Abstract
Introduction
This study explored the value of measuring programmed death 1 (PD-1) in peripheral blood, combined with breast ultrasound using the Breast Imaging Reporting and Data System (BI-RADS) classification, for differentiation between benign and malignant breast tumors.
Materials and methods
We enrolled 113 patients with breast cancer and 66 patients with benign breast tumors who were admitted to Hangzhou First People’s Hospital from September 2014 to August 2017. The mRNA level of PD-1 was detected by quantitative real-time polymerase chain reaction.
Results
The mRNA levels of PD-1 were significantly higher in the peripheral blood of patients with breast cancer than those in patients with benign breast tumors. The diagnostic sensitivity of PD-1 mRNA expression was 0.805, the specificity was 0.788, and the area under the curve (AUC) was 0.848 (P < 0.001); the sensitivity of breast ultrasound-based BI-RADS classification was 0.752, the specificity was 0.909, and the AUC was 0.906 (P < 0.001); and the combined sensitivity, specificity, and AUC of the two assays were 0.920, 0.879, and 0.938, respectively (P < 0.001). Progesterone receptor-positive breast cancer patients exhibited high levels of PD-1 expression (P < 0.001).
Conclusion
This study suggests that the measurement of PD-1 combined with breast ultrasound-based BI-RADS classification represents a significant improvement for breast cancer diagnosis compared with diagnoses based on either method alone.
Keywords: PD-1, breast ultrasound, diagnosis, breast cancer
Introduction
Breast cancer is the most commonly reported cancer in women worldwide, and results in high levels of mortality, although this trend has gradually declined in recent years.1,2 Pathological diagnosis is the gold standard for detection of breast cancer, although puncture sampling is invasive. Moreover, there is a problem of sample errors due to the analysis on portion of tumors that are highly heterogeneous. Therefore, the exploration of less traumatic examination methods for the early diagnosis and treatment of breast cancer is important.
Imaging-based diagnoses and measurements of hematological indicators are the main modes of assessing breast cancer.3–5 Clinical breast examinations and radiological studies are established as essential tools for early detection and are associated with significant improvements in patient outcomes.4,6–8 According to the 5th edition of Breast Imaging Reporting and Data System (BI-RADS) classification, there are BI-RADS 1–6 for tumor diagnosis.9 Breast ultrasound with BI-RADS classification is a common imaging methodology for assessment of breast cancer with a valuable role in distinguishing between benign and malignant breast tumors.10–13 Carbohydrate antigen 153 is a tumor marker for breast cancer, although its diagnostic sensitivity and specificity are limited.14,15 Recently, new indicators for diagnosing breast cancer have been studied, including free DNA in the plasma, microRNA, long-chain non-coding RNA, and vascular endothelial growth factor.5,16–21
Programmed death 1 (PD-1) is an immunosuppressive molecule expressed in T lymphocytes and has an important role in immune escape in cancer patients.22,23 It promotes angiogenesis and suppresses immune responses. PD-1 is expressed in tumor samples and has prognostic value in cancer patients.24–26 Moreover, PD-1 expression in peripheral blood cells increased with tumor stage and correlated with prognosis in various cancer.25,27,28 Thus, we believe that PD-1 expression coupled to medical imaging could impact the way to perform diagnosis and prognosis and to predict therapy outcome in cancer. This study aimed to test the mRNA levels of PD-1 in peripheral blood from patients and controls and to explore its value for making differential diagnoses of benign and malignant breast tumors. Importantly, we found that the combination of breast ultrasound and measurement of PD-1 expression improves the differential diagnosis of breast cancer.
Materials and methods
Study population
The present study enrolled 113 patients with breast cancer and 66 patients with benign breast tumors who were diagnosed and treated in Hangzhou First People’s Hospital from September 2014 to August 2017. All patients had normal liver function; Karnofsky performance status ≥70; and no infections, immune system-related diseases, organ transplant history, or other tumors. All enrolled patients were diagnosed by pathological examination. Clinicopathological information about the 179 enrolled patients was collected using our electronic medical record management system. The following information was collected: age at diagnosis, tumor stage, tumor grade, pathological type, and human epidermal growth factor receptor-2 (HER-2), progesterone receptor (PR), and estrogen receptor status. In addition, 32 healthy people were enrolled as controls. The present study was approved by the Ethics Committee of Hangzhou First People’s Hospital. All patients signed written informed consent forms for this study.
Detection of PD-1 mRNA by quantitative real-time polymerase chain reaction (qRT-PCR)
Venous blood samples (4 mL) were collected from healthy controls and patients before any antitumor treatment and stored in EDTA-anticoagulant tubes. Red blood cells were lysed using lysis solution. Lysates were centrifuged at 2,000 rpm for 5 min. RNA was extracted using the TRIzol method according to the manufacturer’s instructions. Subsequently, RNA was reverse transcribed to cDNA, and qRT-PCR was performed to determine PD-1 mRNA levels. Primer sequences for PD-1 were 5′-GGTGTGAGGCCATCCACAA-3′ and 5′-CCATTCTGTCGGAGCCTCTG-3′. The gene for interferon gamma (IFN-γ) was used as a biological negative control of the procedure. Primer sequences for IFN-γ were 5′-GCCAGTTACTGCCGGTTTGA-3′ and 5′-CTGGAAGCACCAGGCATGA-3′. β-Actin was used as the internal reference to calculate ΔCt values. Primer sequences for β-actin were 5′-ACGTTGCTATCCAGGCTGTG-3′ and 5′-CGCTCGGTGAGGATCTTCAT-3′. The 2−ΔΔCT method was employed for the relative quantification of PD-1 mRNA expression, where ΔΔCT was defined as the difference between the ΔCT value of PD-1 mRNA in patient peripheral blood and that in the blood of healthy volunteers.
BI-RADS classification
According to the 5th edition of the BI-RADS classification, BI-RADS 6 represents a confirmed malignant tumor, BI-RADS 5 represents a highly suspicious malignant tumor with a recommendation for biopsy, BI-RADS 4 represents suspicious findings with a recommendation for biopsy (BI-RADS 4 is classified into 3 types: 4A-low risk, 4B-medium risk, and 4C-high risk), BI-RADS 3 represents a benign tumor with a recommendation for re-examination after 6 months, BI-RADS 2 represents benign findings, and BI-RADS 1 represents negative findings.9
Statistical analysis
We calculated areas under receiver-operating characteristic (ROC) curves to evaluate the differential diagnostic values of specific methods, defined by the area under the curve (AUC), and their specificities and sensitivities. The cutoff values for BI-RADS classification and PD-1 levels were determined using the maximal Youden index value (sensitivity + specificity − 1). Pearson’s chi-squared or Fisher’s exact tests were used to evaluate the associations between PD-1/BI-RADS and clinicopathological information. Binary logistic regression analysis was performed to calculate the combined predictors of BI-RADS and PD-1. P-values <0.05 were considered statistically significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS), Version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
The clinicopathological characteristics of the 113 breast cancer patients are presented in Table 1. All patients and healthy subjects were females. The median ages of patients with breast cancer, those with benign tumors, and healthy subjects were 53 (24–81), 39 (20–60), and 51 (28–75) years, respectively. The mean body weights of the patients with breast cancer (n = 113), patients with benign tumors (n = 66), and healthy subjects (n = 32) were 62.5 ± 7.5, 58.4 ± 9.3, and 61.2 ± 8.9 kg, respectively. The 66 benign breast tumors identified included 42 fibroadenomas, 17 fibroadenosis, and 7 intraductal papillomas. In this study, 3 breast cancer patients were defined as BI-RADS 3, 6 as BI-RADS 4A, 19 as BI-RADS 4B, 42 as BI-RADS 4C, 33 as BI-RADS 5, and 10 as BI-RADS 6; 26 patients with benign tumors were defined as BI-RADS 3, 24 as BI-RADS 4A, 10 as BI-RADS 4B, 5 as BI-RADS 4C, and 1 as BI-RADS 5.
Table 1.
Clinicopathological parameters of 113 breast cancer patients
| Parameter | Number of patients (%) |
|---|---|
| BI-RADS classification | |
| 3 | 3 (2.7) |
| 4A | 6 (5.3) |
| 4B | 19 (16.8) |
| 4C | 42 (37.2) |
| 5 | 33 (29.2) |
| 6 | 10 (8.8) |
| Pathology | |
| Invasive ductal carcinoma | 68 (60.2) |
| Invasive lobular carcinoma | 14 (12.4) |
| Invasive carcinoma | 31 (27.4) |
| Tumor grade | |
| Grade 1 | 8 (7.1) |
| Grade 2 | 70 (61.9) |
| Grade 3 | 17 (15.0) |
| Unknown | 18 (15.9) |
| Tumor stage | |
| T1 | 35 (31.0) |
| T2 | 69 (61.1) |
| T3 | 9 (8.0) |
| Lymph node status | |
| N0 | 65 (57.5) |
| N1 | 33 (29.2) |
| N2 | 6 (5.3) |
| N3 | 9 (8.0) |
| AJCC stage | |
| I | 25 (22.1) |
| II | 71 (62.8) |
| III | 13 (11.5) |
| IV | 4 (3.5) |
| ER status | |
| + | 82 (72.6) |
| − | 31 (27.4) |
| PR status | |
| + | 76 (67.3) |
| − | 37 (32.7) |
| HER-2 status | |
| + | 32 (28.3) |
| − | 81 (71.7) |
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; AJCC, American Joint Committee on Cancer; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor-2.
PD-1 mRNA levels in breast cancer patients were higher than in patients with benign tumors (Figure 1, P < 0.001). Considering the age differences between patients with breast cancer and benign breast tumors, we conducted three comparisons. First, we restricted age of breast cancer patients to a range of 24–60 years and found that there were 76 patients with median age of 41 (24–60) years, which was comparable to the median age of 39 (20–60) years in patients with benign breast tumors. By comparing these two groups, we still found that PD-1 mRNA levels in breast cancer patients were higher than those in patients with benign tumors (Figure S1, P < 0.001). Second, we compared the PD-1 expression in high-age group (age > median age) and low-age group (age ≤ median age) in breast cancer patients and found that there was no significant difference between them (Figure S2, P = 0.700). Third, we compared the PD-1 expression in high-age group (age > median age) and low-age group (age ≤ median age) in patients with benign breast tumors and also found no difference between them (Figure S3, P = 0.785). Expression levels of PD-1 normalized to β-actin for tumor, benign, and healthy subjects were 0.024 ± 0.050, 0.001 ± 0.002, and 0.00004 ± 0.00002, respectively.
Figure 1.
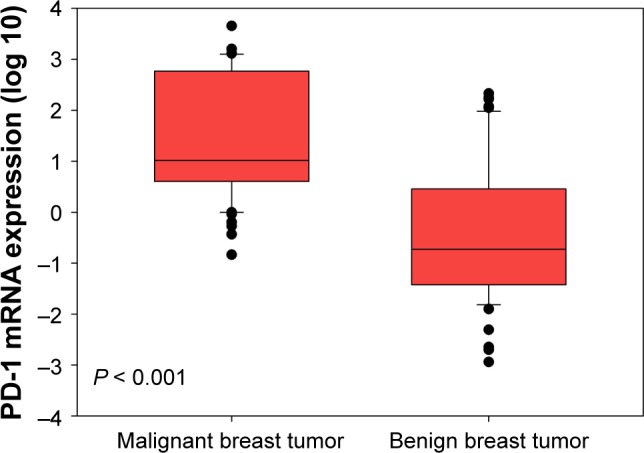
Peripheral PD-1 mRNA expression in patients with malignant and benign breast tumors.
Notes: Outliers were plotted as individual points. The bottom and top of the box are the first and third quartiles. The band inside the box is the second quartile (the median).
Abbreviation: PD-1, programmed death 1.
The associations between BI-RADS/PD-1 and clinicopathological characteristics are shown in Table 2. BI-RADS scores were positively correlated with American Joint Committee on Cancer stage, tumor size, and positive HER-2 status (P = 0.004, 0.001, 0.003, respectively). We also evaluated the correlation between PD-1 mRNA expression and clinicopathological characteristics in patients with breast cancer; PR-positive breast cancer patients showed higher levels of PD-1 expression than PR-negative patients (P < 0.001).
Table 2.
Correlation of clinicopathological parameters with BI-RADS classification and peripheral PD-1 expression in breast cancer patients
| Parameter | BI-RADS
|
PD-1
|
||||
|---|---|---|---|---|---|---|
| 3-4B | 4C-6 | P-value | <3.62 | ≥3.62 | P-value | |
| Age, years (mean ± standard) | 50 ± 11 | 53 ± 12 | 0.634 | 51 ± 11 | 53 ± 14 | 0.463 |
| Tumor grade | ||||||
| Grade 1–2 | 21 | 56 | 0.392 | 19 | 59 | 0.754 |
| Grade 3 | 7 | 11 | 3 | 14 | ||
| AJCC stage (n) | ||||||
| I | 12 | 13 | 0.004 | 4 | 21 | 0.778 |
| II–IV | 16 | 72 | 18 | 70 | ||
| Tumor size (n) | ||||||
| T1 | 16 | 19 | 0.001 | 6 | 29 | 0.800 |
| T2–3 | 12 | 66 | 16 | 62 | ||
| Lymph node status (n) | ||||||
| N0 | 17 | 48 | 0.826 | 15 | 50 | 0.338 |
| N1–3 | 11 | 37 | 7 | 41 | ||
| HER-2 status (n) | ||||||
| + | 2 | 31 | 0.003 | 9 | 22 | 0.181 |
| − | 26 | 54 | 13 | 69 | ||
| ER status (n) | ||||||
| + | 22 | 63 | 0.802 | 13 | 72 | 0.060 |
| − | 6 | 22 | 9 | 19 | ||
| PR status (n) | ||||||
| + | 19 | 58 | 0.970 | 8 | 69 | <0.001 |
| − | 9 | 27 | 15 | 22 | ||
Note: Bold values represent statistical significance, P<0.05.
Abbreviations: BI-RADS, Breast Imaging Reporting and Data System; PD-1, programmed death 1; AJCC, American Joint Committee on Cancer; n, number of patients; HER-2, human epidermal growth factor receptor-2; ER, estrogen receptor; PR, progesterone receptor.
The cutoff value for PD-1 mRNA expression was 3.62, with a maximum Youden index of 0.593 (P < 0.001). A larger percentage of patients with malignant breast tumors expressed PD-1 mRNA (PD-1 ≥ 3.62) than those with benign breast tumors (81% vs 21%, P < 0.001, Figure 2). The differential diagnostic sensitivity and specificity for PD-1 were 0.805 and 0.788, respectively. AUC for PD-1 was 0.848 (0.785–0.911) (Figure 3, Table 3).
Figure 2.
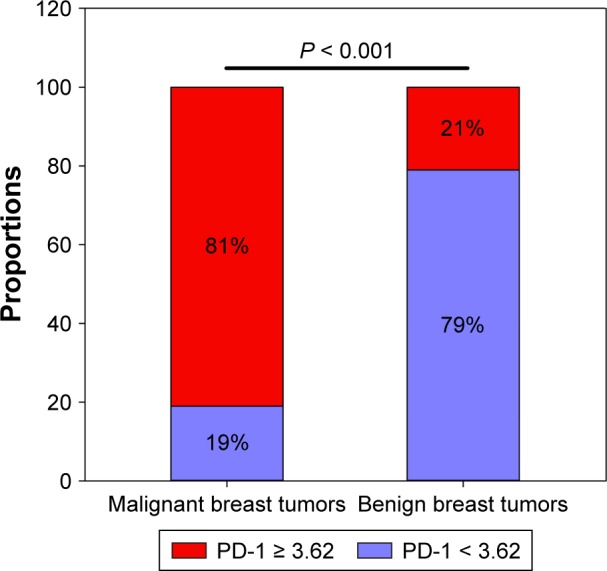
Comparison of PD-1 expression between patients with malignant and benign breast tumors according to cutoff value.
Abbreviation: PD-1, programmed death 1.
Figure 3.
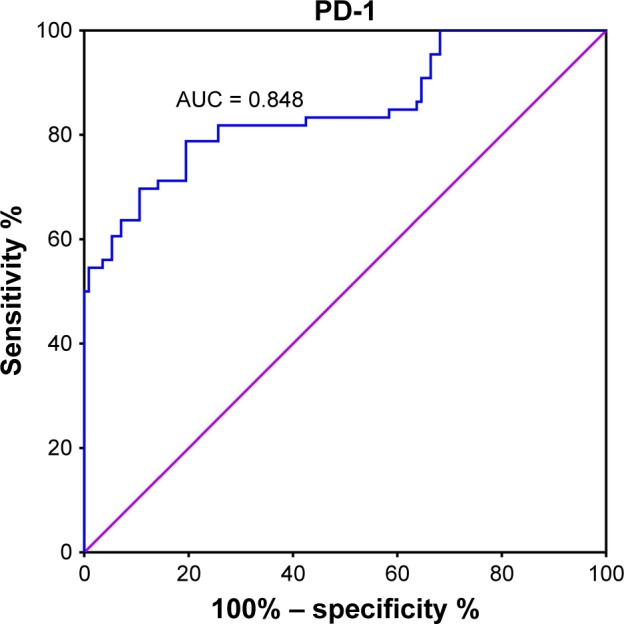
ROC curve for PD-1 detection to differentiate malignant from benign tumors.
Abbreviations: ROC, receiver-operating characteristic; PD-1, programmed death 1; AUC, area under the curve.
Table 3.
Diagnostic value for PD-1 and BI-RADS for ROC curve analysis
| Parameters | Cutoff | Sensitivity | Specificity | Youden index | AUC (95% CI) | P-value |
|---|---|---|---|---|---|---|
| PD-1 | 3.62 | 0.805 | 0.788 | 0.593 | 0.848 (0.785–0.911) | <0.001 |
| BI-RADS | 4C | 0.752 | 0.909 | 0.661 | 0.906 (0.860–0.952) | <0.001 |
| BI-RADS+ PD-1 | NA | 0.920 | 0.879 | 0.799 | 0.938 (0.903–0.973) | <0.001 |
Note: Bold values represent statistical significance, P<0.05.
Abbreviations: PD-1, programmed death 1; BI-RADS, Breast Imaging Reporting and Data System; ROC, receiver-operating characteristic; AUC, area under curve; NA, not available.
BI-RADS 4C, with a maximum Youden index of 0.661 (P < 0.001), was defined as the cutoff value for discrimination between malignant and benign tumors. A larger percentage of patients with malignant breast tumors had a higher BI-RADS classification (BI-RADS 4C-6) than those with benign breast tumors (92% vs 24%, P < 0.001, Figure 4). The differential diagnostic sensitivity and specificity for BI-RADS were 0.752 and 0.909, respectively. AUC was 0.906 (0.860–0.952) (Figure 5, Table 3).
Figure 4.
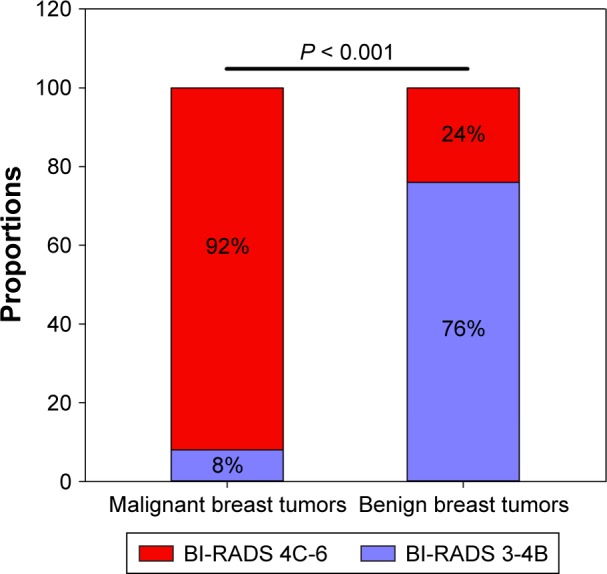
Comparison of BI-RADS between patients with malignant and benign breast tumors according to cutoff value.
Abbreviation: BI-RADS, Breast Imaging Reporting and Data System.
Figure 5.
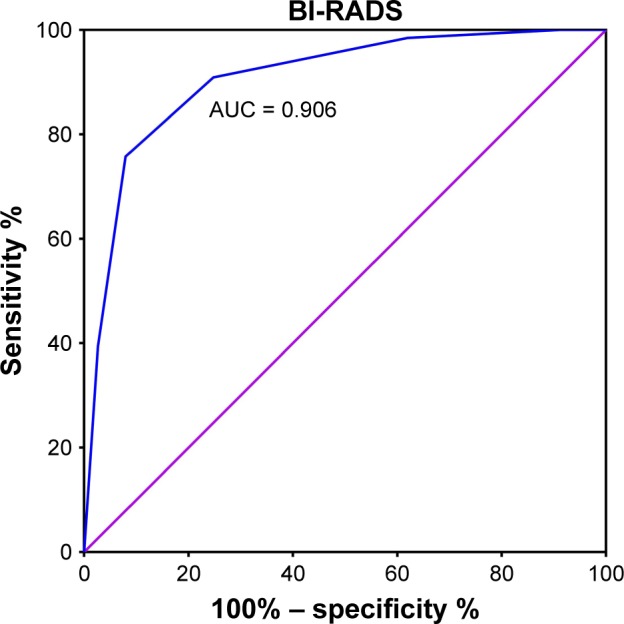
ROC curve for BI-RADS to differentiate malignant from benign tumors.
Abbreviations: ROC, receiver-operating characteristic; BI-RADS, Breast Imaging Reporting and Data System; AUC, area under the curve.
The combined predictive value of PD-1 and BI-RADS was calculated by performing ROC curve and binary logistic regression analysis. The specificity and sensitivity for these methods in combination were 0.879 and 0.920, respectively. AUC was 0.938 (0.903–0.973), which was higher than the separate values for BI-RADS and PD-1 (Figure 6, Table 3).
Figure 6.
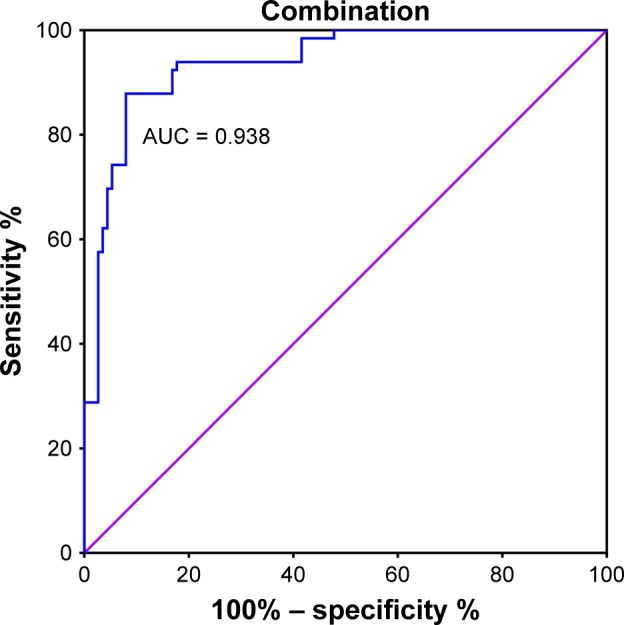
ROC curve for PD-1 detection combined with BI-RADS to differentiate malignant from benign tumors.
Abbreviations: ROC, receiver-operating characteristic; PD-1, programmed death 1; BI-RADS, Breast Imaging Reporting and Data System; AUC, area under the curve.
Discussion
To the best of our knowledge, this is the first study to investigate the diagnostic value of PD-1 combined with breast ultrasound in breast cancer patients. Our study found that breast ultrasound-based BI-RADS scores and PD-1 mRNA expression were individually effective for differentiating malignant and benign breast tumors, with sensitivities of 0.752 and 0.805 and specificities of 0.909 and 0.788, respectively. The combination of these two parameters significantly improved both the sensitivity (0.920) and specificity (0.879), which is clinically valuable. The combination of PD-1 expression levels and imaging increased sensitivity from 0.752 (Imaging alone) to 0.920. Furthermore, our study found that BI-RADS scores were higher in patients with larger tumors and more advanced breast cancer. Patients with PR-positive breast cancer showed higher PD-1 mRNA expression levels than those with PR-negative tumors.
Peripheral immune cells play a central role in the predictive and prognostic value of breast cancer screening.29–34 The immunosuppressive molecule, PD-1, is expressed in T cells and is relevant in the prognosis of cancer patients.23,26,35–42 After T cells are activated by tumor antigens, PD-1 is highly expressed and makes T cells exhausted and consequently inhibits their antitumor immunity.22,24,43 We measured the mRNA levels of PD-1 in patient peripheral blood samples and assessed their correlation with clinical parameters. Our results suggest that PD-1 expression correlates significantly with PR status. The cross talk between hormone receptor-positive tumor cells and the immune system may explain this phenomenon. Previous studies have proven that hormones can enhance PD-1 expression in various immune cells, including macrophages, dendritic cells, and B cells.44 Moreover, several studies have investigated the clinical significance of PD-1+ tumor-infiltrating lymphocytes (TILs) in breast cancer. PD-1+ TILs were expressed at higher levels in the sentinel lymph nodes of patients with triple-negative breast cancer compared with levels in patients with other breast cancer subtypes.45 In addition, levels of PD-1+ TILs correlated negatively with the prognosis of patients with breast cancer.26,46 Nevertheless, reports of the expression levels and diagnostic value of PD-1 in the peripheral blood of patients with breast cancer are scarce. Using a cutoff value of 3.62, we determined that the sensitivity and specificity of PD-1 to differentiate malignant and benign breast tumors were 0.805 and 0.788, respectively; AUC was 0.848 (0.785–0.911).
Breast ultrasound is a routine and valuable measure to differentiate malignant and benign breast tumors.8,47 Jeffers et al4 predicted breast cancer risks by BI-RADS classification, with an AUC of 0.68. Evans et al13 reported that the sensitivity and specificity of ultrasound-based BI-RADS scores to identify benign and malignant breast tumors were 0.95 and 0.69, respectively; however, some studies evaluating the prognostic value of BI-RADS classification reported that it was a negative prognostic indicator.11,48 To evaluate differences related to the ethnicity of patients enrolled in the study and variation related to ultrasonography itself, we determined the cutoff value for ultrasound-based BI-RADS scores and PD-1 mRNA expression using ROC curves. The sensitivity and specificity for BI-RADS scores were 0.752 and 0.909, respectively, and AUC was 0.906 (0.860–0.952), with a cutoff value for BI-RADS 4C, which is consistent with previous studies.8,49
The use of a combination of ultrasound-based BI-RADS scores and PD-1 mRNA expression levels significantly improved the sensitivity (0.920) and specificity (0.879) of discrimination between benign and malignant tumors. In addition, AUC increased to 0.938 (0.903–0.973). Compared with other studies of combined diagnostic indicators, our results exhibit a clinical advantage.5,50
The present study has some limitations. First, this was a single-center investigation. Second, individual differences in BI-RADS scores from mammograms may affect their diagnostic value. Third, some unknown factors, such as depression, sleep, emotion, and eating habits, may influence PD-1 expression. Fourth, the sample size was small. A larger validation cohort is therefore needed to understand the full potential of these markers. Despite these limitations, our study suggests that breast ultrasound-based BI-RADS classification combined with measurement of PD-1 expression levels is clinically valuable for the diagnosis of breast cancer.
Conclusion
Peripheral PD-1 expression combined with BI-RADS classification is effective for differentiating malignant and benign breast tumors. Additional studies are needed to confirm our findings.
Supplementary materials
Peripheral PD-1 mRNA expression in patients with malignant and benign breast tumors (comparable age between two groups).
Notes: Median age of breast cancer patients was 41 (24–60) years. Median age of patients with benign breast tumors was 39 (20–60) years.
Abbreviation: PD-1, programmed death 1.
PD-1 expression in high-age group (age > median age) and low-age group (age ≤ median age) in breast cancer patients.
Abbreviation: PD-1, programmed death 1.
PD-1 expression in high-age group (age > median age) and low-age group (age ≤ median age) in patients with benign breast tumors.
Abbreviation: PD-1, programmed death 1.
Acknowledgments
The study was supported by the Science and Technology Program of Hangzhou (grant no 20150633B18).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Danala G, Patel B, Aghaei F, et al. Classification of breast masses using a computer-aided diagnosis scheme of contrast enhanced digital mammograms. Ann Biomed Eng. 2018 May 10; doi: 10.1007/s10439-018-2044-4. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffers AM, Sieh W, Lipson JA, et al. Breast cancer risk and mammographic density assessed with semiautomated and fully automated methods and BI-RADS. Radiology. 2017;282(2):348–355. doi: 10.1148/radiol.2016152062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ławicki S, Zajkowska M, Glazewska EK, Będkowska GE, Szmitkowski M. Plasma levels and diagnostic utility of VEGF, MMP-9, and TIMP-1 in the diagnosis of patients with breast cancer. Onco Targets Ther. 2016;9:911–919. doi: 10.2147/OTT.S99959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Cao G, Sun X, et al. Intratumoral spatial heterogeneity at perfusion MR imaging predicts recurrence-free survival in locally advanced breast cancer treated with neoadjuvant chemotherapy. Radiology. 2018;288(1):26–35. doi: 10.1148/radiol.2018172462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyante SJ, Lee SS, Benefield TS, Hoots TN, Henderson LM. The association between mammographic calcifications and breast cancer prognostic factors in a population-based registry cohort. Cancer. 2017;123(2):219–227. doi: 10.1002/cncr.30281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao X, Jiang Q, Wu H, Guan X, Qin W, Luo B. Diagnosis of sub-centimetre breast lesions: combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound-a preliminary study in China. Eur Radiol. 2017;27(6):2443–2450. doi: 10.1007/s00330-016-4628-4. [DOI] [PubMed] [Google Scholar]
- 9.Mercado CL. BI-RADS update. Radiol Clin North Am. 2014;52(3):481–487. doi: 10.1016/j.rcl.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Cen D, Xu L, Li N, et al. BI-RADS 3-5 microcalcifications can preoperatively predict breast cancer HER2 and Luminal a molecular subtype. Oncotarget. 2017;8(8):13855–13862. doi: 10.18632/oncotarget.14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badan GM, Piato S, Roveda D, Jr, de Faria Castro Fleury E. Predictive values of BI-RADS(®) magnetic resonance imaging (MRI) in the detection of breast ductal carcinoma in situ (DCIS) Eur J Radiol. 2016;85(10):1701–1707. doi: 10.1016/j.ejrad.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy G, Markert M, Alexander JR, Avisar E. Predictive value of BI-RADS classification for breast imaging in women under age 50. Breast Cancer Res Treat. 2011;130(3):819–823. doi: 10.1007/s10549-011-1669-x. [DOI] [PubMed] [Google Scholar]
- 13.Evans A, Whelehan P, Thomson K, et al. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer. 2012;107(2):224–229. doi: 10.1038/bjc.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang S, Wei L, Sun Y, et al. CA153 in breast secretions as a potential molecular marker for diagnosing breast cancer: a meta analysis. PLoS One. 2016;11(9):e0163030. doi: 10.1371/journal.pone.0163030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu SG, He ZY, Zhou J, et al. Serum levels of CEA and CA15-3 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast. 2014;23(1):88–93. doi: 10.1016/j.breast.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Ye M, Huang T, Ying Y, et al. Detection of 14-3-3 sigma (σ) promoter methylation as a noninvasive biomarker using blood samples for breast cancer diagnosis. Oncotarget. 2017;8(6):9230–9242. doi: 10.18632/oncotarget.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheuk IW, Shin VY, Kwong A. Detection of methylated circulating DNA as noninvasive biomarkers for breast cancer diagnosis. J Breast Cancer. 2017;20(1):12–19. doi: 10.4048/jbc.2017.20.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan M, Liaw CS, Ji SM, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013;19(16):4477–4487. doi: 10.1158/1078-0432.CCR-12-3401. [DOI] [PubMed] [Google Scholar]
- 19.Mishra S, Srivastava AK, Suman S, Kumar V, Shukla Y. Circulating miRNAs revealed as surrogate molecular signatures for the early detection of breast cancer. Cancer Lett. 2015;369(1):67–75. doi: 10.1016/j.canlet.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Xu Y, Jin X, et al. A circulating miRNA signature as a diagnostic biomarker for non-invasive early detection of breast cancer. Breast Cancer Res Treat. 2015;154(2):423–434. doi: 10.1007/s10549-015-3591-0. [DOI] [PubMed] [Google Scholar]
- 21.Uehiro N, Sato F, Pu F, et al. Circulating cell-free DNA-based epigenetic assay can detect early breast cancer. Breast Cancer Res. 2016;18(1):129. doi: 10.1186/s13058-016-0788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertucci F, Finetti P, Birnbaum D, Mamessier E. The PD1/PDL1 axis, a promising therapeutic target in aggressive breast cancers. Oncoimmunology. 2016;5(3):e1085148. doi: 10.1080/2162402X.2015.1085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsuya Y, Horinouchi H, Asao T, et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer. 2016;99:4–10. doi: 10.1016/j.lungcan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Huang W, Fan M. Emerging therapies for breast cancer. J Hematol Oncol. 2017;10(1):98. doi: 10.1186/s13045-017-0466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waki K, Yamada T, Yoshiyama K, et al. PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci. 2014;105(10):1229–1235. doi: 10.1111/cas.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun S, Fei X, Mao Y, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63(4):395–406. doi: 10.1007/s00262-014-1519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen SF, Demuth C, Weber B, Sorensen BS, Meldgaard P. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer. 2016;100:77–84. doi: 10.1016/j.lungcan.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 28.MacFarlane AW, 4th, Jillab M, Plimack ER, et al. PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res. 2014;2(4):320–331. doi: 10.1158/2326-6066.CIR-13-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Huang Z, Wang Q, et al. Usefulness of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in hormone-receptor-negative breast cancer. Onco Targets Ther. 2016;9:4653–4660. doi: 10.2147/OTT.S106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varadan V, Gilmore HL, Miskimen KL, et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperative trastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res. 2016;22(13):3249–3259. doi: 10.1158/1078-0432.CCR-15-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulligan AM, Pinnaduwage D, Tchatchou S, Bull SB, Andrulis IL. Validation of intratumoral T-bet+ lymphoid cells as predictors of disease-free survival in breast cancer. Cancer Immunol Res. 2016;4(1):41–48. doi: 10.1158/2326-6066.CIR-15-0051. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Zhao F, Zhang X, et al. Involved-field irradiation in definitive chemoradiotherapy for T4 squamous cell carcinoma of the esophagus. Curr Oncol. 2016;23(2):e131–e137. doi: 10.3747/co.23.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, Yang J, Jiao S, Li Y, Zhang W, Wang J. Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: implications for prognosis. Cancer Immunol Immunother. 2015;64(7):853–860. doi: 10.1007/s00262-015-1696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi: 10.1186/s12885-016-2352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang BS, Kim IA. A radiosensitivity gene signature and PD-L1 status predict clinical outcome of patients with invasive breast carcinoma in The Cancer Genome Atlas (TCGA) dataset. Radiother Oncol. 2017;124(3):403–410. doi: 10.1016/j.radonc.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Hendrickx W, Simeone I, Anjum S, et al. Identification of genetic determinants of breast cancer immune phenotypes by integrative genome-scale analysis. Oncoimmunology. 2017;6(2):e1253654. doi: 10.1080/2162402X.2016.1253654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckers RK, Selinger CI, Vilain R, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69(1):25–34. doi: 10.1111/his.12904. [DOI] [PubMed] [Google Scholar]
- 40.Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20(10):2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 41.Tsang JY, Au WL, Lo KY, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat. 2017;162(1):19–30. doi: 10.1007/s10549-016-4095-2. [DOI] [PubMed] [Google Scholar]
- 42.Duchnowska R, Peksa R, Radecka B, et al. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. 2016;18(1):43. doi: 10.1186/s13058-016-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229(1):126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dieci MV, Griguolo G, Miglietta F, Guarneri V. The immune system and hormone-receptor positive breast cancer: is it really a dead end? Cancer Treat Rev. 2016;46:9–19. doi: 10.1016/j.ctrv.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Tatara T, Mukohara T, Shimono Y, et al. Expression of programmed death-1 in sentinel lymph nodes of breast cancer. J Surg Oncol. 2018;117(6):1131–1136. doi: 10.1002/jso.24937. [DOI] [PubMed] [Google Scholar]
- 46.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Gao P, Yang J, Yu H, Zhu Y, Si W. Relationship between IL-10 expression and prognosis in patients with primary breast cancer. Tumour Biol. 2014;35(11):11533–11540. doi: 10.1007/s13277-014-2249-6. [DOI] [PubMed] [Google Scholar]
- 48.Kim JY, Jung EJ, Park T, et al. Prognostic importance of ultrasound BI-RADS classification in breast cancer patients. Jpn J Clin Oncol. 2015;45(5):411–415. doi: 10.1093/jjco/hyv018. [DOI] [PubMed] [Google Scholar]
- 49.Jin ZQ, Lin MY, Hao WQ, et al. Diagnostic evaluation of ductal carcinoma in situ of the breast: ultrasonographic, mammographic and histopathologic correlations. Ultrasound Med Biol. 2015;41(1):47–55. doi: 10.1016/j.ultrasmedbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Chang HJ, Yang MJ, Yang YH, Hou MF, Hsueh EJ, Lin SR. MMP13 is potentially a new tumor marker for breast cancer diagnosis. Oncol Rep. 2009;22(5):1119–1127. doi: 10.3892/or_00000544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peripheral PD-1 mRNA expression in patients with malignant and benign breast tumors (comparable age between two groups).
Notes: Median age of breast cancer patients was 41 (24–60) years. Median age of patients with benign breast tumors was 39 (20–60) years.
Abbreviation: PD-1, programmed death 1.
PD-1 expression in high-age group (age > median age) and low-age group (age ≤ median age) in breast cancer patients.
Abbreviation: PD-1, programmed death 1.
PD-1 expression in high-age group (age > median age) and low-age group (age ≤ median age) in patients with benign breast tumors.
Abbreviation: PD-1, programmed death 1.


