Warning signs of low value or unsafe care are detectable within existing data, but often are overlooked. Rather than duplicate efforts and collect more data, healthcare leaders should renew their focus on making better use of available data. An example in which this is readily apparent involves the monitoring of medical devices.
The advent, increase, and decline in the use of the laparoscopic gastric band to treat morbid obesity illustrates when examination of existing data could have changed practice much earlier. The gastric band device (commonly referred to by its brand name, the “Lap-Band”) was approved by the Food and Drug Administration (FDA) in 2001 and peaked in usage by 2008 (Figure 1A). However, use of the device gradually declined as reports emerged describing complications (e.g. band erosion, band slippage) and variable effectiveness (e.g. inadequate weight loss) that required reoperation to revise, replace or remove the device1. A recent study using Medicare claims found that of the $470 million paid for the gastric band device, $223 million (47%) was for reoperations2.
Figure 1A.
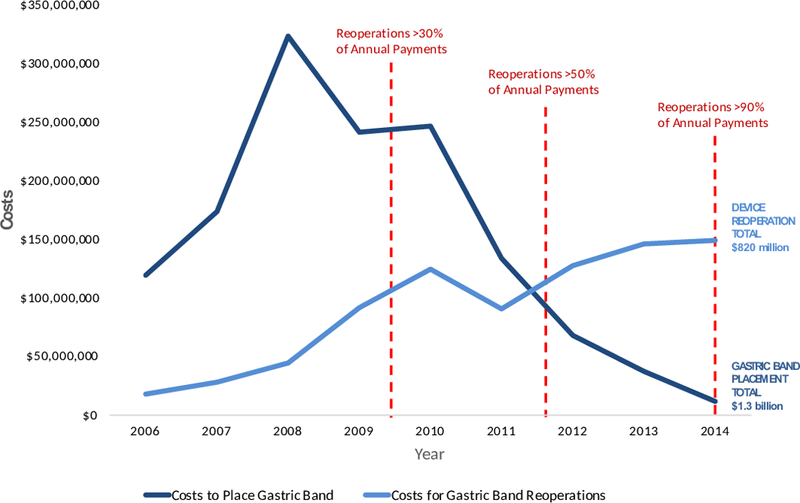
Annual Procedures for Gastric Band Placement and Device Reoperations
While there is broad consensus now that the use of gastric band device should be significantly restricted3, if not eliminated, why did the medical device monitoring system not identify these concerns sooner?
Current Limitations in Monitoring Medical Devices
Before a medical device in the United States can be brought to market, it must undergo a series of pre-market trials that are submitted to the Food and Drug Administration (FDA). These studies often test the device within a homogenous population of patients being treated by experienced clinicians. Even the most well-designed clinical trial might not identify important safety concerns that arise after broad adoption of the device, because of inadequate sample size or limited follow-up time in the clinical trial.
In response to the widely acknowledged limitations of pre-marketing trials for devices, several attempts have been made to support the FDA in post-approval monitoring. This includes requirements from manufacturers and hospitals to provide a timely report of device related complications as they become aware of them to Medical Device Reporting system. Additionally, anyone, including patients, are encouraged to voluntarily report a device related adverse event online through the FDA MedWatch system4.
A recent United States Congress investigation on safety monitoring of medical devices identified several limitations to the current post-approval approach5. First, the report noted that because the system relies heavily on voluntarily reporting, it likely does not capture a significant portion of adverse events. Second, the FDA currently receives more than one million reports annually that are often incomplete and require triage through a limited number of clinical reviewers resulting in significant delays. Third, even with complete reporting and appropriate review, the FDA has no reliable method to determine how often a given device is being used. Without such a denominator, the FDA is unable estimate a frequency of adverse events to determine if further investigation is indicated. The report concluded by recommending legislation that would require any FDA approved device to carry a unique device identifier so that it can be tracked prospectively in insurance claims, electronic health records, and device registries.
The gastric band device illustrates the limitations of the current medical device monitoring process. When the FDA reassessed indications for the gastric band device in 2011, no current population level estimates of device-related adverse events had been published. Instead, the FDA relied on a single-group trial including 149 patients to state that the data, “provide reasonable assurance of the safety and effectiveness” of the gastric band device6. With that evidence the FDA expanded indications for use of the gastric band. Had existing administrative data been utilized to evaluate the device, however, it is possible that the decision may have been different.
Use of Administrative Claims to Monitor the Gastric Band
Although most medical devices currently do not carry a unique device identifier that can be tracked in administrative data, the surgically placed gastric band is a notable exception. Current International Classification of Diseases – 9th Revision, Clinical Modification (ICD-9-CM) contains unique procedure codes for placement of the gastric band (ICD-9-CM code 44.95) as well as for revision or removal of the gastric band (ICD-9 Codes 44.96–98.) When these codes are applied to administrative claims, the power of a unique identifier within existing data to monitor devices for safety and value is illustrative.
Data published by the Healthcare Cost and Utilization Project using claims from the National Inpatient Sample estimates that a total of 216,781 gastric band procedures were performed in the United States between 2006 and 20147. When categorized by those for placing the gastric band device versus those for revising or removing the device (possible because each procedure carries a unique ICD-9 identification code), concerns about safety and expenditures are apparent. Of the $2.1 billion dollars spent on the gastric band device during this time period, $820 million was spent on device-related reoperations. Perhaps of greater concern is that the trends of increasing payments to remove or revise the device appears clear as early as 2009 when payments for reoperations totaled more than 30% of the annual spending on the gastric band device (Figure 1B.) While it is unlikely that this alone would have been compelling enough to remove the device from market at the time, such a decision would have prevented an estimated 104,000 more devices from being placed and potentially saved payers approximately $1 billion between 2010 and 2014.
In 2011, when the FDA was reassessing safety of the gastric band, the available data from clinical trials suggested that device reoperations rates were as low as 4%6. In that same year, nationally representative administrative claims demonstrated that reoperations totaled more than 50% of annual spending on the device (Figure 1B).
In 2017, the gastric band device is still covered by payers and approved by the FDA with even broader indications (now including patients with a body mass index >30 kg/m2 as opposed to only those >35 kg/m2), even though more than 90% of payments in 2014 were for device reoperations.
Making Better Use of Existing Administrative Data
The use of administrative data to track medical devices has several advantages over the current the FDA monitoring system. First, administrative data is required for payment and therefore more comprehensive than voluntary reporting to capture significant device-related hospitalizations. Second, much of the reviewing burden within administrative data can be automated through coding algorithms to limit time delays experienced when human clinical reviewers have to triage each report. Third, administrative data provides both a numerator and denominator for medical device use so that the frequency of adverse device events can be more accurately estimated to inform further investigation. Fourth, administrative claims data includes a measure of resource utilization (i.e. payments) that allows for value to also be integrated into assessing the medical device. While efforts by the FDA to use administrative claims and create detailed registries have been slow to mature8, the example of the gastric band reveals that even crude measures (i.e. payments for device removal) can be a valuable signal within existing data.
The majority of medical devices used in practice today, however, cannot be identified within existing administrative data due to lack of device-specific codes. Legislation that would require all FDA approved devices to be trackable within data that are already collected could be a useful strategy to help ensure that, unlike the gastric band, the warning signs of other widely adopted devices are not missed.
Figure 1B.
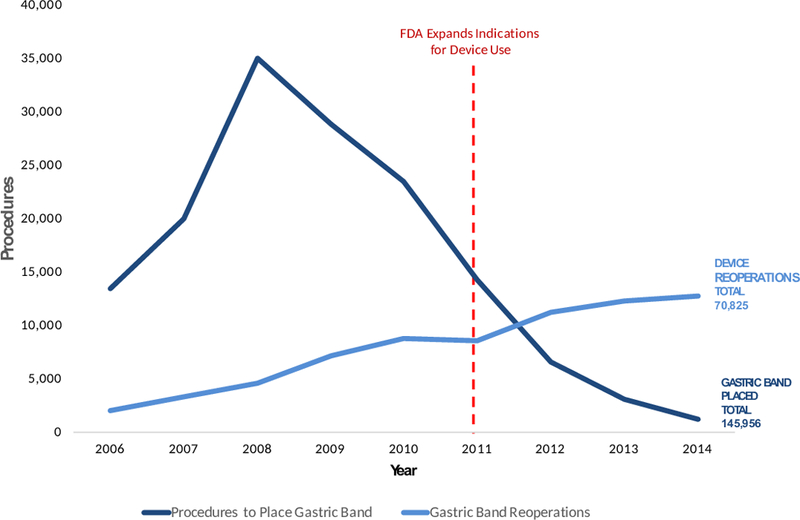
Annual Payments for Gastric Band Placement and Device Reoperations
Acknowledgements
Author AMI acknowledges funding from the Robert Wood Johnson Foundation and the United States Department of Veterans Affairs supporting his role as a Robert Wood Johnson Clinical Scholar.
Author JBD acknowledges funding from the National Institute on Aging under award number R01AG039434-04.
Footnotes
Author JBD has a financial interest in ArborMetrix, Inc., which had no role in the analysis herein.
Data Source: Data for figures extracted from data published by Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project made publically available through their online query tool, HCUPnet ( https://hcupnet.ahrq.gov/ )7. Dashed lines of timeline events and payment percentages added by the authors.
Contributor Information
Andrew M. Ibrahim, Center for Healthcare Outcomes and Policy, University of Michigan, Ann Arbor, Michigan..
Justin B. Dimick, Center for Healthcare Outcomes and Policy, University of Michigan, Ann Arbor, Michigan..
References
- 1.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA surgery March 2014;149(3):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim AM, Thumma JT, Dimick JB. Reoperation and Medicare Expenditures after Laparoscopic Gastric Band Surgery. JAMA surgery 2017;In Press. [DOI] [PMC free article] [PubMed]
- 3.Obeid NR, Pryor AD. Is the Adjustable Gastric Band Dead? Annals of surgery March 2017;265(3):446–447. [DOI] [PubMed] [Google Scholar]
- 4.Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. Jama June 02 1993;269(21):2765–2768. [DOI] [PubMed] [Google Scholar]
- 5.Murray P Preventable Tragedies: Superbugs and How Ineffective Monitoring of Medical Device Safety Fails Patients Senate US, ed. Washington, D.C. 2016. [Google Scholar]
- 6.Food and Drug Adminstration. LAP-BAND; Summary of Safety and Effectiveness (SSED). 2011; http://www.accessdata.fda.gov/cdrh_docs/pdf/P000008S017b.pdf. Accessed 19 APril, 2016.
- 7.HCUPnet. Healthcare Cost and Utilization Project (HCUP). 2006–2014. Agency for Healthcare Research and Quality, Rockville, MD: http://hcupnet.ahrq.gov/ Accessed April 16, 2017, 2017. [PubMed] [Google Scholar]
- 8.Kuehn BM. FDA’s Foray Into Big Data Still Maturing. Jama May 10 2016;315(18):1934–1936. [DOI] [PubMed] [Google Scholar]


