Abstract
Endovascular aortic repair (EVAR) of abdominal aortic aneurysms have been performed successfully since 1991.[1] However, 20 to 50% of these patients may develop an endoleak – or continued aneurysmal sac expansion or perfusion despite stent graft coverage.[4] Current recommendations suggest lifelong surveillance with CTA at least 1 month after intervention and yearly after that. In select patients with a stable aneurysm sac on CT performed 1 year after treatment, future screening could be performed with ultrasonography. However, color Doppler ultrasound can fail to detect as many as 31% of endoleaks.[5–7] Contrast enhanced ultrasound (CEUS) provides an alternative approach to excluded aneurysm sac follow-up imaging. The Society for Vascular Surgery notes a need for further research on the role of CEUS in EVAR surveillance.[5] The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) suggests early results are promising.[12] Meta-analyses report pooled sensitivities and specificities of CEUS compared with CTA for the detection of endoleak between 89 to 98% and 86 to 88%, respectively.[8–11] Owing to the dynamic flow information it provides, CEUS may actually be more sensitive than CTA at detection and characterization in select circumstances.[2 12] Challenges with adoption, patient selection, and operator dependency remain but current and future research suggests a role for CEUS in endoleak surveillance.
Keywords: Contrast Enhanced Ultrasound, Endovascular Aortic Repair, Endoleak
Introduction
Endovascular aortic repair (EVAR) was first successfully described by Dr. Juan Parodi in 1991.[1] Currently, the EVAR technique accounts for over 56% of all abdominal aortic aneurysm (AAA) repairs in the United States.[2] In the appropriately selected patient, an endovascular approach significantly reduces perioperative morbidity and mortality.[3] However, between 20 to 50 % of these patients treated with EVAR may develop endoleaks – some requiring reoperation.[4]
Endoleak is defined as continued aneurysm sac pressurization or expansion despite being covered by an endograft.[4] Endoleaks are classified by the presumed source of continued sac pressurization (Figure 1). Type I endoleaks are seal failures between the graft and the native aorta at the proximal or distal ends.[4] Type II endoleaks occur when a patent arterial vessel retrograde fills the aneurysm sac.[4] Type III endoleaks occur through the body of the graft via gaps between components or actual tears in the graft.[4] Type IV endoleaks occur with extravasation of blood products through the graft wall into the sac as a function of the implant fabric porosity.[4] Finally, there is a subset of patients for whom the aneurysm sac expansion occurs but no perfusion source is identified. These have been labelled type V endoleaks and may be due to a phenomenon called “endotension” in which increased sac pressure is experienced despite no identifiable.[4]
Figure 1.
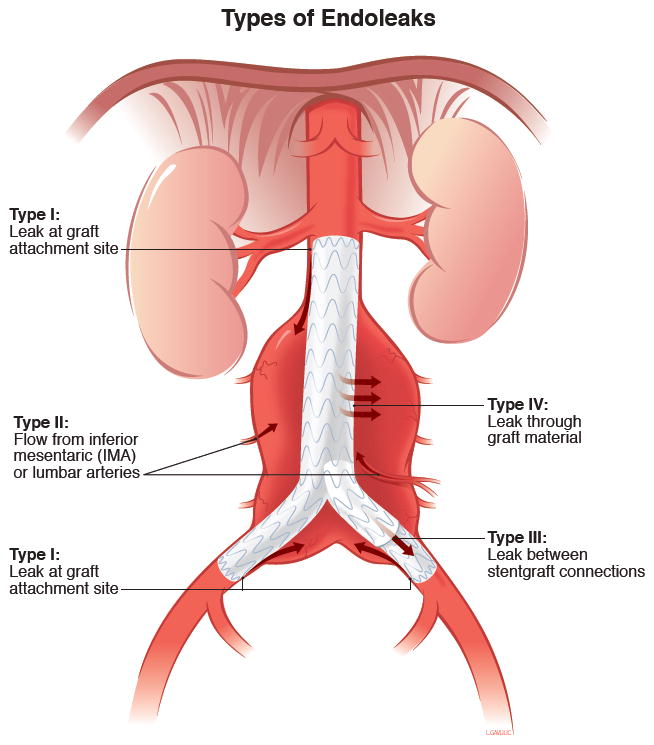
Types of Endoleaks. Type V (not depicted) are endoleaks of unknown origin, potentially due to a poorly understood phenomenon termed “endotension.” Reprinted with permission from University of Michigan Vascular Surgery. Accessed at http://surgery.med.umich.edu/vascular/patient/pdf/EndoLeaks.pdf
Because endoleaks are often asymptomatic and early recognition may allow for elective minimally invasive intervention, the Society of Vascular Surgery (SVS) recommends lifelong surveillance.[5] The gold standard recommended by the SVS is CTA with 3D reconstruction at 1 month post-operation and yearly after that.[5] If an abnormality is noted at the initial post-operative imaging, a second scan is recommended at 6 months to document stability.[5] The SVS concedes that there are concerns with cost, nephrotoxicity, and radiation exposure with lifelong CT imaging and suggest that color Doppler ultrasonography might be substituted in place of CTA if no abnormalities are found in the first year and in patients with contraindications to CTA.[5]
However, color Doppler ultrasound is less sensitive than CTA, failing to detect as many as 31% of endoleaks.[5–7] Instead, in 2009, the SVS practice guidelines noted a need for research on defining the role of contrast-enhanced ultrasound (CEUS) in postoperative surveillance.[5] Subsequent meta-analyses and systematic reviews have been performed showing a comparable diagnostic accuracy to CTA in the detection of endoleaks.[8–11] In their 2011 practice guidelines update on non-hepatic applications of CEUS, the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) suggest that early results are promising and that CEUS may actually be particularly more suited for characterization of endoleaks than CTA.[12] In this narrative review, we highlight the salient body of literature available on the role of CEUS in endoleak identification and categorization.
Technical Considerations of Contrast-Enhanced Ultrasound (CEUS)
Ultrasound contrast agents function by their inherent non-linear scatter of transmit ultrasound frequencies (f0) into subharmonic (1/2 f0) and superharmonic (n * f0) signals.[13] This is unlike the generally linear scatter of surrounding tissue.[13] Using pulse inversion techniques, unenhanced tissue suppression may be achieved – allowing for greater contrast-to-tissue signal ratio.[13]
Multiple agents are available (Table 1), differentiated by their gas core and outer shell.[14] Early agents (“first-generation”) utilized an air gas core.[14] However, these first-generation contrast agents were easily destroyed (even at low mechanical indices) and short lived because of their high blood solubility, limiting use to intermittent pulse imaging.[14] Newer agents (“second-generation”) are characterized by a high density low solubility gas core that allows for real-time imaging and quantitative analysis.[14] Of note, while these agents are not currently approved by the Food and Drug Administration (FDA) for endoleak surveillance, approved indications include echocardiography, the evaluation of focal hepatic masses, and the assessment of ureterovesical reflux (the latter two with regard to only Lumason at this time).
Table 1.
Second Generation Ultrasound Contrast Agents approved for use in the United States[14]
| Agent | Manufacturer | Gas Core | Shell |
|---|---|---|---|
| Optison | GE Healthcare (Princeton, NJ) | Octafluoropropane | Albumin |
| Definity | Lantheus Medical Imaging (N. Billerica, MA) | Octafluoropropane | Phospholipid |
| Lumason | Bracco Diagnostics (Monroe Township, NJ) | Sulfur Hexafluoride | Phospholipid |
CEUS holds several potential advantages over CTA. There is no concern for nephrotoxicity or radiation exposure.[13] Furthermore, CTA may have difficulty in characterizing endoleaks obscured by metallic streak artifact (from the endograft) or of particularly low flow.[15 16] Additionally, ultrasonography is less expensive.[8]
However, several limitations exist. CEUS requires appropriate training and additional expertise in technical application and interpretation.[8] Because of the aorta’s midline retroperitoneal location, interposed bowel gas can make obtaining an adequate window difficult.[8] Abdominal wall hernias, hernia mesh, significant ascites, and an obese body habitus can further worsen visualization.[8] Consequently, we recommend a standardized approach to scanning post-operative EVAR patients when performing CEUS. (Table 2)
Table 2.
Key Steps to Performing Contrast-Enhanced Ultrasonography of the Aorta after EVAR
| Contrast-Enhanced Ultrasound Examination of Aorta after EVAR |
|---|
|
Approach to CEUS in Post-EVAR Surveillance
The patient should be advised to avoid taking anything by mouth the morning of the study to decrease bowel gas and meteorite artifact. Upon arrival, the patient should be positioned supine and a straight, antecubital IV (22 gauge or larger) should be established.
Prior to contrast infusion, the aortic endograft is evaluated in the standard fashion. A low frequency (1–5 MHz) curvilinear array probe is oriented transversely beginning in the epigastrium and moving caudally through the iliac bifurcation to both inguinal ligaments. B-mode should be used to measure maximal aneurysmal sac and iliac diameters. Stent continuity should be documented. Additionally, color Doppler and spectral Doppler is used to assess both distal limb signal waveforms, evaluate for residual sac flow and interrogate flow through a patent inferior mesenteric artery and paired lumbar arteries. Velocities through the stent graft, iliac limbs, renal vessels, and mesenteric vessels should be recorded. The examination should be repeated in the sagittal orientation. Of note, the insonation angle should be less than 60 degrees and gain set as high as possible without creating significant artifact to facilitate detection of smaller low flow vessels.
Next, the contrast-enhanced portion of the exam is performed (Figure 2). While a variety of protocols exist, we use a bolus dose of 0.2 mL Definity (Lantheus, N. Billerica, MA) or 1.5ml of Lumason (Bracco Diagnostics, Princeton, NJ) followed by a 10-mL saline flush for enhancement. Dual screen format is utilized with B-mode display on the left and contrast display on the right to guide anatomic landmarks. The focal zone should be placed just beyond the aorta. The need for increased mechanical index (MI) for adequate signal intensity at this focal distance should be balanced against increased bubble destruction. Consequently, the MI should not exceed 0.2 except in extremely difficult examinations. The gain should be initially set such that the aorta is just outside of view on contrast imaging. Upon arrival of contrast, the gain should be carefully increased to the highest level possible without creating significant artifact.
Figure 2.
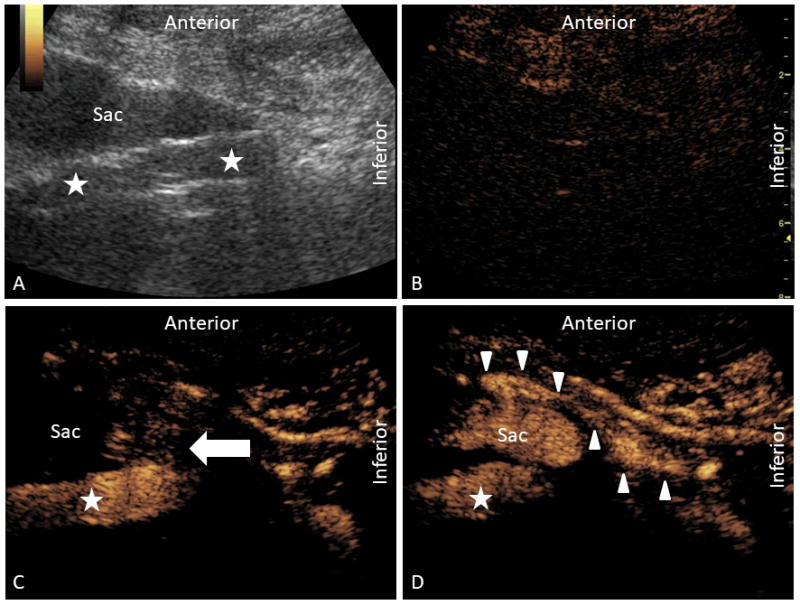
Contrast-enhanced ultrasound examination depicting a complex multi-type endoleak in the sagittal orientation. In panel A, grayscale ultrasound of the aorta (star) is depicted with an anteriorly positioned aneurysmal sac. In panel B, contrast-mode is depicted prior to the administration of contrast. At the current gain setting, the aorta is just out of view. In panel C, a type III endoleak is demonstrated (arrow) at the junction of the body and left iliac extension limb. In panel D, a type II endoleak is demonstrated by reversal of flow within the traced inferior mesenteric artery (arrowhead) and perfusion into the sac.
Examination is attempted in whichever plane provides the best simultaneous view of the aorta and excluded sac based on B-mode imaging. Initial examination should focus on time of enhancement of the aneurysmal sac versus the endograft lumen. Synchronous enhancement suggests an antegrade leak while delayed enhancement suggest a retrograde leak (Type II). Naturally, no sac enhancement suggests against an endoleak.
This examination should continue for at least 10 minutes to ensure that delayed and low-flow endoleaks are identified. If an endoleak is identified, the study should be repeated after disappearance of ultrasound contrast from the circulation, usually within 10 minutes after first injection. Focus should be given to attempting to identify the anatomic source and direction of the endoleak. This should include an examination of the proximal and distal attachment sites, areas of known graft junction, the inferior mesenteric artery, and paired posteriorly oriented lumbar arteries. The key steps of the examination have been outlined in Table 2.
Challenges in examination typically involve difficulty in adequate visualization of the aorta and related structures. Specifically, in patients with extensive abdominal hernias or significant truncal obesity, transabdominal imaging may be difficult. A coronal approach may be attempted with the patient in a lateral decubitus position. Intervening bowel gas can obstruct distal iliac system visualization. The coronal or flank approach can be utilized here as well. While out outside the scope of this article, newer state-of-art probes may utilize high resolution vascular imaging such as Advanced Dynamic Flow (ADF) or Superb Microvascular Imaging (SMI).[17] These tools may be employed with or without contrast and may improve fidelity of low-flow vessels.[17]
Endoleak Detection by Contrast-Enhanced Ultrasound: A Timeline in Meta-Analyses
Early experiences with CEUS suggested sensitivities comparable to CTA in the detection of endoleaks.[18] However, concerns regarding interobserver reliability and inconsistent examination windows remained.[19] Nonetheless, to overcome the limitations of vascular center experiences being published in isolation, several groups have pursued systematic reviews of CEUS for endoleak detection (Table 3).[8–11]
Table 3.
Notable Meta-Analyses Assessing CEUS vs. CTA in Detection of Endoleaks after EVAR
In one of the earliest large meta-analyses evaluating CEUS in endoleak detection compared with CTA, Mirza et al 2010 pooled 7 (of 21 total) studies evaluating CEUS.[8] Using CTA as the gold-standard, they reported a pooled sensitivity of 98% and pooled specificity of 88%.[8] Of the 288 scan pairs evaluated, only 2 instances of endoleaks were seen on CTA but not CEUS.[8] Contrastingly, 24 endoleaks were appreciated on CEUS and not CTA.[8] They comment that this “high false-positive rate” may actually suggest an increased sensitivity compared with CTA (the gold standard) in a particular subset of low-flow endoleaks.[8]
Then, in 2012, Karthikesalingam et al pooled three additional studies comparing CEUS with CTA and reported a pooled sensitivity of 96% and pooled specificity of 85%.[9] Interestingly, they performed a secondary analysis using CEUS as the gold standard instead and reported a CTA pooled sensitivity of only 70% and pooled specificity of 98%, suggesting that the true sensitivity of CEUS may actually exceed that of CTA.[9] However, they concede a major limitation in that significant variation exists in the CT protocols between patients and studies and that protocol delayed-phase imaging might detect the low-flow endoleaks missed on CTA but seen on CEUS.[9]
Similarly, Guo et al 2016 pooled 19 studies comparing CEUS and CTA with a total of 1694 paired scans.[11] They note that CEUS found 138 endoleaks that were not identified by CTA while CTA only identified 51 endoleaks not seen on CEUS.[11] However, they do comment that in examination of only type I and type III endoleaks, just 5 endoleaks were detectable on CEUS alone, compared with 3 endoleaks on CTA alone – suggesting that many of the endoleaks detected by CEUS alone are Type II.[11] More recently, Sun et al 2017 pooled 14 studies (of 42) and noted a pooled sensitivity of 88.9% and pooled specificity of 86.2% (with CTA as the gold standard).[10] Again, they note that in 11 of their 14 pooled studies, CEUS had a higher rate of endoleak detection than CTA.[10]
However, these recent meta-analyses share several common limitations. Notable variability in sonographic technique, CT protocol, and surveillance practice exists.[8] CTA phase protocols for the evaluation of endoleaks can vary between institutions and practitioners.[20] Venous phase delays as long as 300 seconds may be necessarily performed or added to improve the detection of low flow endoleaks on CTA.[21] Newer time-resolved CTA imaging adds low-radiation dynamic resolution to otherwise static CT images and may also further facilitate the detection of small or low-flow endoleaks traditionally missed on static phase CT protocols.[22 23] Additionally, in several of the included studies, the interpreting clinicians were not specifically blinded to the CT findings prior to performing CEUS.[8] Furthermore, there has been significant evolution in microbubble construct, sonographic hardware, and post-processing software in the last decade.[11] Taken together, significant inter-study heterogeneity presents an important limitation of all the presented meta-analyses. Moreover, as Guo et al, 2016 suggests, if many of the unrecognized endoleaks are actually low-flow type II endoleaks for which simply surveillance is advocated, the clinical value in increased sensitivity may be limited.[11]
Endoleak Characterization by Contrast-Enhanced Ultrasound
The ability to differentiate antegrade endoleaks (type I, III and IV) from retrograde endoleaks (type II) is critical to management practice.[4] Antegrade endoleaks result from a mechanical failure of sac exclusion by the graft.[4] Contrastingly, retrograde endoleaks pressurize the sac by backward flow from feeding collateral vessels.[4] While type II endoleaks are frequently observed, type I and all type III endoleaks invariably result in sac expansion and require prompt intervention.[4] Classification provides additional insight into the management of these patients beyond detection.[4]
CEUS may be uniquely positioned for endoleak characterization. Metallic construct is utilized in various degrees (depending on the endograft) at attachment sites along the body of the graft. These may contribute to metallic streak artifact on CTA, potentially impairing endoleak visualization on arterial phase imaging.[24] While a similar echo-artifact is a notable limitation of color Doppler ultrasound, this is minimized in CEUS through utilization of low-intensity sonography and post-processing such as pulse inversion.[25] Additionally, because CEUS provides a real-time depiction of blood flow compared with the static angiographic images of CTA, it is possible to determine speed and direction of flow.[11] This is particularly salient when attempting to differentiate antegrade verse retrograde endoleaks.
Specifically, CEUS is particularly valuable in the characterization of type II endoleaks. CEUS has been shown to correctly characterize retrograde endoleaks that were either misclassified or unclassified by CTA.[24 26–28]. CEUS can identify specific supplying hypertrophic lumbar arteries.[27] Contrastingly, these vessels can be under-characterized by CTA due to low flow.[16 27] With relation to the sac, afferent vessels can be differentiated from efferent vessels – or the lack thereof.[28] Additionally, wash-in and wash-out kinetics might stratify type II endoleaks by flow dynamics.[28] Low flow type II endoleaks with traced inflow vessels and without a well-defined outflow may be at higher risk for sac expansion.[16] Taken together, this may have implications in preventing unnecessary angiographic interventions in purported low risk groups.[24]
The detection and categorization of antegrade endoleaks is important because of greater consensus for intervention. Comparable overall detection rates of antegrade endoleaks by CEUS and CTA are described.[9 11 27] However, CTA-missed type I and type III endoleaks have both been reported.[16 19 24 29] CEUS can identify small antegrade endoleaks at both proximal (type Ia) and distal attachment (type Ib) sites that might otherwise be obscured on CTA by graft metallic artifact.[15 19 24] Though uncharacterized by CTA, these endoleaks actually necessitated intervention.[19]
Investigation into the role of CEUS in type IV (fabric porosity) and type V (endotension or endoleaks of unknown etiology) endoleaks is much more limited. Detection of type IV endoleaks by CEUS have been reported (albeit infrequently, owing to its rarity).[30 31] Contrastingly, previously classified type V endoleaks may actually be re-characterized after CEUS.[16 28 32] While endotension is still incompletely understood, there is certainly a subset of patients for which the actual origin of sac pressurization (and true endoleak type) is simply poorly demonstrated by CTA.[33] Blackwood et al 2016 developed an in vitro model of sac hypertension (simulating endotension) caused by type Ia endoleaks that were only visible on markedly delayed dynamic imaging.[32] Similarly, Millen et al 2013 reported a series of 33 patients for which CEUS was used a problem-solving adjunct imaging modality, adding that temporal flow dynamics allowed for further characterization of endoleaks of uncertain or unknown etiology.[29] As noted earlier, slow or low flow endoleaks may be particularly problematic for CTA and where CEUS can be diagnostic.[16]
Nonetheless, while CEUS appears facile in endoleak classification, CTA is still the preferred modality in assessing actual aneurysmal sac morphology. Additionally, structural facets of aortic implants are difficult to reproducibly assess by ultrasound – particularly when evaluating for implant kinking, migration, and anchorage.[8 16] While operator dependency certainly plays a role, certain configurations of endoleaks (such as posteriorly oriented type II endoleaks) may also be more difficult to detect on CEUS compared with CTA (owing to the interposed graft).[27]
Conclusion
As noted, the early diagnosis and characterization of endoleaks is important in guiding management. Low-risk non-expanding type II endoleaks are frequently observed.[4] Expanding type II endoleaks may require vessel embolization.[4] Contrastingly, whenever possible, type I and type III endoleaks are repaired through endovascular graft extension, angioplasty, or seal of the component / junction defect.[4] Complex endovascular device deployment (with fenestrations or branches) and surgical approaches exist in patients refractory to standard endovascular interventions.[4]
Evidence from large single center experiences to meta-analyses have been published illustrating the efficacy of CEUS in diagnosing and characterizing endoleaks with rates comparable to (or better than) CTA. Yet, notable hurdles to CEUS adoption still exist, including technical limitations (hardware and software), operator-dependency, and patient-related factors (anatomy and pathology). Additionally, CTA still maintains a key role in anatomic and structural characterization of these lesions. However, newer experiences with 4D CEUS (CEUS with 3D reconstruction and real-time interrogation) and CEUS-CT fusion techniques are extremely promising and propose an even more robust characterization of in vivo aortic endografts.[34 35]
Acknowledgments
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R01CA194307. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Because all clinical vignettes contain only de-identified retrospective data and are utilized for educational purposes only, no consent outside standard procedural consent was requested of any patients.
Conflicts of Interest
The authors report non-financial and grant support from GE Healthcare and Toshiba Medical Systems outside the submitted work.
References
- 1.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Annals of Vascular Surgery. 1991;5(6):491–99. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 2.Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. Journal of vascular surgery. 2009;49(3):543–50. doi: 10.1016/j.jvs.2008.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaer RA. Endovascular Repair of Abdominal Aortic Aneurysm. In: Mills JL, Eidt JF, Mohler ER Iii, editors. UpToDate. Waltham, MA: UpToDate Inc; 2017. [Google Scholar]
- 4.Chaer RA, Avgerinos E. Endoleak Following Endovascular Aortic Repair. In: Eidt JF, Mills JL, Collins KA, editors. UpToDate. Waltham, MA: UpToDate Inc; 2017. [Google Scholar]
- 5.Chaikof EL, Brewster DC, Dalman RL, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. Journal of vascular surgery. 2009;50(4):880–96. doi: 10.1016/j.jvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Ashoke R, Brown LC, Rodway A, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. Journal of Endovascular Therapy. 2005;12(3):297–305. doi: 10.1583/04-1479R.1. [DOI] [PubMed] [Google Scholar]
- 7.Zaiem F, Almasri J, Tello M, Prokop LJ, Chaikof EL, Murad MH. Surveillance after endovascular aortic repair. Journal of Vascular Surgery. 2017 doi: 10.1016/j.jvs.2017.04.058. [DOI] [PubMed] [Google Scholar]
- 8.Mirza TA, Karthikesalingam A, Jackson D, et al. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. European Journal of Vascular and Endovascular Surgery. 2010;39(4):418–28. doi: 10.1016/j.ejvs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Karthikesalingam A, Al-Jundi W, Jackson D, et al. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. British Journal of Surgery. 2012;99(11):1514–23. doi: 10.1002/bjs.8873. [DOI] [PubMed] [Google Scholar]
- 10.Sun C, Lin S, Zhao L, Xin S. A meta-analysis of ultrasound imaging in diagnosis of endoleak among patients after endovascular abdominal aortic aneurysm repair. International Journal of Clinical and Experimental Medicine. 2017;10(1):1502–12. [Google Scholar]
- 11.Guo Q, Zhao J, Huang B, et al. A systematic review of ultrasound or magnetic resonance imaging compared with computed tomography for endoleak detection and aneurysm diameter measurement after endovascular aneurysm repair. Journal of Endovascular Therapy. 2016;23(6):936–43. doi: 10.1177/1526602816664878. [DOI] [PubMed] [Google Scholar]
- 12.Piscaglia F, Nolsøe C, Dietrich CFa, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall in der Medizin-European Journal of Ultrasound. 2012;33(01):33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 13.Eisenbrey JR, Sridharan A, Liu J-B, Forsberg F. Recent experiences and advances in contrast-enhanced subharmonic ultrasound. BioMed research international. 2015;2015 doi: 10.1155/2015/640397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paefgen V, Doleschel D, Kiessling F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Frontiers in pharmacology. 2015;6 doi: 10.3389/fphar.2015.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bendick PJ, Bove PG, Long GW, Zelenock GB, Brown OW, Shanley CJ. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. Journal of vascular surgery. 2003;37(2):381–85. doi: 10.1067/mva.2003.17. [DOI] [PubMed] [Google Scholar]
- 16.Napoli V, Bargellini I, Sardella SG, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology. 2004;233(1):217–25. doi: 10.1148/radiol.2331031767. [DOI] [PubMed] [Google Scholar]
- 17.Cantisani V, David E, Ferrari D, et al. Color Doppler Ultrasound with Superb Microvascular Imaging Compared to Contrast-enhanced Ultrasound and Computed Tomography Angiography to Identify and Classify Endoleaks in Patients Undergoing EVAR. Annals of vascular surgery. 2017;40:136–45. doi: 10.1016/j.avsg.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 18.Heilberger P, Schunn C, Ritter W, Weber S, Raithel D. Postoperative color flow duplex scanning in aortic endografting. Journal of Endovascular Therapy. 1997;4(3):262–71. doi: 10.1177/152660289700400305. [DOI] [PubMed] [Google Scholar]
- 19.Ten Bosch JA, Rouwet EV, Peters CTH, et al. Contrast-enhanced ultrasound versus computed tomographic angiography for surveillance of endovascular abdominal aortic aneurysm repair. Journal of Vascular and Interventional Radiology. 2010;21(5):638–43. doi: 10.1016/j.jvir.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Picel AC, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: postprocedure surveillance and complications. American Journal of Roentgenology. 2014;203(4):W358–W72. doi: 10.2214/AJR.13.11736. [DOI] [PubMed] [Google Scholar]
- 21.Iezzi R, Cotroneo AR, Filippone A, Santoro M, Basilico R, Storto ML. Multidetector-row computed tomography angiography in abdominal aortic aneurysm treated with endovascular repair: evaluation of optimal timing of delayed phase imaging for the detection of low-flow endoleaks. Journal of computer assisted tomography. 2008;32(4):609–15. doi: 10.1097/RCT.0b013e31814b271d. [DOI] [PubMed] [Google Scholar]
- 22.Sommer WH, Becker CR, Haack M, et al. Time-resolved CT angiography for the detection and classification of endoleaks. Radiology. 2012;263(3):917–26. doi: 10.1148/radiol.12111217. [DOI] [PubMed] [Google Scholar]
- 23.Lehmkuhl L, Andres C, Lücke C, et al. Dynamic CT angiography after abdominal aortic endovascular aneurysm repair: influence of enhancement patterns and optimal bolus timing on endoleak detection. Radiology. 2013;268(3):890–99. doi: 10.1148/radiol.13120197. [DOI] [PubMed] [Google Scholar]
- 24.Carrafiello G, Lagana D, Recaldini C, et al. Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience. Cardiovascular and interventional radiology. 2006;29(6):969–74. doi: 10.1007/s00270-005-0267-x. [DOI] [PubMed] [Google Scholar]
- 25.Perini P, Sediri I, Midulla M, et al. Single-centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR. European Journal of Vascular and Endovascular Surgery. 2011;42(6):797–802. doi: 10.1016/j.ejvs.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Giannoni MF, Fanelli F, Citone M, Cristina Acconcia M, Speziale F, Gossetti B. Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow-up☆. Interactive cardiovascular and thoracic surgery. 2007;6(3):359–62. doi: 10.1510/icvts.2006.137265. [DOI] [PubMed] [Google Scholar]
- 27.David E, Cantisani V, Grazhdani H, et al. What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. Journal of ultrasound. 2016;19(4):281–87. doi: 10.1007/s40477-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bargellini I, Napoli V, Petruzzi P, et al. Type II lumbar endoleaks: hemodynamic differentiation by contrast-enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. Journal of vascular surgery. 2005;41(1):10–18. doi: 10.1016/j.jvs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Millen A, Canavati R, Harrison G, et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. Journal of vascular surgery. 2013;58(1):18–23. doi: 10.1016/j.jvs.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 30.Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. Journal of vascular surgery. 2009;49(3):552–60. doi: 10.1016/j.jvs.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Grtler VM, Sommer WH, Meimarakis G, et al. A comparison between contrast-enhanced ultrasound imaging and multislice computed tomography in detecting and classifying endoleaks in the follow-up after endovascular aneurysm repair. Journal of vascular surgery. 2013;58(2):340–45. doi: 10.1016/j.jvs.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Blackwood S, Mix D, Chandra A, Dietzek AM. A model to demonstrate that endotension is a nonvisualized type I endoleak. Journal of vascular surgery. 2016;64(3):779–87. doi: 10.1016/j.jvs.2015.04.422. [DOI] [PubMed] [Google Scholar]
- 33.Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. Journal of Vascular Surgery. 2002;35(5):1029–35. doi: 10.1067/mva.2002.123095. [DOI] [PubMed] [Google Scholar]
- 34.Lowe C, Abbas A, Rogers S, Smith L, Ghosh J, McCollum C. Three-dimensional contrast-enhanced ultrasound improves endoleak detection and classification after endovascular aneurysm repair. Journal of vascular surgery. 2017;65(5):1453–59. doi: 10.1016/j.jvs.2016.10.082. [DOI] [PubMed] [Google Scholar]
- 35.Gargiulo M, Gallitto E, Serra C, et al. Could four-dimensional contrast-enhanced ultrasound replace computed tomography angiography during follow up of fenestrated endografts? Results of a preliminary experience. European Journal of Vascular and Endovascular Surgery. 2014;48(5):536–42. doi: 10.1016/j.ejvs.2014.05.025. [DOI] [PubMed] [Google Scholar]


