Abstract
Objectives
Cervical noninvasive vagus nerve stimulation (nVNS) emerged as an adjunctive neuromodulation approach for primary headache disorders with limited responsiveness to pharmacologic and behavioral treatment. This narrative review evaluates the safety and efficacy of invasive and noninvasive peripheral nerve stimulation of the cervical branch of the vagal nerve (afferent properties) for primary headache disorders (episodic/chronic migraine [EM/CM] and cluster headache [ECH/CCH]) and provides a brief summary of the preclinical data on the possible mechanism of action of cervical vagus nerve stimulation (VNS) and trigemino-nociceptive head pain transmission.
Materials and methods
A systematic search of published data was performed in PubMed for randomized controlled trials (RCTs) and prospective cohort clinical studies assessing the efficacy/safety and cost-effectiveness of cervical VNS in primary headache disorders and related preclinical studies.
Results
Three RCTs were identified for ECH/CCH (ACT-1, ACT-2 and PREVA), one RCT for migraine (EVENT) and several prospective cohort studies and retrospective analyses for both headache disorders. In ACT-1, a significantly higher response rate, a higher pain-free rate and a decrease in mean attack duration were found in nVNS-treated ECH/CCH patients compared to sham stimulation. ACT-2 confirmed these findings (e.g., significantly higher pain-free attacks, pain severity decline and increased responder-rate [defined as ≥50% reduction]). The PREVA study demonstrated the superiority of adjunctive nVNS to standard care alone and observed a significantly higher attack reduction (p=0.02) and responder rate (defined as ≥50% reduction). For CM, the EVENT study assessed a significantly higher frequency of decline in the open-label phase. Mostly transient mild/moderate adverse events were recorded, and no severe device-related adverse events occurred.
Conclusion
Cervical nVNS represents a novel, safe and efficient adjunctive treatment option for primary headache disorders. In particular, preliminary observations suggest enhanced nVNS responsiveness in favor of episodic subtypes (EM and ECH). However, preclinical studies are urgently warranted to dissect the mechanism of action.
Keywords: cervical vagus nerve stimulation, migraine, cluster headache, safety/efficacy, trigemino-nociceptive signaling, neuroinflammation
Introduction
In the past two decades, surgically implanted cervical vagal nerve stimulation (iVNS; Cyberonics Inc., TX, USA) was investigated in clinical trials in a broad variety of neurologic disorders such as epilepsy, depression and Alzheimer’s disease.1–3
Chronic daily headache (CDH) and/or migrane occur particularly frequent in an underestimated proportion of seizure patients.4 The impact of iVNS on seizure-associated head pain was first reported >10 years ago.5–7 However, only two pilot studies have been specifically conducted for the treatment of primary headache disorders. Including migraine and cluster headache, both pilot studies demonstrated encouraging efficacy in terms of 50% reduction in severity/frequency with adjunctive iVNS.8,9 More recently, a retrospective, large database analysis found a sustained, clinically meaningful impact of iVNS in seizure-related CDH and migraine.10 In spite of this marked observed effect, iVNS requires surgical implantation and has been associated with a considerable percentage of implantation- and/or stimulation-associated side effects, diminishing the otherwise beneficial VNS outcome.3 In view of the currently available abortive pharmacologic interventions (e.g., triptans) with pain-free response rates in migraine of 30% at 2 h and 20% at 24 h, and in cluster headache of 45% at 15 min after rescue drug intake, novel adjunctive acute and preventive treatment alternatives are urgently needed to counterbalance the long-term pharmacologic side effects and/or limited responsiveness.11
Thus, a cervical non-invasive, equally effective approach has been developed (noninvasive vagus nerve stimulation [nVNS]; gammaCore, NJ, USA), and the capability to perform preventive and abortive neuromodulation therapy for migraine and cluster headache with a distinct lower incidence of adverse events (AEs) has been demonstrated.11–29 Cervical nVNS represents a portable neuromodulation device that received CE mark approval for the acute and preventive treatment of primary headache disorders (migraine, cluster headache) and medication-overuse headache, and was approved by the US Food and Drug Administration for the acute treatment of episodic cluster headache and acute pain associated with migraine.
The scope of this article is to provide a narrative review of randomized controlled trials (RCTs), prospective cohort studies, retrospective analyses and cost-effectiveness assessments in order to determine the impact, safety and tolerability of cervical nVNS as an adjunctive treatment of primary headache disorders with a focus on episodic and chronic migraine (EM/CM) and cluster headache (ECH/CCH). Specified parameters were the following: cervical iVNS (invasive; surgically implanted), cervical nVNS (noninvasive, transcutaneous), primary headache disorders (migraine and cluster headache), severity and frequency, prevention and acute head pain treatment, study year/design, observation period, stimulation paradigm, the safety and tolerability profile of VNS, trigeminal allodynia, trigemino-nociceptive system, trigemino-cervical (vascular) complex, neuroinflammation, experimental head pain model and cortical spreading depression (CSD).
In addition, this review briefly covers experimental studies to highlight the postulated mechanism of action (MOA) of VNS in head pain models with emphasis on the neuroinflammation genesis of primary headache disorders and possible interactions with trigemino-nociceptive headache signaling.
Other VNS neuromodulation approaches targeting, for instance, the auricular branch of the vagal nerve were per definition not part of this review, as well as preliminary reports of otherwise unpublished data.
General characteristics and class of evidence of cervical VNS studies
Between 2000 and 2018, two clinical trials (pilot studies) were published using iVNS specifically addressed to migraine and cluster headache patients. As yet, there exist mainly case reports and/or retrospective assessments of iVNS seizure patients with coincidental CDH, migraine or other forms of headache. Mainly due to the low number of patients investigated and the study design, evidence-based conclusion about iVNS head pain outcome is limited (Table 1).5–10
Table 1.
Summary of clinical studies addressed to invasive cervical vagal nerve stimulation (iVNS) and primary headache disorders
| Year, study design | Headache disorder | Primary indication | Patient no. | Outcome/parameter | Follow-up | Cyclic stimulation paradigm | Efficacy | Safety/tolerability |
|---|---|---|---|---|---|---|---|---|
| 2000, rCS5 Class IV | CM | Seizure | 1 | Severity/frequency | 10 years | 30 sec on/1–5 min off 20 Hz, 200–250 µsec |
R | Not reported |
| 2002, pCS6 Class IV | CM | Seizure | 1 | Severity/frequency | 2 months | Not reported | R | Not reported |
| 2003, rCS7 Class IV | CM | Seizure | 4 | Severity/frequency | 14 years | 30 sec on/1–5 min off 20 Hz, 200–250 µsec |
R-75% | Not reported |
| 2005, pPS8 Class IV | CM/CCH/BM | Headache | 6 3 CM 2 CCH 1 BM |
Severity/frequency Functional impairment (MIDAS) |
6 months | Not reported | R-66% (2 CM) R-100% (2 CCH) | Vomitus BM patient |
| 2009, pPS9 Class IV | CM | Headache Depressive disorder | 4 | Severity/frequency Functional impairment (MIDAS/HRSD) |
4–14 months | 30 sec on/1–5 min off 1.25–2.5 mA, 30 Hz 500 µsec |
R-100% | Voice alteration, dyspnea, cough |
| 2017, rCS10 Class IV | CM | Seizure iVNS+SoC vs. SoC vs. HC | 19 10 iVNS 9 SoC |
Severity/frequency Affective/cognitive head pain perception (MIDAS, PASS-40, FSVA) | 5–13 years | 30 sec on/1–5 min off 0.5–2 mA, 20 Hz, 200–250 µsec |
iVNS (VAS) 5.4 vs. SoC (VAS) 7.8, p=0.03 iVNS (PASS) 21 vs. SoC (PASS) 16, p=0.02 |
Voice disturbance Battery replacement |
Abbreviations: BM, basilar migraine; CCH, chronic cluster headache; CH, cluster headache; CM, chronic migraine; FSVA, questionnaire for pain-associated vigilance and attention; HC, healthy control; HRSD, Hamilton Rating Scale for Depression; iVNS, surgically implanted cervical vagal nerve stimulation; MIDAS, Migraine Disability Scale; PASS, Pain Anxiety Symptoms Scale; pCS, prospective case series; pPS, prospective pilot study; R, responder (≥30%–50% reduction severity/frequency); rCS, retrospective case series; SoC, standard of care; VAS, visual analog scale.
Specifically targeting EM and CM, one RCT (EVENT: Chronic migraine prevention with non-invasive vagus nerve stimulation; Class II study) and five prospective observational, cohort studies have been extracted for the years 2014–2018 (data from PRESTO study not included). All included trials were conducted to determine, in particular, the preventive and abortive impact of adjunctive, cervical nVNS. In some instances, patients were included and classified as not drug resistant (Table 2).4,11–18
Table 2.
Summary of clinical studies addressed to non-invasive cervical vagal nerve stimulation (nVNS) for the treatment of episodic and chronic migraine
| Year, study design | Headache disorder | Primary treatment | Patient no. | Outcome/parameter | Follow-up | Cyclic stimulation paradigm | Efficacy | Safety/tolerability |
|---|---|---|---|---|---|---|---|---|
| 2014, pCS11 Class IV | EM±aura | Acute | 27 | Treatment of four attacks per patient 80 attacks treated (54 moderate/severe) (26 mild/moderate) |
6 weeks | 90 sec dose at a 15 min interval applied two times Unilateral vagus nerve Right sided |
47% pain relief+21% pain free for the first attack (moderate/severe) 63% pain free for the first attack (mild/moderate) 43% pain relief+22% pain free for all attacks (moderate/severe) 38% pain free for all attacks (mild/moderate) |
No SAE Mild AE (four patients) Stiff neck Raspy voice Dizziness |
| 2015, pCS12 Class IV | HFEM CM | Acute | 48 14 HFEM 34 CM |
Treatment of one to five attacks per patient 131 attacks treated (all mild/moderate) |
2 weeks | 120 sec dose at a 3 min interval applied two times Unilateral vagus nerve Right sided |
38% pain relief at 1 h 51% pain relief at 2 h 18% pain free at 1 h 23% pain free at 2 h R-65%=pain relief at 2 h R-40%=pain free at 2 h |
No SAE |
| 2015, pCS13 Class IV | EM±aura CM±aura | Acute Prevention | 20 10 EM 10 CM |
Severity/frequency Functional impairment (MIDAS, PSQI, BDI) |
3 months | 120 sec dose at a 3 min interval applied bilaterally two times/day (prevention) 120 sec dose at a 3 min interval applied bilaterally two times/day (acute) Bilateral vagus nerve |
Severity reduction 8±0.5 vs 4±0.5 VAS Frequency (headache days/attack) reduction 14.7±0.9 vs. 8.9±0.8 7.3±0.9 vs. 4.5±0.6 MIDAS, PSQI (all p values <0.001) |
No SAE |
| 2016, RCT14 Event 1-month baseline phase 2-month double- blind, sham- controlled phase 6-month open- label phase Class II for safety Class III for efficacy |
CM | Prevention | Randomized phase n=59 nVNS=30 Sham=29 Open-label phase n=48 |
Severity/frequency Safety, tolerability and efficacy |
8 months | Two 120 sec doses at a 5–10 min interval applied unilaterally three times/day Unilateral vagus nerve Right sided |
Randomized phase Frequency (headache days per month) nVNS=1.4 days reduction Sham=0.2 days reduction (p=0.56) Open-label phase Frequency (headache days per month) nVNS=3.6 days reduction Sham=2.5 days reduction p<0.05 vs. baseline |
No device-related discontinuations Randomized phase No SAE n=6 nVNS AE n=5 sham AE Open-label phase Two nVNS-unrelated SAE n=5 nVNS AE |
| 2016, pCS15 Class IV | MM | Prevention | 51 | Severity/frequency Functional Impairment (MIDAS/HIT) |
3 months | 120 sec dose at a 3 min interval bilaterally applied three times/day (prevention) Three days prior to 3 days after menstruation (6 days treatment) Bilateral vagus nerve |
Severity reduction −0.5±0.2, p=0.002 Frequency (headache days) reduction 7.2±0.7 vs 4.7±0.5, p<0.001 MIDAS −11.9±3.4, p<0.001 HIT −3.1±0.7, p<0.001 R-39% (secondary endpoint) |
No SAE |
| 2017, pCS16 Class IV | EM-aura | Acute | 9 Adolescents (13–18 years of age) |
47 attacks treated | 4 weeks | 120 sec dose unilaterally plus additional 120 sec dose within 1 h allowed (if not pain free) Right-sided vagus nerve |
47% without medication 53% with medication 40% became pain free at 1 h 6% achieved pain relief at 2 h |
No SAE |
Abbreviations: AE, adverse event; BDI, Beck Depression Inventory; CM, chronic migraine; DAE, device-related adverse event; EM, episodic migraine; HFEM, high-frequency episodic migraine; HIT, Headache Impact Test; MIDAS, Migraine Disability Scale; MM, menstrual migraine; nVNS, noninvasive vagus nerve stimulation; pCS, prospective case series, PSQI, Pittsburgh Sleep Quality Index; R, responder; RCT, randomized controlled trial; SAE, serious adverse event; SDAE, serious device-related adverse event; VAS, visual analog scale.
Clinical trials conducted to assess nNVS for ECH or CCH (e.g., preventive and abortive capability) exist in higher numbers and on a higher level of evidence. Three RCTs (ACT-1: Non-invasive vagus nerve stimulation for the acute treatment of cluster headache; ACT-2: Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache, PREVA: Non-invasive vagus nerve stimulation for prevention and acute treatment of chronic cluster headache) and two prospective case series were published in the past 3 years (2015–2018).23,25–29 Distinct study designs were conceptualized in order to separately screen the acute (ACT-1, ACT-2) and the preventive (PREVA) outcome of ECH/CCH patients treated with cervical nVNS.25,26,28,29 Additionally, a post hoc analysis and a cost-effectiveness evaluation were performed according to the PREVA data (Table 3).
Table 3.
Summary of clinical studies addressed to non-invasive cervical vagal nerve stimulation (nVNS) for the treatment of episodic and chronic cluster headache
| Year, study design | Headache disorder | Primary treatment | Patient no. | Outcome/parameter | Follow-up | Cyclic stimulation paradigm | Efficacy | Safety/tolerability |
|---|---|---|---|---|---|---|---|---|
| 2015, pCS23 Class IV | ECH CCH |
Acute Prevention |
19 8 ECH 11 CCH |
Severity/frequency Safety/tolerability |
12 months | 120 sec dose at a 15 min interval applied two times (prevention) 120 sec dose at a 3 min interval applied unilaterally two to three times/day (acute) Unilateral vagus nerve Predominant head pain |
48% overall improvement in 15 patients 47% overall abortive rate within 11±1 min 55% oxygen reduction in 10 patients 48% triptan intake reduction in nine patients Significant attack frequency reduction (4.5/24 vs. 2.6/24 h) p<0.0005 |
No SAE Mild AE (two patients) Skin reaction Side shift of attacks |
| 2015, pCS24 Class IV | CTS | Acute Prevention |
1 | Severity/frequency Functional impairment (MIDAS, BDI) |
12 weeks | 90 sec dose at a 15 min interval applied two times (prevention) 90 sec dose at a 15 min interval applied at attack onset (acute) Unilateral vagus/right sided |
45% overall improvement 53% significant MIDAS improvement |
No SAE |
| 2016, RCT25 ACT-1 study 1-month double- blind, sham- controlled phase 3-month open- label phase Class I |
ECH CCH Subgroup analysis |
Acute | Randomized phase 150 73 nVNS 77 sham Open-label phase 128 59 nVNS 69 sham |
Primary endpoint (response rate at 15 min treatment) Secondary endpoint (sustained response rate at 15–60 min treatment) ECH vs. CCH subtype analysis |
4 months | Three to five doses of 120 sec duration at premonitory symptoms or pain onset Unilateral vagus nerve Right sided |
Primary endpoint ITT analysis p=0.1 nVNS-ECH vs. sham-ECH p=0.008 Secondary endpoint ITT analysis p=0.04 nVNS-ECH vs sham-ECH p=0.008 |
Randomized phase ≥1 AE in 72/150 (48%) ADE in 35/150 (23%) Open-label phase ≥1 AE in 42/128 (33%) ADE in 18/128 (14%) No SDAE |
| 2017, RCT26 ACT-2 study 2-week double- blind, sham- controlled phase 2-week open-label phase Class I | ECH CCH Subgroup analysis |
Acute | Randomized phase 102 50 nVNS 52 sham Open-label phase 83 45 nVNS 38 sham |
Primary endpoint (pain free at 15 min treatment) Secondary endpoint (pain-relief rate + pain-free rate + mean change in pain intensity at 30 min treatment) ECH vs. CCH subtype analysis |
4 weeks | Three to six doses of 120 sec duration at premonitory symptoms or pain onset Unilateral vagus nerve Predominant head pain |
Primary endpoint ITT analysis p=0.71 nVNS-ECH vs. sham-ECH p<0.01 Secondary endpoint pain relief ITT analysis p=0.05 nVNS-ECH vs sham-ECH p=0.07 nVNS-CCH vs sham-CCH p=0.34 |
Randomized phase ≥1 AE in 34/102 (33%) ≥1 ADE 18% Open-label phase ≥1 AE in 23/83 (27%) ≥1 ADE 12% No SDAE |
| 2015, RCT27 PREVA study 4-week randomized phase 4-week extension phase Class III |
CCH | Prevention Acute nVNS+SoC vs. SoC alone |
Randomized phase 97 48 nVNS+SoC 49 SoC alone Extension phase 92 nVNS+SoC |
Primary endpoint (reduction in mean of CH attacks/week) Secondary endpoint (≥50% response rate, acute medication use, duration/intensity of CH attacks) |
8 weeks | Three doses of 120 sec duration at a 5 min interval twice per day plus three additional doses for acute use Unilateral vagus nerve Right sided |
Primary endpoint ITT analysis p=0.02 nVNS+SoC (−5.9) vs. SoC (−2.1) Secondary endpoint ≥50% response rate nVNS+SoC (40%) vs. SoC (8.3%) p<0.001 |
Randomized phase ≥1 AE 38% (nVNS+SoC) ≥1 AE 27% (SoC) Extension phase ≥1 AE 25% (nVNS+SoC) ≥1 AE 24%(SoC) No SDAE |
| 2017, RCT28 PREVA study post hoc analysis 4-week randomized phase 4-week extension phase Class III |
CCH | Prevention Acute nVNS+SoC vs. SoC alone | Randomized phase 97 48 nVNS+SoC 49 SoC alone Extension phase 92 nVNS+SoC |
Primary endpoint (reduction in mean of CH attacks/week + global changes) Secondary endpoint (response rates at cut-offs of ≥25%, ≥50%, ≥75%, ≥100% frequency reduction) |
8 weeks | Three doses of 120 sec duration at a 5 min interval twice per day plus three additional doses for acute use Unilateral vagus nerve Right sided |
Primary endpoint ITT analysis p<0.02 nVNS+SoC vs. SoC at all study time points Secondary endpoint ≥25%, ≥50% (p<0.001) ≥75% (p=0.009) nVNS+SoC vs. SoC |
Randomized phase ≥1 AE 38% (nVNS+SoC) ≥1 AE 27% (SoC) Extension phase ≥1 AE 25% (nVNS+SoC) ≥1 AE 24% (SoC) No SDAE |
| 2016, RCT21 Cost-effectiveness analysis PREVA data |
Pharmacoeconomic model using PREVA data nVNS+SoC vs. SoC alone |
1-year cost- effectiveness analysis CCH |
Preventive treatment | Health care cost Short-/long-term response QALY Abortive medication use Adjunctive therapies |
12 months | ------------- | nVNS=7,096 Euro SoC=7,511 Euro nVNS+SoC vs. SoC 23% reduction in abortive use nVNS (QALY) 0.607 vs. SoC (QALY) 0.522 |
Adjunctive nVNS more effective and cost saving than SoC alone |
| 201622 Cost-effectiveness analysis |
Pharmacoeconomic nVNS+SoC vs. SoC alone |
1-year cost- effectiveness analysis ECH |
Acute treatment | Health care cost QALY Adjunctive therapies |
12 months | ------------- | nVNS=$9,510 SoC=$10,040 nVNS (QALY) 0.83 vs. SoC (QALY) 0.74 |
Adjunctive nVNS more effective and cost saving than SoC alone |
Abbreviations: ADE, adverse device effect; AE, adverse event; BDI, Beck Depression Inventory; CCH, chronic cluster headache; CH, cluster headache; CTS, cluster tic syndrome; ECH, episodic cluster headache; ITT, intent-to-treat; MIDAS, Migraine Disability Scale; nVNS, noninvasive vagus nerve stimulation; pCS, prospective observational cohort studies, QALY, quality-adjusted life years; RCT, randomized controlled trial; SAE, serious adverse event; SDAE, serious device-related adverse event; SoC, standard of care.
Cervical invasive (surgically implanted) VNS and primary headache disorders
The majority of the published iVNS data consist of case series or retrospective analyses, predominantly in patients with refractory focal seizures with co-occurring chronic headache (commonly migraine) with follow-up varying from 3 months to 14 years.3,5–10 For surgically implanted VNS, a multicenter, randomized controlled study has not yet been carried out, as seizure mostly represented the primary indication. Thus, a sufficient and comparative analysis of stimulation patterns and surgical- and/or stimulation-induced side effects addressed to head pain outcome is limited.
The majority of the 26 patients (basilar migraine, CCH, CDH, CM) were treated by iVNS.3,5–7 Two pilot studies dedicated to refractory headache in 10 patients have been published.8,9 The first included six patients (three CM, two CCH and one basilar migraine). Two of three patients with CM and both CCH patients improved markedly with respect to severity and functional impairment. The second pilot trial enrolled four patients (solely CM) and observed a 100% responder rate, as all four patients (CM) responded (defined as at least 50% reduction in severity or frequency).
The largest previous study was a retrospective analysis including 325 seizure patients (6% with coincidental CDH or CM [19/325]) that compared iVNS/standard medical care (SMC) vs. SMC alone vs. an age-/gender-matched healthy control (HC) group with an observation period ranging from 5 to 13 years (mean 8 years).10
iVNS was applied utilizing the following cyclic stimulation paradigms: 1.3 mA (0.5–2 mA), 20 Hz, 250 μs, 30 sec on/1.9 min off (0.5–5 min). iVNS outcome parameters were headache severity/frequency and functional capacity (mood changes, sleep, cognitive pain perception, pain-associated anxiety and fear behavior). The iVNS group experienced a significantly lower headache severity (visual analog scale [VAS] scores [iVNS 5.4, SMC 7.8; p=0.03]) accompanied by functional responsiveness measured by the Pain Anxiety Symptom Score (cognitive/anxiety subscores [iVNS 21, SMC 16; p=0.02]) compared to SMC and HC. Other functional state parameters were not significantly different (Table 1).
However, the earlier data (Class IV studies) suggest that iVNS attenuates seizure-associated head pain and primary headache disorders. Although not determined on a high evidence level, in some instances, reduction of head pain severity/frequency was superior to seizure improvement.
Cervical noninvasive VNS and primary headache disorders
Episodic and chronic migraine
Several prospective cohort studies have been published assessing the preventive and acute usefulness of cervical nVNS for EM and CM and migraine-associated comorbidities.11–14 In addition migraine subtypes such as menstrual-related migraine and migraine in young adolescents have been under clinical investigation.15–17
Goadsby et al first introduced nVNS as an adjunctive alternative for abortive treatment in EM with/without aura.11 In an open-label study design, cervical nVNS was assessed for its adjunctive and abortive impact in 27 EM patients (19 with moderate-severe head pain and 8 with mild-moderate head pain), who in total treated 80 attacks. nVNS was unilaterally (right sided) applied with a 90 sec dose at a 15 min interval. Participants were permitted to treat up to four attacks (Table 2). Adjunctive nVNS for the first treated attack demonstrated a pain-free rate, at 2 h after treatment, of 21% (4/19) and a pain relief rate of 47% (9/19) in the moderate–severe classified group. Also, 63% (5/8) in the mild–moderate rated group experienced a pain-free state. For all mild–moderate attacks, 38% (10/26) achieved freedom from pain, and for all attacks classified as moderate–severe, 22% (12/54) were pain free at 2 h and 43% (23/54) achieved pain relief. Thus, the abortive effect of cervical nVNS for mild–moderate attacks as well as for moderate–severe classified head pain was comparably effective with first-line pharmacologic interventions. These initial observations were extended and confirmed toward EM and CM and migraine-related impaired functional capacity such as mood changes and sleep quality.12,13 In the first clinical trial, both migraine subtypes (EM and CM) were evaluated.
Adjunctive cervical nVNS was applied abortively over 2 weeks with an acute treatment protocol (120 sec dose, unilateral, right sided, at 3 min intervals, two times/day) and achieved a pain relief rate of 38% at 1 h and 51% at 2 h after treatment and a pain-free rate of 18% at 1 h and 23% at 2 h. Out of the 48 EM/CM patients, 56% reported pain relief at 1 h and 65% at 2 h.12 The second EM/CM cohort study extended the use of nVNS toward prevention and abortive use and found a significant decline of severity (8±0.5 vs 4±0.5 VAS, p<0.001) and frequency (headache days: 14.7±0.9 vs. 8.9±0.8, attacks: 7.3 0.9 vs. 4.5±0.6; p<0.001) and improved functional capacity (e.g., sleep quality, mood, and migraine disability, p<0.001) after 3 months adjunctive nVNS (prevention protocol: 120 sec dose, bilateral, at 3 min interval; two times/day plus acute protocol: 120 sec dose, bilateral, at 3 min interval two times for abortive use).13 Both studies found nVNS to be effective in EM compared to chronic migraine and that nVNS may improve head pain-related disability. Notably and contrary to the pilot study of Goadsby et al, the nVNS paradigm was slightly modified as nVNS was applied at a 120 sec dose for a shorter interval.
A randomized double-blind, sham-controlled multicenter trial (EVENT) solely evaluated the preventive value and safety/tolerability in chronic migraine with a slightly different stimulation protocol compared to the previous migraine/nVNS studies.14 In total, 48 CM patients were enrolled and a meaningful frequency reduction was observed in the randomized phase (nVNS −1.4 days vs. 0.2 days), while the open-label phase nVNS (120 sec dose, right sided, at 5–10 min interval) was associated with significantly decreased headache days/month (nVNS −3.6 vs. −2.5 days, p<0.05). Of note, the EVENT study failed to achieve its primary endpoint, as a 50% reduction was only confirmed in 39% of nVNS-treated subjects. Most of the observed AEs (treatment related or device related) were of mild character and transient. No device-associated discontinuation and/or device-associated severe AEs were recorded.14
Furthermore, menstrual-related migraine and migraine in young adolescents have been under nVNS investigation, as both migraine subtypes are limited either in preventive/abortive responsiveness to conventional pharmacotherapy or in the availability of conventional interventions in the case of young migraine patients.15,16 The first study assessed 51 menstrual/menstrual-associated migraine patients treated with 3 months adjunctive, prophylactic nVNS (prevention protocol: 120 sec dose, bilateral, at 3 min interval, three times/day, nVNS initiation 3 days prior to 3 days after menstruation onset). Thirty-nine percent (20/51) perceived a ≥50% reduction with a significant decline in frequency (headache days/month: 7.2±0.5 vs 4.7±0.5, p<0.001), severity (VAS reduction: −0.5±0.2, p=0.002) and functional responsiveness (migraine disability score: −11.9±0.5, headache impact test: −3.1±0.7; p<0.001).15
The second study included nine adolescent migraineurs (EM; age: 13–18 years), who treated 47 attacks in total within 4 weeks adjunctive nVNS (acute protocol: a single 120 sec dose plus additional single 120 sec dose within 1 h if not pain free, unilateral, right sided). Interestingly, in 47% of all nVNS-treated attacks, adjunctive rescue medication was not required and 53% of all attacks required rescue medication. A pain-free state was observed after 1 h of treatment in 40% (19/47) and pain relief was observed in 6% (3/47).16 Given these facts, nVNS deserves clinical attention as a considerable and safe alternative in migraine subtypes with impaired therapy options like menstrual-related migraine or migraine in young adolescents.
Although not published, based upon the results of the multicenter, randomized, double-blind, sham-controlled trial, termed PRESTO study (prospective study of nVNS for the acute treatment of migraine), nVNS received the US Food and Drug Administration clearance for abortive therapy in migraine (Class I study). Briefly, nVNS acute treatment resulted in significantly higher rates of pain freedom at 30, 60 and 120 min and mean head pain reduction compared to sham stimulation, with a comparable safety/tolerability profile as previously reported.18
Episodic and chronic cluster headache
Nesbitt et al pioneered the prophylactic and acute administration of cervical nVNS for ECH and CCH in a 1-year observational study.23 In this initial pilot study, the prophylactic and acute impact of nVNS was observed for CH (cluster headache) in 19 CH patients (8 ECH and 11 CCH; nVNS prevention protocol: 120 sec dose, predominant head pain side, at 3 min interval 2–3 times/daily 120 sec dose; acute protocol: single 120 sec dose for acute use). A significant decline in attack frequency was observed after 12 months (4.5/24 vs. 2.6/24 h, p<0.0005). Fifteen of 19 participants reported an overall improvement of 48%, and 47% of all treated attacks were aborted within an average of 11±1 min. In 10 out of 14 patients (71%), oxygen demand was decreased by 55%±8%, with one patient increasing the oxygen uptake; in nine out of 12 patients (75%), triptan intake declined by 48%±6%, with none increasing the triptan demand. Of note, nVNS was unilaterally administered on the predominant head pain side and encompassed a combined preventive/acute nVNS protocol.
The ACT-1/ACT-2 studies (Class II studies) represent the first RCT-designed studies for the acute treatment in ECH/CCH with adjunctive nVNS with the following stimulation parameters: 120 sec dose, unilateral, predominant head pain side, 3–6 times at premonitory symptoms or at pain onset.25,26 ACT-1 and ACT-2 confirmed the abortive impact of nVNS with a more pronounced responsiveness for ECH patients. In ACT-1, the randomized phase response rate (defined as the proportion of subjects with pain intensity score of 0 or 1 within 15 min) in the total sample was not significantly different (p=0.1) compared to sham. However, interestingly, in the ECH subgroup, the response rate was significantly higher after verum stimulation than sham (p=0.008), while there was no significant difference for CCH patients (p=0.48). In the open-label phase, significantly higher response rates were found in the entire study population (p=0.04) and in the ECH subgroup (p=0.008).25 The ACT-2 study confirmed and extended the findings from the ACT-1 study. No significant differences were found in the total sample (p=0.71), while the ECH patients differed significantly (pain-free rate p<0.01) compared to sham in the randomized 2-weeks phase. In the open-label phase, nVNS was associated with a significantly higher pain-free rate for the total cohort (p=0.05), but not for the ECH group (p=0.07) and not for the CCH group (p=0.34).26 A pooled assessment of ACT-1 and ACT-2 outcome parameters demonstrated a significantly improved responsiveness for ECH patients treated with nVNS compared to sham stimulation.27
The PREVA study (Class III study) was solely addressed to CCH patients and compared nVNS plus standard care vs. standard care alone for both prevention and abortive administration. For primary and secondary endpoints, nVNS plus standard care demonstrated significant differences in favor of adjunctive use of nVNS.28 The utilized nVNS parameters were as follows: prevention protocol: three doses of 120 sec, predominant head pain side, at 5 min interval, twice/day; acute protocol: 120 sec dose (three times) for acute use. In the randomized phase, the reduction of CH attacks/week was significantly different between the nVNS+standard of care (SoC) and SoC groups (−5.9 vs. −2.1, p=0.02) and achieved a ≥50% response rate in the extended phase (nVNS+SoC 40% vs. Soc 8.3%, p<0.001). A post hoc analysis of the PREVA data evaluated the mean reduction of CH attacks/week, global changes (primary endpoint), and as a secondary endpoint, the response rate at cut-offs of ≥25%, ≥50% and ≥75% and 100% frequency reduction.29 At all study time points, nVNS+SoC was superior to SoC alone (p<0.02) and the mean weekly attack frequency was significantly decreased within 2 weeks of the randomized phase. nVNS combined with standard care performed significantly better at all study time points and at the response rates defined as cut-offs of ≥25%, ≥50% and ≥75% reduction, with a comparable safety/tolerability outcome between both the groups.28 A 1-year cost-effectiveness analysis was in addition performed on the PREVA data (CCH) including the following parameters: health care cost, short-/long-term response, quality-adjusted life years, abortive medication use and additional therapies required. Using these parameters, a pharmacoeconomic model demonstrated lower costs for nVNS+SoC (7096 Euro) vs. SoC alone (7511 Euro), 23% reduction in abortive medication requirement and a higher quality-adjusted life years index (nVNS+SoC 0.607 vs. SoC 0.522).20,21 Mwamburi et al extended the cost-effectiveness analysis toward ECH and the socioeconomic burden of nVNS vs. SoC alone and found similar results comparable to the PREVA cost-effectiveness assessment.22 Table 3 gives a summary of the published literature.
The safety and tolerability profile of cervical nVNS
The most common AEs of iVNS have been associated with either the surgical implantation procedure (cardiac bradyarrhythmia, infection, bleeding, hardware malfunction) or stimulation-induced complications (cough, voice disturbances, pain) in a considerable proportion of surgical VNS procedures. Contrary to noninvasive VNS, invasive VNS uses a permanent cyclic stimulation paradigm, which may explain in part the higher incidence of stimulation-associated complications observed with iVNS in the past.
According to the current literature, nVNS has not been associated with serious AEs or serious device-related AEs and the majority of reported AEs remained transient and of mild character (e.g., skin irritation, stiff neck). With respect to the nVNS RCTs (ACT-1/ACT-2, PREVA, EVENT) and the available prospective cohort studies, a low rate of AEs or adverse device-associated events (ADEs) occurred with nVNS treatment in particular, with a similar safety/tolerability profile for nVNS compared to sham stimulation.
MOA of cervical VNS in migraine (human and preclinical studies)
The neuroinflammatory pathways have been linked to the genesis and maintenance of primary headache disorders such as migraine and cluster headache. Specifically, interactions of interleukins may lead to a disturbed neuroimmune balance.
An increasing body of experimental evidence suggests that VNS modulates the immune response and systemic inflammation by influencing pro- and anti-neuroinflammatory cytokine release (e.g., interleukin [IL]-1β, IL-10, IL-6, tumor necrosis factor [TNF]-α, HMGB-1, oxytocin) through the cholinergic anti-inflammatory reflex.30–37
Perini et al compared the plasma concentrations of pro-and anti-inflammatory cytokines in migraine patients and HCs.38 Significantly elevated concentrations of intraictal proinflammatory mediators (IL-1β, TNF-α) and anti- inflammatory cytokines such as IL-10 were observed compared to postictal values. Interestingly, increased postictal levels declined after acute head pain onset, over time. In healthy subjects, nVNS significantly decreased the plasma levels of proinflammatory IL-1β, TNF-α, IL-8, MCP-1 and MIP-1 and significantly increased the anti-inflammatory marker IL-10 compared to sham stimulation, indicating that nVNS may downregulate neuroinflammation and thus effectively acts in migraine by restoring the neuroimmune communication.39 So far, there exists no human data addressed to possible interactions of nVNS with the peripheral markers of neuroinflammation in migraine patients.
In order to establish a preclinical model, which parallels the state of recurrent headache attacks or chronic trigeminal nociceptive hypersensitivity, Oshinsky and Gomonchareonsiri exposed dural nociceptors repetitively to an inflammatory infusion.40 Quantitative sensory testing (von Frey hair/monofilaments) of the periorbital region and microdialysis screening in the trigeminal nucleus caudalis (TNC) were implemented in order to assess glyceryl nitrate-evoked changes. After repeated inflammatory infusion, a chronic hypersensitive status along with significantly elevated extracellular glutamate levels was observed. In a later preclinical migraine setting, nVNS (120 sec dose) significantly suppressed extracellular glutamate concentrations without hemodynamic and cardiac side effects.41 Serotonin or nor-epinephrine remained unchanged, indicating that nVNS may be a reasonable treatment approach for trigeminal allodynia.
The impact of preventive migraine drugs (topiramate, valproate, propranolol, amitryptiline) on CSD frequency and the electrical threshold required to initiate CSD propagation was investigated by Ayata et al in another preclinical study using topical application of potassium (applied locally [dura]) or incremental cathodal stimulation.42 Chronic administration of prophylactic migraine drugs decreased CSD frequency by 40%–80% and increased cathodal stimulation thresholds for CSD induction. In contrast, acute drug delivery remained without any effect, suggesting CSD analysis to be a suitable approach to determine and develop preventive treatment alternatives. Based on this model, invasive and noninvasive VNS equally suppressed CSD susceptibility in the occipital cortex and increased electrical thresholds by nearly 2-fold either in the ipsilateral or the contralateral hemisphere. Of note, CSD suppression lasted >3 h after a 240 sec dose of nVNS application. These observations indicate that nVNS may interact with the development and propagation of CSD as the electrophysiological correlate of migraine aura.43–45
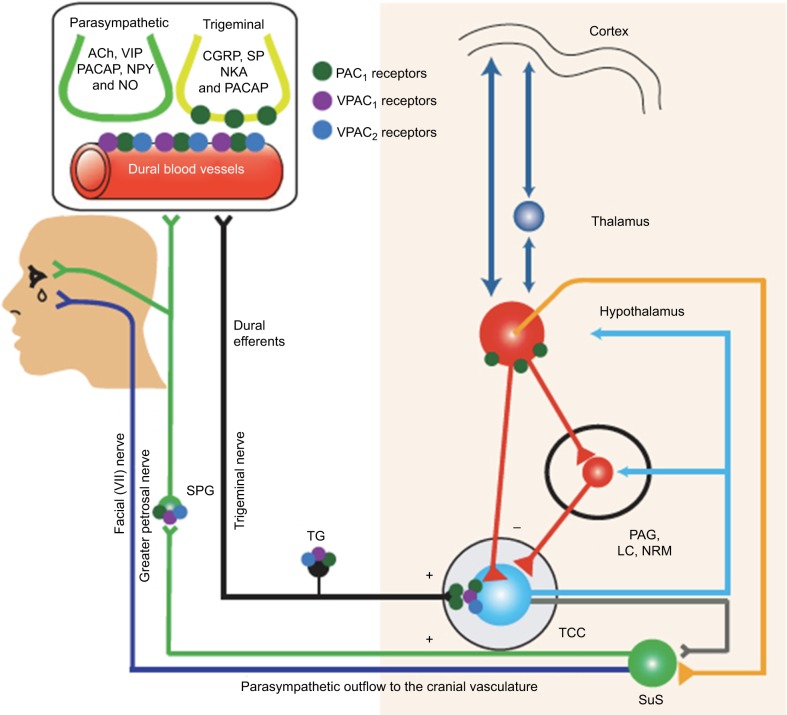
The genesis of acute migraine pain has been linked with primary afferents activation of the trigeminal nerve/ganglion (TNC) promoted by increased firing of dural nociceptors (vasculature of the dura). The TNC itself projects to the trigemino-cervical complex (TCC), brainstem/medulla oblongata, hypothalamic/thalamic and cortical associated networks (Figure 1). In order to assess the impact of peripheral and central mechanisms in migraine onset, Akerman and Goadsby measured dural vasculature changes and TCC firing response after intravenous and intracerebroventricular administration of the sensory and parasympathetic neuropeptides, vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide 38 (PACAP-38), with receptor-subtype analysis (VPAC1/2, PAC1).46 Briefly, PACAP-38 and VIP induced dural vessel dilatation via VPAC2-receptor, but VIP was not associated with changes in TCC neuron firing pattern. Neurogenic dural vasodilatation evoked by dural terminals of trigeminal nerve fibers was suppressed by PAC1-receptor antagonist. Intracerebral–ventricular application of PACAP-38, but not of VIP, caused delayed activation and central sensitization of spontaneous TCC firing response (mainly via VPAC1 and more pronounced PAC1) along with increased responsiveness to intracranial (dural-evoked) and extracranial (cutaneous) stimulation. In conclusion, these observations suggest the involvement of endogenous mechanisms in migraine onset rather than dural vascular dilatation. Of note, preclinical nVNS models determined possible interactions with some of these components of the trigemino-nociceptive head pain circuits (Figure 1).46
Figure 1.
Schematic drawing of suspected distribution of VPAC1/2 and PAC 1 receptors subtype within the trigeminovascular complex and associated brain circuits.
Notes: The parasympathetic neuropeptides vasoactive intestinal peptide (VIP) and pituitary adenylate cylase-activating peptide 38 (PACAP-38) interacts with receptor-subtype analysis (VPAC1/2, PAC 1) on a central and peripheral level. Neurogenic dural vasodilatation evoked by dural terminals of trigeminal nerve fibers was suppressed via PAC1-receptor subdomain. Intra-cerebral-ventricular application of PCAP-38, but not VIP, caused delayed activation and central sensitization of spontaneous TCC ring response (mainly via PAC1) along with increased responsiveness to intra- (dural-evoked) and extracranial (cutaneous) stimulation. From Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci Transl Med. 2015;7(308):308ra157. Reprinted with permission from AAAS.46
Abbreviations: SuS, nucleus salivatorius superior; TG, trigeminal ganglion; TCC, trigeminocervical complex; SPG, ganglion sphenopalatinum; PAG, periaquaeductal grey; LC, locus coeruleus; NRM, nucleus raphe magnus; Ach, acetylcholine; NKA, neurokrinin; CGRP, calcitonin gene-related peptide; SP, substance P.
MOA of cervical VNS in cluster headache (human and preclinical data)
With respect to cluster headache, several human studies determined serum concentrations of pro- and anti-neuroinflammatory cytokines/chemokines compared to HCs. Utilizing a sensitive enzyme-linked immunosorbent assay, a decreased IL-2 receptor expression on the lymphocyte was found in episodic cluster patients. Furthermore, recombinant IL-2 and interferon-β were found to counteract this peripheral downregulation.47–49 In a later work, Martelletti et al observed that the serum IL-1β level was significantly higher in ECH patients compared to HCs, intraictally as well as postictally.50 In addition, intraictal concentrations were higher than postictal values. IL-1β binds on the hypothalamic receptors, induces corticosteroid secretion and increases substance P synthesis, which itself sensitizes neurons of the autonomic nervous system (sympathetic branch). These multiple reciprocal interactions are believed to be part of a possible feedback loop involved in CH attack onset and suppression.
In cluster head pain, the trigemino-autonomic reflex is suspected to contribute to cluster attack onset and autonomic symptoms (lacrimation flow, nasal congestion). Activation of the nucleus salivatorius superior induces the parasympathetic vasodilatation pathway involving the modulation of TCC neurons and related circuits (Figure 2). Thus, Akerman and Goadsby developed an acute preclinical approach with the capability to screen migraine-like head pain (dural vascular activation) and cluster-like head pain (trigemino-autonomic reflex). In earlier electrophysiological, preclinical head pain studies, administration of triptans significantly inhibited spontaneous and evoked firing response rates of TCC neurons.51
Figure 2.
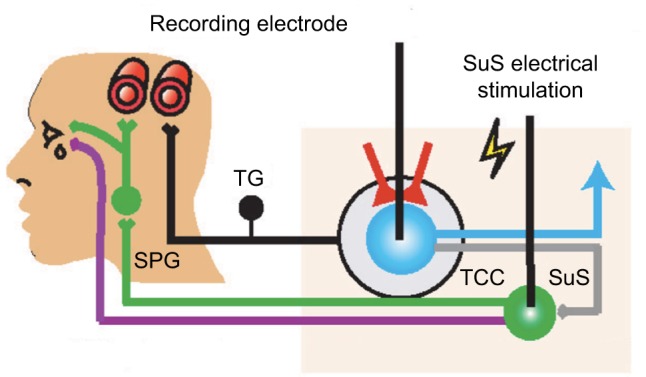
Schematic drawing illustrating the preclinical setting for cluster-like head pain.
Notes: The trigemino-autonomic reflex is suspected to contribute to cluster attack onset and autonomic symptoms (lacrimation flow, nasal congestion). Activation of the nucleus salivatorius superior induces the parasympathetic vasodilatation pathway involving the modulation of TCC neurons and related circuits and evokes autonomic features of cluster headache. From Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci Transl Med. 2015;7(308):308ra157. Reprinted with permission from AAAS.46
Abbreviations: SuS, nucleus salivatorius superior; TG, trigeminal ganglion; TCC, trigeminocervical complex; SPG, ganglion sphenopalatinum.
In order to validate the postulated abortive impact of VNS, spontaneous and nociceptive-evoked firing rates of TCC neurons were recorded ipsilaterally and in the contra-lateral hemisphere.52 Dose-dependent changes were observed using a single vs. two 120 sec doses of direct VNS applied at the following parameters: 1 ms pulse of 5 kHz sine waves repeated at 25 Hz. The VNS dose–response was more pronounced with two 120 sec doses.
After both ipsi- and contralateral stimulation, a dose-dependent prolonged decline of spontaneous TCC firing rates was observed after 3 h (60%). Likewise, a suppression of dural-evoked TCC firing by 22% (Aα fiber mediated; fast response) and by 55% (C fiber mediated; slow response) was evident in the VNS-treated group compared to sham stimulation. There were no significant differences between both hemispheres. In the same experimental approach, the nucleus (ncl.) salivatorius superior–induced response (TCC firing rate) was suppressed by 22% for 2.5 h after VNS, compared to ipsilateral sham stimulation, and was significantly diminished for ongoing spontaneous TCC neuronal firing. The observed contralateral effects may be indicative of head pain modulation by descending pathways involving the ncl. paraventricularis of the hypothalamus, locus coeruleus and dorsal raphe nucleus. Furthermore, TCC neuron suppression by VNS decreases the likelihood of an attack due to sustained lower TCC thresholds and may explain partly the observed preventive clinical VNS responsiveness.52
Conclusion
Currently published clinical nVNS data demonstrate promising clinical effects for the abortive use in episodic migraine and cluster headache. The interpretation of the findings in this narrative review may be hindered due to several considerations. Although, most of the abortive and preventive trials have been determined as Class I–IV studies (Class I studies for the acute treatment and Class II–IV studies for the preventive use), comparative and reproduc ible conclusions are limited by the different stimulation protocols and/or outcome parameter measures. Secondly, a more systematic review-based approach including multiple comparative correlation analysis of primary and secondary endpoint classified as significantly different or nearly-significant should re-examine the positive findings of our narrative review. So far, the episodic subtypes of migraine and cluster headache have responded superiorly compared to the chronic forms. Due to its noninvasive character along with the reported tolerability, cervical nVNS may be justified in the pre-refractory state of migraine and cluster headache, and probably in a migraine subpopulation with limited available options (e.g., adolescents with migraine, menstruation-associated migraine). The afferent properties of the vagus nerve are well connected via the ncl. tractus solitarii to the locus coeruleus, the dorsal raphe nucleus, the parabrachial plexus, the paraventricular nucleus of the hypothalamus and directly to the TNC and the cervical spinal cord. Given these anatomic reciprocal projections of the vagus nerve, electrical noninvasive modulation of the cervical vagal afferents may impact trigeminovascular nociceptive transmission.
Along with a rising number of targeted preclinical studies supporting the observed clinical VNS responsiveness in primary headache disorders, the authors strongly believe that VNS constitutes an emerging issue of ongoing headache treatment and research.
Acknowledgments
The authors want to express their thanks to Bruce J Simon (PhD) for giving important intellectual input.
Footnotes
Disclosure
Thomas M Kinfe has received training support, and works as a consultant for St Jude Medical, Inc. and Medtronic Inc. The authors report no other conflicts of interest in this work.
References
- 1.Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014;1(2):64–73. doi: 10.1007/s40473-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27(2):130–138. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22(9):1260–1268. doi: 10.1111/ene.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 5.Lenaerts ME, Oommen KJ, Couch JR, Skaggs V. Can vagus nerve stimulation help migraine? Cephalalgia. 2008;28(4):392–395. doi: 10.1111/j.1468-2982.2008.01538.x. [DOI] [PubMed] [Google Scholar]
- 6.Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia. 2002;22:482–484. doi: 10.1046/j.1468-2982.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 7.Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagal nerve stimulation on migraine. J Pain. 2003;4:530–534. doi: 10.1016/j.jpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82–86. doi: 10.1111/j.1468-2982.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 9.Cecchini AP, Mea E, Tullo V, et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci. 2009;30(1):101–104. doi: 10.1007/s10072-009-0073-3. [DOI] [PubMed] [Google Scholar]
- 10.Pintea B, Hampel K, Boström J, et al. Extended long-term effects of cervical vagal nerve stimulation on headache intensity/frequency and affective/cognitive headache perception in drug resistant complex-partial seizure patients. Neuromodulation. 2017;20(4):375–382. doi: 10.1111/ner.12540. [DOI] [PubMed] [Google Scholar]
- 11.Goadsby PJ, Grosberg BM, Mauskop A, Cady R, Simmons KA. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34(12):986–993. doi: 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 12.Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Noninvasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 2015;16:61. doi: 10.1186/s10194-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinfe TM, Pintea B, Muhammad S, et al. Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: a prospective observational cohort study. J Headache Pain. 2015;16:101–107. doi: 10.1186/s10194-015-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silberstein SD, Calhoun AH, Lipton RB, et al. Chronic migraine headache prevention with non-invasive vagus nerve stimulation: the EVENT study. Neurology. 2016;87:1–10. doi: 10.1212/WNL.0000000000002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grazzi L, Egeo G, Calhoun AH, McClure CK, Liebler E, Barbanti P. Non-invasive Vagus Nerve Stimulation (nVNS) as mini-prophylaxis for menstrual/menstrually related migraine: an open-label study. J Headache Pain. 2016;17(1):91. doi: 10.1186/s10194-016-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grazzi L, Egeo G, Liebler E, et al. Non-invasive Vagus Nerve Stimulation (nVNS) as symptomatic treatment for migraine in young patients: a preliminary safety study. Neurol Sci. 2017;38(Suppl 1):S197–S199. doi: 10.1007/s10072-017-2942-5. [DOI] [PubMed] [Google Scholar]
- 17.electroCore News. Electrocore receives FDA approval for chronic migraine study. [Accessed September 14, 2014]. Available from: http://www.electrocore.com/elec-trocore-receives-fda-approval-for-chronic-migraine-study.
- 18.electroCore News. Electrocore receives FDA clearance for acute treatment of pain associated with migraine headache in adult patients. [Accessed January 29, 2018]. Available from http://www.electrocore.com/3518-2.
- 19.Holle-Lee D, Gaul C. Noninvasive vagus nerve stimulation in the management of cluster headache: clinical evidence and practical experience. Ther Adv Neurol Disord. 2016;9(3):230–234. doi: 10.1177/1756285616636024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenks M, Davis S, Amato F, Errico J, Strickland I. A preliminary cost-utility analysis of non-invasive vagusnerve stimulation therapy in patients suffering with headache and functional disorder multi-morbidity. ISPOR 2016: 19th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR); Health Economics Consortium; 2016 Oct 29 – Nov 2; Vienna, Austria. [Google Scholar]
- 21.Morris J, Straube A, Diener HC, et al. Cost-effectiveness analysis of non-invasive vagus nerve stimulation for the treatment of chronic cluster headache. J Headache Pain. 2016;17:43. doi: 10.1186/s10194-016-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwamburi M, Liebler E, Tenaglia AT. Cost-effectiveness of gammaCore (non-invasive vagus nerve stimulation) for acute treatment of episodic cluster headache. Am J Manag Care. 2017;23(16 Suppl):S300–S306. [PubMed] [Google Scholar]
- 23.Nesbitt AD, Marin JC, Tompkins E, Ruttledge MH, Goadsby PJ. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84(12):1249–1253. doi: 10.1212/WNL.0000000000001394. [DOI] [PubMed] [Google Scholar]
- 24.Kinfe TM, Pintea B, Güresir E, Vatter H. Partial response of intractable clustertic syndrome treated by cervical non-invasive vagal nerve stimulation (nVNS) Brain Stimul. 2015;8(3):669–671. doi: 10.1016/j.brs.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317–1332. doi: 10.1111/head.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goadsby PJ, de Coo IF, Silver N, et al. ACT2 Study Group. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38(5):959–969. doi: 10.1177/0333102417744362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coo I, Marin JCA, Silberstein SD, et al. Non-invasive Vagus Nerve Stimulation (nVNS): Acute Treatment of Episodic and Chronic Cluster Headache: Pooled Analysis of ACT1 and ACT2 Studies. Paper presented at: AAN2017; Boston. [Google Scholar]
- 28.Gaul C, Diener HC, Silver N, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomized controlled study. Cephalalgia. 2015;36(6):534–546. doi: 10.1177/0333102415607070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaul C, Magis D, Liebler E, Straube A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post-hoc analysis of the randomized, controlled PREVA study. J Headache Pain. 2017;18(1):22. doi: 10.1186/s10194-017-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemper RH, Meijler WJ, Korf J, Ter Horst GJ. Migraine and function of the immune system: a meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia. 2001;21:549–557. doi: 10.1046/j.1468-2982.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 32.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19(6):493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex -linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasko G, Szabo C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem Pharmacol. 1998;56:1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 35.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neural excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 37.Vida G, Peña G, Deitch EA, Ulloa L. α7- cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186(7):4340–4346. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perini F, D’Andrea G, Galloni E, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- 39.Lerman I, Hauger R, Sorkin L, et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation. 2016;19(3):283–290. doi: 10.1111/ner.12398. [DOI] [PubMed] [Google Scholar]
- 40.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47(7):1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshinsky ML, Murphy AL, Hekierski H, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155:1037–1042. doi: 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 43.Chen SP, Ay I, de Morais AL, et al. Vagus nerve stimulation inhibits cortical spreading depression susceptibility. Pain. 2016;157(4):797–805. doi: 10.1097/j.pain.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen SP, Ayata C. Spreading depression in primary and secondary headache disorders. Curr Pain Headache Rep. 2016;20(7):44. doi: 10.1007/s11916-016-0574-8. [DOI] [PubMed] [Google Scholar]
- 45.Karatas H, Erdender SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339:1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 46.Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Sci Transl Med. 2015;7(308):308ra157. doi: 10.1126/scitranslmed.aaa7557. [DOI] [PubMed] [Google Scholar]
- 47.Martelletti P, Stirparo G, De Stefano L, Di Sabato F, Giacovazzo M, Rinaldi-Garaci C. Defective expression of IL-2 receptors on peripheral blood lymphocytes from patients with cluster headache. Headache. 1990;30:228–231. doi: 10.1111/j.1526-4610.1990.hed3004228.x. [DOI] [PubMed] [Google Scholar]
- 48.Stirparo G, Martelletti P, Morrone S, Savarese A, Giacovazzo M. Impaired natural killer activity in PBLs from cluster headache patients is restored by interleukin 2. Int J Immunother. 1990;6:181–186. [Google Scholar]
- 49.Martelletti P, Stirparo G, De Stefano L, Rinaldi-Garaci C, Giacovazzo M. Studies on lymphokine-activated killer cells and natural killer cells in cluster headache: increased sensitivity to RIL-2 and IFN-B. In: Rose C, editor. New Advances in Headache Research. London: Smith Gordon; 1989. pp. 139–144. [Google Scholar]
- 50.Martelletti P, Granata M, Giacovazzo M. Serum interleukin-1 beta is increased in cluster headache. Cephalalgia. 1993;13(5):343–350. doi: 10.1046/j.1468-2982.1993.1305343.x. [DOI] [PubMed] [Google Scholar]
- 51.Akerman S, Goadsby PJ. A novel translational animal model of trigeminal autonomic cephalalgias. Headache. 2015;55(1):197–203. doi: 10.1111/head.12471. [DOI] [PubMed] [Google Scholar]
- 52.Akerman S, Simon B, Romero-Reyes M. Vagus nerve stimulation suppresses acute noxious activation of trigeminiocervical neurons in animal models of primary headache. Neurobiol Dis. 2017;102:96–104. doi: 10.1016/j.nbd.2017.03.004. [DOI] [PubMed] [Google Scholar]