Abstract
Objective
To evaluate provider responses to a narrowly targeted “Best Practice Advisory” (BPA) alert for the intensification of blood pressure medications for persons with diabetes before and after implementation of a “chart closure” hard stop, which is non-interruptive but demands an action or dismissal before the chart can be closed.
Materials and Methods
We designed a BPA that fired alerts within an electronic health record (EHR) system during outpatient encounters for patients with diabetes when they had elevated blood pressures and were not on angiotensin receptor blocking medications. The BPA alerts were implemented in eight primary care practices within UCLA Health. We compared data on provider responses to the alerts before and after implementing a “chart closure” hard stop, and we conducted chart reviews to adjudicate each alert’s appropriateness.
Results
Providers responded to alerts more often after the “chart closure” hard stop was implemented (P < .001). Among 284 alert firings over 16 months, we judged 107 (37.7%) to be clinically unnecessary or inappropriate based on chart review. Among the remainder, which represent clear opportunities for treatment, providers ordered the indicated medication more often (41% vs 75%) after the “chart closure” hard stop was implemented (P = .001).
Discussion
The BPA alerts for diabetes and blood pressure control achieved relatively high specificity. The “chart closure” hard stop improved provider attention to the alerts and was effective at getting patients treated when they needed it.
Conclusion
Targeting specific omitted medication classes can produce relatively specific alerts that may reduce alert fatigue, and using a “chart closure” hard stop may prompt providers to take action without excessively disrupting their workflow.
Keywords: guideline adherence, decision support systems, clinical, diabetes mellitus, hypertension, drug prescriptions
BACKGROUND AND SIGNIFICANCE
Control of blood pressure has been shown to substantially delay or prevent the microvascular and macrovascular complications of diabetes,1 but in the United States, about 40% of adults with a diabetes diagnosis have poorly controlled hypertension.2 Although standards of medical care in diabetes urge the timely treatment of hypertension using an angiotensin converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB),3 there is evidence of delayed intensification of blood pressure medications in clinical practice.4–6 Many factors can lead to failure to intensify antihypertension treatment, such as deficiencies in providers’ comfort with escalating antihypertensive therapies or in their ability to consistently identify and act on elevated blood pressures.7,8 Other factors include polypharmacy, medication interactions, contraindications, and patient preferences.9 Clinical decision support (CDS) systems are a promising solution to address provider-specific barriers to hypertension management in patients with diabetes. CDS systems, for example, can trigger electronic alerts to providers when deviations from accepted diabetes care standards are detected. A systematic review found that CDS systems can increase visits for blood pressure checks and improve measurement and documentation of blood pressure values during visits.10 There is limited evidence, however, for the effect of CDS systems on subsequent intensification of medications for patients with diabetes and hypertension.
A major challenge with CDS systems is that their designers often favor sensitivity over specificity, but the resulting high frequency of false alerts can lead providers to ignore or override all alerts without considering their appropriateness. This phenomenon is known as “alert fatigue.”11 Studies estimate that providers override between 49% and 96% of all medication-related alerts,12,13 which limits CDS system effectiveness. An important step in addressing “alert fatigue” is to reduce the number of inappropriate alerts by improving specificity.13,14 Another approach to reduce the annoyance of alerting systems is to use passive, non-interruptive alerts. However, the challenge with these types of alerts is that providers may not notice them. Less than 40% of providers reported noticing passive recommendations triggered by CDS systems for diabetes care.15
The Epic electronic health record (EHR) system has a commonly used decision support tool called the “Best Practice Advisory” (BPA) that displays alerts to providers during patient encounters when pre-programmed conditions are met. BPAs in Epic Ambulatory can be configured with different levels of interruption. A “passive” BPA creates a visual flag but does not require providers to act on alerts. A “hard stop” BPA does not allow providers to continue using Epic until they act on the alert. A third level of interruption is to use a “chart closure” hard stop in which the alert is passive while the patient’s chart is open, but it becomes a hard stop if it has not been addressed when the provider attempts to exit the workspace. While there are published studies of Epic’s BPA that utilize all levels of interruption,16–20 we could not find a study evaluating the impact of the “chart closure” hard stop feature on provider responses.
The objective of this study was to assess provider responses to a focused BPA alert for the intensification of blood pressure medications before versus after implementation of the “chart closure” hard stop and to evaluate the specificity of the alerts and provider responses to appropriate alerts. This study explores a novel alert intended to reduce alert fatigue by focusing on a specific clinical situation in which a class of evidence-based medications is indicated and appears to have been omitted. The results of the study advance the design of CDS systems for diabetes care by testing a new approach to improve alert visibility for providers while minimizing interruption via Epic’s “chart closure” hard stop feature.
MATERIALS AND METHODS
Best practice advisory alerts for diabetes and blood pressure control
In 2013, UCLA Health launched CareConnect, its implementation of the Epic EHR system. A driving principle in implementing CareConnect was to minimize provider interruptions with alerts. However, as part of UCLA’s response to the Centers for Medicare and Medicaid funded Delivery System Reform Incentive Payment Program, UCLA Health chose to implement a narrowly targeted BPA in primary care practice locations to alert providers of clear opportunities to treat elevated blood pressures among patients with diabetes by starting an ACEI or ARB. The BPA was implemented in the context of Managing Your Medication for Education and Daily Support (MYMEDS), a larger primary care team-based pharmacist medication management program designed to improve medication adherence and cardiovascular risk factor control.21 The responsibilities of MYMEDS pharmacists consist of collaborating with primary care physicians to conduct medication therapy management, provide education to patients, help patients address cost-related issues, conduct medication reconciliation, and correct potential medication problems. The pharmacists were present in all practices that implemented the BPA during the time period of the present study. In regard to BPA implementation, the responsibilities of the pharmacists were to provide education to primary care physicians on the alerts and occasionally follow up with those who received alerts. These responsibilities did not change with the addition of the “chart closure” hard stop.
During a patient encounter, the BPA fired an alert if the patient met the following criteria: (1) diabetes diagnosis on the problem list, (2) last blood pressure value in a primary care office setting exceeded 140/90, (3) average of last three blood pressure values in a primary care office setting exceeded 140/90, (4) no active prescription for an ACEI or ARB, (5) no documented allergy or intolerance to an ACEI and ARB, (6) age between 18 and 75 years, and (7) not pregnant. Blood pressures recorded in the hospital, urgent care, or emergency room visits were excluded. The BPA prompted primary care providers to respond in one of two ways: (1) ordering an ACEI or ARB directly within the BPA, or (2) dismissing the alert by clicking a reason for dismissal.
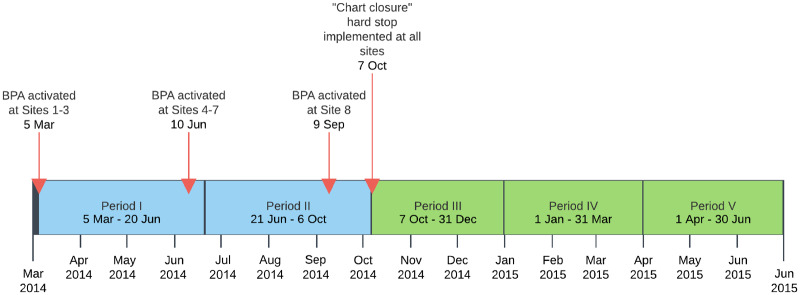
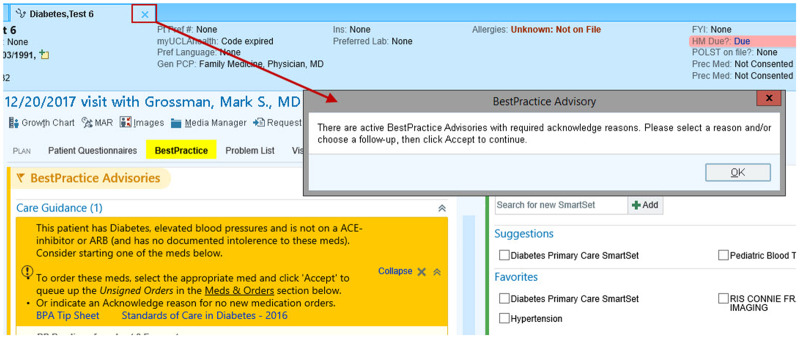
The passive BPA for diabetes and blood pressure control was activated across eight primary care practice locations within UCLA Health between May and October of 2014 (see Figure 1). When the BPA fired an alert, the “BestPractice” section header in the Epic visit navigator turned yellow (see Figure 2) and providers needed to click on the section header or scroll to the BestPractice navigator section to see the alert. On October 7, 2014, the BPA began using a “chart closure” hard stop, which meant that, if the BPA fired an alert, providers could not close the patient’s chart without acting on the alert (i.e., using the BPA to order a prescription or manually dismissing the alert by clicking a dismissal reason). After the “chart closure” hard stop was implemented, providers could still escape from responding to alerts by modifying the data that fired the alert (e.g., deleting diabetes from the problem list, entering a new blood pressure value that lowered the average, or ordering an ACEI or ARB outside the BPA). While the “chart closure” hard stop prevented providers with a pending BPA alert from logging out of CareConnect by clicking on the “Log Out” button, they could escape from responding to the BPA by being automatically logged off due to time-out.
Figure 1.
Study timeline and BPA activation across UCLA Health primary care practice locations.
Figure 2.
The figure shows a screenshot of what primary care providers began seeing in CareConnect after implementation of the “chart closure” hard stop when they tried to close a patient’s chart without acting on the alert. It also shows that the BestPractice section header turned yellow when the BPA fired an alert.
Study data and variables
To examine the impact of the BPA for diabetes and blood pressure control, we extracted data from CareConnect on all BPA alerts that fired between March 5, 2014, and June 30, 2015. CareConnect captured in user action logs when alerts fired, whether providers dismissed the alert, the dismissal reason providers selected (if any), and whether providers used the BPA to order an ACEI or ARB. From these data, we categorized provider responses in the following ways: (1) no response to alert, (2) response to alert by selection of a dismissal reason, or (3) response to alert by using the BPA to prescribe an ACEI or ARB medication. Medications that providers ordered outside the BPA—which MYMEDS pharmacists identified through review of patients’ charts—were categorized as “no response to alert.”
To evaluate alert specificity and provider responses to appropriate alerts, MYMEDS pharmacists reviewed patients’ charts and, when necessary, spoke directly with providers who received the alerts. They determined whether the provider actually prescribed an ACEI or ARB during the encounter, or if not, any reasons that it might have been an appropriate omission based on the patient’s clinical situation (but not based on any reasons the providers selected in dismissing the BPAs). In the early periods, pharmacists also spoke with providers who did not respond to alerts to assess whether the providers had noticed the alerts, and if so, what their clinical reasoning was for not prescribing an ACEI or ARB. We categorized these assessments to indicate whether (1) the alert firing was clinically unnecessary or inappropriate (e.g., due to an ACEI or ARB intolerance that failed to suppress the alert), (2) there was possibly an acceptable reason to withhold treatment (e.g., deferring decision to the patient’s nephrologist), or (3) there was no reason to withhold treatment, and the provider either did or did not prescribe an ACEI or ARB. We determined whether a provider prescribed a medication within the BPA through user action logs in CareConnect. We determined whether a provider prescribed a medication outside the BPA through pharmacist review of patients’ charts.
Data analysis
Descriptive statistics were performed to summarize provider responses to alerts, alert specificity, and provider responses to appropriate alerts. Fisher’s exact tests were used to assess the changes in the proportion of provider responses to alerts, alert specificity, and provider responses to appropriate alerts in the time periods before and after implementation of the “chart closure” hard stop.
RESULTS
From March 5, 2014, to June 30, 2015, the BPA fired an alert for 89 providers during 284 encounters with 219 distinct patients in eight UCLA Health primary care practices. Encounters with an alert firing made up 3.3% (284/8498) of all encounters with a patient with diabetes that happened during the part of the study period for which the BPA was active in the eight practices. The median number of alerts that each provider received during the study period was two (range: 1-17), and the median number of alerts associated with each patient was one (range: 1-7). MYMEDS pharmacists discussed responses to alert firings with the providers who received the alerts in 10.2% (29/284) of alert firings.
Provider responses to alerts before and after “chart closure” hard stop
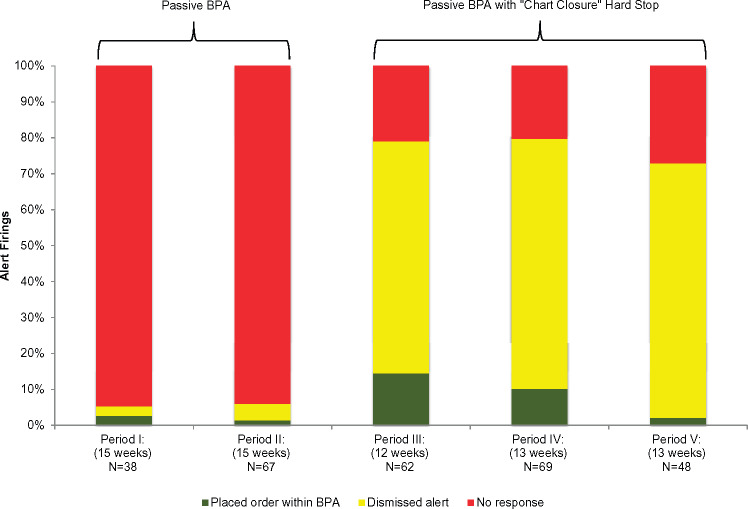
Table 1 shows provider responses to alerts across five 12-15 week time periods before and after implementation of the “chart closure” hard stop. The change in the proportion of provider responses to alerts (no response versus response) in the time periods before and after implementation of the “chart closure” hard stop was significantly different (P < .001). Prior to implementation of the “chart closure” hard stop, providers almost never responded (94% with no response) to the passive alerts. Discussions with providers indicated that they rarely noticed the alerts during the passive period. After the “chart closure” hard stop was implemented, providers responded to alert firings more often, and only 20% to 27% did not respond.
Table 1.
Provider responses to alerts across sequential time periods before and after implementation of the “chart closure” hard stop
| Provider Responses to Alerts | Passive BPA** |
Passive BPA with “Chart Closure” Hard Stop |
|||
|---|---|---|---|---|---|
| Period I 03/05/14–06/20/14 (15 weeks) N = 38 | Period II 06/21/14–10/06/14 (15 weeks) N = 67 | Period III 10/07/14–12/31/14 (12 weeks) N = 62 | Period IV 01/01/15–03/31/15 (13 weeks) N = 69 | Period V 04/01/15–06/30/15 (13 weeks) N = 48 | |
| No response* | 36 (94.7%) | 63 (94.0%) | 13 (21.0%) | 14 (20.3%) | 13 (27.1%) |
| Response | 2 (5.3%) | 4 (5.9%) | 49 (79.0%) | 55 (79.7%) | 35 (72.9%) |
| Dismissed alert | 1 (50.0%) | 3 (75.0%) | 40 (81.6%) | 48 (87.3%) | 34 (91.9%) |
| Placed prescription order*** | 1 (50.0%) | 1 (25.0%) | 9 (18.4%) | 7 (12.7%) | 1 (2.7%) |
After the BPA was configured as passive with a “chart closure” hard stop, providers could still escape from responding in the following ways: passively dismissing alert by modifying data that fired alert (e.g., deleting diabetes from problem list, entering new blood pressure values that lowered the average, or ordering an ACEI or ARB outside BPA) or by being automatically logged off from CareConnect due to time-out.
Alerts were active by the end of Period I in seven primary care practice locations and by the end of Period II in all 8 locations.
Considers only ACEI or ARB prescriptions that were ordered within the BPA.
Although 87.8% of providers’ responses were dismissals after the “chart closure” hard stop implementation, overall, they used the BPA more frequently to place an ACEI or ARB order (two of 105 alert firings before [1.9%] vs 17 of 179 alert firings after [9.5%], P = .013). Figure 3 shows these results graphically.
Figure 3.
Provider responses within alerts before and after implementation of the “chart closure” hard stop.
Specificity of alert firings
On pharmacist review of patients’ charts for the 284 alert firings, 107 (37.7%) were judged to be unnecessary or inappropriate. The most frequent cause of unnecessary alert firings was patients having an isolated blood pressure elevation, for example, due to pain, which was high enough to raise the average blood pressure above the 140/90 threshold (N = 18) (Table 2). This category also included patients whose initial blood pressure reading raised the average above threshold, but whose blood pressure decreased in subsequent readings during the same encounter (N = 26). The next most frequent cause was that the BPA exclusion criteria did not specify all legitimate exceptions to prescribing an ACEI or ARB, such as creatinine elevation (N = 19), planned pregnancy (N = 3), or limited life expectancy (N = 1). Creatinine lab values can be obtained in the EHR, but planned pregnancy and limited life expectancy are not discretely captured in the EHR. Another cause was inaccurate or incomplete documentation in the EHR of data needed to evaluate the BPA’s exclusion criteria of allergy or intolerance to ACEI or ARB (N = 15), diabetes on the problem list (N = 4), and currently active ACEI or ARB prescription (N = 2). Furthermore, the BPA fired unnecessary alerts when providers changed the dose of an existing ACEI or ARB prescription or renewed it (N = 15). These alerts would fire momentarily in between the provider discontinuing the prior ACEI or ARB and entering a new prescription. In this case, the alerts fired because the medication orders temporarily did not represent whether the patient’s medication regimen included an ACEI or ARB. The proportion of alert firings in this category increased significantly after implementation of the “chart closure” hard stop (P = .002). A final reason for inappropriate alerts was programming error (N = 4). The BPA misfired alerts, especially in the early periods, because of mistakes in programming the criteria that the BPA used to determine whether to fire an alert. No new ACEI or ARB prescriptions were ordered after alert firings that were categorized as unnecessary or inappropriate.
Table 2.
Reasons for unnecessary or inappropriate alert firings before and after implementation of the “chart closure” hard stop
| Reasons for Unnecessary or Inappropriate Alert Firings* | Passive BPA N = 46 | Passive BPA with “Chart Closure” Hard Stop N = 61 |
|---|---|---|
| Sensitivity to transient increases in blood pressure | 26 (56.5%) | 18 (29.5%) |
| Criteria do not cover all appropriate exclusions | 6 (13.0%) | 17 (27.9%) |
| Inaccurate or incomplete data documented in the EHR | 10 (21.7%) | 11 (18.0%) |
| Orders misrepresent the true medication regimen | 1 (2.2%) | 14 (23.0%) |
| Programming error | 3 (6.5%) | 1 (1.6%) |
No ACEI or ARB prescriptions were ordered.
Provider responses to appropriate alerts
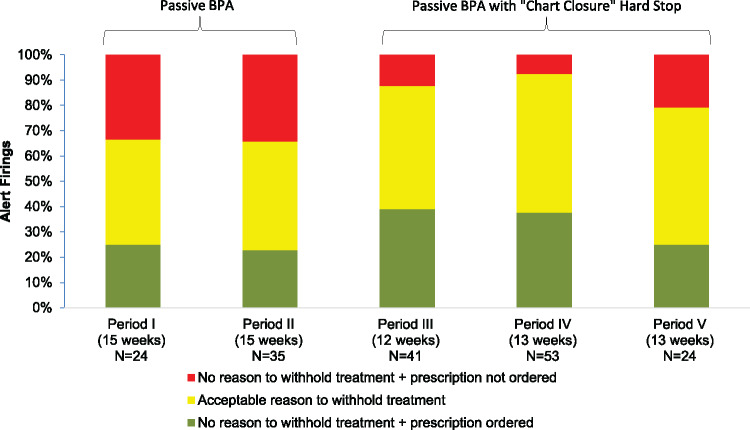
For the alerts that were deemed clinically appropriate, Table 3 shows our adjudication of the ultimate appropriateness of provider responses. For the group of alert firings that did not result in a new ACEI or ARB prescription, we determined whether providers had a possibly acceptable clinical reason for withholding treatment. Overall, the most frequent reason was deferring the ACEI or ARB prescription due to the blood pressure average being close to the threshold level. Another reason was patients declining the recommendation to use an ACEI or ARB to treat their hypertension. A final reason was that providers who received alerts thought it was more appropriate to defer the decision to prescribe the medication to another provider (e.g., the patient’s primary care provider or nephrologist). The proportion of alert firings in these categories did not change significantly after implementation of the “chart closure” hard stop (P = .126). Figure 4 shows these results graphically across the five time periods.
Table 3.
Provider responses to appropriate alert firings before and after implementation of the “chart closure” hard stop
| Provider Responses to Appropriate Alert Firings | Passive BPA N = 59 | Passive BPA with “Chart Closure” Hard Stop N = 118 |
|---|---|---|
| Acceptable reason to withhold treatment* | 25 (42.4%) | 62 (52.5%) |
| Blood pressure close to threshold | 14 (56.0%) | 22 (35.5%) |
| Patient declined | 4 (16.0%) | 22 (35.5%) |
| Decision deferred to another provider | 7 (28.0%) | 18 (29.0%) |
| No reason to withhold treatment | 34 (57.6%) | 56 (47.5%) |
| Prescription ordered** | 14 (41.2%) | 42 (75.0%) |
| Prescription not ordered | 20 (58.8%) | 14 (25.0%) |
No ACEI or ARB prescriptions were ordered.
ACEI or ARB prescription was ordered during the encounter, either within or outside of the BPA.
Figure 4.
Provider responses to appropriate alert firings before and after implementation of the “chart closure” hard stop.
When we determined based on a patient’s specific clinical situation that there was no reason for a provider to withhold treatment (i.e., it was a clear opportunity for treatment), the overall frequency of an ACEI or ARB order (either within or outside BPA) increased significantly, from 41.2% to 75.0%, after the “chart closure” hard stop was implemented (P = .001).
DISCUSSION
We found that a narrowly targeted BPA alert for the intensification of blood pressure medications for persons with diabetes achieved a relatively high specificity and that changing the alert from being passive to having a “chart closure” hard stop significantly improved the frequency of providers responding to the alerts. When a patient’s clinical situation represented a clear opportunity for treatment, we found that a high proportion of alert firings resulted in providers prescribing the indicated medication after the “chart closure” hard stop was implemented.
Addressing the problem of false alerts in CDS systems is critically important if systems are to reach their promise of improving diabetes quality of care.22–24 In contrast to studies reporting that providers override the majority of alerts because the alerts are inappropriate,13,22 our study found upon chart review that over 60% of alerts were clinically appropriate when considering patients’ complete clinical situation. This demonstrates our success in developing an alert with relatively high specificity for the intensification of blood pressure medications for persons with diabetes. Our findings also indicate that suppressing alerts for patients with creatinine elevations, planned pregnancies, and limited life expectancy could further improve alert specificity by eliminating a large proportion of false alerts. Efforts to improve alert specificity can also focus on improving the alert criteria’s sensitivity to isolated blood pressure elevations that raise the average above the threshold, for example, by applying statistical quality control algorithms to identify when elevations are truly meaningful.25,26 This methodology has been applied successfully to monitor changes in blood pressure among patients with hypertension.27,28
In addition, our findings point to a “gray area” of provider responses to appropriate alert firings: instances when providers deferred treatment because the patient’s specific clinical situation did not represent a clear opportunity to prescribe a medication. Future research should focus on investigating how to deal with these gray areas in order to prevent patients from being overlooked. CDS systems could include a feature to allow the alert-receiving provider to assign responsibility to another provider. The feature could, for example, allow the provider to route the encounter note to patients’ primary care physicians, prompt assistants to follow up with patients, or enable them to schedule visits with primary care physicians. Integrating a mechanism into the CDS systems for active followup would help to ensure that the appropriate provider is discussing with patients the opportunity to initiate treatment with an ACEI or ARB.
Furthermore, the results of our study demonstrate that providers’ attention to alerts can be improved using a “chart closure” hard stop. That the majority of providers do not notice passive CDS system recommendations for diabetes care15 suggests the need for a different method to get providers’ attention. The prescription rate observed in our study before the “chart closure” hard stop was implemented was similar to that reported in another study testing passive reminders for providers delivering diabetes care.29 A randomized controlled trial also demonstrated that passive alerts are not effective at improving provider compliance with alert recommendations.30 Conversely, CDS systems utilizing hard stop alerts are generally successful at improving provider response to alerts and have been studied in a variety of care areas, including treatment planning and implementation,31–33 diagnosis,17 and preventive care.34,35 However, these types of alerts may be too disruptive for providers. The “chart closure” hard stop used in this study appears to represent an important middle ground.
Finally, even though most alert firings did not result in new orders of blood pressure medications for persons with diabetes, our findings reveal that when the situation was a clear opportunity for treatment, the “chart closure” hard stop alert was better than a passive alert at achieving its intended function of increasing prescriptions. Ultimately, three quarters of patients got treatment when they needed it. Therefore, coupling high alert specificity with a “chart closure” hard stop may be an effective design characteristic for strengthening the impact of CDS systems on processes of care for patients with diabetes and high blood pressure. In our future research, we will test this hypothesis, as well as investigate effects on clinical outcomes.
Our study is limited by its single-arm, time series design. It is possible that providers’ increased awareness of the alerts over time may account for some of the improvements in provider response to alerts that we observed after implementation of the “chart closure” hard stop. Consequently, our study may have overestimated the true effect of the hard stop on provider responses. However, since each provider saw only about two alerts over the whole study period, a learning effect seems quite unlikely. It is also possible that our observed increase in provider response to alerts after the “chart closure” hard stop was implemented could have been due to a simultaneous decrease in provider use of escape routes (i.e., modifying data that fired the alert or being automatically logged off due to time-out). We were unable to analyze these data on escape routes because Epic does not record them in user action logs. However, our anecdotal evidence suggests that providers rarely noticed alert firings before the “chart closure” hard stop was implemented. This would suggest that the reason providers were not responding to alerts in the periods prior to the “chart closure” hard stop was because they simply did not notice the alerts. In addition, before the “chart closure” hard stop, there would be little reason for providers to escape from responding to alerts since the alerts were passive and therefore did not require a response. Furthermore, the study is limited by a small sample size and study period. The sample size of eight primary care practices was necessary for feasible chart review. Moreover, we noted that the proportion of providers who ordered medications within the BPA appeared to decline in periods IV and V compared to period III, which would suggest an extinguishing effect of the “chart closure” hard stop. Our sample size did not support formally testing this hypothesis, but our future research will investigate this using data that include additional primary care practice locations and a longer study period. Finally, our study is limited by a lack of qualitative data to gain insight into providers’ responses to alert firings and their overall perception of the BPA alerts. Future qualitative research is needed to understand why providers did not prescribe an ACEI or ARB when patients’ clinical situation clearly represented an opportunity. This understanding could lead to identification of problems with the BPA design that were not picked up through pharmacist chart review and that could further improve alert specificity.
CONCLUSION
We achieved relatively high specificity in a BPA alert for the intensification of blood pressure medications for primary care patients with diabetes. Specificity may be further improved in the future by suppressing alerts for patients with creatinine elevations, planned pregnancies, or limited life expectancy and by improving the alert criteria’s sensitivity to isolated blood pressure elevations. Alerts that were configured with a “chart closure” hard stop were more effective at getting a response from providers than passive alerts. Finally, a narrowly focused alert with a “chart closure” hard stop feature was effective at improving primary care provider adherence to intensifying treatment when a patient’s specific clinical situation represented a clear opportunity. Future research is needed to evaluate effects on clinical outcomes such as blood pressure control.
FUNDING
Dr Ramirez’s efforts were supported by grant number T32HS00046 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr Mangione holds the Barbara A. Levey and Gerald S. Levey Endowed Chair in Medicine, which partially supported her work. Dr Moreno’s efforts were supported by a National Institute on Aging Paul B. Beeson Career Development Award K23 AG042961-01. The project was also supported by grant number UL1TR001881 from the National Center for Advanced Translational Science (NCATS) and by funding from the California Medicare/Medicaid Delivery System Reform Incentive Program (DSRIP).
Conflict of interest statement. The authors have no competing interests to declare.
CONTRIBUTORS
Drs. Bell, Moreno, and Mangione conceptualized and led the development of the Best Practice Advisory (BPA) under study. Dr Bell, Mr Maranon, Mr Fu, Ms Chon, and Ms Chen contributed to the development and testing of the BPA and to data acquisition for the current study. Drs. Ramirez and Bell designed the study, analyzed and interpreted the data, and drafted the manuscript. All authors revised the manuscript critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENTS
We acknowledge UCLA Primary Care Innovation Model (PCIM) leadership for facilitating the implementation of the best practice advisory alerts.
REFERENCES
- 1. Group UPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 3177160: 703–13. [PMC free article] [PubMed] [Google Scholar]
- 2. Saydah SH, Fradkin J, Cowie CC.. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004; 2913: 335. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diabetes advocacy. Sec.15. In Standards of Medical Care in Diabetes–2016. Diabetes Care 2017;40(Suppl. 1):S128–S129.
- 4. Bolen SD, Samuels TA, Yeh H-C, et al. Failure to intensify antihypertensive treatment by primary care providers: a cohort study in adults with diabetes mellitus and hypertension. J Gen Intern Med 2008; 235: 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grant RW, Buse JB, Meigs JB; University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care 2005; 282: 337–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berlowitz DR, Ash AS, Hickey EC, Glickman M, Friedman R, Kader B.. Hypertension management in patients with diabetes. Diabetes Care 2003; 262: 355.. [DOI] [PubMed] [Google Scholar]
- 7. Heisler M, Hofer TP, Klamerus ML, et al. Study protocol: the Adherence and Intensification of Medications (AIM) study–a cluster randomized controlled effectiveness study. Trials 2010; 111: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corriere MD, Minang LB, Sisson SD, Brancati FL, Kalyani RR.. The use of clinical guidelines highlights ongoing educational gaps in physicians’ knowledge and decision making related to diabetes. BMC Med Educ 2014; 141: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khatib R, Schwalm J-D, Yusuf S, et al. Patient and healthcare provider barriers to hypertension awareness, treatment and follow up: a systematic review and meta-analysis of qualitative and quantitative studies. PLoS One 2014; 91: e84238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali SM, Giordano R, Lakhani S, Walker DM.. A review of randomized controlled trials of medical record powered clinical decision support system to improve quality of diabetes care. Int J Med Inform 2016; 87: 91–100. [DOI] [PubMed] [Google Scholar]
- 11. Kesselheim AS, Cresswell K, Phansalkar S, Bates DW, Sheikh A.. Clinical decision support systems could be modified to reduce “alert fatigue” while still minimizing the risk of litigation. Health Aff (Millwood) 2011; 3012: 2310–7. [DOI] [PubMed] [Google Scholar]
- 12. Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc 2014; 213: 487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Sijs H, Aarts J, Vulto A, Berg M.. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006; 132: 138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown CL, Mulcaster HL, Triffitt KL, et al. A systematic review of the types and causes of prescribing errors generated from using computerized provider order entry systems in primary and secondary care. J Am Med Informatics Assoc 2016; 242: 432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sequist TD, Gandhi TK, Karson AS, et al. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc 2005; 124: 431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jenssen BP, Shelov ED, Bonafide CP, Bernstein SL, Fiks AG, Bryant-Stephens T.. Clinical decision support tool for parental tobacco treatment in hospitalized children. Appl Clin Inform 2016; 72: 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cecchini M, Framski K, Lazette P, Vega T, Strait M, Adelson K.. Electronic intervention to improve structured cancer stage data capture. J Oncol Pract 2016; 1210: e949–56. [DOI] [PubMed] [Google Scholar]
- 18. Zelig A, Harwayne-Gidansky I, Gault A, Wang J.. Effect of educational and electronic medical record interventions on food allergy management. Allergy Asthma Proc 2016; 375: 404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pell JM, Cheung D, Jones MA, Cumbler E.. Don’t fuel the fire: decreasing intravenous haloperidol use in high risk patients via a customized electronic alert. J Am Med Inform Assoc 2014; 216: 1109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Behav Med Pract Policy Res 2014; 43: 324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreno G, Lonowski S, Jeffery F, et al. Physician experiences with clinical pharmacists in primary care teams. J Am Pharm Assoc 2017; 576: 686–691. [DOI] [PubMed] [Google Scholar]
- 22. Berner ES. Clinical Decision Support Systems: State of the Art. AHRQ Publication No. 09-0069-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2009.
- 23. McCoy AB, Thomas EJ, Krousel-Wood M, Sittig DF.. Clinical decision support alert appropriateness: a review and proposal for improvement. Ochsner J 2014; 142: 195–202. doi: 10.1043/1524-5012-14.2.195 [PMC free article] [PubMed] [Google Scholar]
- 24. Phansalkar S, Edworthy J, Hellier E, et al. A review of human factors principles for the design and implementation of medication safety alerts in clinical information systems. J Am Med Inform Assoc 2010; 175: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tennant R, Mohammed MA, Coleman JJ, Martin U.. Monitoring patients using control charts: a systematic review. Int J Qual Heal Care 2007; 194: 187–94. [DOI] [PubMed] [Google Scholar]
- 26. Montgomery Douglas C. Introduction to statistical Quality Control. New York: John Wiley & Sons, 2009: 179–213. [Google Scholar]
- 27. Solodky C, Chen H, Jones PK, Katcher W, Neuhauser D.. Patients as partners in clinical research: a proposal for applying quality improvement methods to patient care. Med Care 1998; 368: AS13. [DOI] [PubMed] [Google Scholar]
- 28. Hebert C, Neuhauser D.. Improving hypertension care with patient‐generated run chart: physician, patient, and management perspectives. Q Manag Heal Care 2004; 133: 174–7. [DOI] [PubMed] [Google Scholar]
- 29. O’Connor P. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Ann Fam Med 2011; 91: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lo HG, Matheny ME, Seger DL, Bates DW, Gandhi TK.. Impact of non-interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Informatics Assoc 2009; 161: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strom BL, Schinnar R, Aberra F, et al. Unintended effects of a computerized physician order entry nearly hard-stop alert to prevent a drug interaction. Arch Intern Med 2010; 17017: 1578–83. [DOI] [PubMed] [Google Scholar]
- 32. Scott GPT, Shah P, Wyatt JC, Makubate B, Cross FW.. Making electronic prescribing alerts more effective: scenario-based experimental study in junior doctors. J Am Med Inform Assoc 2011; 186: 789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Myers JS, Gojraty S, Yang W, Linsky A, Airan-Javia S, Polomano RC.. A randomized-controlled trial of computerized alerts to reduce unapproved medication abbreviation use. J Am Med Inform Assoc 2011; 181: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schnall R, Liu N.. The effect of an electronic “hard-stop” alert on hiv testing rates in the emergency department. Nurs Res Pract 2008; 8612: 3279–88. [Google Scholar]
- 35. Pevnick JM, Li X, Grein J, Bell DS, Silka P.. A retrospective analysis of interruptive versus non-interruptive clinical decision support for identification of patients needing contact isolation. Appl Clin Inform 2013; 0404: 569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]