Abstract
Developmental patterning is a complex biological phenomenon, involving integrated cellular and molecular signaling across diverse tissues. In Astyanax cavefish, the lateral line sensory system is dramatically expanded in a region of the cranium marked by significant bone abnormalities. This system provides the opportunity to understand how facial bone patterning can become altered through sensory system changes. Here we investigate a classic postulation that mechanosensory receptor neuromasts seed intramembranous facial bones in aquatic vertebrates. Using an in vivo staining procedure across individual life history, we observed infraorbital canal neuromasts serving as sites of ossification for suborbital bones. The manner in which cavefish departed from the stereotypical and symmetrical canal neuromast patterns of closely-related surface-dwelling fish were associated with specific changes to the suborbital bone complex. For instance, bony fusion, rarely observed in surface fish, was associated with shorter distances between canal neuromasts in cavefish, suggesting that closer canal neuromasts result in bony fusions. Additionally, cavefish lacking the sixth suborbital bone (SO6) uniformly lacked the associated (sixth) canal neuromast. This study suggests that patterning of canal neuromasts may impact spatial position of suborbital bones across development. The absence of an eye and subsequent orbital collapse in cavefish appears to influence positional information normally inherent to the infraorbital canal. These alterations result in coordinated changes to adult neuromast and bone structures. This work highlights complex interactions between visual, sensory and bony tissues during development that explain certain abnormal craniofacial features in cavefish.
Keywords: Intramembranous ossification, dermal bone, lateral line, canal neuromast
1. Introduction
The vertebrate skull is comprised of bone and cartilage, with many tissue progenitors originating from the cranial neural crest (Abzhanov et al. 2007). Aquatic vertebrates are distinct from their terrestrial relatives with respect to the presence of a mechanosensory lateral line system, originating from ectodermal neural placodes (Webb & Noden 1993; Raible & Kruse 2000). A relationship between sensory neuromast organs (that comprise the lateral line) and positioning of dermatocranial bones has long been suspected, based on their close proximity within the cranium. Parrington (1949) hypothesized that neuromasts may be responsible for the early appearance of osteoblasts (bone depositing cells) or, alternatively, early ossification points may recruit lateral line primordia toward them. Further, Nelson (1969) noted that the patterning of sensory neuromast organs was consistent with infraorbital bone position within Osteoglossomorph fishes. Jollie (1975), studying Esox, suggested that the canal-associated bones develop either from neuromasts or deeper membranous activity.
Mechanoreceptor sensation is an important feature of life in aquatic environments. In 1904, Parker described the lateral line as the ‘sixth sense’ of aquatic vertebrates, and Dijkgraaf (1962) regarded the system as a “touch-at-a-distance” sense. These neuromast organs are made up of a cupula that covers sensory hair cells, which manifest cilia that respond to deflections in water flow (Ghysen et al. 2007). Studies have revealed that neuromasts have diverse adaptive functions which facilitate navigation in aquatic environments, mediating foraging (Schwartz, 1971; Coombs & Conley 1997; Coombs 2001; Montgomery & Saunders 1985), escape (Korn & Faber 1975; McHenry et al. 2009), and schooling (Ritz et al. 2011) behaviors. For instance, flatfish use their lateral line system to orient to tidal flows (Harden James et al. 1979), brook trout employ this system to hold stationary positions within stream channels (Sutterlin & Waddy 1975), while African cichlids use their lateral line sense to engage in social behaviors (Butler & Maruska 2015).
An extreme adaptation involving the lateral line system is seen in subterranean fish. Astyanax mexicanus cavefish have a dramatic increase in both the number and size of neuromasts compared to a closely-related surface-dwelling morph (Teyke 1990). These morphological changes have evolved in response to life in the dark and nutrient-poor cave environment without visual input, facilitating vibration attraction behavior (Yoshizawa et al. 2010; 2012; 2015) and hydrodynamic imaging (Windsor et al. 2008; Herzog et al. 2017).
Interestingly, variation and asymmetry in cranial neuromast positions mirror alterations to the suborbital bones in cavefish (Gross & Powers 2015). These bones harbor a variety of abnormalities, such as fusions and fragmentations of the dermal bones. Suborbital bone anomalies in Astyanax cavefish were first documented in the holotype specimen (then referred to as Anoptichthys jordani) by Hubbs and Innes in 1936. Since this discovery, suborbital bone fusion and fragmentation has been observed in at least 10 different cavefish populations (Mitchell et al. 1977; Jeffery 2000; reviewed in Gross & Powers 2015). All of these populations demonstrate similar cranial alterations alongside a dramatic expansion of the sensory lateral line. Therefore, we performed morphological comparisons between cavefish and extant surface-dwelling fish to characterize the developmental patterning of the suborbital bones and the lateral line system.
Using an in vivo double-labeling approach, we characterized the development of infraorbital canal neuromasts (CNs) and the canal-associated bones directly inferior to the eye. These bones, called the suborbital (SO; synonomous with infraorbital) series of dermal bones, are present in both cave- and surface-dwelling Astyanax. We found that CNs are situated near sites of early ossification, as predicted nearly 70 years ago (Parrington, 1949). We discovered the fusion of neighboring bones in the SO complex is associated with CNs that are set close to one another. Moreover, individuals lacking the terminal CN in the infraorbital series (CN6) did not develop the associated suborbital bone (SO6). This apparent structural relationship between sensory expansion and aberrant bone development in cavefish may indicate an evolutionary trade-off of bone abnormalities in exchange for a sensory adaptation to life in the extreme cave environment. This interaction further illustrates the complexity of tissue interactions operating during the development of the vertebrate cranium.
2. Materials and Methods
2.1. Fish Husbandry and Breeding
Animals used in this study were maintained in a satellite aquatic facility at the University of Cincinnati (Cincinnati, OH, USA). All experiments were approved by the University of Cincinnati Institutional Animal Care and Use Committee (IACUC). Adult Astyanax mexicanus surface and cavefish were housed in 5- or 10-gallon glass tanks (depending on density) fed from an aquatic husbandry system that circulates reverse-osmosis water with a pH of 7.3 (±0.2) and a conductivity of ~800μS (±50). All system water is circulated through an automatic filtration system equipped with mechanical, biological, micron and UV filters. All fish were subjected to a 12hr:12hr light/dark cycle with a system water temperature (23 ± 2°C) and fed once daily with flake food (TetraMin Pro) mixed in system water.
Larvae were reared in glass finger bowls with filtered system water with 0.1% Methylene blue and kept in an incubator at 28°C. Juveniles were collected at ~15mm standard length (SL) from n= 30 surface fish and n= 30 cavefish and transferred to individual 1L BPA-free plastic tanks off of the system to facilitate staining, and maintain individual identity for the duration of the longitudinal study. Tank cleaning and system water changes were administered weekly.
Cavefish originating from the Pachón cave locality (Sierra de El Abra region of NE Mexico) were used from two breeding pedigrees: ‘Asty-138’ (n= 11) and ‘Asty-163’ (n= 19). Surface fish used in this study (pedigree ‘Asty-155’) were relatives of individuals collected from Rio Sabinas and Rio Valles near Ciudad Valles, Mexico. Adult breeding fish from these pedigrees were provided by Dr. Richard Borowsky (NYU).
2.2. In vivo co-labeling of the lateral line and cranial bone
The lateral line begins to develop from neurogenic placodes at 1.5 days post fertilization or ~2.7mm SL (Hinaux et al. 2011), well before ossification of cranial bones. Neuromasts embedded in the infraorbital canal arise by 72 hours post fertilization (hpf) in zebrafish (Raible & Kruse 2000). Components of the lateral line, both canal and superficial neuromasts, can be visualized in vivo using 2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide (DASPEI; Sigma Aldrich D3418; Raible & Kruse 2000; Fig. 1A). Because DASPEI can be visualized under fluorescent light ranging from 488–600nm (green and red filters), we performed co-labeling for dermal facial bones using Calcein (Sigma Aldrich C0875) under the same light filter (GFP) at 488nm (Fig. 1A–F).
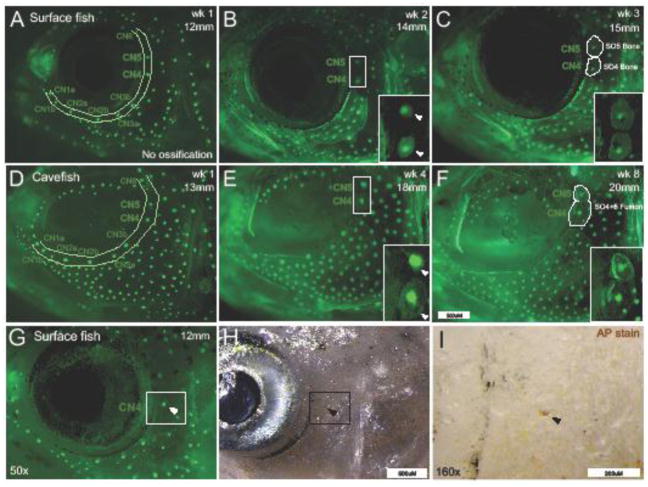
Figure 1. Canal neuromast positioning corresponds with suborbital bone ossification centers.
During the first week of imaging (wk 1) of imaging with DASPEI and calcein (12mm SL, 8 wpf), surface fish (A) display a complete canal neuromast (CN) chain, but the SO4 and SO5 bones cannot yet be detected with calcein staining. One week later (14mm SL, 9wpf) within the same surface fish individual, bony matrix condensations form around CN4 and CN5 (positive calcein staining indicated by white arrowheads). At week 3 (15mm, 10wpf), the SO4 and SO5 bones are visible with CN4 and CN5 residing at the center of the bony matrix. Cavefish at the same stage (13mm SL, 9 wpf) also have a complete CN chain before ossification is detected by calcein staining (B). Cavefish suborbital bones ossify at a slower rate compared to surface fish. By week 4 (18mm SL, 13 wpf) in the same cavefish individual, ossification centers formed around CN4 and CN5 (white arrowheads). By week 8 (20mm SL, 17wpf), SO4 and SO5 bones fused together with both CN4 and CN5 fixed within the bony matrix. Alkaline Phosphatase (AP) staining (black arrowheads) revealed that CN positioning serves as a marker location for early osteoblast activity (C). Images in A and B were captured at 35x magnification and the white scale bar represents 500μm. Images in C were captured at 50x and 160x with scale bars representing 500μm and 200μm, respectively.
Juvenile fish were stained overnight in 2μM Calcein, buffered with NaOH in treated system water, in individual 1L tanks. Calcein is a compound that binds to calcified bony matrix (Jun Du et al. 2001) and is a more inclusive method for bone staining compared to other chromatic stains (i.e. Alizarin red). Following overnight staining, fish were placed in clean 1L tanks with system water to rinse for ~1 hour. Fish were then placed in individual glass bowls and immersed in a solution of 0.5μM DASPEI in system water for approximately 5 minutes.
2.3. Fluorescent Imaging
Fish were anesthetized for imaging by placing them in ice-cold system water for approximately 15 seconds. Fish were mounted on a 2% agarose bed at 35x magnification under the GFP (488nm) fluorescent filter, and imaged on a Leica stereomicroscrope (M205FA) using Leica Application Suite software (LAS v3.8; Leica, Wetzlar, Germany). Images were collected with varying exposure levels (300ms–600ms) owing to slight differences in calcein and DASPEI staining sensitivity per individual. After lateral images were collected from the right and left sides of the face, the fish was measured for SL and revived in system water.
2.4. Chromatic Staining for Osteoblast Activity
Alkaline Phosphatase (AP) staining was performed to detect osteoblast activity (Albertson & Yelick 2009; Edsall & Odendaal 2010). Fish reared to approximately 12mm in SL were sacrificed by anesthetic overdose with 1% MS222 and preserved with 4% PFA in PBS for 2 hours (hrs) at room temperature (RT). Specimens were digested overnight in a solution of 3% trypsin and 50% sodium borate. Whole-mount AP staining was performed according to Edsall & Odendaal (2010). Accordingly, specimens were incubated in 0.05M Tris-maleate buffer (pH 8.3) for 1 hr at RT. Next, specimens were incubated in AP substrate detection solution for 1 hr in the dark at RT. The staining reaction was terminated with 5% saturated sodium borate. Background staining was cleared using 1% KOH solution. Positive label was detected under bright field using a Leica stereomicroscope (M205FA) at 50x and 160x magnification (Fig. 1H–I).
2.5. Statistical Analyses
We defined stereotypical patterning of CN by recording the number of CNs associated with each suborbital bone in surface fish (Fig. 2A) and cavefish (Fig. 2B) on both the right and left sides of the face. To explain variation in patterning, we divided the number of individuals exhibiting the stereotypical CN pattern across the suborbital series of bones by the total number of individuals and multiplied by 100 to get a percentage. This calculation was used across the suborbital series (total CN) and for CN associated with individual suborbital bones (Fig. 2C). A similar calculation was used to describe variation in bone fusion and the loss of the SO6 bone in cavefish (Fig. 2D).
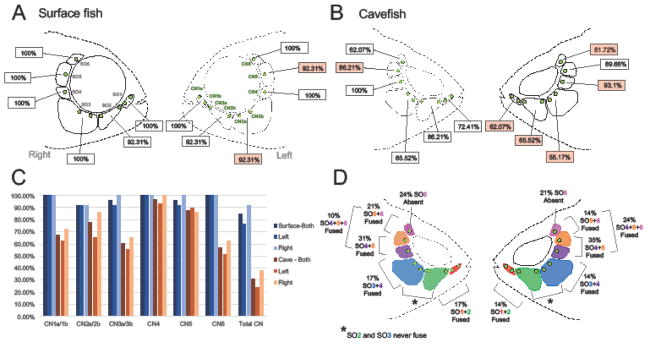
Figure 2. Stereotypical patterning of infraorbital canal neuromasts and suborbital bones in the Astyanax mexicanus cranial complex.
Surface fish (A) typically follow a pattern of nine canal neuromasts (CN) embedded in the infraorbital canal that runs through each of six suborbital bones (SO). Generally, surface fish display a normal sequence of canal neuromasts, but in rare instances (~8% of individuals) a deviation in number of CN per bone occurred. For example, on the left side of surface fish, 92.31% of individuals had two CN (CN2a and CN2b) associated with the SO3 bone (A). This same pattern was scored on the right and left sides of the face for both surface and cavefish with red boxes denoting lateral differences in each morph (A–B). Cavefish exhibited a larger deviation from the typical CN pattern and the sequence was frequently asymmetric. For example, only 55.17% of individuals conformed to the normal pattern on the SO3 bone on the left side, compared to 65.52% on the right side (B). Surface fish display symmetric CN sequences on the lateral sides with the of slight deviations on the SO3 and SO5 bones on the left side (A–C). Cavefish, however, were on average asymmetric for each of the SO bones (B–C). Interestingly, there was a decrease in the proportion of cavefish individuals that follow the CN pattern on the left side for each of the SO bones with the exception of SO5 on the right (C). In addition to alterations in CN patterning, cavefish frequently display both bone loss (SO6) and fusion events within the suborbital series (D). These events also occur asymmetrically across the lateral axis of the face. In comparison, SO bone loss and fusion rarely occur in surface fish. The second and third SO bones never fuse in either morph (Fig. 3).
Inter-canal neuromast distances (mm; Fig. 4A–D) and bone area (mm2; Supp. Fig. 1A–D) were measured using the freehand line and polygon tools in FIJI (v1.0; NIH Bethesda, MD; Schindelin et al. 2012). Data analyses were performed using Microsoft Excel for Mac (v15.38), StatPlus:mac LE (v6.2.21) and JMP (v.13.2). Pairwise t-tests were used to compare means across measurements. Nonparametric analyses (Wilcoxon/Kruskal-Wallis tests for rank sums) were used to detect statistical differences between datasets with unequal n values.
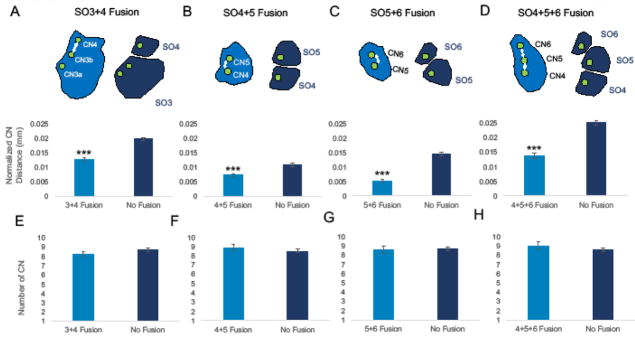
Figure 4. A decrease in inter-canal neuromast distance influences facial bone fusion in cavefish.
Inter-canal neuromast distance was measured between CN3b and CN4 in cavefish individuals exhibiting SO3 and SO4 fusion and was compared to inter-canal neuromast distance (mm) in individuals with separate SO3 and SO4 bones (A). The normalized distance (inter-canal neuromast distance divided by standard length in mm) was significantly lower in cases of SO3+SO4 fusion compared to cases where bones were separate. This was the case for each bone event analyzed: SO4+5 fusion (B), SO5+6 fusion (C), and SO4-6 fusion (D). The total number of canal neuromasts was scored for both individuals with bone fusion events and normal bones. Interestingly, there was no significant difference between the number of canal neuromasts in the infraorbital chain for SO3+4 fusion (E), SO4+5 fusion (F), SO5+6 fusion (G), and SO4-6 fusion (H). Due to varying n numbers, the Wilcoxon/Kruskal-Wallis test for rank sums was used to determine significance. Asterisks indicate significance where p<0.0001.
3. Results
3.1. Infraorbital canal neuromasts serve as ossification sites for suborbital bones
It has long been postulated that canal neuromasts (CNs) serve as ossification centers for dermal bones in aquatic organisms (Parrington, 1949; Nelson 1969; Jollie, 1975). Yamamoto et al. (2003) echoed this notion by suggesting that neuromasts may serve as ossification centers for suborbital bones in Astyanax cavefish. To test this hypothesis, we utilized in vivo staining techniques in cave and surface fish to detect the presence of CNs and developing suborbital 4 and 5 (SO4 and SO5) bones across ontogeny.
At ~12mm SL, both surface and cavefish illustrate a fully developed infraorbital CN chain, but no ossification of the SO4 and SO5 was detected at this point of development using calcein staining (Fig. 1A, D). Within a week of rapid growth, the same surface fish (14 mm SL) displayed condensations of bony matrix directly surrounding both CN4 and CN5 (Fig. 1B). By 15mm SL the SO4 and SO5 bones were clearly visible at the CN4 and CN5 positions (Fig. 1C). Cavefish also displayed a complete CN chain by ~12mm SL, however, there was a delay in SO ossification compared to surface fish (Fig. 1D). By 18mm SL, two weeks after surface fish, bony condensations began to form around CN4 and CN5 in cavefish individuals (Fig. 1E). By 20mm SL, the SO4 and SO5 had fused together, encompassing both CN4 and CN5, in the cavefish specimen shown in Fig. 1F. We did not detect ossification of the infraorbital canal until after SO bones had formed. In both morphs, it was clear that SO bones specifically formed in the area directly surrounding CN position.
Calcein is useful for visualizing the presence of mature bony matrix, with limited detection of early bony condensations. In order to determine if CNs serve as ossification centers for intramembranous bone, we utilized Alkaline Phosphatase (AP) staining to detect early osteoblast (bone deposition) activity (Edsall & Franz-Odendaal 2010). We found an aggregation of AP at the position of CN4 (Fig. 1G–I), suggesting that CNs serve as sites of early bone deposition. In addition, we found that CN patterning occurs before AP-positive staining was detected. In Fig. 1G, we found AP-positive staining at CN4, but not at CN5 or CN6, which is consistent with the order in which the suborbital series of bones ossify as described by Yamamoto et al. (2003). Together with calcein staining, these results suggest that CN position prefigures dermal bone ossification in Astyanax.
3.2. In surface fish, nine neuromasts embedded in the infraorbital canal predict positional arrangement of six suborbital bones
Surface fish demonstrate stereotypical patterning of the infraorbital CN chain composed of nine CNs imbedded within the infraorbital canal (Fig. 2A). The infraorbital canal sits in a fixed position, associated with six suborbital (SO) bones encircling the eye orbital. Each suborbital bone has associated CNs: two CNs reside on the SO1 bone (CN1a and CN1b), two CNs on SO2 (CN2a and CN2b), two CNs on SO3 (CN3a and CN3b), and one CN on each SO4-6 (CN4, CN5, and CN6; Fig. 2A). Surface fish also illustrate bilateral symmetry of CN patterning, with slight deviations in the number of CNs per bone between the right and left sides of the face (<8% of individuals). For example, 100% of individuals (n= 13) followed the stereotypical pattern of CN number on the SO3 for the right side, while 92.3% demonstrated this pattern on the left (Fig. 2C). The presence of slight asymmetry may reflect natural variation across individuals, or occur as a consequence of normative CN cycling, wherein neuromasts periodically die and regenerate (Song et al. 1995).
This pattern differs from the infraorbital CN chain in closely-related Danio rerio zebrafish, which is made up of 4 CNs (Raible & Kruse 2000). Further, five infraorbital bones make up the orbital series in zebrafish (Cubbage & Mabee 1996; Chang & Franz-Odendaal 2014), compared to six suborbital bones in Astyanax surface fish (Valdez-Moreno & Contreras-Balderas 2003). Thus, considerable variation exists in both mechanosensory receptor neuromasts and cranial bone structure across aquatic organisms.
3.3. Cavefish depart from stereotypical cranial patterning observed in surface fish
Cavefish exhibit alterations in the organization of the infraorbital CN chain including changes in the number and positioning of CNs. Nine CNs typically make up the infraorbital chain in surface fish, however, cavefish can have as many as 13, or as few as 6, total CNs (Fig. 2B). In total, only 31% of cavefish adhered to a nine CN pattern, compared to > 92% in surface fish (Fig. 2C). For example, surface fish displayed a typical pattern of two CNs associated with the SO3 bone (CN3a and CN3b; Supp. Fig. 2A). Cavefish, however, exhibited between 1 – 4 CNs on the SO3 bone (Supp. Fig. 2B–D). Interestingly, cavefish SO3 bones frequently fragment into separate bony elements (Gross & Powers 2015). Despite this numerical variation in CNs, we found no evidence of a correlation between CN number and SO3 bone fragmentation in cavefish.
Other common alterations to the SO bone series in cavefish included bone fusion (Fig. 1F; Fig. 2D) and bone loss (Fig. 5D). This resulted in a departure from the stereotypical pattern of six distinct SO bones present in surface-dwelling Astyanax. Bone fusion was scored by visual observation and confirmed by area measurements (mm2). We compared the area of fused bones to the sum of the individual bony areas of individuals that did not display fusions (Supp. Fig. 1A–D). Specifically, we observed SO1 + SO2 fusion, SO3 + SO4 fusion, SO4-6 fusion, SO5 + SO6 fusion and the complete absence or loss of the SO6 bone (Fig. 2D). For each fusion event (with the exception of SO5 + SO6 fusion), the area of fused bones was not significantly different from the sum of the two separate bones (Supp. Fig. 1A–D). Interestingly, the area of SO5 + SO6 fused bones was less than the average combined areas of separate SO5 and SO6 bones (Supp. Fig. 1C). This may reflect restriction in space for SO bone development due to altered orbit morphology, particularly at in the anterior region where the supraorbital meets the SO6 bone.
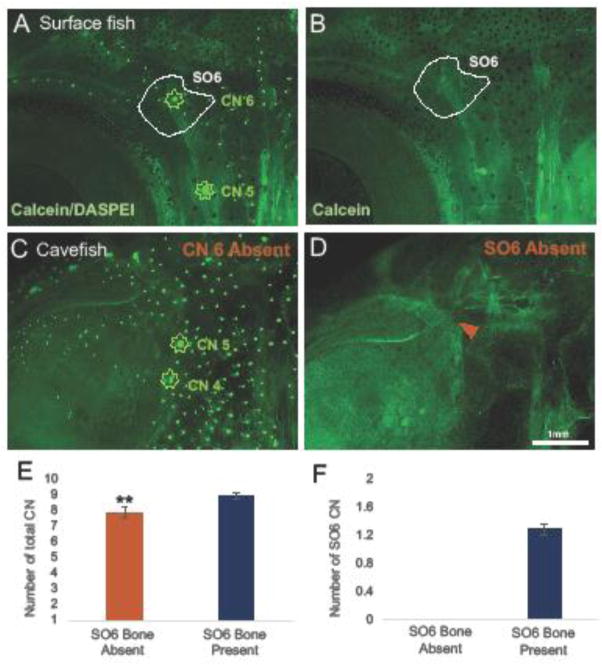
Figure 5. The presence of CN6 is necessary for SO6 bone development.
In surface fish, the CN6 resides in the center of the SO6 bone as illustrated by DASPEI and calcein co-labeling (A–B). Coincidentally, in each instance where CN6 was absent (C) in cavefish, the SO6 bone was also absent (D; orange arrowhead). There were no observations of missing CN6 or the SO6 bone in surface fish. In cavefish, there was a significant decrease in the total number of CN when the SO6 bone was absent (E; p=0.0051). The number of CN within the SO6 bone varied in cavefish, but in every instance of CN6 absence, the SO6 bone was also absent (F). This suggests that CN6 is required for ossification of the SO6 bone. Images were taken at 25x magnification and the scale bar represents 1mm.
In addition to alterations in SO bone development, cavefish exhibit lateral asymmetry in both neuromast and cranial bone patterning. The overall percentage of cavefish that demonstrated the typical CN pattern was low (31%). However, at the level of individual SO bones, deviations from CN patterning were more common on the left side of the face. For instance, on the right side of the face, 86.21% of cavefish individuals had two CNs (CN2a and CN2b) residing on the SO2 bone, compared to 65.52% on the left side. Each of the SO bones had similar results (a larger deviation in CN patterning on the left), with the exception of the SO5 bone, which showed a slightly larger deviation on the right side of the cranium (Fig. 2B).
Asymmetry in CN patterning was mirrored by left-right differences in spontaneous and random alterations to the SO bones in cavefish. Virtually every individual cavefish specimen exhibited some form of bone fusion, loss or combination of features on one side of the face. For example, the SO4-6 bones were fused in 10% of individuals on the right side, and 24% on the left side (Fig. 2D). Unlike asymmetry in CN patterning, SO bone fusion occurred with relatively equal frequency across cavefish (with the exception of SO4-6 fusion) without a clear bias on either side of the face.
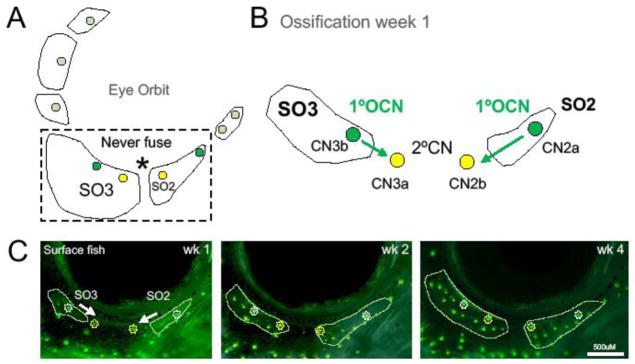
3.4. The SO2 and SO3 bones fail to fuse in Astyanax cavefish
While SO bone fusion has been frequently observed in cavefish, two bones in the SO chain, the SO2 and SO3, never fuse together (Mitchell et al. 1977; reviewed in Gross & Powers 2015; Fig. 2D). We investigated this finding by utilizing in vivo imaging to characterize the direction of ossification in relation to CN position (Fig. 3C). We found that the SO2 bone began to ossify from the anterior aspect of CN2a and grew towards CN2b in the posterior direction (Fig. 3B). The SO3 bone ossifies in the opposite direction, initiating ossification from the posterior aspect of CN3b and grew toward CN3a in the anterior. This suggests that primary ossifying canal neuromasts (1°OCNs) may initiate ossification, and secondary canal neuromasts (2°CNs) may serve as “land marks” for the direction of bone growth (Fig. 3B).
Figure 3. Ossification centers of suborbital bones 2 and 3 migrate from primary ossifying canal neuromasts toward secondary canal neuromasts.
A fusion event between the SO2 and SO3 bones was never observed in surface or cavefish (A). The ossification center for the SO2 bone forms at CN2a and SO3 ossifies from CN3b. These are the primary ossifying canal neuromasts (1°OCNs). The ossification of both bones continues in the direction of the secondary canal neuromast (2°CN). The ossification of the SO2 moves in an anterior-posterior direction from the 1°OCN CN2a toward CN2b. In contrast, the ossification of the SO3 continues in a posterior-anterior direction from the 1°OCN CN3b toward CN3a (B). Surface fish SO2 and SO3 bones start to ossify around the 1°OCN at ~12mm SL as shown by DASPEI and calcein co-labeling (C). By the next week the SO2 and SO3 grew towards the 2°CNs. At week 4, both bones have extended beyond the 2°CNs, but do not fuse. This may indicate that 1°OCNs and direction of ossification may influence bony fusion. Images were taken at 35x magnification and the white scale bar represents 500μM.
The infraorbital CN chain is made up of nine CNs in Astyanax, with six 1°OCNs (CN1a, CN2a, CN3b, CN4, CN5, and CN6) and three 2°CNs (CN1b, CN2b, and CN3a). In each instance of SO bone fusion, the 1°OCNs are in close proximity to one another. For the SO2 and SO3 bones, there are two 2°CNs located between the 1°OCNs, which may limit the ability of these bones to fuse to one another.
3.5. Suborbital bone fusion events are associated with a shorter distance between canal neuromasts
Cavefish SO bones frequently and spontaneously fuse together with the exception of SO2 and SO3 (Fig. 2D). Bone fusion occurs asymmetrically across the lateral axis of the face in individual cavefish. This fusion reflected the bilateral asymmetry observed in variations of infraorbital CN number (Fig. 2B). Previously, it was unclear whether variable numbers of CNs influenced bone fusion events. The number of CNs in the infraorbital chain were scored and binned for individuals with fused or unfused SO bones. There was no significant difference in the total number of infraorbital CNs between fused and unfused bones for SO3-SO4 (Fig. 4E; Wilcoxon/Kruskal-Wallis test p=0.3803), SO4-SO5 (Fig. 4F; p=0.3593), SO5-SO6 (Fig. 4G; p=0.8231) and SO4-SO6 (Fig. 4H; p=0.3489).
In cavefish, eye degradation and subsequent orbital collapse (Yamamoto et al. 2003) results in a departure from normal infraorbital canal morphology, which may in turn displace CN positions within the ossified canal. To determine if CN displacement is associated with bone fusion, the average distance between CNs was compared for fused and separate (unfused) SO bones in cavefish. For SO3+4 fusion, the distance between CN3b and CN4 was significantly smaller than in unfused SO3 and SO4 bones (p<0.0001, n=9; Fig. 4A). The same was true for CN distance measured in examples of SO4+5 fusion (p<0.0001, n=21; Fig. 4B), SO5+6 fusion (p<0.0001, n=10; Fig. 4C) and SO4+5+6 fusion (p<0.0001, n=10; Fig. 4D). Therefore, the spatial distribution of CNs, and not the number (Fig. 4E–H), is correlated with dermal bone fusion in cavefish.
3.6. CN6 absence predicts SO6 bone loss
Yamamoto et al. (2003) reported a decrease in the number of bones within the SO4-6 complex in cavefish, noting that the SO6 bone was usually absent. The authors suggested that the ossification of the SO6 bone could be physically restricted by active growth of the supraorbital bone, which is directly superior to the SO6 (Yamamoto et al. 2003). Eye degradation causes extreme morphological changes in cavefish, resulting in the invasion of the supraorbital bone into the empty orbit (Powers et al. 2017). To determine the extent of SO6 bone loss, we used in vivo bone-labeling to characterize SO6 ossification in surface and cavefish.
In surface fish, the SO6 was always present on both sides of the face (Fig. 5B; n=13). We observed the loss of the SO6 bone in ~41% of cavefish in this study (Fig. 5D; n=12/29). In each case of bone loss, the supraorbital bone did not appear to impede the ossification of the SO6 bone. At this stage in juvenilehood (<1 year, 35 mm SL), the supraorbital has not yet invaded the orbital region to the extent observed in adult cavefish (> 1 year), illustrating the lability of the skull over life history.
Because CNs appear to serve as ossification centers for SO bones, we reasoned that CN6 would also be absent in instances of SO6 bone loss in cavefish. Indeed, in every case of SO6 bone loss, CN6 was also absent (Fig. 5C–D; n=13/13). There was a significant decrease in the total number of CNs in the infraorbital chain among individuals missing an SO6 bone (Fig. 5E; p=0.0051). The number of CNs within the SO6 bone can range from 1–2 in cavefish, however CN6 was always absent in the case of SO6 bone loss (Fig. 5F). In contrast, neither the SO6 nor CN6 were missing in any surface fish (Fig. 2A; Fig. 5A).
The loss of the SO6 bone occurred asymmetrically across the lateral axis of the face, at a frequency of 24% on the right and 21% on the left (Fig. 2D). One individual cavefish was missing an SO6 bone on both sides of the face. This illustrates a common theme in cavefish: a rampant decoupling of left-right symmetry, in sensory and bony development, across the facial midline.
4. Discussion
4.1. Canal neuromasts as ossification sites for dermal bones
Using a combination of in vivo labeling and histological staining, we observed that suborbital bone ossification in Astyanax is initiated at locations specific to infraorbital CNs. Our findings are consistent with Parrington (1949), who suggested that the presence of neuromasts may be responsible for the early appearance of osteoblast cells. Indeed, we found an aggregation of alkaline phosphatase-positive staining for osteoblast activity specific to CN positioning (Fig. 1H–I). This result confirms predictions by several researchers over the last century (Devillers 1947; Lekander 1949; Parrington 1949; Branson & Moore 1962; Reno 1966; Nelson 1969; Kapoor 1970; Jollie 1975) – that neuromasts serve as ossification sites for dermal bones, although future studies utilizing ablation methods are necessary to determine if CNs are necessary for canal-associated bone formation.
Although intramembranous ossification of the suborbital series is consistent with CN position, the role of the semi-ossified infraorbital canal, in which CNs are embedded, is presently unknown. Lekander (1949) described the canal and canal-related facial bones as separate components that fail to fuse in Ostariophysans, suggesting that the canal and underlying dermal bone form independently. Chang & Franz-Odendaal (2014) showed that laser ablation of canal neuromasts in zebrafish resulted in reduced canal wall mineralization, but did not affect canal-associated bones, suggesting two developmental mechanisms of ossification. In contrast, Tarby et al. (2003) characterized canal-bearing bones in cichlids as ‘composite structures’ (Webb & Noden 1993) generated by the fusion of an ossified canal and underlying dermatocranial bone.
The mechanism and timing of canal ossification are unclear in Astyanax cave- and surface-dwelling fish. However, we did not detect ossification of the canal tube until after the SO bones had formed during our longitudinal study. It is possible that the canal tube forms within associated dermal bones through a bone-remodeling mechanism. Wada et al. (2014) demonstrated that in zebrafish, the trunk lateral line canals originate from scales through a bone-remodeling process involving both osteoblast and osteoclast cells. This study suggests that the ossified canal and dermal bone may not be a fused, composite structure. Rather, the canal itself may develop from bone-remodeling processes within the underlying dermal bone already present around the CNs. This notion remains to be tested within the cranial lateral line, however this work suggests that there is a clear association between the lateral line and bony progenitors during development (Wada et al. 2014).
Pehrson (1944) suggested that it is unlikely that bones are dependent on sensory organs for their origin since higher terrestrial vertebrates with similar facial morphology lack lateral line organs (Raible & Kruse 2000). While it is true that terrestrial vertebrates lack mechanoreceptor neuromasts, the nerves that innervate these sensory organs are broadly conserved across vertebrates. For example, the mandibular ramus of the facial nerve innervates CNs in Astyanax – the same nerve is found broadly across vertebrate taxa (Sumi et al. 2015). Further, researchers have compared the lateral line organs to hair cells of the inner ear of higher vertebrates since the late 1800’s (Parker 1904). These studies suggest that complex interactions between sensory tissues and bony structures occur during facial development across vast vertebrate lineages.
4.2. Disruption of developmental patterning within the cavefish cranium
Despite conservation in canal patterning, the number of CNs and associated dermal bones varies dramatically across aquatic vertebrates (Montgomery et al. 1995). The cephalic lateral line canal system can even be used to discriminate between different species of Opsariichthyin cyprinid fishes (Ito et al. 2017). Here, we show that Astyanax surface fish display a stereotypical and symmetric pattern of 9 CNs enclosed within the infraorbital canal (Fig. 2A). In contrast, cavefish harbor asymmetries and numerical variation in CN patterning, ranging from 6 – 13 CNs embedded in the infraorbital canal (Fig. 2B). It is perhaps not surprising, given the vast morphological differences between the morphs, that surface- and cave-dwelling fish display differences in lateral line patterning. It is interesting, however, that cavefish (unlike other aquatic vertebrates) do not exhibit a consistent and stereotypical CN pattern. Rather, cave morphs display dramatic differences in CN number and positioning across individuals. These deviations also occur asymmetrically across the midline of the face within individuals, which may reflect differences in cranial morphology such as eye loss and orbital collapse. Such alterations in form may be at the root of several global shape changes in the cranium (Powers et al. 2017). The infraorbital canal runs directly inferior to the eye forming a stereotypical “curve” shape in surface fish. The loss of eyes in cavefish may result in an irregular morphology of the canal system, which in turn may impact the developmental program of CNs. While this may explain the irregular spatial distribution of CNs, it does not fully explain differences in CN number. This may be related to the function, rather than morphology, of the canal lateral line system in aquatic organisms.
CNs can mitigate “noise” produced by fish while swimming and facilitate sensing surrounding water flows, including those produced by nearby conspecifics (Montgomery et al. 1995). For example, African cichlids use the lateral line system for social communication and initiating aggressive behavior (Butler & Maruska 2015). Surface-dwelling Astyanax also engage in social interactions such as shoaling (Gregson & de Perera 2007) and schooling (Kowalko et al. 2013) behavior. Cavefish have completely lost these behaviors, swimming alone rather than in groups, due to the lack of visual input (Kowalko et al. 2013). Rather than using the lateral line as a means of social interaction, cavefish may have co-opted lateral line changes for cave-associated behaviors. Stomiiform fishes have reduced canals and an increase in neuromast proliferation for flow sensing in the dimly lit, deep sea environment (Marranzino & Webb 2018). Similarily, the increase in number and size of neuromasts in cavefish (Teyke 1990), as well as degradation and irregular morphology of the canals (Schemmel, 1967; Wilkens, 1988; Montgomery et al. 1995), may facilitate sensory navigation within the cave environment in the absence of visual cues.
4.3. Bone fusion is associated with aberrant spatial distribution of CN in cavefish
Here we report that SO bone fusion (SO1 + SO2, SO3 + SO4, SO5 + SO6, SO4-SO6) in cavefish is associated with a decrease in linear distance between CNs (Fig. 4A–D). The altered spatial distribution of the CNs may reflect morphological changes to the orbital area as a result of eye loss. Yamamoto et al. (2003) used lentectomy experiments to characterize the effect of eye degeneration on cranial bone development. In their approach, a surface fish lens was transplanted onto one side of a cavefish embryo to induce the growth of an eye, restoring orbital shape. On the control side (eyeless) the cavefish demonstrated fused SO bones, while on the transplant side (restored eye), there was no bone fusion, suggesting that that a rounded orbital shape may be critical for maintaining canal morphology and position of CNs.
An alternative explanation may involve genetic changes influencing both sensory organ and bone patterning. Through genetic association analyses, Gross et al. (2014) discovered that bone fusion has a complex genetic basis, likely involving multiple genetic influences. Furthermore, the genetic signals were asymmetric, with significant associations on the right side for SO1 + SO2 fusion and on the left for SO4 + SO5 fusion (Gross et al. 2014). Although bone fusion is under genetic control, whether the same genetic loci impact CNs is yet to be determined. Other studies, however, have demonstrated that genetic changes can affect both bone and neuromast development. For example, endothelin-1 knockdowns in zebrafish result in shape and positioning changes in the opercle bone, which leads to patterning errors in neuromast proliferation and distribution (Wada et al. 2010). This study, however, focused on the developmental interactions between the opercle bone and accessory, superficial neuromasts, rather than CNs and canal-associated bones. In our longitudinal study, we found CN patterning occurs before evidence of bone fusion in cavefish, thus the fusion of two SO bones doesn’t not appear to “pull” two CN’s closer together. For example, in Fig. 1D–F, CN4 and CN5 maintain their position over 8 weeks of growth in the cavefish individual and it was not until week 8 that the SO4 and SO5 bones begin to fuse together.
Bony fusion events between SO2 and SO3 bones have not been reported in wild populations (Mitchell et al. 1977), nor observed in laboratory fish (Gross & Powers 2015). In this study, we found that the SO2 and SO3 bones demonstrate a different direction of ossification that is correlated with specific CNs. This may indicate that primary ossifying CNs are responsible for recruiting osteoblasts to deposit bone, while secondary CNs may act as spatial or boundary markers for growing bone. This also suggests that other SO bones spontaneously fuse due to the close proximity of primary ossifying CNs.
Secondary CNs may also serve to prevent fusion of growing bones, although this remains to be tested. In surface fish, we did not observe any AP-positive staining at secondary CNs (CN3a; Fig. 1H) in fixed juveniles, however, staining across multiple developmental stages is necessary to confirm whether osteoblast activity is observed at secondary CNs at later stages. Merrilees (1975) demonstrated through transplantation experiments that canals and CNs can have inhibiting properties, possibly via morphogens, preventing the formation of scales, cartilage and bone. Future studies investigating potential signaling differences between primary and secondary CNs (and the tube canal itself) are necessary in order to determine if secondary CNs can inhibit bone formation.
4.4. Canal neuromast loss is correlated with suborbital bone loss
Along with the belief that neuromasts may serve as ossification centers for dermal bones came the prediction that additional neuromasts could induce the formation of ectopic bones (Branson & Moore 1962). We found that cavefish can have up to 4 extra CNs compared to the stereotypical pattern in surface fish, but no ectopic SO bones were observed. These ancillary CNs are likely secondary CNs, as opposed to primary ossifying CNs, and therefore may lack the ability to induce bone growth. The presence of additional neuromasts, however, may be adaptive and serve to amplify sensory signals in the cave environment.
Nelson (1972) noted that in two species of Esox, the infraorbital canal is discontinuous and the canal-associated bones are incomplete. Later, Jollie (1975) who also studied Esox, suggested that absent canal-associated bones could be the result of absent CN. Cavefish have fewer bones within the SO4-SO6 complex (Yamamoto et al. 2003). We demonstrate that the decrease in SO bone number in cavefish is caused by both fusion events and the (frequent) absence of the SO6 bone. We attribute the failure of SO6 to develop to the absence of CN6. In addition to the absence of CN6, the infraorbital canal failed to ossify beyond the SO5 bone in cases of missing CN6 and SO6 bones. This further supports the notion that the canal may form via bone-remodeling processes (Wada et al. 2014) initiated within SO bones later in development.
5. Conclusions
In this study, we show that patterning of the SO bony complex in Astyanax cave- and surface-dwelling fish is consistent with infraorbital CN patterning. Osteoblasts aggregate at CNs to form centers for intramembranous ossification of canal-associated bones. The spatial distribution of the CNs plays an important role in the patterning of separate SO bones in the series surrounding the eye. In cavefish, the degradation of the eye and subsequent orbital collapse influences canal morphology such that a decrease in the distance between CNs is correlated with bone fusion. Missing CNs are associated with the absence of SO bones, including the frequent absence of the SO6 bone in cavefish. While further analyses are needed to characterize the precise mechanisms of these interactions between sensory tissues and bone, this work underscores the importance of longitudinal studies in understanding developmental patterning of the cranium. These results indicate that a trade-off between sensory expansion and bone development may have evolved as a mechanism for survival in the extreme cave environment.
Supplementary Material
Scoring bone fusion events can be difficult due to the lability of the morphology in the cavefish suborbital chain. We measured individual bony area (mm2) as well as area of fused bones in order to determine if fused bones encompassed the same area as unfused bones. There was no significant difference in the total bony area for SO3+SO4 fusion (light blue) compared to the sum of SO3 bone area (dark blue) and SO4 area (gray; A; p=0.6543). There was also no significant difference for SO4+SO5 fusion (B; p=0.3709) and SO4-SO6 fusion (D; p=0.2293). Interestingly, fused SO5+SO6 bones were significantly smaller than unfused bones (C; p=0.0091). This may be due the variability of SO6 size and positioning in cavefish.
Surface fish display a typical pattern of two canal neuromasts within the SO3 bone: CN3a and CN3b (A). Cavefish SO3 bones frequently fragment into multiple, discrete elements. The SO3 bone for cavefish individual 163_07 is made up of 3 fragments and has only one canal neuromast, CN3b, with an absence CN3a (B). The SO3 bone for cavefish individual 163_03 is fragmented into 2 elements, but the canal neuromast pattern is normal with both CN3a and CN3b present (C). The SO3 bone for cavefish individual 138_04 is also fragmented into 2 separate elements, but has an aberrant pattern of canal neuromasts with the CN3a, CN3b and two ectopic CNs (D). Taken together, the pattern of canal neuromasts does not appear to impact SO3 bone fragmentation in cavefish. Neuromasts and bone were co-labeled with DASPEI and calcein. Images were taken at 30x and the white scale bar represents 1mm.
highlights.
Cranial canal neuromasts serve as ossification sites for suborbital bones in Astyanax cave- and surface-dwelling fish
Surface fish conform to a stereotypical pattern of canal neuromasts, while cavefish display variable and asymmetrical patterning, mirroring commonly observed aberrations in canal-associated facial bones
In cavefish, shorter distances between canal neuromasts result in fusion of suborbital bones and the absence of particular canal neuromasts is associated with the loss of suborbital bones
The patterning of the infraorbital canal neuromasts may impact spatial positioning of suborbital bones across development
Acknowledgments
Funding: The authors would like to acknowledge members of the Gross lab, especially Stephanie Hacker, Shane Kaplan, Daniel Berning and Mia Burris, for helpful discussions, assistance in imaging, and animal care. This work was funded by the US National Institute of Health (NIDCR R01-DE025033) and US National Science Foundation (DEB-1457630) to J.B.G., University of Cincinnati GSGA, URC and Sigma Xi funds to A.K.P. and UC Department of Biological Sciences STEM funds to T.E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abzhanov A, McMahon SJ, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–3144. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- Albertson RC, Yelick PC. Fgf8 haploinsufficiency results in distinct craniofacial defects in adult zebrafish. Dev Biol. 2009;306:505–515. doi: 10.1016/j.ydbio.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson BA, Moore GA. The lateralis components of the acoustico-lateralis system in the sunfish family centrarchidae. Copeia. 1962;1962:1–108. [Google Scholar]
- Butler JM, Maruska KP. The mechanosensory lateral line is used to assess opponents and mediate aggressive behaviors during territorial interactions in an African cichlid fish. J Exp Biol. 2015;218:3284–3294. doi: 10.1242/jeb.125948. [DOI] [PubMed] [Google Scholar]
- Chang CT, Franz-Odendaal TA. Perturbing the developing skull: using laser ablation to investigate the robustness of the infraorbital bones in zebrafish (Danio rerio) BMC Dev Biol. 2014;14:44. doi: 10.1186/s12861-014-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs S, Conley RA. Dipole source localization by the mottled sculpin II. The role of lateral line excitation patterns. J Comp Physiol A. 1997;180:401–415. doi: 10.1007/s003590059958. [DOI] [PubMed] [Google Scholar]
- Coombs S. Smart skins: information procession by lateral line flow sensors. Autonomous Robots. 2001;11:255. doi: 10.1023/A:1012491007495. [DOI] [Google Scholar]
- Devillers C. Recherches dur le crane dermique des teleosteens. Ann Paleontol. 1947;33:1–94. [Google Scholar]
- Dijkgraaf S, Kalmijn AJ. Behavioral tests on the function of ampullae of Lorenzini. Naturwissenschaften. 1962;49:400–400. doi: 10.1007/BF00632257. [DOI] [Google Scholar]
- Edsall SC, Franz-Odendaal TA. A quick whole-mount staining protocol for bone deposition and resorption. Zebrafish. 2010;7:275–280. doi: 10.1089/zeb.2009.0641. [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudière C. The lateral line microcosmos. Gene Dev. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- Gregson JNS, de Perera TB. Shoaling in eyed and blind morphs of the characin Astyanax fasciatus under light and dark conditions. J Fish Biol. 2007;70:1615–1619. doi: 10.1111/j.1095-8649.2007.01430.x. [DOI] [Google Scholar]
- Gross JB, Krutzler AJ, Carlson BM. Complex craniofacial changes in blind cave-dwelling fish are mediated by genetically symmetric and asymmetric loci. Genetics. 2014;196:1303–1319. doi: 10.1534/genetics.114.161661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JB, Powers AK. Biology of the Mexican Cavefish. Academic Press; Cambridge: 2015. The evolution of the cavefish craniofacial complex. [Google Scholar]
- Harden Jones FR, Arnold GP, Greer Walker M, Scholes P. Selective tidal stream transport and the migration of plaice (Pleuronectes platessa L.) in the southern North Sea. ICES J Mar Sci. 1979;38:331–337. doi: 10.1093/icesjms/38.3.331. [DOI] [Google Scholar]
- Herzog H, Klein B, Ziegler A. Form and function of the teleost lateral line revealed using three-dimensional imaging and computation fluid dynamics. J Royal Soc Inter. 2017;14 doi: 10.6084/m9.figshare.c.3746690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinaux H, Pottin K, Chalhoub H, Père S, Elipot Y, Legendre L, Rétaux S. A developmental staging table for Astyanax mexicanus surface fish and Pachón cavefish. Zebrafish. 2011;8:155–165. doi: 10.1089/zeb.2011.0713. [DOI] [PubMed] [Google Scholar]
- Ito T, Fukuda T, Morimune T, Hosoya K. Evolution of the connection patterns of the cephalic lateral line canal system and its use to diagnose opsariichthyin cyprinid fishes (Teleostei, Cyprinidae) Zookeys. 2017;718:115–131. doi: 10.3897/zookeys.718.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR, Strickler AG, Guiney S, Heiser DG, Tomarev SI. Prox 1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax. Dev Genes Evol. 2000;210:223–230. doi: 10.1007/s004270050308. [DOI] [PubMed] [Google Scholar]
- Jollie Development of the head skeleton and pectoral girdle in Esox. J Morphol. 1975;147:61–68. doi: 10.1002/jmor.1051470106. [DOI] [PubMed] [Google Scholar]
- Jun Du S, Frenkel V, Kindschi G, Zohar Y. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Development. 2001;238:239–246. doi: 10.1006/dbio.2001.0390. [DOI] [PubMed] [Google Scholar]
- Kapoor AS. Development of dermal bones related to sensory canals of the head in the fishes Ophicephalus punctatus Bloch (Ophicephalidae) and Wallago attu Bl. & Schn. (Siluridae) Zool J Linn Soc-Lond. 1970;49:69–97. doi: 10.1111/j.1096-3642.1970.tb00731.x. [DOI] [Google Scholar]
- Korn H, Faber DS. An electrically mediated inhibition in goldfish medulla. J Neurophysiol. 1975;38 doi: 10.1152/jn.1975/38.2.452. [DOI] [PubMed] [Google Scholar]
- Kowalko JE, Rohner N, Rompani SB, Peterson BK, Linden TA, Yoshizawa M, Key EH, Weber J, Hoekstra HE, Jeffery WR, Borowsky R, Tabin CJ. Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms. Curr Biol. 2013;23:1874–1883. doi: 10.1016/j.cub.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander B. The sensory line system and the canal bones in the head of some ostariophysi. Acta Zool-Stockholm. 1949;30:1–131. doi: 10.1111/j.1463-6395.1949.tb00503.x. [DOI] [Google Scholar]
- Mabee PM, Olmstead KL, Cubbage CC. An experimental study of intraspecific variation, developmental timing, and heterochrony in fishes. Evolution. 2000;54:2091–2106. doi: 10.1111/j.0014-3820.2000.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Marranzino AN, Webb JF. Flow sensing in the deep sea: the lateral line system of stomiiform fishes. Zool J Linn Soc-Lond. 2018:zlx090. doi: 10.1093/zoolinnean/zlx090. [DOI]
- McHenry MJ, Feitl KE, Strother JA, Van Trump WJ. Larval zebrafish rapidly sense the water flow of a predator’s strike. Biol Letters. 2009;5 doi: 10.1098/rsbl.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrilees MJ. Tissue interaction: Morphogenesis of the lateral-line system and labyrinth of vertebrates. J Exp Zool. 1975;192:113–118. doi: 10.1002/jez.1401920112. [DOI] [Google Scholar]
- Mitchell RW, Russell WH, Elliot WR. Mexican eyeless characin fishes, genus Astyanax: environment, distribution, and evolution. Texas Tech Press; Lubbock: 1977. [Google Scholar]
- Montgomery JC, Saunders AJ. Functional morphology of the piper Hyporhamphus ihi with references to the role of the lateral line in feeding. Proc Royal Soc B. 1985;224 doi: 10.1098/rspb.1985.0029. [DOI] [PubMed] [Google Scholar]
- Montgomery J, Coombs S, Halstead M. Biology of the mechanosensory lateral line in fishes. Rev Fish Biol Fisher. 1995;5:399–416. [Google Scholar]
- Nelson G. Infraorbital bones and their bearing on the phylogeny and geography of osteoglossomorph fishes. Am Mus Novit. 1969;2394 [Google Scholar]
- Nelson G. Cephalic sensory canals, pitlines, and the classification of esocoid fishes, with notes on galaxiids and other teleosts. Am Mus Novit. 1972;2492 [Google Scholar]
- Parker GH. The function of the lateral-line organs in fishes. Bulletin of the Bureau of Fisheries. 1904;24:1–183. [Google Scholar]
- Parrington FR. A theory of the relations of lateral lines to dermal bones. J Zool. 1949;119:65–78. doi: 10.1111/j.1096-3642.1949.tb00868.x. [DOI] [Google Scholar]
- Pehrson T. The development of laterosensory canal bones in the skull of esox litcius. Acta Zool-Stockholm. 1944;25:135–157. doi: 10.1111/j.1463-6395.1944.tb00350.x. [DOI] [Google Scholar]
- Powers AK, Davis EM, Kaplan SA, Gross JB. Cranial asymmetry arises later in the life history of the blind Mexican cavefish, Astyanax mexicanus. PLos One. 2017;12:e0177419. doi: 10.1371/journal.pone.0177419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. doi: 10.1002/(SICI)1096-9861(20000529)421:2<189::AID-CNE5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Reno HW. The infraorbital canal, its lateral line ossicles and neuromasts, in the minnows Nortropis volucellus and N. buchanani, Copeia. 1966;1966:403–413. [Google Scholar]
- Ritz DA, Hobday AJ, Montgomery JC, Ward AJW. Chapter Four – Social aggregation in the pelagic zone with special reference to fish and invertebrates. Adv Marine Biol. 2011;60:161–227. doi: 10.1016/B978-0-12-385529-9.00004-4. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kayning V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J, White DJ, Hartenstein V, Eliceiri K, Tomancak P. Fiji: an open-source platform for biological-image analysis. Nat Method. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemmel C. Vergleichende untersuchunger an den hautsinnesorganene ober- und unterirdisch lebender Astyanax-formen. Z Morphol Tiere. 1967;61:255–316. doi: 10.1007/BF00400988. [DOI] [Google Scholar]
- Schwartz E. Die ortung von wasserwellen durch oberflächenfische. Z Vergl Physiol. 1971;74:64–80. doi: 10.1007/BF00297791. [DOI] [Google Scholar]
- Song J, Yan HY, Popper AN. Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hearing Res. 1995;91:63–71. doi: 10.1016/0378-5955(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Sumi K, Asaoka R, Nakae M, Sasaki K. Innervation of the lateral line system in the blind cavefish Astyanax mexicanus (Characidae) and comparisons with the eyed and surface-dwelling form. Ichthyol Res. 2015;62:420–430. doi: 10.1007/s10228-015-0458-7. [DOI] [Google Scholar]
- Sutterlin AM, Waddy S. Possible Role of the Posterior Lateral Line in Obstacle Entrainment by Brook Trout (Salvelinus fontinalis) J Fish Res Board Can. 1975;32:2441–2446. doi: 10.1139/f75-281. [DOI] [Google Scholar]
- Tarby ML, Webb JF. Development of the supraorbital and mandibular lateral line canals in the cichlid, archocentrus nigrofasciatus. J Morphol. 2003;255:44–57. doi: 10.1002/jmor.10045. [DOI] [PubMed] [Google Scholar]
- Teyke T. Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain Behav Evolut. 1990;35:23–30. doi: 10.1159/000115853. [DOI] [PubMed] [Google Scholar]
- Valdez-Moreno M, Contreras-Balderas S. Skull osteology of the characid fish Astyanax mexicanus (teleostei: charaidae) P Biol Soc Wash. 2003;116:341–355. [Google Scholar]
- Wada H, Ghysen A, Satou C, Higashijima S, Kawakami K, Hamaguchi S, Sakaizumi M. Dermal morphogenesis controls lateral line patterning during postembryonic development of teleost. Dev Biol. 2010;340:583–594. doi: 10.1016/j.ydbio.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Wada H, Iwasaki M, Kawakami K. Development of the lateral line canal system through a bone remodeling process in zebrafish. Dev Biol. 2014;392:1–14. doi: 10.1016/j.ydbio.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Webb JF, Noden DM. Ectodermal placodes: contributions to the development of the vertebrate head. Am Zool. 1993;33:434–477. doi: 10.1093/icb/33.4.434. [DOI] [Google Scholar]
- Wilkens H. Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces) Evol Biol. 1988;23:271–367. [Google Scholar]
- Windsor SP, Tan D, Montgomery JC. Swimming kinematics and hydrodynamic imaging in the blind Mexican cave fish (Astyanax fasciatus) J Exp Biol. 2008;211:2950–2959. doi: 10.1242/jeb.020453. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Espinasa L, Stock DW, Jeffery WR. Development and evolution of craniofacial patterning is mediated by eye-dependent and – independent processes in the cavefish Astyanax. Evolution & Development. 2003;5:435–446. doi: 10.1046/j.1525-142X.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Goricki S, Soares D, Jeffery WR. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr Biol. 2010;20:1631–1636. doi: 10.1016/j.cub.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Yamamoto Y, O’Quin KE, Jeffery WR. Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish. BMC Biol. 2012;10:108. doi: 10.1186/1741-7007-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M. Behaviors of cavefish offer insight into developmental evolution. Mol Reprod Dev. 2015;82:268–280. doi: 10.1002/mrd.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scoring bone fusion events can be difficult due to the lability of the morphology in the cavefish suborbital chain. We measured individual bony area (mm2) as well as area of fused bones in order to determine if fused bones encompassed the same area as unfused bones. There was no significant difference in the total bony area for SO3+SO4 fusion (light blue) compared to the sum of SO3 bone area (dark blue) and SO4 area (gray; A; p=0.6543). There was also no significant difference for SO4+SO5 fusion (B; p=0.3709) and SO4-SO6 fusion (D; p=0.2293). Interestingly, fused SO5+SO6 bones were significantly smaller than unfused bones (C; p=0.0091). This may be due the variability of SO6 size and positioning in cavefish.
Surface fish display a typical pattern of two canal neuromasts within the SO3 bone: CN3a and CN3b (A). Cavefish SO3 bones frequently fragment into multiple, discrete elements. The SO3 bone for cavefish individual 163_07 is made up of 3 fragments and has only one canal neuromast, CN3b, with an absence CN3a (B). The SO3 bone for cavefish individual 163_03 is fragmented into 2 elements, but the canal neuromast pattern is normal with both CN3a and CN3b present (C). The SO3 bone for cavefish individual 138_04 is also fragmented into 2 separate elements, but has an aberrant pattern of canal neuromasts with the CN3a, CN3b and two ectopic CNs (D). Taken together, the pattern of canal neuromasts does not appear to impact SO3 bone fragmentation in cavefish. Neuromasts and bone were co-labeled with DASPEI and calcein. Images were taken at 30x and the white scale bar represents 1mm.