Abstract
CCN proteins are secreted into the extracellular environment where they interact with both components of the extracellular matrix and with cell surface receptors to regulate cellular function. Through these interactions, CCNs act as extracellular ligands to activate intracellular signal transduction pathways. CCN4/WISP-1, like other CCNs, plays multiple physiologic roles in development and also participates in pathogenesis. CCN4 is of particular interest with respect to cancer, showing promise as a biomarker or prognostic factor as well as a potential therapeutic target. This review focuses on recent work addressing the role of CCN4 in cancer. While CCN4 has been identified as an oncogene in a number of cancers, where it enhances cell migration and promoting epithelial–mesenchymal transition, there are other cancers where CCN4 appears to play an inhibitory role. The mechanisms underlying these differences in cellular response have not yet been delineated, but are an active area of investigation. The expression and activities of CCN4 splice variants are likewise an emerging area for study. CCN4 acts as an autocrine factor that regulates the cancer cells from which it is secreted. However, CCN4 is also a paracrine factor that is secreted by stromal fibroblasts, and can affect the function of vascular endothelial cells. In summary, current evidence is abundant in regard to establishing potential roles for CCN4 in oncogenesis, but much remains to be learned about the functions of this fascinating protein as both an autocrine and paracrine regulator in the tumor microenvironment.
Keywords: matricellular proteins, oncogenesis, extracellular matrix, tumor microenvironment, signal transduction
Introduction
Cancer is a condition in which a group of cells, for some entirely unknown reason, commences to grow and multiply at a tremendous rate, invading and destroying surrounding tissues, spreading through the lymphatic system [&] to distant parts of the body, where the destructive growth is repeated.1
The words above, written in 1937, have withstood the test of time with the exception of one phrase. Today we appreciate that the “unknown reason” underlying cancer growth actually consists of many “reasons.” The interaction of tumor cells with their microenvironment is a critical aspect of cancer growth and metastasis.
CCN4, also known as WISP-1, is a member of the WNT1 inducible signaling pathway protein (WISP) subfamily of the connective tissue growth factor/CCN family of matricellular proteins. CCN4 is the preferred nomenclature and will be used herein except in reference to the WISP-1 gene. The roles of CCNs were first recognized with respect to mammalian development, consistent with the initial naming of CCN4 in recognition of its induction through the WNT1 pathway (WISP-1 = Wnt1-induced secreted protein-1). CCN proteins, which are secreted, interact with cell surface receptors (eg, integrins) and extracellular matrix components to modulate cellular functions. Although CCN proteins activate signal transduction events when added exogenously to cells, they are not classical agonists with a single receptor. Rather, CCN proteins interact with multiple proteins, including integrins, to modulate cellular functions.
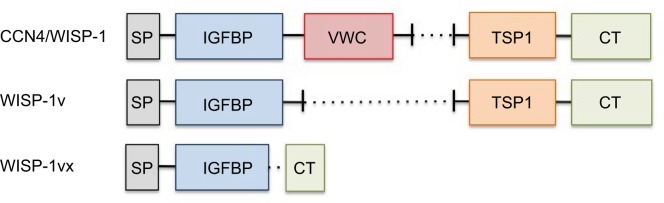
Full-length human CCN4 has a molecular mass of 40 kD. Like other CCNs, CCN4 contains four conserved cysteine-rich domains that interact with other proteins.2,3 Within the CCN family, CCN4 is most similar to CCN3/WISP-3.3 CCN proteins are characterized by four highly conserved domains (Figure 1). The differentiating, nonconserved region is a protease-sensitive “hinge” located after exon 3, the von Willebrand factor type C domain.4 CCNs are differentially expressed in different tissues, and appear to play distinct, nonredundant roles. However, considering the complexity of CCN protein–protein interactions as well as the regulation of their expression, it is not yet clear to what extent CCNs may be complementary or even antagonistic.
Figure 1.
Domain structure of CCN4/WISP-1 and splice variants.
Notes: The following are the highly conserved domains in CCN proteins: SP, IGFBP, VWC, TSP1, and CT. WISP-1v is a splice variant that lacks exon 3, which contains the VWC domain. WISP-1vx contains a truncated IGFBP domain, creating a frameshift that results in loss of the VWC and TSP1 domains.
Abbreviations: SP, signaling peptide; IGFBP, insulin-like growth factor binding protein; VWC, von Willebrand factor type C; TSP1, thrombospondin 1; CT, C-terminal.
It is important to note that CCN mRNAs are subject to alternative splicing, and that the protein products can be post-translationally modified. Thus, there are a number of CCN variants, including truncated forms (Figure 1). As truncation can remove or disrupt conserved domains, CCN4 splice variants can have different biologic functions. This situation has been reviewed previously.5 CCN4 also contains multiple glycosylation sites; glycosylation patterns differ between fibroblasts and cancer cells.6
The six CCN family members play distinct roles with respect to cancer; the role of CCN4 has been previously reviewed in comparison to that of other CCN proteins.7,8 CCN4 has been reported to either enhance or inhibit tumor cell function in different cancers. It should be noted that cancer is not the only disease in which CCN4 has been implicated.9,10 As the roles of CCN4 in oncogenesis have been reviewed recently by others,8,11,12 this review will emphasize findings published since 2015; it will be organized according to cancer type.
Breast cancer
The role of CCN4 in breast cancer has been controversial, with different studies reporting different results. The roles of CCN proteins in breast cancer were reviewed by Kleer in 2016.13 This author compared CCN family members, emphasizing that although CCN proteins share structural motifs, the differences between them result in very different functions. In the case of CCN4, the predominant evidence supports a pro-oncogenic role in breast cancer.
A study published in 2001 by Xie et al provided evidence that CCN4 is highly expressed in primary breast cancer, with a positive correlation between mRNA levels and more advanced disease parameters such as stage, patient age, and tumor size.14 In contrast, a 2007 report published suggested that CCN4 acts as a tumor suppressor in breast cancer, based on an examination of mRNA levels in human breast tumors as compared to normal tissues.15
In a gene profiling study, Taghavi et al identified differences in CCN4 mRNA expression between tissue samples from breast cancer patients with nonmetastatic vs metastatic disease.16 Interestingly, expression of CCN4 mRNA was lower in the metastatic group than in the healthy control group, with no differences between the nonmetastatic group and controls. The authors acknowledge that their results differ from those published previously by others, but do suggest that a decline in CCN4 mRNA could indicate metastatic disease. Expression levels of CCN4 protein were not examined in this study.
In a paper published in 2015, Chiang et al examined the role of CCN4 in breast cancer using several approaches.17 They demonstrated that CCN4 mRNA was increased in human breast cancer tissue as compared to normal breast tissue. They further demonstrated that recombinant exogenous CCN4 enhances proliferation of breast cancer cells in culture, and that transfection of CCN4 into MCF-7 cells increased growth both in cell culture and in mouse xenografts. Finally, the authors showed that transfection of CCN4 in MCF-7 increases epithelial–mesenchymal transition (EMT) as evidenced by changes in expression of a panel of genes and gene products. The authors concluded that CCN4 is an oncogene with respect to breast cancer.
Prostate cancer
In 2013, Ono et al published a comprehensive analysis of the role of CCN4 in prostate cancer.18 They first screened prostate cancer tissue and serum from patients for CCN4 protein expression, comparing the levels to normal controls and also stratifying the cancer patients by Gleason Grade. Immunohistochemistry (IHC) studies showed staining mainly in the tumor stroma. Serum levels were analyzed by quantitative immunob-lotting, using an antibody generated by the investigators. The results indicated that CCN4 protein is higher in low-grade tumors than in control subjects, but that CCN4 serum levels decrease as tumor grade increases, and also as prostate specific antigen (PSA) levels increase. In patients with lymph node+/seminal vesicle+ disease, serum CCN4 protein levels were not elevated compared to those in control patients. The authors then showed, using IHC, that CCN4 protein was also increased in prostate tumors in the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model. Finally, the investigators used a xenograft mouse model in which luciferase-expressing PC-3 human prostate tumor cells were introduced into athymic mice. Anti-CCN4 antibody inhibited growth of the xenograft tumors, and also inhibited migration of the PC-3 cells in a Transwell assay. IHC studies showed that CCN4 protein was present at the tumor–bone interface in the xenograft model. The authors concluded that CCN4 is a potential therapeutic target, as well as a biomarker, in prostate cancer.
A 2014 study presented novel insights into the role of CCN4 in prostate cancer.19 Bone metastasis, a particular problem in advanced prostate cancer, can cause severe pain. Osteoblasts secrete paracrine factors that attract prostate tumor cells to migrate to bone. In their study, Tai et al showed that osteoblast conditioned medium-induced migration and invasion of PC-3 and Du145 human prostate cancer cells in a Transwell assay, that exogenous CCN4 further increased migration and invasion, and that an antibody to CCN4 inhibited the response to the conditioned medium. When osteoblasts were treated with a silencing RNA directed against CCN4, the response of PC-3 and DU145 to the conditioned medium was reduced although not entirely eliminated. The investigators continued to explore the signaling pathways involved in CCN4-induced migration. They identified inte-grins, VCAM-1, FAK, and p38 mitogen-activated protein kinase (MAPK) as components of these pathways. They next examined the role of microRNA-126 (miR-126), which suppresses VCAM-1 expression. CCN4 was shown to inhibit miR-126 expression in PC-3 cells, thereby causing upregulation of VCAM-1. In conclusion, the authors concluded that CCN4 is a potential therapeutic target in prostate cancer.
Ovarian cancer
There is limited work published on the role of CCN4 in ovarian cancer. In their 2015 review, Gurbuz and Chiquet-Ehrismann used the Oncomine database to analyze differences in CCN4 expression between normal and tumor tissues for many types of cancers.11 In the compiled data, these authors noted a slight elevation of CCN4 mRNA in ovarian tumor samples. Another role for CCN4 emerged from a study published in 2017, in which the researchers were seeking a molecular signature for lymphatic invasion of ovarian cancer.20 They found that a decrease in CCN4 mRNA expression was associated with lymphatic invasion and poorer survival. Additional studies on the role of CCN4 in ovarian cancer are needed before any conclusions can be drawn.
Oral cancer
The role of CCN4 has been particularly intensively studied in oral squamous cell carcinoma (OSCC), in which Wnt signaling is known to be abnormal.21 Hence, research concerning CCN4 in oral cancer has moved to a more mechanistic level in recent years. The insights gained are potentially applicable to other types of cancers.
Tang et al, some of whom had previously characterized CCN4 as being important for migration and angiogenesis in OSCC,22 specifically examined angiogenesis in a report published in 2016.23 Using human patient specimens, they found that expression of CCN4 was positively correlated with expression of CCN4, although it is not clear whether mRNA or protein expression was quantified. The authors also showed that conditioned medium from CCN4-treated OSCC cells enhanced migration and tube formation of human lymphatic endothelial cells. Using blocking antibodies, the investigators further demonstrated that the response to conditioned medium was mediated by vascular endothelial growth factor C (VEGF-C) and integrins ανβ3 and α5β1. Finally, this study presented evidence that CCN4 enhances VEGF-C production by inhibiting expression of miR-300 in OSCC.
A very interesting study was published by Clausen et al in 2016.24 These investigators conducted a genome-wide methylation assessment using OSCC samples from patients with and without lymph node metastasis. WISP-1 showed the most marked hypomethylation of any gene in the metastatic group. Hypomethylation was associated with high levels of CCN4 expression. This study not only suggested that CCN4 may be a biomarker for metastatic oral cancer, but also showed that epigenetic methylation of WISP-1 is likely involved in regulating its expression.
Work reported in 2017 by Jung et al confirmed that expression of CCN4 protein is elevated in human OSCC, and also showed that high CCN4 was associated with treatment failure.25 In this study, siRNA-mediated partial knockdown of CCN4 in an OSCC cell line reduced cell invasion as assessed in a Transwell assay, and also increased apoptosis.
In 2017, Lau et al presented the results of their analysis of single-nucleotide polymorphisms (SNPs) in WISP-1 in OSCC patient samples.26 These investigators found that the rs2929970 SNP was associated with increased risk of OSCC progression. Patients carrying this SNP had later-stage OSCC and larger tumors. The identified SNP is in the 3′ untranslated region (UTR), in an area involved in splicing variations. While this study did not test whether different splice variants were generated in the patients with the SNP, the results are certainly tantalizing in view of the developing work on CCN4 splice variants mentioned earlier in this review.
Esophageal cancer
Moving farther down the alimentary tract, the role of CCN4 in esophageal cancer has received some attention. In 2011, Nagai et al examined expression of CCN4 in esophageal squamous cell carcinoma (ESCC) using IHC.27 They reported that CCN4 expression was correlated with lymph node metastasis and poor prognosis. The investigators also tested ESCC cell lines, finding that only one (TE8) expressed CCN4 mRNA. Transfection of CCN4 into a nonexpressing ESCC cell line resulted in enhanced growth, but not invasion, in a Transwell assay. The authors concluded that CCN4 represents a potential biomarker as well as a therapeutic target in ESCC.
A novel aspect of CCN4 was explored in a study published by Zhang et al in 2016.28 This group examined radiation resistance, a serious problem in ESCC. Radioresistant ESCC cells have an EMT-like phenotype in which CCN proteins are implicated. The investigators showed that a radioresistant ESCC cell line, KYSE-150, exhibited high levels of CCN4. Radioresistance was decreased in KYSE-150 cells treated with an anti-CCN4 antibody. Further overexpression of CCN4 in these cells led to development of larger tumors following radiotherapy in a mouse xenograft model. Moreover, CCN4 was an independent prognostic factor in human ESCC patients following radiotherapy, and was associated with an EMT-like phenotype. The authors concluded that CCN4 is a potential prognostic factor as well as a therapeutic target for reversing radiotherapy resistance in ESCC.
Gastric cancer
Based on the involvement of CCN4 in many other cancers, Jia et al tested whether there was an association of CCN4 with gastric cancer.29 This group determined that CCN4 mRNA levels were elevated in gastric cancer specimens, and that CCN4 was expressed in gastric carcinoma cell lines. The investigators showed that knockdown of CCN4 resulted in decreased proliferation, migration, and invasion of two cell lines that expressed high endogenous levels of CCN4. Cyclin D1 was implicated signaling pathways downstream of CCN4, because knockdown of CCN4 reduced expression of cyclin D1. In addition, CCN4 knockdown reduced levels of multiple proteins involved in EMT progression. From these studies, the authors concluded that WISP-1 acts as an oncogene in gastric cancer.
Colon cancer
Several early studies of the role of CCN4 in colorectal cancer yielded conflicting conclusions. Therefore, in a report published in 2016, Wu et al took a fresh look at the issue.30 They found that higher levels of CCN4 expression in human colon cancer cases were associated with a poor prognosis. Using colon cancer cell lines, these investigators showed that knockdown of CCN4 decreased proliferation and invasion and increased apoptosis. Interestingly, they also presented evidence that CCN4 binds directly to β-catenin, and that loss of CCN4 alters mRNA expression of multiple genes in cell adhesion and cytokine–cytokine receptor interaction pathways (eg, decrease in ICAM-1 and CCL-18; increase in TGFBR1). Based on their findings, the investigators concluded that CCN4 acts as an oncogene in colon cancer.
In 2009, Wang et al reported that CCN4 was expressed by several human colon cancer cell lines, including Caco2 cells.31 The focus of the study was on inflammatory signaling in the colon, rather than on cancer. The authors showed that CCN4 levels were higher in human ulcerative colitis, and that nitric oxide increased CCN4 mRNA and protein levels. This result was interesting because, aside from the induction of CCN4 through the WNT1 pathway, the signaling pathways regulating CCN4 expression have been relatively understudied.
With respect to colon cancer proliferation, our group examined the effects of lysophosphatidic acid (LPA) in Caco2 cells. LPA is a lipid growth factor that is mitogenic for many types of cancer cells,32 including Caco2 cells.33 Some CCN proteins are rapidly induced in response to extracellular ligands. For example, in prostate cancer cells, we showed that LPA induces expression of CCN1 within 60 minutes.34 However, in Caco2 cells, LPA did not alter levels of CCN4 protein at times up to 4 hours (L Fix, J Zhang, and K Meier, unpublished data.). This was in contrast to the prominent activation of p70S6K and AKT within 30 minutes of LPA treatment in Caco2 cells. The effects of various extracellular ligands on CCN4 levels are worthy of further examination in colon cancer cells as well as in other cell types. Information of this nature will provide insight into additional signaling pathways regulating CCN4 expression.
Liver cancer
CCN proteins have received considerable attention with respect to liver cancer, as reflected in a review article on the subject published in 2015.35 This review, which focused on the role of CCNs in the interplay between inflammation and oncogenesis, concluded that CCN4 suppresses liver tumor growth, while CCN2 promotes growth and is a therapeutic target. Key recent findings regarding CCN4 are discussed below.
In 2015, Zhang et al examined expression levels of mRNAs encoding CCN proteins in paired HCC and normal liver samples from the same patients.36 They reported a decrease in CCN4 expression in HCC as compared to normal liver tissue, suggesting that CCN4 serves as a tumor suppressor in the liver.
Ge et al, in 2015, assessed the role of CCN4 in a mouse hepatocellular carcinoma cell line.37 This cell line, Hca-F, expressed endogenous CCN4. Cells treated with silencing RNA for WISP-1 showed inhibition of proliferation, migration, and adhesion along with enhanced apoptosis. In the silenced cells, there were decreases in phospho-AKT, p53, and matrix metalloproteinase-2 (MMP-2). Thus, in contrast to other studies, these authors suggest that CCN4 plays an oncogenic role in liver cancer.
Of particular note, an article published in 2004 analyzed the expression of CCN4 in human hepatocellular carcinoma cell lines.38 The authors identified multiple splice variants, and showed that their levels varied between cell lines. While the cellular roles of individual CCN4 variants were not explored, this study further emphasizes the need to consider such variants.
The existing studies are provocative but somewhat limited in scope; further studies of the role of CCN4 in hepatocellular carcinoma are warranted.
Cholangiocarcinoma
Tanaka et al discovered that a splice variant of WISP-1, termed WISP-1v, was overexpressed in human cholangiocarcinomas (bile duct tumors).39 This variant lacks the second von Willebrand factor type C (VWC) domain of CCN4 (Figure 1). WISP-1v expression in human tumor samples was positively associated with lymphatic and perineural invasion and poor prognosis. Conditioned medium, collected from WISP-1v-transfected NIH3T3 fibroblasts, stimulated migration of HUCTT1 cholangiocarcinoma cells, and activated the p38 and ERK MAPK pathways in these cells. A p38 pharmacologic inhibitor inhibited the WISP-1v-induced migration. This was a key study with respect to characterizing biologic effects of a CCN4 splice variant.
Pancreatic cancer
There are very few published studies concerning the role of CCN4 in pancreatic cancer. In pancreatic ductal adenocarcinoma, Yang et al demonstrated that expression of WISP-1 mRNA was elevated in tumor tissues as compared to adjacent normal tissue.40 Expression of CCN4 was positively correlated with clinical stage and liver metastasis. The biologic activity of CCN4 was not tested in this report. The authors concluded that CCN4 has potential utility as a biomarker in pancreatic cancer.
Lung cancer
Lung cancer is another cancer in which the role of CCN4 has been controversial. An early study on this topic showed that overexpression of CCN4 in the H460 human lung cancer cell line inhibited motility and invasion as assessed in cell culture.6 However, another 2003 study indicated that WISP-1 mRNA was elevated in metastases of D122 mouse lung cancer cells in a xenograft model.41 Subsequently, an examination of mRNA expression in primary human lung cancers and matched normal lung tissues revealed upregulation of WISP-1 mRNA in tumor tissue, although there was no association of CCN4 levels with patient survival.42
In 2015, Chen et al published an interesting analysis of WISP-1 genetic polymorphisms (SNPs) in lung cancer patients, also with respect to their response to platinum-based chemo-therapy.43 The investigators identified a total of seven SNPs associated either with lung cancer risk, or with response to chemotherapy. As all of these SNPs were located in the promoter, 3′ UTR, or introns, it is likely that they are involved in modulating expression levels and/or splicing of CCN4. The biology underlying the effects of the SNPs was not examined in this study, which was primarily designed to identify potential biomarkers for diagnosis and chemotherapy response, but the results emphasize an important area for continuing research.
Melanoma
An intriguing study, published in 2016, examined the production of CCN4 in cancer-associated fibroblasts with respect to melanoma.44 Shao et al examined how fibroblasts regulate melanoma metastasis in the tumor microenvironment. In particular, they focused on the Notch pathway in mesenchymal stem cells-derived fibroblasts (MSC-DF), using an elegant mouse xenograft model in which melanoma cells are co-grafted with MSC-DF. The authors showed that the Notch pathway inhibits melanoma metastasis in fibroblasts, and that the inhibition can be attributed to CCN4 secreted from stromal MSC-DF. This conclusion was reached based on the results of studies in which the Notch1 pathway was silenced in fibroblasts, and was then “reconstituted” by overexpression of CCN4 in the Notch1 fibroblasts. In summary, this study demonstrated that CCN4 acts to suppress tumor metastasis in a mouse xenograft model of paracrine interactions between fibroblasts and melanoma cells.
With respect to the normal physiologic role of CCN4 in fibroblasts, a 2018 report showed that CCN4 is upregulated during skin wound healing, and enhances both migration and proliferation in dermal fibroblasts.45 This positive influence of CCN4 is generally consistent with an early report that CCN4 is antiapoptotic and activates AKT in a fibroblast cell line, NRK-49F.46 Thus, CCN4 plays autocrine roles in normal fibroblasts, as well as the paracrine role discussed above for fibroblasts in the tumor microenvironment.
Osteosarcoma
Investigators in Taiwan have been studying the role of CCN4 in osteosarcoma, a bone cancer. Their 2013 report showed a strong positive correlation of CCN4 protein expression with tumor stage in human osteosarcoma patients.47 In the same study, the researchers used osteosarcoma cell lines to demonstrate that CCN4 enhanced cell migration and invasion. This response was accompanied by upregulation of MMP-2 and MMP-9, as well as activation of αvβ3 integrin receptor, the ERK MAPK pathway, and the nuclear factor κB (NF-κB) pathway.
In a subsequent study published in 2017, the same group examined the role of CCN4 in angiogenesis in osteosar-coma.48 They found that exogenous CCN4 increased VEGF-A expression at the transcriptional level in an osteosarcoma cell line, MG-63. Conditioned medium from these cells stimulated migration and tube formation when applied to human endothelial progenitor cells (EPCs). Additional studies using pharmacologic inhibitors suggested that the RAK, JNK, and HIF-1a signaling pathways were involved in mediating CCN4 response in EPCs. The authors also implicated downregulation of miR-381 in response to CCN4. In summary, the cumulative work from this research group has established that CCN4 acts as an autocrine factor to simulate migration of osteosarcoma cells, and also as a paracrine factor to stimulate angiogenesis.
Chondrosarcoma
Chondrosarcoma is a highly invasive form of bone cancer. In 2007, a Japanese group examined the expression and function of CCN4 splice variants in a chondrosarcoma-derived chondrocytic cell line, HCS-2/8.49 The researchers found that HCS-2/8 cells expressed full-length WISP-1 mRNA, as well as two splice variants. The first variant, WISP-1v, had been described previously,39 and was discussed earlier in this review. The second was a new variant, termed WISP-1vx, which lacks the second and third CCN modules and a small part of the first module (Figure 1). WISP-1vx was detected only in the chondrosarcoma cell line, based on a small panel including other cell lines. The investigators then utilized primary rabbit growth cartilage chondrocytes, which can progress to mineralized “terminally differentiated” cells in culture, to explore the role of WISP-1v in differentiation. They found that expression of WISP-1v increased as differentiation progressed, and that overexpression of WISP-1v in HCS-2/8 cells resulted in expression of an alkaline phosphatase that serves as a marker for terminal differentiation in chondrocytes. Interestingly, the authors note that CCN4 orthologs are expressed solely in animals with a calcified skeleton, while other CCN proteins are expressed in both invertebrates and vertebrates. However, full-length CCN4 did not appear to be associated with chondrocyte differentiation. The role of WISP-1vx in the chondrosarcoma cell line was not delineated in this study, which focused largely on chondrocyte differentiation and not on cancer. The study identified an interesting biologic role for a CCN4 splice variant.
In 2011, Hou et al examined effects of CCN4 on JJ012 human chondrosarcoma cells.50 This work was from the same group that had studied CCN4 in osteosarcoma.47,48 With respect to human chondrosarcoma, the investigators found that CCN4 expression was elevated in chondrosarcoma tissues as compared to normal cartilage. In JJ012 cells, they showed that CCN4 stimulated migration, upregulated MMP-2, and activated the signaling proteins FAK, ERK, and NF-κB. An antibody against α5β1 integrin blocked CCN4-induced migration. In summary, this study established a potential role for full-length CCN4 in enhancing metastasis of chondrosarcoma cells.
Glioblastoma
Jing et al published results concerning CCN4 in glioblastoma in 2017.51 CCN4 was overexpressed in glioblastoma tissues and cell lines as compared to normal tissues and cells. Higher expression levels of CCN4 were associated with more advanced tumors and poor prognosis. Knockdown of CCN4 in cell lines inhibited proliferation, MMP9 activation, and EMT, while also inhibiting growth of these cells in xenograft tumors, and increasing sensitivity of the tumors to temozolimide. On the basis of these experimental results, the authors concluded that CCN4 is a potential biomarker and therapeutic target in glioblastoma.
Neurofibromatosis
Neurofibramatosis type 1 (NF1) is an inherited disorder that confers predisposition to tumor formation. Pasmant et al published a study in 2010 examining four CCN family members in NF1.52 In the complex analysis of the various types of tissue abnormalities in NF1, the authors ultimately focused primarily on CCN1, which was upregulated in plexiform neurofibromas. However, the researchers also discovered that CCN4 was upregulated in biopsy samples of malignant peripheral nerve sheath tumors, consistent with its role in oncogenesis in other types of tumors.
Hematopoietic cancers
A 2015 report addressed the role of CCN4 in the Jurkat cell line, which is derived from a human acute lymphoblastic leukemia.53 The authors examined the expression of CCN4 mRNA and protein in several leukemia cell lines, showing highest expression in Jurkat. They then conducted a proliferation/viability assay in Jurkat cells with and without siRNA knockdown of CCN4. Silencing of WISP-1 resulted in lower cell numbers, G1 cell cycle arrest, increased apoptosis, and increased reactive oxygen species. Loss of CCN4 also decreased levels of phosph-AKT, phospho-ERK, and Bcl2 among other proteins studied. Based on these results, the authors suggest that CCN4 is important for proliferation and apoptosis in Jurkat cells.
Our research group became interested in CCN4 as a result of genomic studies carried out in EL4 mouse lymphoma cell lines. These clonal lines, which were developed from naturally occurring variants and then characterized in our lab, differed in multiple aspects that include signal transduction, protein expression, and metastatic vs nonmetastatic phenotypes.54–58 The metastasis assay involved tail vein injection of EL4 cells into syngeneic mice, resulting in subsequent development of solid liver tumors for metastatic lines.57,58 Metastatic cell lines were also differentiated from nonmetastatic variants by their enhanced adhesion to cell culture substrates,58,59 noticeable because EL4 cells are able to grow in suspension. The meta-static/adherent phenotype is characterized by expression of FAK, a tyrosine kinase that regulates focal adhesion function. In 2016, we published the results of an Affymetrix microarray analysis comparing gene expression between metastatic and nonmetastatic EL4 cell lines.60 Some of the results recapitulated those we had already published previously studying the levels of expression of signal transduction proteins.57,58 However, to our surprise, WISP-1 emerged as a new protein of interest, being one of the top-ranked genes with respect to differences in mRNA expression. This result was confirmed by immunoblotting for protein expression, which revealed that CCN4 was highly expressed in the metastatic EL4 cell line tested, and was not detectable in the nonmetastatic variant. CCN4 expression in metastatic EL4 cells was also confirmed by IHC. In this study, we did not investigate whether loss of CCN4 altered the behavior of metastatic EL4 cells, and did not determine the mechanism underlying differences in CCN4 expression. However, expression of CCN4 is consistent with the overall metastatic phenotype in EL4 cells, because enhanced cell adhesion and migration in metastatic cells suggest differences in interaction with extracellular matrix.56,59 This was the first study to identify a potential role for a CCN family member in a lymphoma model.
Conclusion
This overview of the role of CCN4 in cancer cells has focused largely on data published in the last few years. The goal was to be comprehensive in including all types of cancers that had been studied; any omissions were unintentional. Findings from the studies cited herein are summarized in Table 1.
Table 1.
CCN4 expression in various tumors
| Cancer type | Increase/decrease in tumor activity | CCN4 splice variant | Mechanism/pathway (if known) | Reference |
|---|---|---|---|---|
| Breast |
  
|
– | – |
14 15 17 |
| Prostate |
 
|
– | Paracrine secretion factors |
18 19 |
| Ovarian |
 
|
– | – |
11 20 |
| Oral |
   
|
– – – SNP |
VEGF-C; integrins ανβ3 and α5β1 Hypomethylation |
22,23 24 25 26 |
| Esophageal |
 
|
– | – |
27 28 |
| Gastric |

|
– | Cyclin D1; EMT proteins | 29 |
| Liver |
   – |
Multiple | pAKT, p53, MMP-2 |
35 36 37 38,39 |
| Cholangiocarcinoma |

|
WISP-1v | P38, ERK MAPK | 40 |
| Pancreatic |

|
– | – | 41 |
| Lung |
   a a– |
SNPs | – |
6 42 43 44 |
| Melanoma |

|
Stromal secretion | 45 | |
| Osteosarcoma |
 
|
– | MMP-2, MMP-9, ανβ3 integrin; activates ERK-MAPK, NF-κB VEGF-A; RAK, JNK, HIF-1a |
48 49 |
| Chondrosarcoma | –
|
WISP-1v, WISP-1vx |
MMP-2; FAK, ERK, NF-κB |
50 51 |
| Glioblastoma |

|
– | – | 52 |
| Neurofibromatosis |

|
– | – | 2 |
| Hematopoietic |
 
|
– | pAKT, pERK, Bcl2 |
54 58–60 |
Notes: The findings derived from the references cited in this review article are summarized in tabular form. In column 2, the green arrows indicate an increase in tumor cell proliferation, migration, or other pro-oncogenic activity, while the red arrows indicate a decrease in these activities. “
 ” indicates an increase in tumor cell proliferation, migration, or other pro-oncogenic activity, while “
” indicates an increase in tumor cell proliferation, migration, or other pro-oncogenic activity, while “
 ” indicates a decrease in these activities.
” indicates a decrease in these activities.
No association with patient survival.
Abbreviations: EMT, epithelial–mesenchymal transition; SNPs, single-nucleotide polymorphisms; MAPK, mitogen-activated protein kinase; MMP-2, matrix metalloproteinase-2; NF-κB, nuclear factor κB; VEGF-C, vascular endothelial growth factor C.
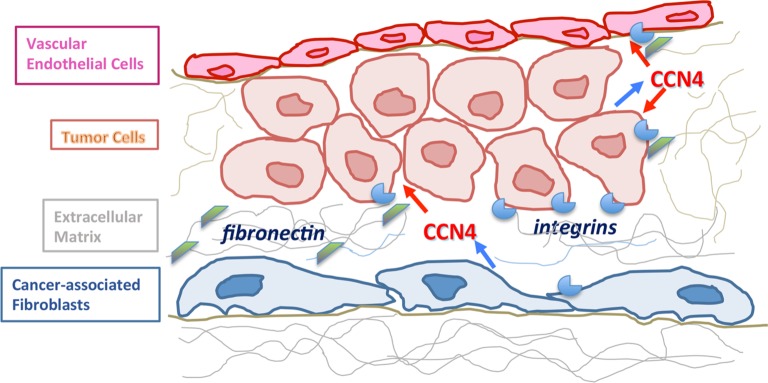
Figure 2 depicts both autocrine and paracrine roles of CCN4 in the tumor environment. Multiple studies have focused on the role of autocrine CCN4, demonstrating that CCN4 is produced by cancer cells, and can elicit effects from the same cells. However, additional studies discussed herein have shown that CCN4 produced by stromal fibroblasts can regulate cancer cells in a paracrine fashion. Other reports presented in this review have shown that CCN4 secreted by tumor cells can influence the tumor vasculature. It should be noted that there are additional potential paracrine interactions in the tumor environment that can be explored in the future.
Figure 2.
CCN4 as an autocrine and paracrine mediator within the tumor microenvironment.
Notes: CCN4 is produced and secreted (blue arrows) by tumor cells as well as by stromal fibroblasts. Secreted CCN4 is then available to exert effects (red arrows) on tumor cells and vascular endothelial cells through both paracrine and autocrine mechanisms. CCN4 is able to bind to extracellular matrix proteins (eg, fibronectin; green shapes), as well as to integrins (blue shapes) in the cell membrane, thereby modulating cell interactions with the extracellular matrix. Integrins are expressed by all of the cell types depicted.
Several major questions arise from the data presented herein. The first concerns the findings showing that, while CCN4 acts as an oncogene in most types of cancer, it can inhibit the proliferation or migration of other types. The positive and negative effects of CCN4 on the tumor cell types discussed in this review are summarized in Table 1. It will be informative to understand the mechanisms responsible for negative vs positive effects of CCN4 on cancer cells. In cell types where signal transduction pathways have been studied, CCN4 predictably activates promitogenic and survival pathways (eg, ERK, AKT) in cells where it promotes tumor growth and/or metastasis. However, the pathways involved in the inhibitory actions of CCN4 have not been clearly defined. It is possible that such effects are mediated at the level of the cell membrane, where protein–protein interactions mediated by CCN4 may suppress the actions of receptors critical for proliferation of certain types of tumors. In some cases, it may be too early to conclude that CCN4 plays “dual roles” in cancer, although it clearly does play complex roles. Specifically, the results of mRNA expression analyses of human tumors need to be complemented by other studies that explore the effects of CCN4 in more mechanistic detail, and with consideration of the expression of CCN4 partners by the cells of interest. It is likely that we have not yet been sufficiently comprehensive in delineating all of the cellular responses of interest and pathways involved. For example, most studies of CCN4 action have focused on its role in migration and invasion, and not in proliferation or metabolism. As CCN4 activates multiple signaling pathways, and interacts with multiple protein partners, at this point we may be missing details that would provide us greater insight into the mechanisms by which CCN4 regulates tumor cells.
A second area for further work lies in the existence of CCN4 splice variants. To date, these have only been examined in a few cancer cell types, and their cellular activities have only been explored in a few instances as cited in Table 1. CCN4 splice variants have been previously reviewed by others.5 The existing data are very provocative, and suggest that the roles of the splice variants need to be considered and explored in more detail, in more types of cancers. The products of CCN proteolysis are also of interest.10
A third area of interest is not specific to the role of CCN4 in cancer. CCN proteins are very unique ligands in their ability to bind to multiple protein partners. They can be viewed as “docking ligands” that help extracellular proteins to interact with cell surface receptors such as integrins. While we have considerable understanding of how docking proteins work within cells to facilitate signal transduction, there is much to be learned about how CCNs modulate cell function. Further studies of the roles of specific CCN4 domains in its biologic function will be very helpful. Ideally, these studies will include consideration of the activities of splice variants lacking some of these domains. Further analysis of the expression and biologic activity of splice variants could potentially explain some of the results regarding varying expression of CCN4 through cancer progression, and/or the paradox of why CCN4 appears to promote some tumors and inhibit others.
Finally, CCN4 is not the only CCN protein that has been shown to play an important part in tumor cell regulation. The functional differences and similarities between CCNs are only beginning to be elucidated. It is possible, for example, that an excess or deficiency of one CCN impacts the activities of other CCNs in the same cell. The abilities of CCN proteins to antagonize or synergize with each other are a promising avenue for future research.
In the end, the goal of research concerning the role of CCN4 in cancer is to fill gaps in our knowledge regarding the “unknown reason”, posed in 1937 by Carlson,1 of why cancer cells behave as they do. CCN proteins are particularly attractive as potential targets for therapeutic intervention because these protein ligands act from within the extracellular space. It is, therefore, conceivable that pharmacologic approaches can be developed to interfere with CCN4 action, thereby impairing cancer cell metastasis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Carlson AJ, Johnson V. The Machinery of the Body. Chicago: University of Chicago Press; 1937. [Google Scholar]
- 2.Planque N, Perbal B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int. 2003;3(1):15. doi: 10.1186/1475-2867-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng M, Jia S. Dual effect of WISP-1 in diverse pathological processes. Chin J Cancer Res. 2016;28(6):553–560. doi: 10.21147/j.issn.1000-9604.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10(12):945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perbal B. Alternative splicing of CCN mRNAs &. it has been upon us. J Cell Commun Signal. 2009;3(2):153–157. doi: 10.1007/s12079-009-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278(13):11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- 7.Dhar A, Ray A. The CCN family proteins in carcinogenesis. Exp Oncol. 2010;32(1):2–9. [PubMed] [Google Scholar]
- 8.Barreto SC, Ray A, Ag Edgar P. Biological characteristics of CCN proteins in tumor development. J Buon. 2016;21(6):1359–1367. [PubMed] [Google Scholar]
- 9.Berschneider B, Königshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int J Biochem Cell Biol. 2011;43(3):306–309. doi: 10.1016/j.biocel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Krupska I, Bruford EA, Chaqour B. Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases: highlights of their structural likenesses and functional dissimilarities. Hum Genomics. 2015;9:24. doi: 10.1186/s40246-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurbuz I, Chiquet-Ehrismann R. CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): a focus on its role in cancer. Int J Biochem Cell Biol. 2015;62:142–146. doi: 10.1016/j.biocel.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Yeger H, Perbal B. CCN family of proteins: critical modulators of the tumor cell microenvironment. J Cell Commun Signal. 2016;10(3):229–240. doi: 10.1007/s12079-016-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleer CG. Dual roles of CCN proteins in breast cancer progression. J Cell Commun Signal. 2016;10(3):217–222. doi: 10.1007/s12079-016-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61(24):8917–8923. [PubMed] [Google Scholar]
- 15.Davies SR, Watkins G, Mansel RE, Jiang WG. Differential expression and prognostic implications of the CCN family members WISP-1, WISP-2, and WISP-3 in human breast cancer. Ann Surg Oncol. 2007;14(6):1909–1918. doi: 10.1245/s10434-007-9376-x. [DOI] [PubMed] [Google Scholar]
- 16.Taghavi A, Akbari ME, Hashemi-Bahremani M, et al. Gene expression profiling of the 8q22-24 position in human breast cancer: TSPYL5, MTDH, ATAD2 and CCNE2 genes are implicated in oncogenesis, while WISP1 and EXT1 genes may predict a risk of metastasis. Oncol Lett. 2016;12(5):3845–3855. doi: 10.3892/ol.2016.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang K-C, Yeh C-N, Chung L-C, et al. WNT-1 inducible signaling pathway protein-1 enhances growth and tumorigenesis in human breast cancer. Sci Rep. 2015;5(1):8686. doi: 10.1038/srep08686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono M, Inkson CA, Sonn R, et al. WISP1/CCN4: a potential target for inhibiting prostate cancer growth and spread to bone. PLoS One. 2013;8(8):e71709. doi: 10.1371/journal.pone.0071709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai HC, Chang AC, Yu HJ, et al. Osteoblast-derived WNT-induced secreted protein 1 increases VCAM-1 expression and enhances prostate cancer metastasis by down-regulating miR-126. Oncotarget. 2014;5(17):7589–7597. doi: 10.18632/oncotarget.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paik ES, Choi HJ, Kim TJ, et al. Molecular signature for lymphatic invasion associated with survival of epithelial ovarian cancer. Cancer Res Treat. 2018;50(2):461–473. doi: 10.4143/crt.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5(7):e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang JY, Chang AC, Chiang IP, Tsai MH, Tang CH. Apoptosis signal-regulating kinase 1 is involved in WISP-1-promoted cell motility in human oral squamous cell carcinoma cells. PLoS One. 2013;8(10):e78022. doi: 10.1371/journal.pone.0078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CC, Chen PC, Lein MY, et al. WISP-1 promotes VEGF-C-dependent lymphangiogenesis by inhibiting miR-300 in human oral squamous cell carcinoma cells. Oncotarget. 2016;7(9):9993–10005. doi: 10.18632/oncotarget.7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clausen MJ, Melchers LJ, Mastik MF, et al. Identification and validation of WISP1 as an epigenetic regulator of metastasis in oral squamous cell carcinoma. Genes Chromosomes Cancer. 2016;55(1):45–59. doi: 10.1002/gcc.22310. [DOI] [PubMed] [Google Scholar]
- 25.Jung EK, Kim SA, Yoon TM, et al. WNT1-inducible signaling pathway protein-1 contributes to tumor progression and treatment failure in oral squamous cell carcinoma. Oncol Lett. 2017;14(2):1719–1724. doi: 10.3892/ol.2017.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau HK, Wu ER, Chen MK, et al. Effect of genetic variation in microRNA binding site in WNT1-inducible signaling pathway protein 1 gene on oral squamous cell carcinoma susceptibility. PLoS One. 2017;12(4):e0176246. doi: 10.1371/journal.pone.0176246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai Y, Watanabe M, Ishikawa S, et al. Clinical significance of Wnt-induced secreted protein-1 (WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer Res. 2011;31(3):991–998. [PubMed] [Google Scholar]
- 28.Zhang H, Luo H, Jiang Z, et al. Fractionated irradiation-induced EMT-like phenotype conferred radioresistance in esophageal squamous cell carcinoma. J Radiat Res. 2016;57(4):370–380. doi: 10.1093/jrr/rrw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia S, Qu T, Feng M, et al. Association of Wnt1-inducible signaling path way protein-1 with the proliferation, migration and invasion in gastric cancer cells. Tumour Biol. 2017;39(6):1–9. doi: 10.1177/1010428317699755. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Long Z, Cai H, et al. High expression of WISP1 in colon cancer is associated with apoptosis, invasion and poor prognosis. Oncotarget. 2016;7(31):49834–49847. doi: 10.18632/oncotarget.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Zhang R, Wen S, Mccafferty DM, Beck PL, Macnaughton WK. Nitric oxide increases Wnt-induced secreted protein-1 (WISP-1/CCN4) expression and function in colitis. J Mol Med. 2009;87(4):435–445. doi: 10.1007/s00109-009-0445-4. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, Gibbs TC, Mukhin YV, Meier KE. Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells. J Biol Chem. 2002;277(36):32516–32526. doi: 10.1074/jbc.M203864200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Bialkowska A, Rusovici R, et al. Lysophosphatidic acid facilitates proliferation of colon cancer cells via induction of Krüppel-like factor 5. J Biol Chem. 2007;282(21):15541–15549. doi: 10.1074/jbc.M700702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Hopkins MM, Zhang Z, et al. Omega-3 fatty acids and other FFA4 agonists inhibit growth factor signaling in human prostate cancer cells. J Pharmacol Exp Ther. 2015;352(2):380–394. doi: 10.1124/jpet.114.218974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia Q, Dong Q, Qin L. CCN: core regulatory proteins in the micro-environment that affect the metastasis of hepatocellular carcinoma? Oncotarget. 2015;7(2):1203–1214. doi: 10.18632/oncotarget.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Li W, Huang P, Pinbo H, et al. Expression of CCN family members correlates with the clinical features of hepatocellular carcinoma. Oncol Rep. 2015;33(3):1481–1492. doi: 10.3892/or.2015.3709. [DOI] [PubMed] [Google Scholar]
- 37.Ge J, Zhang XH, Wang F, et al. Effect of siRNA on Wisp-1 gene expres-sion, proliferation, migration and adhesion of mouse hepatocellular carcinoma cells. Asian Pac J Trop Med. 2015;8(10):821–828. doi: 10.1016/j.apjtm.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Cervello M, Giannitrapani L, Labbozzetta M, et al. Expression of WISPs and of their novel alternative variants in human hepatocellular carcinoma cells. Ann NY Acad Sci. 2004;1028:432–439. doi: 10.1196/annals.1322.051. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka S, Sugimachi K, Kameyame T, et al. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- 40.Yang JY, Yang MW, Huo YM, et al. High expression of WISP-1 correlates with poor prognosis in pancreatic ductal adenocarcinoma. Am J Transl Res. 2015;7(9):1621–1628. [PMC free article] [PubMed] [Google Scholar]
- 41.Margalit O, Eisenbach L, Amariglio N, et al. Overexpression of a set of genes, including WISP-1, common to pulmonary metastases of both mouse D122 Lewis lung carcinoma and B16-F10.9 melanoma cell lines. Br J Cancer. 2003;89(2):314–319. doi: 10.1038/sj.bjc.6600977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen PP, Li WJ, Wang Y, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2(6):e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Yin JY, Li XP, et al. Association of Wnt-inducible signaling pathway protein 1 genetic polymorphisms with lung cancer susceptibility and platinum-based chemotherapy response. Clin Lung Cancer. 2015;16304(4):298–304. e1–2. doi: 10.1016/j.cllc.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Shao H, Cai L, Moller M, et al. Notch1-WISP-1 axis determines the regulatory role of mesenchymal stem cell-derived stromal fibroblasts in melanoma metastasis. Oncotarget. 2016;7(48):79262–79273. doi: 10.18632/oncotarget.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ono M, Masaki A, Maeda A, et al. CCN4/WIST1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts viaα5β1 and TNFα. Matrix Biol. 2018;68–69:533–546. doi: 10.1016/j.matbio.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu CL, Tsai HC, Chen ZW, et al. Ras activation mediates WISP-1-in-duced increases in cell motility and matrix metalloproteinase expression in human osteosarcoma. Cell Signal. 2013;25(12):2812–2822. doi: 10.1016/j.cellsig.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Tsai HC, Tzeng HE, Huang CY, et al. WISP-1 positively regulates angiogenesis by controlling VEGF-A expression in human osteosarcoma. Cell Death Dis. 2017;8(4):e2750. doi: 10.1038/cddis.2016.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanagita T, Kubota S, Kawaki H, et al. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. Febs J. 2007;274(7):1655–1665. doi: 10.1111/j.1742-4658.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- 50.Hou CH, Chiang YC, Fong YC, Tang CH. WISP-1 increases MMP-2 expression and cell motility in human chondrosarcoma cells. Biochem Pharmacol. 2011;81(11):1286–1295. doi: 10.1016/j.bcp.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Jing D, Zhang Q, Yu H, Zhao Y, Shen L. Identification of WISP1 as a novel oncogene in glioblastoma. Int J Oncol. 2017;51(4):1261–1270. doi: 10.3892/ijo.2017.4119. [DOI] [PubMed] [Google Scholar]
- 52.Pasmant E, Ortonne N, Rittié L, et al. Differential expression of CCN1/CYR61, CCN3/NOV, CCN4/WISP-1, and CCN5/WISP2 in neurofibromatosis type 1 tumorigenesis. J Neuropathol Exp Neurol. 2010;69(1):60–69. doi: 10.1097/NEN.0b013e3181c79bff. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Chen X, Liu J, et al. Knockdown of WISP1 inhibit proliferation and induce apoptosis in ALL Jurkat cells. Int J Clin Exp Pathol. 2015;8(11):15489–15496. [PMC free article] [PubMed] [Google Scholar]
- 54.Sansbury HM, Wisehart-Johnson AE, Qi C, Fulwood S, Meier KE. Effects of protein kinase C activators on phorbol ester-sensitive and -resistant EL4 thymoma cells. Carcinogenesis. 1997;18(9):1817–1824. doi: 10.1093/carcin/18.9.1817. [DOI] [PubMed] [Google Scholar]
- 55.Ku H, Meier KE. Phosphorylation of paxillin via the ERK mitogen-activated protein kinase cascade in EL4 thymoma cells. J Biol Chem. 2000;275(15):11333–11340. doi: 10.1074/jbc.275.15.11333. [DOI] [PubMed] [Google Scholar]
- 56.Han S, Knoepp SM, Hallman MA, Meier KE. RasGRP1 confers the phorbol ester-sensitive phenotype to EL4 lymphoma cells. Mol Pharmacol. 2007;71(1):314–322. doi: 10.1124/mol.106.028639. [DOI] [PubMed] [Google Scholar]
- 57.Knoepp SM, Chahal MS, Xie Y, et al. Effects of phospholipase D2 expression on adhesion and tumorigenesis in EL4 thymoma cells. Mol Pharm. 2008;74:574–584. doi: 10.1124/mol.107.040105. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Knoepp SM, Sansbury HM, et al. Differential expression of FAK and Pyk2 in phorbol ester-sensitive and -resistant EL4 thymoma cells. Clin Exp Metastasis. 2011;28:551–565. doi: 10.1007/s10585-011-9391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chahal MS, Brauner DJ, Meier KE. Phospholipase D2 enhances epidermal growth factor-induced Akt activation in EL4 lymphoma cells. Pharmaceuticals. 2010;3(7):2045–2058. doi: 10.3390/ph3072045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chahal MS, Ht K, Zhang Z, et al. Differential expression of WISP1/CCN4 and other genes between metastatic, non-metastatic, and PLD2-expressing metastatic EL4 mouse lymphoma cell lines. Cancer Genomics Proteomics. 2016;13:437–442. doi: 10.21873/cgp.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]