Abstract
Objective
Prior reports suggested that infection with Helicobacter pylori was associated with respiratory diseases; pathogenetic mechanisms however, were not defined. We tested the hypothesis that VacA, an exotoxin of Helicobacter pylori, a gastric pathogen, was aspirated into the lung and could stimulate secretion of inflammatory cytokines by lung epithelial cells.
Methods
The presence of VacA was determined by immunohistochemistry in surgical lung biopsy tissue samples from 72 patients with interstitial pneumonia. The effects of VacA on A549 human alveolar epithelial adenocarcinoma cells and normal human bronchial epithelial cells were determined. After incubation with VacA, the secretions of cytokines were measured by Multiplex Luminex® Assays.
Results
VacA was detected with anti-VacA antibodies in bronchial epithelial cells and alveolar epithelial cells from 10 of 72 patients with interstitial pneumonia. VacA was more prevalent in lungs of patients with collagen vascular disease-associated interstitial pneumonia than in those of patients with idiopathic pulmonary fibrosis, nonspecific interstitial pneumonia and cryptogenic organizing pneumonia. Incubation of A549 cells and normal human bronchial epithelial cells with VacA for 24 h was cytotoxic, and resulted in vacuolation. VacA induced interleukin-8 production by A549 cells and normal human bronchial epithelial cells and interleukin-6 production by A549 cells. Based on multiplex screening, interleukin-8 and interleukin-6 were the primary secretory products induced by VacA.
Conclusions
Helicobacter pylori VacA is present in human lung and can induce interleukin-8 and interleukin-6 production by human lung cells. VacA could have a role in the pathogenesis of respiratory diseases by its cytotoxic effects and by inducing the secretion of interleukin-8 and interleukin-6 by targeted airway epithelial cells.
Keywords: Helicobacter pylori, VacA, A549 cells, normal human bronchial epithelial cells, interleukin-8, interleukin-6
Introduction
Helicobacter pylori (H. pylori) has been shown to be causally related to some digestive diseases, such as gastritis, peptic ulcer, gastric mucosa-associated lymphoid tissue lymphoma, and gastric carcinoma. Recently, however, H. pylori infection has been reported to be associated with extra-digestive diseases [1]. A high seroprevalence of H. pylori was observed in patients with bronchiectasis [2] and chronic bronchitis [3]. Further, patients with idiopathic pulmonary fibrosis (IPF) having anti-H. pylori antibodies in serum showed significantly lower pulmonary function and increased mortality compared to patients with IPF but without anti-H. pylori antibodies in serum [4]. These reports suggest that H. pylori could be associated with respiratory diseases, however pathogenetic mechanisms for the effects of H. pylori were not defined.
It is possible that H. pylori could be aspirated into the respiratory tract from oropharynx or the gastric reservoir and cause respiratory disease. This hypothesis, however, appears unlikely since H. pylori deoxyribonucleic acid (DNA) was not detected by polymerase chain reaction (PCR) in bronchoalveolar lavage fluids and lung tissue samples [5], and an anti-H. pylori antibody was not reactive with bronchial mucosa [6]. Furthermore, it appears unlikely that H. pylori could proliferate in the environmental conditions found in human lung. Therefore, we hypothesized that H. pylori infection raises the risk of respiratory diseases indirectly, rather than directly, by means of systemic inflammatory and/or autoimmune responses, and/or by aspiration into the lung of H. pylori products, such as exotoxins. Vacuolating cytotoxin (VacA) is the major exotoxin of H. pylori [7,8], and induces vacuolation in the cytoplasm of susceptible mammalian cells. Further, VacA has pleiotropic effects on target cells, including membrane depolarization, mitochondrial dysfunction, autophagic process, activation of mitogen-activated protein kinases, inhibition of T cell function and cell death [9].
We hypothesized that H. pylori VacA could be aspirated into the lung and stimulate secretion by lung epithelial cells of inflammatory cytokines. Here, we investigated the presence of VacA in human lung and whether inflammatory cytokine production could be induced by VacA in A549 human alveolar epithelial adenocarcinoma cells and normal human bronchial epithelial (NHBE) cells.
Methods
Study population
The protocol of this retrospective study was approved by the Institutional Review Board of Nagasaki University Hospital and the Ethics Committee, Nagasaki University Graduate School of Biomedical Sciences, IRB00009218. We evaluated 72 patients with interstitial pneumonia who underwent surgical lung biopsy in Nagasaki University Hospital between 1983 and 2011. Twenty-two patients were diagnosed with IPF, 19 with nonspecific interstitial pneumonia (NSIP), 8 with cryptogenic organizing pneumonia (COP) and 23 with collagen vascular disease-associated interstitial pneumonia (CVD-IP). The idiopathic interstitial pneumonias were diagnosed according to the consensus criteria of the American Thoracic Society/European Respiratory Society [10]. The diagnoses associated with CVD-IP were rheumatoid arthritis (RA; n = 6), systemic sclerosis (SSc; n = 5), Sjogren’s syndrome (SjS; n = 5), polymyositis/dermatomyositis (PM/DM; n = 3), mixed connective tissue disease (MCTD; n = 2), RA/SjS overlap (n = 1), and SjS/DM overlap (n = 1).
Immunohistochemistry
Immunohistochemistry was performed in 4-μm paraffin sections. The primary antibodies used for the immunohistochemical studies included an anti-Helicobacter pylori antibody (Dako, Glostrup, Denmark) and rabbit anti-VacA antibodies, which were prepared as described previously [11]. Control studies were performed using rabbit immunoglobulin (Dako) as the primary antibodies. Immunohistochemistry was performed using the EnVision™ System (Dako) according to the manufacturer’s instructions.
Incubation of cultured cells with VacA
A549 cells (American Type Culture Collection, Rockville, MD, USA) and NHBE cells (CC-2541, Lonza, Walkersville, VA, USA) were maintained in, respectively, Dulbecco’s modified minimal essential medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen) and bronchial epithelial growth medium (BEGM; Lonza) in a humidified incubator (5% CO2 at 37°C).
The toxin-producing H. pylori strain, ATCC 49503, was the source of VacA, which was purified as described previously [12].
To quantify cytokine production, A549 cells were seeded in 6-well plates at a density of 3.0 × 105 per well and NHBE cells in 12-well plates at a density of 1.0 × 105 per well. At subconfluence, serum-free medium was added to the wells. Cells were subsequently stimulated for 3, 6, 12, and 24 h with 1, 10, 30, 60, or 120 nM VacA or heat-inactivated VacA (100°C, 10 min; iVacA control). These VacA concentrations have been used for the purpose of investigating the effects of toxin on gastric epithelial cells [13]. Changes in cell morphology were assessed by phase-contrast light microscopy.
Cytokine levels in supernatants from cells incubated with VacA
Cytokines in supernatants were measured by Multiplex Luminex® Assays. The Cytokine Human 10-Plex Panel (granulocyte macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and tumor necrosis factor (TNF)-α) was purchased from Life Technologies (LHC0001). 96-well plates, reagents and wash solutions provided or recommended by the manufacturer were used and all data were analysed as recommended by the manufacturers. All measurements were performed in duplicate, and the values reported are the means of 4 experiments.
Statistical analysis
The values of continuous variables were expressed as median (range). Differences between 2 groups were determined by Mann-Whitney’s U-test. Differences among groups were determined by one-factor analysis of variance for continuous variables, and Pearson’s chi-square test for categorical variables, as appropriate. If a significant difference was found by one-factor analysis of variance, pair-wise comparison was performed using the Scheffë method. Statistical analysis was performed using Stat ViewJ-5.0 (SAS Institute, Cary, NC, USA). A p-value < 0.05 was considered statistically significant.
Results
Histology and immunohistochemistry
To verify the effectiveness of the antibodies, we performed immunohistochemistry with anti-VacA and anti-H. pylori antibodies using gastric paraffin sections from patients with H. pylori infection (representative results are shown in Fig. 1a–c). Anti-H. pylori antibodies recognized H. pylori in gastric sections (Fig. 1b). Similarly, VacA was identified in H. pylori as well as in the vicinity of the bacteria (Fig. 1c). Control studies using rabbit immunoglobulin did not reveal any immunoreactive cells (Data not shown).
Figure 1. Photomicrographs of histological and immunohistochemical studies of representative gastric and surgical lung biopsy specimens.
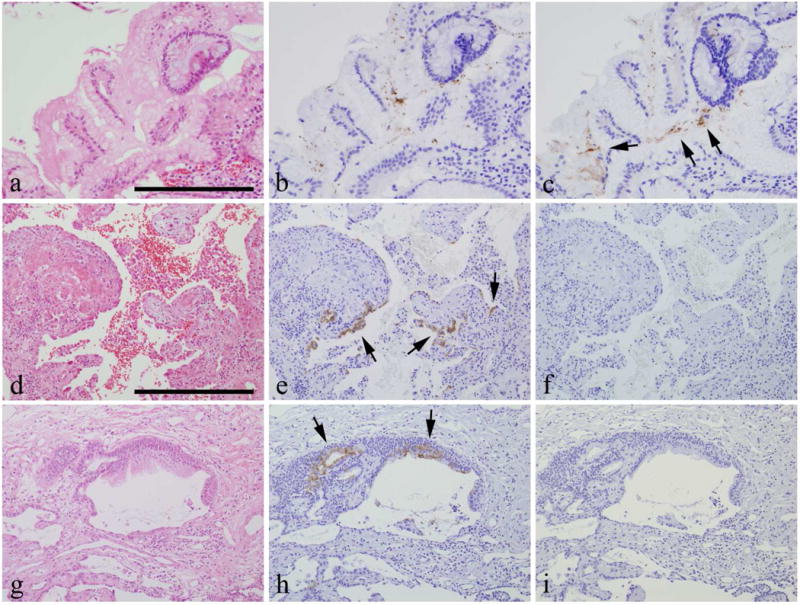
a–c are sequential sections, a: Hematoxylin-eosin staining, b: Immunohistochemistry with anti-Helicobacter pylori antibody, c: Immunohistochemistry with anti-VacA antibody. Arrows show VacA. Scale bar: = 200 μm.
d–f and g–i are sequential sections obtained from 2 patients. Lung tissues in d–f and gastric tissues in a–c were obtained from the same patient, d, g: hematoxylin-eosin staining, e, h: immunohistochemistry with anti-VacA antibody. Arrows show VacA. f, i: control studies using rabbit immunoglobulin as the primary antibodies. Scale bar: = 400 μm.
To identify and localize VacA in human lung tissues, we performed immunohistochemical analysis for VacA in lung paraffin sections from patients with different types of interstitial pneumonias. VacA was detected in the lung tissues in 10 of 72 patients (13.9%) by immunohistochemistry (representative results are shown in Fig. 1 d–i). VacA was identified in alveolar epithelial cells (Fig. 1e) and in bronchial epithelial cells (Fig. 1h). Control studies using rabbit immunoglobulin did not reveal any immunoreactive cells (Fig. 1f, 1i). In this study, gastric (Fig. 1a–c) and lung tissues (Fig. 1 d–f) were obtained from the same patient.
Comparison of patient characteristics between the VacA-positive and -negative groups
Patient demographic data and diagnosis in the VacA-positive (n = 10) and VacA-negative (n = 62) groups are shown in Table 1. The diagnoses of VacA-positive cases were IPF (n = 1), NSIP (n = 2) and CVD-IP (n = 7). The diagnoses of collagen vascular diseases in the VacA-positive group included SjS (n = 2), and PM/DM, SSc, RA, MCTD, and SjS/DM overlap (n = 1, respectively). There was a significant difference in the diagnoses between the 2 groups (p=0.04), VacA was more prevalent in the lungs of patients with CVD-IP than those of patients with IPF, NSIP and COP. Detailed chart review revealed that there were no significant differences in other clinical characteristics, including survival, results of pulmonary function tests, arterial blood gas analysis, serological findings and bronchoalveolar lavage fluid findings (Data not shown).
Table 1.
Comparison of patient characteristics by immunohistochemistry between the VacA–positive and negative groups.
| VacA–positive | VacA–negative | P-value | |
|---|---|---|---|
| Subjects | 10 | 62 | |
| Age (years) | 54.5 (40−69) | 60 (28−74) | NS |
| Sex (male:female) | 4:6 | 33:29 | NS |
| Diagnosis | < 0.05 | ||
| IPF | 1 | 21 | |
| NSIP | 2 | 17 | |
| COP | 0 | 8 | |
| CVD-IP | 7 | 16 |
IPF: idiopathic pulmonary fibrosis, NSIP: nonspecific interstitial pneumonia, COP: cryptogenic organizing pneumonia, CVD-IP: collagen vascular disease-associated interstitial pneumonia
VacA-induced vacuolation
Incubation of A549 and NHBE cells with 120nM VacA resulted in vacuolation (Fig. 2a, 2c), which was not observed in cells incubated with 120 nM iVacA (Fig. 2b, 2d), suggesting that vacuolation in cells was a specific response to VacA protein.
Figure 2. VacA-induced vacuolation.
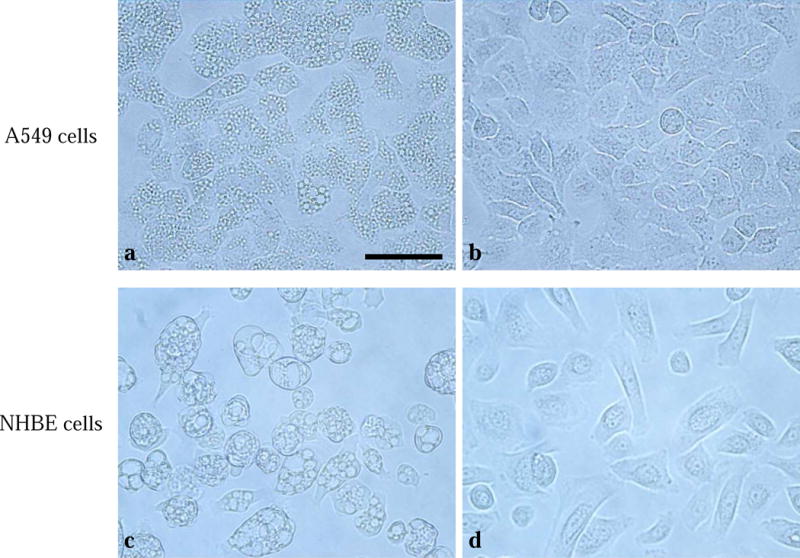
Cells were incubated with 120nM of VacA or heat-inactivated VacA (iVacA, control) for 24 h; incubation with 120nM-VacA resulted in vacuolation of A549 cells (a) and normal human bronchial epithelial (NHBE) cells (c). Vacuolation were not observed in cells incubated with 120nM-iVacA (b, d). Scale bar: = 50 μm.
VacA-induced cytokine production
A549 and NHBE cells were incubated with 120 nM VacA or iVacA for 24 h. As shown in Fig. 3a, after a 24-h incubation with 120 nM VacA or iVacA, 1.9- and 3.6-fold increase were observed in IL-8 and IL-6 concentrations in the medium of A549 cells, respectively. IL-1β and GM-CSF production were also induced by VacA although these were only a slight induction. After a 24-h incubation with 120 nM VacA or iVacA, 17.2-fold increase was observed in IL-8 concentration in the medium of NHBE cells (Fig. 3b).
Figure 3. VacA-induced cytokine production.
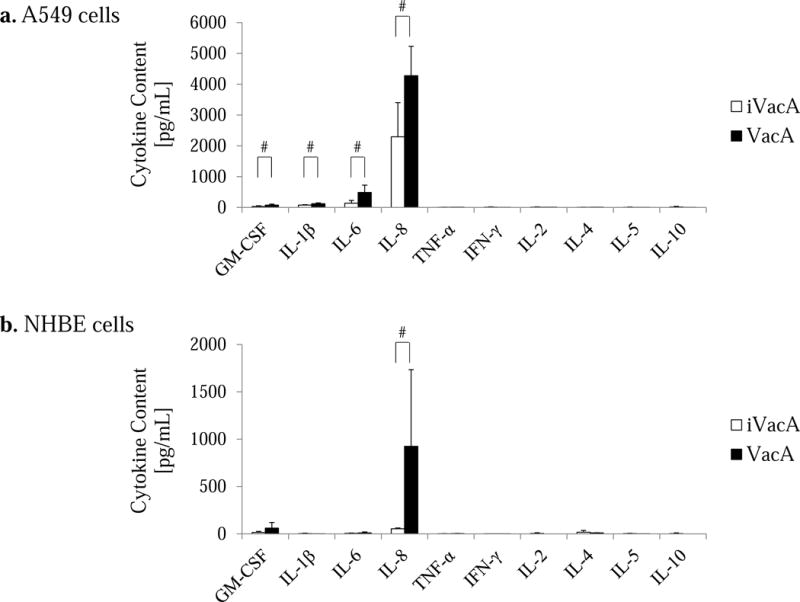
a: A549 cells were incubated with 120 nM VacA or heat-inactivated VacA (iVacA, control) for 24 h. After a 24-h incubation with 120 nM VacA or iVacA, 1.9- and 3.6-fold increase were observed in IL-8 and IL-6 concentrations in the medium of A549 cells, respectively. b: Normal human bronchial epithelial (NHBE) cells were incubated with 120 nM VacA or iVacA for 24 h. After a 24-h incubation with 120 nM VacA or iVacA, 17.2-fold increase was observed in IL-8 concentration in the medium of NHBE cells. All measurements were performed in duplicate, and the values reported are the means of 4 experiments. Statistical significance: #, p < 0.05.
A549 cells were incubated with the indicated concentrations of VacA or iVacA (0–120 nM) for 24 h, VacA increased IL-8 (Fig. 4a) and IL-6 (Fig. 4b) in a concentration-dependent manner. Additionally, A549 cells were incubated with 120 nM VacA or iVacA for the indicated times (0–24 h). Stimulation with 120 nM VacA resulted in a time-dependent increase in IL-8 (Fig. 4d) and IL-6 (Fig. 4e) production. Regarding IL-1β and GM-CSF, there were no concentration- and time-dependent changes (Data not shown).
Figure 4. VacA-induced IL-8 and IL-6 production.
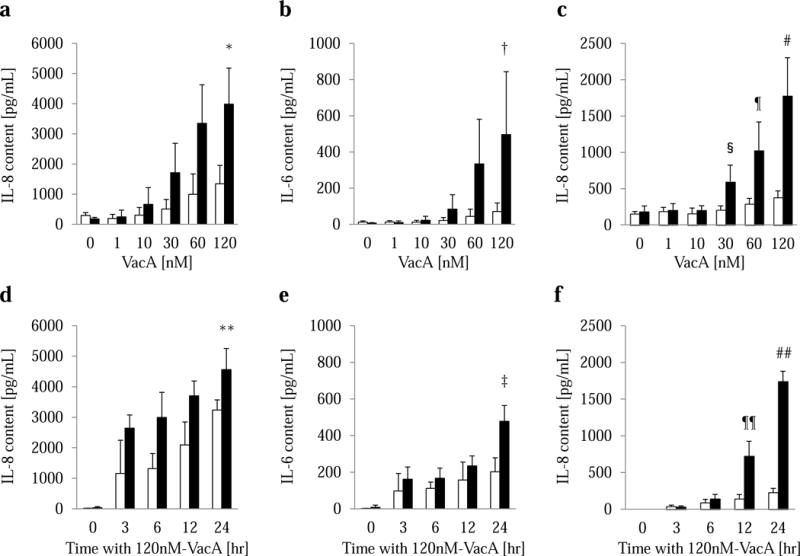
a–c: A549 cells and normal human bronchial epithelial (NHBE) cells were incubated with the indicated concentrations of VacA (■) or heat-inactivated VacA (iVacA, control, □) for 24 h (0–120 nM); incubation with VacA for 24 h resulted in concentration-dependent increases in IL-8 (a) and IL-6 (b) in the medium of A549 cells and IL-8 (c) in that of NHBE cells. * denotes a statistically significant difference as compared with 0, 1, 10, or 30 nM VacA and 120 nM iVacA (p < 0.05), † with 0, 1, or 10 nM VacA and 120 nM iVacA (p < 0.05), # with 0, 1, 10, 30, 60 nM VacA and 120 nM iVacA (p < 0.05), f with 0, 1, 10 nM VacA and 60 nM iVacA (p < 0.05) and § with 30 nM iVacA (p < 0.05). d–f: A549 and NHBE cells were incubated with 120nM VacA (■) or iVacA (□) for the indicated times (0–24 h). There were time-dependent increases in IL-8 (d) and IL-6 (e) contents in the supernatants of A549 cells and IL-8 (f) in that of NHBE cells following incubation with 120 nM VacA. ** denotes a statistically significant difference as compared with 0, 3, 6 h with VacA and 24 h with iVacA (p < 0.05), ‡ with 0, 3, 6 h with VacA and 24 h with iVacA (p < 0.05), ## with 0, 3, 6,12 h VacA and 24 h iVacA (p < 0.05) and ¶¶ with 0, 3, 6 h VacA and 12 h iVacA (p < 0.05). All measurements were performed in duplicate, and the values reported are the means of 4 experiments.
NHBE cells were also incubated with the indicated concentrations of VacA or iVacA (0–120 nM) for 24 h and with 120 nM VacA or iVacA for the indicated times (0–24 h). VacA increased IL-8 in a concentration-(Fig. 4c) and time-dependent manner (Fig. 4f).
Discussion
This is the first study to demonstrate that H. pylori VacA is present in human lung tissues and that VacA induces IL-8 production by A549 and NHBE cells and IL-6 production by A549 cells. The present study also showed that VacA was more prevalent in lungs of patients with CVD-IP than those of patients with IPF, NSIP and COP.
Association between respiratory diseases and gastro-esophageal reflux (GER) has long been recognized [14]. GER has been documented to be highly prevalent in patients with a variety of lung diseases including patients with IPF, cystic fibrosis and CVDs [15]. Chronic microaspiration (i.e., tracheobronchial aspiration of small amounts of gastric secretions) secondary to GER has been hypothesized to cause repetitive subclinical injury to the lung, leading to pulmonary fibrosis [16]. Previous reports showed the presence of high levels of pepsin [17–21] and bile acids [22,23] in the bronchoalveolar lavage fluid of patients with lung transplants and children with GER or cystic fibrosis, suggesting that gastric contents could be aspirated into the lung by microaspiration without any distinct clinical episode. It has been suggested that pepsin or bile acids aspirated through GER may be a potential contributor to lung allograft dysfunction [20–22]. Moreover, suppression of GER in patients with IPF was associated with a smaller decrease in forced vital capacity [24] and a lower radiological fibrosis score on high-resolution computed tomography, and was an independent predictor of longer survival [25]. Other substances in gastric juice as yet unidentified may also contribute to the pathogenesis of respiratory dysfunction and disease. Numerous studies have also shown that H. pylori can be detected in dental plaque and saliva of human subjects, suggesting that the oral cavity may be an extra-gastric reservoir of H. pylori [26]. These findings suggest that VacA could be aspirated into the lung from stomach and/or oral cavity. In fact, in the present study, VacA was detected in the bronchial and alveolar epithelial cells, which are easily accessible following aspiration. Further studies are needed to elucidate the VacA entering mechanisms.
VacA is known to cause vacuoles in human gastric cancer cell lines AZ-521 and AGS, but not in human promyeloblastic cell line HL-60 [11], VacA-induced vacuolation thus appears to be cell-type specific. We showed that VacA causes vacuoles in A549 and NHBE cells. In addition, vacuolation were not observed with heat-inactivated VacA, indicating that these vacuolation were specific to the native VacA protein. However, there was no evidence of vacuolation in VacA immunoreactive cells in human lung tissues and clinical significance of this phenomenon still remains unknown.
Because of its prevalence and ability to affect human immune function, many researchers have hypothesized that H. pylori might contribute to the systemic rheumatic diseases development. Previous reports have suggested a positive relationship between H. pylori infection and various autoimmune diseases [27]. Some evidences suggest that eradication of H. pylori may lead to an improvement of autoimmune disorders, such as immune thrombocytopenia [28], SSc [29] and autoimmune thyroid disease [30]. The present study showed that VacA was more prevalent in lungs of patients with CVD-IP than that of patients with IPF, NSIP and COP. Although the exact role of H. pylori in the pathogenesis of these diseases is still unclear, these findings imply the possible association of H. pylori and autoimmune disorders.
In the present study, we investigated whether several kinds of cytokines (GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and TNF-α) could be induced in A549 and NHBE cells by VacA. Interestingly, VacA induced IL-8 production by A549 and NHBE cells and IL-6 production by A549 cells. Other cytokines were not induced by VacA. These findings suggest that induction of IL-8 and IL-6 in airway epithelial cells are specific reaction against VacA stimulation. IL-8 is expressed in a variety of inflammatory respiratory diseases, including IPF [31], CVD-IP [32] and bronchiectasis [33]. Interestingly, increased plasma IL-8 levels were associated with significantly worse outcomes in IPF [31]. IL-6 is also known to induce interstitial pneumonia [34–36]. The prolonged interaction between the VacA and lung epithelial cells could trigger the release of inflammatory mediators and consequently lead to respiratory disease in predisposed individuals.
Some limitations to this study should be noted. The most important limitation of this study is that only 72 patients were enrolled. We were also not able to evaluate the serum H. pylori antibodies and the concentrations of IL-8 and IL-6 in bronchoalveolar lavage fluid in the enrolled patients because the volume of preserved samples was not sufficient. Further clinical studies involving larger cohorts of patients are warranted to elucidate whether VacA was associated with the pathogenesis of pulmonary diseases and CVDs.
In conclusion, our identification of VacA in human lung tissues of patients with interstitial pneumonia, and the demonstration that VacA induced vacuolation and production of IL-8 and IL-6 by airway epithelial cells, together with previous reports of a high H. pylori seroprevalence in several types of respiratory diseases, suggest that VacA could play a role in the pathogenesis of respiratory diseases and/or CVDs. Further studies of the immunological response to H. pylori VacA and its role in the pathogenesis of interstitial pneumonias and CVDs are warranted.
H. pylori VacA was detected in bronchial and alveolar epithelial cells of human lung.
Incubation of A549 and NHBE cells with VacA resulted in vacuolation.
Interleukin-8 and interleukin-6 production were induced by VacA in A549 cells.
Interleukin-8 production was induced by VacA in NHBE cells.
Acknowledgments
The authors thank Dr. Y. Morinaga and Prof. K. Yanagihara (Department of Laboratory Medicine, Nagasaki University Graduate School of Biomedical Sciences) for performing the Multiplex Luminex® Assays and Mr. A. Yokoyama (Nagasaki University Hospital) for technical assistance.
B.G., A.S. and J.M. were supported by the Intramural Research Program, National Institutes of Health, NHGRI, CC and NHLBI, respectively.
Abbreviations
- H. pylori
Helicobacter pylori
- NHBE
normal human bronchial epithelial
- IPF
idiopathic pulmonary fibrosis
- DNA
deoxyribonucleic acid
- PCR
polymerase chain reaction
- NSIP
nonspecific interstitial pneumonia
- COP
cryptogenic organizing pneumonia
- CVD-IP
collagen vascular disease-associated interstitial pneumonia
- RA
rheumatoid arthritis
- SSc
systemic sclerosis
- SjS
Sjögren’s syndrome
- PM
polymyositis
- DM
dermatomyositis
- MCTD
mixed connective tissue disease
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IFN
interferon
- IL
interleukin
- TNF
tumor necrosis factor
- GER
gastro-esophageal reflux
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20. doi: 10.1111/j.1523-5378.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsang KW, Lam SK, Lam WK, et al. High seroprevalence of Helicobacter pylori in active bronchiectasis. Am J Respir Crit Care Med. 1998;158:1047–1051. doi: 10.1164/ajrccm.158.4.9712104. [DOI] [PubMed] [Google Scholar]
- 3.Caselli M, Zaffoni E, Ruina M, et al. Helicobacter pylori and chronic bronchitis. Scand J Gastroenterol. 1999;34:828–830. doi: 10.1080/003655299750025787. [DOI] [PubMed] [Google Scholar]
- 4.Bennett D, Bargagli E, Refini RM, et al. Helicobacter pylori seroprevalence in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014;43:635–638. doi: 10.1183/09031936.00104813. [DOI] [PubMed] [Google Scholar]
- 5.Gulhan M, Ozyilmaz E, Tarhan G, et al. Helicobacter pylori in bronchiectasis: a polymerase chain reaction assay in bronchoalveolar lavage fluid and bronchiectatic lung tissue. Arch Med Res. 2007;38:317–321. doi: 10.1016/j.arcmed.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Angrill J, Sanchez N, Agusti C, et al. Does Helicobacter pylori have a pathogenic role in bronchiectasis? Respir Med. 2006;100:1202–1207. doi: 10.1016/j.rmed.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Palframan SL, Kwok T, Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. doi: 10.3389/fcimb.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim IJ, Blanke SR. Remodeling the host environment: modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA) Front Cell Infect Microbiol. 2012;2:37. doi: 10.3389/fcimb.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers MG, Pyburn TM, Gonzalez-Rivera C, et al. Structural Analysis of the Oligomeric States of Helicobacter pylori VacA Toxin. J Mol Biol. 2013;425:524–535. doi: 10.1016/j.jmb.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 11.Yahiro K, Niidome T, Hatakeyama T, et al. Helicobacter pylori vacuolating cytotoxin binds to the 140-kDa protein in human gastric cancer cell lines, AZ-521 and AGS. Biochem Biophys Res Commun. 1997;238:629–632. doi: 10.1006/bbrc.1997.7345. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama M, Hisatsune J, Yamasaki E, et al. Clustering of Helicobacter pylori VacA in lipid rafts, mediated by its receptor, receptor-like protein tyrosine phosphatase beta, is required for intoxication in AZ-521 Cells. Infect Immun. 2006;74:6571–6580. doi: 10.1128/IAI.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yahiro K, Niidome T, Kimura M, et al. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J Biol Chem. 1999;274:36693–36699. doi: 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- 14.Belcher JR. The pulmonary complications of dysphagia. Thorax. 1949;4:44–56. doi: 10.1136/thx.4.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweet MP, Patti MG, Hoopes C, et al. Gastro-oesophageal reflux and aspiration in patients with advanced lung disease. Thorax. 2009;64:167–173. doi: 10.1136/thx.2007.082719. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Collard HR, Raghu G, et al. Does chronic microaspiration cause idiopathic pulmonary fibrosis? Am J Med. 2010;123:304–311. doi: 10.1016/j.amjmed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Song JW, Wolters PJ, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:352–358. doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell S, McMaster C, Gibson D, et al. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastro-oesophageal reflux-related pulmonary aspiration. J Pediatr Surg. 2006;41:289–293. doi: 10.1016/j.jpedsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 19.McNally P, Ervine E, Shields MD, et al. High concentrations of pepsin in bronchoalveolar lavage fluid from children with cystic fibrosis are associated with high interleukin-8 concentrations. Thorax. 2011;66:140–143. doi: 10.1136/thx.2009.130914. [DOI] [PubMed] [Google Scholar]
- 20.Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med. 2007;175:1298–1303. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 21.Ward C, Forrest IA, Brownlee IA, et al. Pepsin like activity in bronchoalveolar lavage fluid is suggestive of gastric aspiration in lung allografts. Thorax. 2005;60:872–874. doi: 10.1136/thx.2004.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–1152. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Starosta V, Kitz R, Hartl D, et al. Bronchoalveolar pepsin, bile acids, oxidation, and inflammation in children with gastroesophageal reflux disease. Chest. 2007;132:1557–1564. doi: 10.1378/chest.07-0316. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1:369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand PS, Kamath KP, Anil S. Role of dental plaque, saliva and periodontal disease in Helicobacter pylori infection. World J Gastroenterol. 2014;20:5639–5653. doi: 10.3748/wjg.v20.i19.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyk DS, Koutsoumpas AL, Mytilinaiou MG, et al. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol. 2014;20:613–629. doi: 10.3748/wjg.v20.i3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber MR, Kumar S, Tefferi A. Treatment advances in adult immune thrombocytopenic purpura. Ann Hematol. 2003;82:723–737. doi: 10.1007/s00277-003-0732-z. [DOI] [PubMed] [Google Scholar]
- 29.Gasbarrini A, Massari I, Serricchio M, et al. Helicobacter pylori eradication ameliorates primary Raynaud’s phenomenon. Dig Dis Sci. 1998;43:1641–1645. doi: 10.1023/a:1018842527111. [DOI] [PubMed] [Google Scholar]
- 30.Bertalot G, Montresor G, Tampieri M, et al. Decrease in thyroid autoantibodies after eradication of Helicobacter pylori infection. Clin Endocrinol (Oxf) 2004;61:650–652. doi: 10.1111/j.1365-2265.2004.02137.x. [DOI] [PubMed] [Google Scholar]
- 31.Richards TJ, Kaminski N, Baribaud F, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujimori Y, Kataoka M, Tada S, et al. The role of interleukin-8 in interstitial pneumonia. Respirology. 2003;8:33–40. doi: 10.1046/j.1440-1843.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- 33.Angrill J, Agusti C, De Celis R, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. 2001;164:1628–1632. doi: 10.1164/ajrccm.164.9.2105083. [DOI] [PubMed] [Google Scholar]
- 34.Moodley YP, Misso NL, Scaffidi AK, et al. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol. 2003;29:490–498. doi: 10.1165/rcmb.2002-0262OC. [DOI] [PubMed] [Google Scholar]
- 35.Knight DA, Ernst M, Anderson GP, et al. The role of gp130/IL-6 cytokines in the development of pulmonary fibrosis: critical determinants of disease susceptibility and progression? Pharmacol Ther. 2003;99:327–338. doi: 10.1016/s0163-7258(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, Sakuma J, Hayashi S, et al. A histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor beta 1, or platelet-derived growth factor B gene. Proc Natl Acad Sci U S A. 1995;92:9570–9574. doi: 10.1073/pnas.92.21.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]


