Abstract
The mechanisms responsible for the development of the impaired awareness of hypoglycemia often seen in insulin-treated patients with diabetes remain uncertain, but cerebral adaptations to recurrent hypoglycemia are frequently hypothesized. In this issue of the JCI, Ma et al. demonstrate that neuropeptide Y (NPY) secretion from adrenal chromaffin cells persists during exposure to recurrent hypoglycemia and activation of the sympathetic nerves at the same time that epinephrine secretion is reduced. This results in the inhibition of tyrosine hydroxylase, the rate-limiting enzyme for catecholamine synthesis. These observations suggest that a peripheral mechanism downstream from the brain contributes to the development of impaired awareness of hypoglycemia.
Impaired hypoglycemia awareness
Impaired awareness of hypoglycemia, which is a diminished ability to perceive the onset of hypoglycemia, typically coexists with a reduction in the counterregulatory hormone responses to hypoglycemia and is a major problem for patients with insulin- or secretagogue-treated diabetes. It triples the risk of experiencing severe hypoglycemia (1), which can be fatal, and prevents patients from achieving the glycemic control necessary to minimize the risk of developing the microvascular complications of the disease. Impaired awareness of hypoglycemia exists in up to 25% of patients with type 1 diabetes and up to 10% of patients with insulin- or secretagogue-treated type 2 diabetes (2). Thus, millions of people with diabetes around the globe suffer from this problem, making it a very significant public health issue.
Healthy humans have a robust and redundant counterregulatory response that prevents the development of hypoglycemia. This response is triggered by the detection of a fall in blood sugar by glucose-sensing neurons in the ventral medial hypothalamus and other areas and mediated by the activation of hormonal and neural networks that ultimately increase hepatic glucose production and feeding behavior to restore normoglycemia (3). In patients with type 1 diabetes and advanced type 2 diabetes, this response is impaired (4). Such patients are unable to prevent a fall in glucose by reducing their own insulin secretion because of the β cell failure associated with their disease. They are also unable to release glucagon in response to hypoglycemia, probably because of a failure to reduce insulin levels within the islet in response to the falling glucose levels (5). They come to rely on hypoglycemia-induced secretion of epinephrine from the adrenal medulla and norepinephrine from the sympathetic nerves to cause the adrenergic symptoms that signal the need to ingest carbohydrates. However, the glucose level required to elicit this response is reduced following exposure to hypoglycemia, and, with frequent exposure to hypoglycemia, it drops into the range at which humans experience neuroglycopenia. Patients with impaired awareness of hypoglycemia do not know they are experiencing hypoglycemia until they become confused or lose consciousness — a situation in which they cannot help themselves recover from the drop in blood sugar.
The mechanisms responsible for the development of impaired awareness of hypoglycemia remain uncertain. Many investigators have focused on determining how glucose sensing is altered in the brain. Some have suggested that cerebral glucose transport is increased following an episode of hypoglycemia, such that the brain can obtain sufficient energy during a subsequent episode to maintain normal function (6, 7). Others have suggested that transport of lactate and other monocarboxylic acids is upregulated following hypoglycemia, such that the brain has ready access to alternative fuels should brain glucose levels fall again (8). Still other investigators have focused on how hypoglycemia might alter neurotransmitter signaling in the brain during subsequent hypoglycemia, and changes in both GABAergic (9, 10) and opioidergic (11, 12) signaling have been identified. Relatively little attention has been paid to how recurrent hypoglycemia might directly impact peripheral systems that participate in the generation of the counterregulatory response, despite the elegant work of Sivtiz et al. (13) in 2001, which demonstrated that adrenal sympathetic nervous activity (SNA) was unchanged by recurrent episodes of hypoglycemia in rats and that epinephrine responses were reduced at the same time.
Elucidation of mechanisms for impaired hypoglycemia awareness
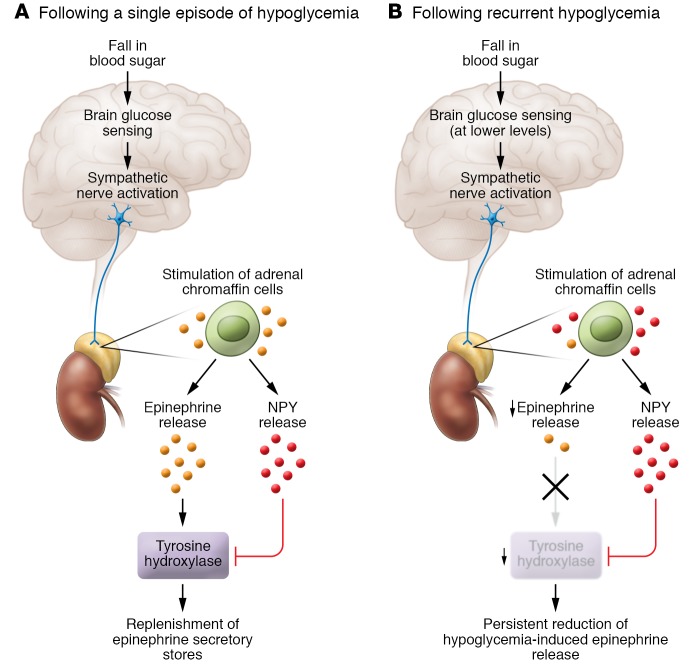
In the current issue of the JCI, Ma et al. (14) examined how recurrent episodes of hypoglycemia might directly act on the adrenal medulla to reduce epinephrine secretion. They observed that a single episode of hypoglycemia in a mouse increased urine epinephrine secretion and, as expected, that this measure of the counterregulatory response was reduced following recurrent episodes of hypoglycemia or after recurrent activation of the counterregulatory response using designer receptors exclusively activated by designer drugs (DREADD) technology. They then used optogenetics to selectively depolarize chromaffin cells in vitro and found that cells derived from mice exposed to a single episode of hypoglycemia before sacrifice demonstrated an increase in the number of evoked events, whereas cells derived from mice exposed to recurrent episodes of hypoglycemia before sacrifice had a reduction in the number of evoked events. Using this in vitro model, they also demonstrated that a single episode of hypoglycemia prior to sacrifice was associated with an increase in immunoreactivity of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis, whereas recurrent episodes of hypoglycemia prior to sacrifice were associated with a reduction in this measure. Interestingly, they also found that neuropeptide Y (NPY) immunoreactivity was significantly elevated in the adrenal glands derived from mice exposed to both single and recurrent episodes of hypoglycemia. NPY is known to be cosecreted from the adrenal medulla with epinephrine and through binding to Y1 receptors, tonically inhibiting expression of tyrosine hydroxylase. In NPY-KO mice, the levels of tyrosine hydroxylase immunoreactivity and urine catecholamine were not reduced by recurrent episodes of hypoglycemia. The authors conclude that NPY release mediates hypoglycemia-induced adrenal impairment of catecholamine secretion (Figure 1).
Figure 1. Proposed mechanism responsible for inhibition of the epinephrine response to recurrent hypoglycemia.
(A) In response to an isolated fall in blood sugar, the brain senses hypoglycemia at a glucose concentration of approximately 65 mg/dl and activates the sympathetic nervous system. This activation results in stimulation of the adrenal chromaffin cells, which in turn leads to the release of epinephrine and NPY. Epinephrine release following a single episode of hypoglycemia increases the activity of tyrosine hydroxylase, resulting in the replenishment of epinephrine secretory stores. (B) In response to repeated episodes of hypoglycemia, the brain senses hypoglycemia at a lower glucose concentration than that following exposure to a single episode. This results in activation of the sympathetic nervous system, followed by stimulation of adrenal chromaffin cells. Because NPY released during previous episodes of hypoglycemia has reduced tyrosine hydroxylase activity, the epinephrine secretory capacity of the chromaffin cells is reduced, resulting in a blunted counterregulatory response.
What significance do these findings have for patients with diabetes who develop impaired awareness of hypoglycemia following exposure to recurrent episodes of hypoglycemia? Is it possible that hypoglycemia-induced adrenal NPY secretion is responsible for the reduced catecholamine response seen in these patients? Future studies will be necessary to answer this question, but the work of Ma et al. opens up a new area in which therapeutic approaches to this critical complication of diabetes can be considered.
Consequences of recurrent hypoglycemia versus a single hypoglycemic event
Before additional translational work is done in this area, it will be important for these results to be confirmed by others, particularly since work by Senthilkumaran and Bobrovskaya (15) recently reported that recurrent hypoglycemia actually increased tyrosine hydroxylase protein while reducing the epinephrine response in a rat model, a response that is opposite to that seen by Ma et al. In humans, recurrent hypoglycemia not only reduces the amount of epinephrine released from the adrenal medulla in response to hypoglycemia, but also reduces the glucose threshold that elicits that response (4). Presumably, that change in threshold is based on alterations present in the glucose-sensing neurons, but future work should determine whether changes in adrenal SNA occurs in a glucose concentration–dependent fashion and whether the glucose concentration required to elicit chromaffin cell catecholamine release is higher in animals exposed to a single episode of hypoglycemia as opposed to recurrent episodes prior to sacrifice.
Targeting the periphery for impaired awareness of hypoglycemia
Impaired awareness of hypoglycemia affects many patients with insulin- and secretagogue-treated diabetes and causes personal (16) and family distress (17) associated with the fear of hypoglycemia. It has long been appreciated that impaired awareness of hypoglycemia can be reversed by scrupulous avoidance of hypoglycemia for three or more weeks (18, 19), but given the very high prevalence of hypoglycemia (20), this is very challenging for most patients to do in real life. The development of new therapies ensuring that the full counterregulatory response to a fall in glucose is elicited every time the plasma glucose falls below 65 mg/dl would be welcome. The work of Ma et al. may encourage some investigators to move out of the brain and into the periphery to find new targets for the prevention of impaired awareness of hypoglycemia in diabetes.
Acknowledgments
ERS thanks Amir Moheet and Gulin Oz (University of Minnesota, Minneapolis, MN, USA) for editorial assistance and the NIH for grant support (R01NS035192).
Version 1. 08/06/2018
Electronic publication
Version 2. 08/31/2018
Print issue publication
Footnotes
Conflict of interest: The University of Minnesota has received funding from Lilly to support the research efforts of ERS.
Reference information: J Clin Invest. 2018;128(9):3739–3741. https://doi.org/10.1172/JCI122449.
See the related article at Recurrent hypoglycemia inhibits the counterregulatory response by suppressing adrenal activity.
References
- 1.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517–522. doi: 10.2337/diacare.18.4.517. [DOI] [PubMed] [Google Scholar]
- 2.Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87(1):64–68. doi: 10.1016/j.diabres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2010;31(1):32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryer PE. Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J Clin Invest. 2006;116(6):1470–1473. doi: 10.1172/JCI28735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooperberg BA, Cryer PE. β-Cell-mediated signaling predominates over direct alpha-cell signaling in the regulation of glucagon secretion in humans. Diabetes Care. 2009;32(12):2275–2280. doi: 10.2337/dc09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res. 2005;79(1–2):42–47. doi: 10.1002/jnr.20296. [DOI] [PubMed] [Google Scholar]
- 7.Herzog RI, et al. Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J Clin Invest. 2013;123(5):1988–1998. doi: 10.1172/JCI65105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes. 2006;55(4):929–934. doi: 10.2337/diabetes.55.04.06.db05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan O, et al. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes. 2008;57(5):1363–1370. doi: 10.2337/db07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedrington MS, Tate DB, Younk LM, Davis SN. Effects of antecedent GABA A receptor activation on counterregulatory responses to exercise in healthy man. Diabetes. 2015;64(9):3253–3261. doi: 10.2337/db15-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caprio S, et al. Opiate blockade enhances hypoglycemic counterregulation in normal and insulin-dependent diabetic subjects. Am J Physiol. 1991;260(6 pt 1):E852–E858. doi: 10.1152/ajpendo.1991.260.6.E852. [DOI] [PubMed] [Google Scholar]
- 12.Vele S, Milman S, Shamoon H, Gabriely I. Opioid receptor blockade improves hypoglycemia-associated autonomic failure in type 1 diabetes mellitus. J Clin Endocrinol Metab. 2011;96(11):3424–3431. doi: 10.1210/jc.2011-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivitz WI, Herlein JA, Morgan DA, Fink BD, Phillips BG, Haynes WG. Effect of acute and antecedent hypoglycemia on sympathetic neural activity and catecholamine responsiveness in normal rats. Diabetes. 2001;50(5):1119–1125. doi: 10.2337/diabetes.50.5.1119. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Wang Q, Joe D, Wang M, Whim MD. Recurrent hypoglycemia inhibits the counterregulatory response by suppressing adrenal activity. J Clin Invest. 2018;128(9):3866–3871. doi: 10.1172/JCI91921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senthilkumaran M, Bobrovskaya L. The effects of recurrent hypoglycaemia and opioid antagonists on the adrenal catecholamine synthetic capacity in a rat model of HAAF. Auton Neurosci. 2018;210:76–80. doi: 10.1016/j.autneu.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Anderbro T, et al. Fear of hypoglycemia: relationship to hypoglycemic risk and psychological factors. Acta Diabetol. 2015;52(3):581–589. doi: 10.1007/s00592-014-0694-8. [DOI] [PubMed] [Google Scholar]
- 17.Gonder-Frederick LA, et al. Predictors of fear of hypoglycemia in adolescents with type 1 diabetes and their parents. Pediatr Diabetes. 2006;7(4):215–222. doi: 10.1111/j.1399-5448.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 18.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43(12):1426–1434. doi: 10.2337/diab.43.12.1426. [DOI] [PubMed] [Google Scholar]
- 19.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344(8918):283–287. doi: 10.1016/S0140-6736(94)91336-6. [DOI] [PubMed] [Google Scholar]
- 20.Khunti K, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18(9):907–915. doi: 10.1111/dom.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]