specific ICS used. In the 2009 meta-analysis, two of 18 trials evaluated budesonide at 800 µg/day, and 16 of 18 trials evaluated fluticasone propionate at dosages of 1,000 µg/day (N=10) or 250 µg/day (N=6). In a separate meta-analysis of seven large budesonide studies in COPD, no significant increased risk of pneumonia was determined with budesonide vs control of either placebo or formoterol (overall risk 1.05, 95% CI 0.81–1.37).72 The Investigation of the Past 10 Years Health Care for Primary Care Patients With Chronic Obstructive Pulmonary Disease (PATHOS) study investigated the incidence of pneumonia in patients with COPD using data from national Swedish health registries, comparing propensity-matched populations treated with budesonide–formoterol (N=2,734) and fluticasone–salmeterol (N=2,734).73 Patients in the fluticasone–salmeterol group experienced an approximately 75% greater occurrence of pneumonia, including pneumonia requiring hospitalization, compared with the budesonide–formoterol group (P<0.001). Additionally, among patients using ICSs at baseline and followed for 4 years in the Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) study, the risk of pneumonia was increased by >20% compared with those who did not use ICSs, but this increased risk was noted only in patients receiving fluticasone propionate and not in those using other ICSs (Table 2).74 Fluticasone was also associated with a higher risk of any pneumonia, but not serious pneumonia, compared with budesonide in a Cochrane database review of 43 randomized controlled trials (26 fluticasone studies and 17 budesonide studies).75 Differences in outcome between ICSs and placebo may be due to uneven distribution of baseline characteristics because patients with more severe disease received more intensive treatment; however, patient subgroups based on the ICS they received were well matched, such that baseline characteristics cannot explain differences between fluticasone and other ICSs.74 Fluticasone differs structurally from beclomethasone and budesonide because it has a fluorine moiety, which leads to distribution in the lipid membranes and slower clearance from lungs and other tissue, which may impact lung immunity and inflammatory responses.74,76 This study also suggested that the use of LAMA therapy may ameliorate some of the respiratory adverse effects of ICSs, supporting their use in combination.74 The absolute risk of pneumonia is low,77 and the authors’ experience suggests that few physicians or patients avoid ICS therapy as a result of this risk. In summary, the physician and patient must consider the benefit of ICSs in reducing the future risk of exacerbations in relation to the increased pneumonia risk of ICS-containing therapy.70,72
Table 2.
Distribution of pneumonia events and incidence rates by treatment in the UPLIFT study
| Treatment | Events | Years in study | Incidence rate | Incidence-rate ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| Any ICS vs no ICS | |||||
| No ICS | 383 | 6,885 | 0.056 | Reference | |
| ICS | 738 | 10,836 | 0.068 | 1.22 (1.08–1.38) | 0.012 |
| FP vs other ICS and no ICS | |||||
| FP | 437 | 5,685 | 0.077 | 1.38 (1.20–1.58) | ,0.001 |
| Other ICS | 301 | 5,151 | 0.058 | 1.05 | 0.52 |
| No ICS | 383 | 6,885 | 0.056 | Reference | |
| Effect of Tio on pneumonia rate, irrespective of ICS | |||||
| FP/Pbo | 230 | 2,720 | 0.081 | 1.45 (1.19–1.77) | ,0.001 |
| FP/Tio | 217 | 2,964 | 0.073 | 1.31 (1.08–1.60) | 0.006 |
| Other ICS/Pbo | 153 | 2,461 | 0.062 | 1.12 (0.90–1.38) | 0.29 |
| Other ICS/Tio | 148 | 2,690 | 0.055 | 0.99 (0.79–1.23) | 0.94 |
| No ICS/Pbo | 184 | 3,317 | 0.055 | Reference | |
| No ICS/Tio | 199 | 3,567 | 0.056 | 1.00 (0.82–1.22) | 0.95 |
Note: Reproduced with permission from Morjaria JB, Rigby A, Morice AH. Inhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT study. Lung. 2017;195(3):281–288. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.74
Abbreviations: FP, fluticasone propionate; ICS, inhaled corticosteroid; Pbo, placebo; Tio, tiotropium.
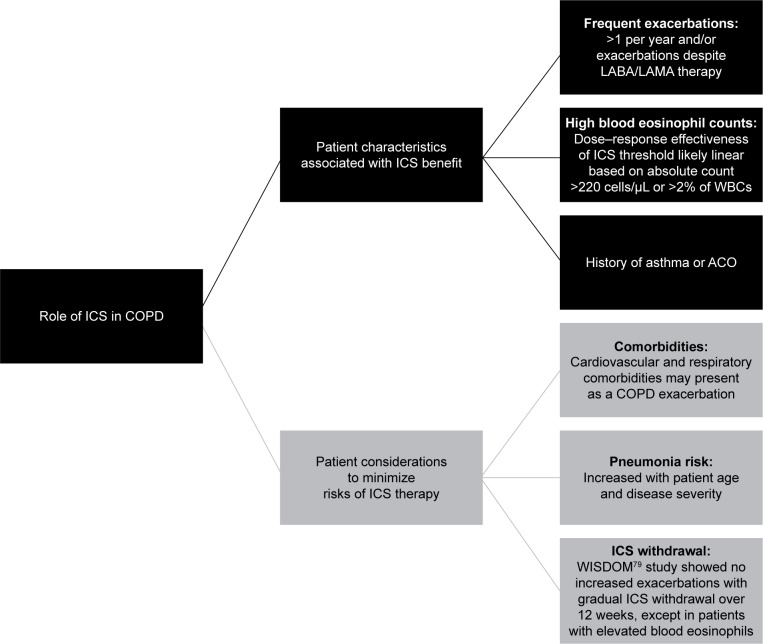
Role of ICSs in current COPD treatment
Large clinical trials and systematic reviews/meta-analyses of clinical trial data provide evidence for the development of COPD-treatment guidelines.1 Currently, GOLD guidelines provide recommendations for drug selection based on a patient’s symptom intensity and disease severity assessed by prior and future risk of exacerbations.1,45 ICS-LABA combinations have been shown to reduce exacerbations, improve lung function, and improve health status.14,26,28–30 ICS-LABA combinations are thus recommended as a treatment option for COPD patients with a history of frequent exacerbations,1 but they are commonly prescribed as first-line treatments, regardless of COPD severity.38,74 Over the past 5 years, there has been an increase in new drugs and delivery devices, adding to the complexity of COPD treatment options.45 However, the data reviewed herein provide information to identify patient groups that may benefit from ICS use (Figure 2). Concerns with ICS use are related to the safety considerations described in the previous section and the potential risks associated with withdrawal of ICS therapy.45,74,78 Long-term adverse events described in some studies are complicated by concomitant oral CS use and confounding disease severity, as well as comorbidities. It is important that exacerbations be differentiated from other events that may be related to common comorbidities of COPD, including acute coronary syndrome, worsening congestive heart failure, pulmonary embolism, and pneumonia.1 Although for many years it was believed that stopping ICS use could trigger an exacerbation, it has recently been shown that withdrawal of ICSs is possible, particularly when other medications are introduced concomitantly.45 In the Withdrawal of Inhaled Steroids during Optimized Bronchodilator Management (WISDOM) study, ICS therapy was withdrawn gradually over 12 weeks from a triple combination, without an overall increased risk of exacerbations compared with the group that remained on triple therapy (HR 1.06, 95% CI 0.94–1.19).79 However, in the group in which ICS therapy was withdrawn, FEV1 declined and health status tended to worsen significantly, albeit modestly, compared with patients who remained on ICS therapy. Moreover, in a subset of the overall patient population (~20%) with eosinophil counts ≥4% or ≥300 cells/µL, withdrawal of ICSs was associated with an increased risk of exacerbations.46 Still, for patients who are at low risk of exacerbation (ie, FEV1 >50% predicted, fewer than two exacerbations/year), ICSs can be withdrawn safely as long as maintenance LABA therapy is continued.80
Figure 2.
The role of ICS in patients with COPD.
Notes: Eosinophil thresholds for beneficial ICS effect are discussed in the text. Data regarding the effect of ICS on comorbidity presence or course are limited.
Abbreviations: ACO, asthma–COPD overlap; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; WBCs, white blood cells.
Future directions: triple therapy and beyond
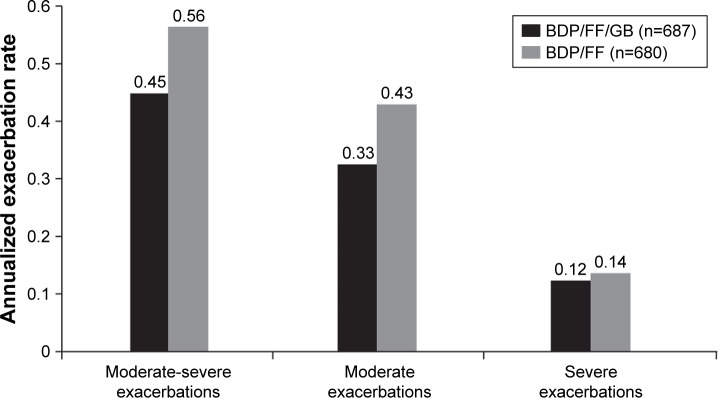
The step up to triple therapy with ICS + LABA + LAMA may improve lung function and patient-reported outcomes for COPD patients (eg, the addition of LAMA to ICS-LABA reduces exacerbation risk).1 In the Single Inhaler Triple Therapy vs Inhaled Corticosteroid Plus Long-acting β2-Agonist Therapy for Chronic Obstructive Pulmonary Disease (TRILOGY) study of COPD patients with severe or very severe airflow limitation (ie, FEV1 <50% predicted and at least one moderate or severe exacerbation in the previous 12 months), triple therapy (beclomethasone dipropionate–formoterol fumarate–glycopyrronium bromide) in a single inhaler significantly improved predose and postdose FEV1 and SGRQ total score and also reduced the exacerbation rate by 23% compared with beclomethasone dipropionate–formoterol fumarate (Figure 3).81 Patients enrolled in this study could have been receiving ICS-LABA, ICS-LAMA, or LABA-LAMA combination therapy or LAMA monotherapy and entered a 2-week run-in phase in which they received ICS-LABA prior to randomization. As such, escalation from LABA-LAMA therapy directly to triple therapy needs to be evaluated. The recently published Lung Function and Quality of Life Assessment in COPD with Closed Triple Therapy (FULFIL) study results support a benefit of single-inhaler triple therapy compared with ICS-LABA for lung function, health status, and exacerbation rate at 24 weeks.82 Moreover, results of the Informing the Pathway of COPD Treatment (IMPACT) study demonstrated that the triple combination of fluticasone furoate–umeclidinium–vilanterol reduced the rate of moderate or severe exacerbations more effectively than both the ICS-LABA (fluticasone furoate–vilanterol, 15% difference, P<0.001) and the LABA-LAMA (umeclidinium–vilanterol, 25% difference, P<0.001) combinations.83 Interestingly, these results imply that ICS-LABA might be superior to LABA-LAMA in reducing exacerbations in this >10,000-patient study, in direct contrast to the findings from the FLAME study.39 A number of factors may have contributed to the disparate findings in the IMPACT and FLAME studies, including different patient populations, study-design differences for the run-in period and ICS withdrawal, and different methods of statistical analyses of end points. More data are needed to determine appropriate patient selection criteria for ICS-LABA, LABA-LAMA, and triple-therapy regimens.
Figure 3.
COPD exacerbations in the TRILOGY study.
Notes: Unadjusted annual rate of COPD exacerbations of differing severity in the intent-to-treat population for the TRILOGY study. Reprinted from The Lancet, 388(10048), Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial, Pages 963–973, Copyright 2016, with permission from Elsevier.81
Abbreviations: BDP, beclomethasone dipropionate; FF, fluticasone furoate; GB, glycopyrronium bromide.
In contrast to these positive results for triple therapy, a 2015 systematic review and meta-analysis of seven trials of triple therapy using tiotropium and an ICS-LABA fixed-dose combination in a separate inhaler vs tiotropium monotherapy showed no significant benefit for triple therapy on mortality or exacerbations.84 Improvement in FEV1 and SGRQ score was greater with triple therapy, but lower than the minimal clinically important difference. Six of the seven trials were ≤24 weeks in duration. These findings highlight potential differences in triple-therapy regimens, the need for studies of longer duration, and the impact of single inhalers (presumably as a result of increased adherence) compared with multiple inhalers. Indeed, the use of multiple inhalers adds complexity to the treatment regimen, especially when considering the different types of inhalers currently available and patient preferences.85
An analysis of prescribing patterns in the UK indicated that 32% of patients received triple therapy between 2002 and 2010, regardless of GOLD severity category.86 Few patients in GOLD groups A, B, C, or D (as defined in the 2011 GOLD report) received triple therapy prior to or at initial diagnosis, but after initial diagnosis, prescriptions for triple therapy occurred in 19%, 28%, 37%, and 46% of patients in GOLD groups A, B, C, and D, respectively.86 The most frequent treatment-escalation pathway was from ICS-LABA to triple therapy. Therefore, the majority of COPD patients may be overtreated compared with GOLD guidelines, with 75% of those receiving triple therapy having only mild or moderate COPD.87,88 However, it is important to note that there have been no studies to evaluate the use of aggressive treatment to reduce COPD exacerbations followed by de-escalation of the therapy. Triple therapy may allow the assumption that a patient is receiving optimal treatment for COPD with optimal bronchodilation via two mechanisms plus anti-inflammatory effects.86 In addition, triple therapy does not appear to be associated with a greater risk of adverse events compared with ICS-LABA or LAMA monotherapy.81,84
Fluticasone furoate–umeclidinium–vilanterol inhalation powder (Trelegy Ellipta; GlaxoSmithKline, Research Triangle Park, NC, USA) was recently approved by the FDA as the first once-daily single-inhaler triple therapy for COPD.89 Combination triple therapy in a single inhaler may increase the likelihood of better adherence,85 but it also increases the expense of the product in the absence of generic equivalents.45 National Institute for Health and Care Excellence guidelines state that triple therapy is cost-effective only in patients who have FEV1 <50% predicted and frequent exacerbations (two or more in past 12 months).86,90 In this regard, it is interesting that only 2.1% of patients in the SPIROMICS cohort, of whom 30% had severe airflow obstruction, had two or more exacerbations in each year over a 3-year period.91 Therefore, the frequent exacerbator phenotype appears to be much less common than reported in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study.92 Accurate assessment of exacerbation history and future risk is thus necessary to guide individualized therapy decisions in patients with COPD.
For patients with COPD who continue to experience exacerbations while receiving LABA-LAMA-ICS therapy, the addition of a phosphodiesterase 4 inhibitor or macrolide antibiotic has been recommended.1 Moreover, therapeutic agents targeting eosinophils, namely anti-IL5 and anti-IL5-receptor monoclonal antibodies, have been evaluated to reduce the risk of exacerbations in COPD patients with high eosinophil counts and a history of exacerbations despite optimized standard-of-care therapy;43 however, results have been inconsistent.93–95
Overall, the use of ICSs in dual and triple therapy for COPD has been shown to reduce exacerbations and improve symptoms. Furthermore, individualizing therapies based on each patient’s phenotype, including risk factors and comorbidities, has the potential to maximize the benefit:risk ratio of COPD treatment.
Acknowledgments
Medical writing support was provided by Katie Gersh, PhD of MedErgy (Yardley, PA, USA), in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE, USA). AstraZeneca reviewed the manuscript for medical accuracy.
Footnotes
Disclosure
DPT has served on advisory boards for AstraZeneca, Novartis, and Sunovion; as a speaker for Boehringer Ingelheim, AstraZeneca, and Sunovion; and as a consultant for Theravance/Innoviva. CS has current, past, or pending grants in COPD from Adverum, the Alpha-1 Foundation, BTG, CSL Behring, Grifols, MatRx, NIH, Novartis, PneumRx, and Shire. He consults for Abeona, AstraZeneca, CSA Medical, CSL Behring, GlaxoSmithKline, Grifols, and Uptake Medical on COPD. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management, and Prevention of COPD. Bethesda (MD): GOLD; 2018. [Google Scholar]
- 2.Saetta M, Finkelstein R, Cosio MG. Morphological and cellular basis for airflow limitation in smokers. Eur Respir J. 1994;7(8):1505–1515. doi: 10.1183/09031936.94.07081505. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1666–1672. doi: 10.1164/ajrccm.152.5.7582312. [DOI] [PubMed] [Google Scholar]
- 4.Thompson AB, Mueller MB, Heires AJ, et al. Aerosolized beclomethasone in chronic bronchitis: improved pulmonary function and diminished airway inflammation. Am Rev Respir Dis. 1992;146(2):389–395. doi: 10.1164/ajrccm/146.2.389. [DOI] [PubMed] [Google Scholar]
- 5.Confalonieri M, Mainardi E, della Porta R, et al. Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(7):583–585. doi: 10.1136/thx.53.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. Lancet. 1998;351(9105):773–780. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 7.Wise R, Connett J, Weinmann G, Scanlon P, Skeans M. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343(26):1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels RA, Löfdahl C-G, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340(25):1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353(9167):1819–1823. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 10.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Highland KB, Strange C, Heffner JE. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease: a meta-analysis. Ann Intern Med. 2003;138(12):969–973. doi: 10.7326/0003-4819-138-12-200306170-00008. [DOI] [PubMed] [Google Scholar]
- 12.Soriano JB, Sin DD, Zhang X, et al. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest. 2007;131(3):682–689. doi: 10.1378/chest.06-1696. [DOI] [PubMed] [Google Scholar]
- 13.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272(19):1497–1505. [PubMed] [Google Scholar]
- 14.Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA. 2003;290(17):2301–2312. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations: a systematic review and metaregression of randomized controlled trials. Chest. 2010;137(2):318–325. doi: 10.1378/chest.09-1305. [DOI] [PubMed] [Google Scholar]
- 16.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(4):672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 19.Cheng SL, Su KC, Wang HC, Perng DW, Yang PC. Chronic obstructive pulmonary disease treated with inhaled medium- or high-dose corticosteroids: a prospective and randomized study focusing on clinical efficacy and the risk of pneumonia. Drug Des Devel Ther. 2014;8:601–607. doi: 10.2147/DDDT.S63100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boardman C, Chachi L, Gavrila A, et al. Mechanisms of glucocorticoid action and insensitivity in airways disease. Pulm Pharmacol Ther. 2014;29(2):129–143. doi: 10.1016/j.pupt.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Powell H, Gibson PG. High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children. Cochrane Database Syst Rev. 2004;2(2):CD004109. doi: 10.1002/14651858.CD004109.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food US, Administration Drug. Chronic obstructive pulmonary disease: developing drugs for treatment. 2016. [Accessed July 12, 2018]. Available from: https://www.gpo.gov/fdsys/pkg/FR-2016-05-20/pdf/2016-11855.pdf.
- 23.Usmani OS, Ito K, Maneechotesuwan K, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172(6):704–712. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- 24.Haque R, Hakim A, Moodley T, et al. Inhaled long-acting β2 agonists enhance glucocorticoid receptor nuclear translocation and efficacy in sputum macrophages in COPD. J Allergy Clin Immunol. 2013;132(5):1166–1173. doi: 10.1016/j.jaci.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J. 2002;19(1):182–191. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 26.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 µg) or salmeterol (50 µg) on COPD exacerbations. Respir Med. 2008;102(8):1099–1108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting β2-agonist in one inhaler versus long-acting β2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829. doi: 10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting β2-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826. doi: 10.1002/14651858.CD006826.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oba Y, Lone NA. Comparative efficacy of inhaled corticosteroid and long-acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:469–479. doi: 10.2147/COPD.S48492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miravitlles M, D’Urzo A, Singh D, Koblizek V. Pharmacological strategies to reduce exacerbation risk in COPD: a narrative review. Respir Res. 2016;17(1):112. doi: 10.1186/s12931-016-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calverley PM, Eriksson G, Jenkins CR, et al. Early efficacy of budesonide/formoterol in patients with moderate-to-very-severe COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:13–25. doi: 10.2147/COPD.S114209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calverley PM, Postma DS, Anzueto AR, et al. Early response to inhaled bronchodilators and corticosteroids as a predictor of 12-month treatment responder status and COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2016;11:381–390. doi: 10.2147/COPD.S93303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 35.Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533. doi: 10.1016/S2213-2600(13)70158-9. [DOI] [PubMed] [Google Scholar]
- 36.Trudo F, Kern DM, Davis JR, et al. Comparative effectiveness of budesonide/formoterol combination and tiotropium bromide among COPD patients new to these controller treatments. Int J Chron Obstruct Pulmon Dis. 2015;10:2055–2066. doi: 10.2147/COPD.S90658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. [Google Scholar]
- 39.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 40.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi: 10.1164/rccm.201502-0235LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bafadhel M, Peterson S, de Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6(2):117–126. doi: 10.1016/S2213-2600(18)30006-7. [DOI] [PubMed] [Google Scholar]
- 43.Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349. doi: 10.2147/COPD.S152291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng SL, Lin CH. Effectiveness using higher inhaled corticosteroid dosage in patients with COPD by different blood eosinophilic counts. Int J Chron Obstruct Pulmon Dis. 2016;11:2341–2348. doi: 10.2147/COPD.S115132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calverley P, Vlies B. A rational approach to single, dual and triple therapy in COPD. Respirology. 2016;21(4):581–589. doi: 10.1111/resp.12690. [DOI] [PubMed] [Google Scholar]
- 46.Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi: 10.1016/S2213-2600(16)00100-4. [DOI] [PubMed] [Google Scholar]
- 47.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 48.Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap: clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamada T, Sugiura H, Takahashi T, et al. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015;10:2169–2176. doi: 10.2147/COPD.S88274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alshabanat A, Zafari Z, Albanyan O, Dairi M, Fitzgerald JM. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One. 2015;10(9):e0136065. doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tashkin DP, Murray RP. Smoking cessation in chronic obstructive pulmonary disease. Respir Med. 2009;103(7):963–974. doi: 10.1016/j.rmed.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Simmons MS, Connett JE, Nides MA, et al. Smoking reduction and the rate of decline in FEV1: results from the Lung Health Study. Eur Respir J. 2005;25(6):1011–1017. doi: 10.1183/09031936.05.00086804. [DOI] [PubMed] [Google Scholar]
- 54.Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med. 2012;106(3):319–328. doi: 10.1016/j.rmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Hoonhorst SJ, ten Hacken NH, Vonk JM, et al. Steroid resistance in COPD? Overlap and differential anti-inflammatory effects in smokers and ex-smokers. PLoS One. 2014;9(2):e87443. doi: 10.1371/journal.pone.0087443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sohal SS, Reid D, Soltani A, et al. Changes in airway histone deacetylase2 in smokers and COPD with inhaled corticosteroids: a randomized controlled trial. PLoS One. 2013;8(5):e64833. doi: 10.1371/journal.pone.0064833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Overveld FJ, Demkow U, Górecka D, de Backer WA, Zieliński J. Differences in responses upon corticosteroid therapy between smoking and non-smoking patients with COPD. J Physiol Pharmacol. 2006;57(Suppl 4):273–282. [PubMed] [Google Scholar]
- 58.Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients: results from Lung Health Study II. Chest. 2004;126(4):1123–1133. doi: 10.1016/S0012-3692(15)31287-3. [DOI] [PubMed] [Google Scholar]
- 59.Eichenhorn MS, Wise RA, Madhok TC, et al. Lack of long-term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest. 2003;124(1):57–62. doi: 10.1378/chest.124.1.57. [DOI] [PubMed] [Google Scholar]
- 60.Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337(1):8–14. doi: 10.1056/NEJM199707033370102. [DOI] [PubMed] [Google Scholar]
- 61.Wang JJ, Rochtchina E, Tan AG, Cumming RG, Leeder SR, Mitchell P. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116(4):652–657. doi: 10.1016/j.ophtha.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Miller DP, Watkins SE, Sampson T, Davis KJ. Long-term use of fluticasone propionate/salmeterol fixed-dose combination and incidence of cataracts and glaucoma among chronic obstructive pulmonary disease patients in the UK General Practice Research Database. Int J Chron Obstruct Pulmon Dis. 2011;6:467–476. doi: 10.2147/COPD.S14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–565. doi: 10.2165/00003495-200969050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva DR, Coelho AC, Dumke A, et al. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care. 2011;56(7):961–968. doi: 10.4187/respcare.01056. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson GT, Calverley PMA, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the Towards a Revolution in COPD Health study. Chest. 2009;136(6):1456–1465. doi: 10.1378/chest.08-3016. [DOI] [PubMed] [Google Scholar]
- 66.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66(8):699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 67.Scanlon PD, Connett JE, Wise RA, et al. Loss of bone density with inhaled triamcinolone in Lung Health Study II. Am J Respir Crit Care Med. 2004;170(12):1302–1309. doi: 10.1164/rccm.200310-1349OC. [DOI] [PubMed] [Google Scholar]
- 68.Romme EA, Geusens P, Lems WF, et al. Fracture prevention in COPD patients: a clinical 5-step approach. Respir Res. 2015;16:32. doi: 10.1186/s12931-015-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourbeau J, Aaron SD, Barnes NC, Davis KJ, Lacasse Y, Nadeau G. Evaluating the risk of pneumonia with inhaled corticosteroids in COPD: retrospective database studies have their limitations SA. Respir Med. 2017;123:94–97. doi: 10.1016/j.rmed.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 70.DiSantostefano RL, Sampson T, Le HV, Hinds D, Davis KJ, Bakerly ND. Risk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort study. PLoS One. 2014;9(5):e97149. doi: 10.1371/journal.pone.0097149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169(3):219–229. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 72.Sin DD, Tashkin D, Zhang X, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet. 2009;374(9691):712–719. doi: 10.1016/S0140-6736(09)61250-2. [DOI] [PubMed] [Google Scholar]
- 73.Janson C, Larsson K, Lisspers KH, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS) BMJ. 2013;346:f3306. doi: 10.1136/bmj.f3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morjaria JB, Rigby A, Morice AH. Inhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT study. Lung. 2017;195(3):281–288. doi: 10.1007/s00408-017-9990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janson C, Stratelis G, Miller-Larsson A, Harrison TW, Larsson K. Scientific rationale for the possible inhaled corticosteroid intraclass difference in the risk of pneumonia in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3055–3064. doi: 10.2147/COPD.S143656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang HH, Lai CC, Wang YH, et al. Severe exacerbation and pneumonia in COPD patients treated with fixed combinations of inhaled corticosteroid and long-acting β2 agonist. Int J Chron Obstruct Pulmon Dis. 2017;12:2477–2485. doi: 10.2147/COPD.S139035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh D, Miravitlles M, Vogelmeier C. Chronic obstructive pulmonary disease individualized therapy: tailored approach to symptom management. Adv Ther. 2017;34(2):281–299. doi: 10.1007/s12325-016-0459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- 80.Rossi A, Guerriero M, Corrado A. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO) Respir Res. 2014;15:77. doi: 10.1186/1465-9921-15-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi: 10.1016/S0140-6736(16)31354-X. [DOI] [PubMed] [Google Scholar]
- 82.Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi: 10.1164/rccm.201703-0449OC. [DOI] [PubMed] [Google Scholar]
- 83.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 84.Kwak MS, Kim E, Jang EJ, Kim HJ, Lee CH. The efficacy and safety of triple inhaled treatment in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis using Bayesian methods. Int J Chron Obstruct Pulmon Dis. 2015;10:2365–2376. doi: 10.2147/COPD.S93191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi: 10.1016/j.rmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217. doi: 10.2147/COPD.S91694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2017;12:73–83. doi: 10.2147/COPD.S122013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mannino D, Yu TC, Zhou H, Higuchi K. Effects of GOLD-adherent prescribing on COPD symptom burden, exacerbations, and health care utilization in a real-world setting. Chronic Obstr Pulm Dis. 2015;2(3):223–235. doi: 10.15326/jcopdf.2.3.2014.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol inhalation powder) [package insert] Research Triangle Park (NC): GlaxoSmithKline; 2017. [Google Scholar]
- 90.National Institute for Health and Care Excellence . Chronic Obstructive Pulmonary Disease: Management of Adults With Chronic Obstructive Pulmonary Disease in Primary and Secondary Care. London: NICE; 2009. [Google Scholar]
- 91.Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 93.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 94.AstraZeneca AstraZeneca provides update on GALATHEA Phase III trial for Fasenra in chronic obstructive pulmonary disease [press release] 2018. May 11, [Accessed July 12, 2018]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2018/astrazeneca-provides-update-on-galathea-phase-iii-trial-for-fasenra-in-chronic-obstructive-pulmonary-disease-11052018.html.
- 95.AstraZeneca Update on TERRANOVA Phase III trial for Fasenra in chronic obstructive pulmonary disease [press release] 2018. May 30, [Accessed July 12, 2018]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2018/update-on-terranova-phase-iii-trial-for-fasenra-in-chronic-obstructive-pulmonary-disease-30052018.html.