Abstract
Type 2 diabetes (T2D) patients have an increased fracture risk, which may be partly explained by compromised bone microarchitecture within the cortical bone compartment. Data on trabecular bone parameters in T2D are contradictory. By high-resolution peripheral quantitative computed tomography (HR-pQCT), trabecular microarchitecture is preserved, yet larger trabecular holes are detected in T2D by MRI and DXA-based trabecular bone scores are abnormal. To determine if there are differences in trabecular microstructure, connectivity, and alignment in postmenopausal women with T2D as compared with controls, we performed an individual trabecula segmentation (ITS) analysis on HR-pQCT scans of the distal radius and tibia in 92 women with (n=42) and without (n=50) T2D. Unadjusted analyses showed that T2D subjects had greater total trabecular bone volume, trabecular plate volume fraction, plate number density, plate junction density, and axial alignment at the radius and tibia, and increased plate tissue fraction, but decreased rod tissue fraction and rod length at the radius (p<0.05 for all). After adjustments for clinical covariates, plate number density and plate junction density remained higher at the radius and tibia, whereas total trabecular bone volume was increased and trabecular rod length was decreased at the radius. These differences remained significant after adjustment for hip BMD and trabecular volumetric bone density. Notably, the increased plate-like ITS qualities were seen in those with T2D duration of <10 years, whereas ITS parameters in subjects with T2D duration ≥10 years did not differ from those of control subjects. In conclusion, postmenopausal women with early T2D had a greater plate-like and less rod-like trabecular network. This early advantage in trabecular plate quality does not explain the well-established increased fracture risk in these patients and does not persist in the later stage of T2D.
Keywords: TYPE 2 DIABETES, INDIVIDUAL TRABECULA SEGMENTATION, HIGH-RESOLUTION PERIPHERAL QUANTITATIVE COMPUTED TOMOGRAPHY, TRABECULAR MICROSTRUCTURE
Introduction
Diabetes mellitus is a metabolic disease that impacts over 425 million individuals.(1) The major consequences of diabetes are well-known, namely microvascular complications including retinopathy and nephropathy. Recently, skeletal fragility has become recognized as another major complication of type 2 diabetes (T2D).(2–6) Fracture risk is increased at multiple skeletal sites,(2) including a 40% to 70% increased fracture risk at the hip.(3,6) Given that a quarter of older adults in the United States have T2D,(7) the age group with the highest fracture risk,(8) understanding the underlying mechanisms for diabetic fracture is essential.
Structural bone abnormalities likely contribute to T2D skeletal fragility. Areal bone density by DXA is normal in T2D,(9) but cross-sectional bone area by central and peripheral QCT is reduced.(10,11) Moreover, microarchitectural assessment by high-resolution peripheral quantitative computed tomography (HR-pQCT) of the distal radius and tibia suggests the presence of diabetes-specific skeletal defects. Cortical porosity is increased in T2D, particularly in subjects with fractures or microvascular complications.(12–17)
Despite consistent findings on cortical bone, data on trabecular bone structure are contradictory. Using HR-pQCT, we and others have found that trabecular microarchitecture is preserved in T2D patients.(12–14,18,19) However, in studies using MRI, postmenopausal women with T2D were found to have larger holes within the trabecular network at the distal radius than nondiabetic women.(20) Moreover, trabecular bone score (TBS), a technique that uses spine DXA images to perform a gray-level textural analysis of trabecular bone microarchitecture, was abnormal in T2D and predictive of fractures in a number of reports.(21–23) It thus is unclear whether trabecular bone morphology is altered and how it relates to bone strength in T2D.
Individual trabecula segmentation (ITS) is an approach to characterizing the morphology of trabecular bone, including the orientation, connectivity, and plate- and rod-like structure of individual trabeculae. Previous studies have shown that variations in plate and rod characteristics may help explain skeletal differences between Chinese and Caucasian women,(24) premenopausal women with idiopathic osteoporosis,(25) and amenorrheic female athletes.(26) In particular, alterations in plate and rod structure were found to discriminate postmenopausal women with prevalent fragility fracture despite no differences in DXA-derived BMD T-scores.(27,28) This tool has not been used to study the T2D population. Therefore, the goal of this studywas to characterize the trabecular rod- and plate-like structure of the radius and tibia of postmenopausal women with T2D using ITS analysis and to determine whether alterations in these variables may explain the increased fracture risk observed in this patient population.
Subjects and Methods
Study population
Forty-two postmenopausal women with T2D and 50 postmenopausal controls were studied. Participants were recruited by advertisement at the Columbia University Medical Center. The diagnosis of T2D was established as defined according to the American Diabetes Association criteria(29): fasting plasma glucose concentration ≥126mg/dL, HbA1c ≥6.5%, or current treatment by oral antidiabetic drugs or insulin. We excluded subjects with type 1 diabetes (T1D) defined by a history of ketoacidosis, age of onset of diabetes before 25 years, BMI below 20 kg/m2, and use of insulin without a concomitant oral hypoglycemic agent. Exclusion criteria for both T2D and controls included renal or hepatic abnormalities (glomerular filtration rate <60 mL/min · 1.73m2 as calculated by the modification of diet in renal disease calculation or liver function tests above the upper limit of normal); therapy with rosiglitazone or pioglitazone; disorders of bone or mineral metabolism (primary hyperparathyroidism, Paget’s disease, osteomalacia, or osteogenesis imperfecta); abnormal thyroid function tests; medical history or clinical findings consistent with Cushing’s syndrome; continuous glucocorticoid use at any dose for>3months over the past 3 years; history of intestinal disorders (celiac disease, exocrine pancreatic insufficiency, or inflammatory bowel disease); current use of anticonvulsants, anticoagulants, loop diuretics, or methotrexate; current use of estrogen preparations, raloxifene, calcitonin, denosumab, or teriparatide; and current or previous use of a bisphosphonate within 36 months. The study was approved by the Columbia University Medical Center Institutional Review Board; written informed consent was obtained from all participants.
Clinical evaluation
Information regarding medical history, including fracture history, calcium intake, and medication use was collected. Weight and height were measured by a balance-beam scale and a wall-mounted, calibrated Harpenden stadiometer, respectively (Holtain Ltd, Crymych, UK).
Biochemical evaluation
PTH was measured by immunoradiometric assay (Scantibodies Laboratories, Santee, CA, USA; coefficient of variation [CV]=6.8%) and 25-hydroxyvitamin D was measured by UPLC/tandem mass spectrometry, validated against National Institute of Standards and Technology reference material (interassay CV=2.9% for 25-hydroxyvitamin D2 and 5.4% for 25-hydroxyvitamin D3). All biochemical markers of bone turnover were measured in duplicate from a morning fasting serum specimen. Amino-terminal propeptide of type I procollagen (P1NP) was measured by radioimmunoassay (Immunodiagnostic Systems [IDS]; CV=7.0%). Serum C-telopeptide (CTX) was measured by ELISA (IDS; CV <10%).
DXA and HR-pQCT
DXA (Hologic QDR 4500, Waltham, MA, USA) was performed using our standard methods.(18) HR-pQCT (Scanco Medical, Bruttisellen, Switzerland) scans were acquired for all subjects using the manufacturer’s in vivo imaging protocol.(30,31) Each subject was scanned on their nondominant forearm and ankle. The wrist or ankle was placed in a carbon-fiber cast to minimize limb motion during scan acquisition. If the subject had a history of fracture in the nondominant limb, then the dominant limb was scanned. A standard anteroposterior scout view was taken (using fixed settings of the machine), and the operator placed the reference line at the endplate of the distal radius or distal tibia.(32) Cortical and trabecular volumetric BMD (vBMD) and microarchitecture was assessed from HR-pQCT images, including cortical porosity; estimated bone stiffness was determined from finite element analysis. Cortical load fraction at the proximal and distal ends of the scan region was obtained by generating different material IDs for trabecular and cortical compartments to provide the stress distribution for both compartments. At the initiation of the study, subjects were scanned using a first-generation HR-pQCT scanner with a nominal isotropic resolution of 82 μm. Toward the end of the study, we transitioned to a second-generation HR-pQCT scanner (XCT2), from the same manufacturer, with a higher nominal isotropic resolution of 61 μm.(33) Ultimately, 76 subjects were scanned on the XCT1 scanner (44 controls, 32 T2D) and 16 subjects (6 controls, 10 T2D) on the XCT2 scanner. Using the XCT1 scanner, a 9.02-mm section (110 slices) was scanned beginning at 9.5-mm proximal to the radius reference line and 22.5-mm proximal to the tibia reference line. Using the XCT2 scanner, a 10.24-mm section (168 slices) was scanned beginning at 9.0-mm proximal to the radius reference line and 22.0-mm proximal to the tibia reference line, thus centered on the XCT1 region. As most of our subjects were scanned on the XCT1, the XCT2 data were calibrated to the XCT1 scanner. The calibration was based on a previously published study in which we compared XCT1 and XCT2 measurements in 51 patients and controls selected in our laboratory to cover the range of bone size and mineral content, with regression equations developed from duplicate scans on each machine, and found excellent agreement between the scanners (R2≥0.91 for almost all measurements).(33) The standard HR-pQCT results presented for the XCT2-scanned subset are based on our analysis of the 61-μm data using the direct analysis technique, then adjusted based on the calibration.
Individual trabecula segmentation-based morphological analyses of HR-pQCT images
The trabecular bone compartment was extracted from each HR-pQCT image using an autosegmentation method that accurately separates trabecular bone from cortical bone.(34,35)
Trabecular bone images were then analyzed using the ITS technique that yields a number of parameters representing the detailed morphology of each trabeculae. A complete volumetric decomposition technique was applied to segment the trabecular network into individual plates and rods. Detailed methods describing this technique and ITS-based measurements can be found in our prior publications.(36–38) Based on the 3D evaluations of each individual trabecular plate and rod, bone volume and plate and rod number were evaluated by plate and rod bone volume fraction as well as plate and rod number densities (1 per mm). Intactness of trabecular network was characterized by plate–plate, plate–rod, and rod–rod junction density (1 permm3), calculated as the total number of junctions between trabecular plates and rods normalized by the bulk volume. Orientation of the trabecular bone network was characterized by axial bone volume fraction, defined as axially aligned bone volume divided by the bulk volume. To compare ITS measurements across scanner generation, we used our calibration study,(33) although the ITS calibration data have not been published yet. Specifically, we downscaled the XCT2 scans to XCT1 resolution for the ITS analysis, slice matched to the same scan region as the XCT1 scanner, and calibrated the results to the XCT1 scanner based on our cohort of 51 adults.
Statistical analyses
Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA) and R (2015; Vienna, Austria). Categorical data were compared using the χ2 test and Fisher’s exact test; continuous data were compared by t tests. Missing data were addressed by multiple imputations. Multivariable linear regression was used for the adjustment of clinical covariates (age, years postmenopause, weight, race, calcium supplements, D supplements), total hip BMD, and trabecular volumetric bone density (radius or tibia); all clinical covariates were analyzed as continuous measures. ANOVA was used for comparisons based on T2D duration (controls; T2D <10 years; T2D ≥10 years) with post hoc Tukey’s test. The 10-year cut-off was selected based on a sensitivity analysis that examined different splits between shorter and longer durations of T2D for each HRpQCT scan and ITS measure in 5-year groupings as follows: 0 to 4 versus ≥5 years, 0 to 9 versus ≥10 years, 0 to 14 versus ≥15 years, 0 to 19 versus≥20 years, and 0 to 24 versus ≥25 years. At the radius and tibia, trabecular volumetric bone density, stiffness, trabecular plate number density, and plate–plate junction density had the greatest differences at the 10- and 15-year cut-points. The 10-year cut-off was specifically selected as a clinically meaningful time point because fracture risk has been shown to increase after 10 years of diabetes duration.(39) Correlations of ITS analysis with standard HR-pQCT measures were obtained with Spearman correlation coefficients. For ITS predictors of stiffness, standardized variables for ITS parameters underwent stepwise multiple regression analysis to select significant predictors of stiffness for the radius and tibia, with diabetes status subsequently added to the model. A P value <0.05 was considered significant.
Results
Cohort characteristics, aBMD, and standard HR-pQCT results
The clinical characteristics of the T2D (n=42) and control (n=50) subjects are summarized in Table 1. Participants did not differ in age (overall age 61.3±7.7 years), although T2D patients had more years since menopause. The T2D group included more Hispanic patients, whereas the controls were predominantly Caucasian. The T2D patients tended to weigh more, with a trend towards a higher BMI. The incidence of prior fracture and the use of proton pump inhibitors, selective serotonin reuptake inhibitors, and tobacco were similar between groups. Most (85%) of the T2D patients were on metformin and 35% were on insulin. T2D patients had higher levels of fasting glucose and HbA1c (mean 8.4%) as expected, and a trend toward lower 25 (OH)D levels. T2D patients had significantly lower levels of P1NP and s-CTX, but higher total alkaline phosphatase levels. T2D women had significantly higher areal bone mineral density (aBMD) at the femoral neck and total hip (Table 2). Standard HR-pQCT measures showed that in T2D patients, trabecular bone volume fraction, trabecular vBMD, and stiffness were higher at the radius and tibia; trabecular thickness was also higher at the radius. Cortical load fraction tended to be lower at the radius (proximal and distal site) and tibia (proximal site), although cortical porosity and cortical vBMD did not differ (Table 2). With adjustment for clinical covariates (years postmenopause, race, and weight), the differences by aBMD persisted; using HR-pQCT, differences persisted at the radius, whereas at the tibia trabecular bone volume fraction remained increased, but trabecular vBMD and stiffness did not.
Table 1.
Characteristics of Type 2 Diabetic and Control Subjects±SD
| Demographics | Control (n=50) | T2D (n=42) | Normal values | p value |
|---|---|---|---|---|
| Age (years) | 60.6±6.8 | 61.9±8.5 | 0.54 | |
| Race | 0.001 | |||
| Caucasian (%) | 27 (54) | 11 (26) | ||
| Hispanic (%) | 19 (38) | 31 (74) | ||
| Asian (%) | 2 (4) | |||
| Indian (%) | 1 (2) | |||
| Native American (%) | 1 (2) | |||
| Duration of T2D (years) | N/A | 11.2±7.6 | N/A | |
| Postmenopause (years) | 11.6±6.6 | 15.5±9.3 | 0.04 | |
| History of smoking cigarettes, n (%) | 6 (12) | 7 (16) | 0.55 | |
| Previous fractures, n (%) | 13 (26) | 13 (30) | 0.65 | |
| PPI Use, n (%) | 5 (10) | 7 (16) | 0.37 | |
| SSRI use, n (%) | 13 (26) | 12 (28) | 0.86 | |
| Height (cm) | 158.3±7 | 157.0±6 | 0.32 | |
| Weight (kg) | 72.6±13.9 | 78.5±19.1 | 0.09 | |
| BMI (kg/m2) | 29.1±5.7 | 31.6±7.3 | 0.06 | |
| Waist circumference (cm) | 92.5±13.5 | 100.9±13.9 | 0.05 | |
| Calcium supplements (total daily dose, mg) | 401±640 [range, 0–3000; median, 0] | 238±430 [range, 0–1285; median, 0] | 0.16 | |
| Vitamin D supplements (total daily dose, IU) | 1,078±2242 [range: 400–10,000; median: 400] | 440±1037 [range: 0–5000; median: 0] | 0.1 | |
| Nephropathy, n (%) | N/A | 6 (13) | N/A | |
| Retinopathy, n (%) | N/A | 8 (17) | N/A | |
| Neuropathy, n (%) | N/A | 6 (13) | N/A | |
| Diabetes therapy with insulin, n (%) | N/A | 16 (35) | N/A | |
| Diabetes therapy with metformin, n (%) | N/A | 39 (85) | N/A | |
| Diabetes therapy with other oral agents, n (%) | N/A | 17 (37) | N/A | |
| Biochemistries | ||||
| Fasting glucose, mmol/L | 4.95±0.5 | 8.61±4.4 | 3.61–5.49 | <0.0001 |
| HbA1c, % | 5.8±0.3 | 8.4±1.6 | <5.7 | <0.0001 |
| Cr Cl, mL/min | 89±19 | 87±25 | 88–128 | 0.69 |
| TSH, mIU/L | 2.15±2.01 | 2.51±3.46 | 0.40–4.50 | 0.41 |
| Alkaline phosphatase, U/L | 76.3±26.9 | 93.0±33.6 | 33–130 | 0.009 |
| Aspartate aminotransferase, U/L | 22.6±14.1 | 21.7±11.5 | 10–35 | 0.76 |
| Alanine aminotransferase, U/L | 24.7±25.5 | 23.1±11.6 | 6–29 | 0.69 |
| Parathyroid hormone, ng/L | 40.7±21.4 | 42.3±26 | 14–64 | 0.57 |
| Calcium, mmol/L | 2.35±0.1 | 2.38±0.1 | 2.15–2.60 | 0.47 |
| Phosphorus, mmol/L | 1.20±0.1 | 1.20±0.2 | 0.81–1.45 | 0.10 |
| 25-hydroxyvitamin D, nmol/L | 82.1±47.7 | 65.4±31.7 | 74.9–250 | 0.05 |
| Albumin, g/L | 43±2 | 43±2 | 34–54 | 0.36 |
| P1NP, μg/L | 57.4±25.3 | 40.4±11.2 | 19–83 | 0.0007 |
| s-CTX, ng/mL | 0.595±0.295 | 0.394±0.170 | 0.112–0.738 | 0.0007 |
| Urinary pentosidine, pmol/mg Cr | 84.8±66 | 99.4±130.6 | 0.53 | |
T2D=type 2 diabetes; PPI=proton pump inhibitors; SSRI=selective serotonin reuptake inhibitors; TSH=thyroid-stimulating hormone.
Table 2.
DXA and HRpQCT in Type 2 Diabetic and Control Subjects±SD
| Control (n=50) | T2D (n=42) | P value | |
|---|---|---|---|
| aBMD by DXA | |||
| Lumbar spine (L1–L4) (g/cm2) | 0.946±0.109 | 0.965±0.143 | 0.47 |
| T-score | −0.9±1 | −0.7±1.3 | 0.47 |
| Femoral neck (g/cm2) | 0.679±0.078 | 0.730±0.131 | 0.004a |
| T-score | −1.4±0.7 | −0.8±1.1 | 0.009a |
| Total hip (g/cm2) | 0.851±0.093 | 0.940±0.168 | 0.003a |
| T-score | −0.8±0.7 | −0.1±1.3 | 0.005a |
| 1/3 radius (g/cm2) | 0.658±0.057 | 0.667±0.068 | 0.48 |
| T-score | −0.6±1.0 | −0.5±1.2 | 0.66 |
| HR-pQCT radius | |||
| Cortical porosity (%) | 2.0±1.3 | 1.9±1.1 | 0.51 |
| Cortical volumetric BMD (mgHA/cm3) | 844.2±77.6 | 840.7±100.6 | 0.85 |
| Cortical thickness (mm) | 0.93±0.17 | 0.99±0.21 | 0.16a |
| Cortical area (mm2) | 53.0±8.2 | 56.1±9.9 | 0.10 |
| Cortical load fraction (%) | |||
| Proximal | 92.4±7 | 89.8±6 | 0.06 |
| Distal | 40.7±9 | 38.6±9 | 0.06 |
| Trabecular bone volume fraction | 0.101±0.025 | 0.118±0.030 | 0.004a |
| Trabecular number (1/mm) | 1.77±0.32 | 1.90±0.39 | 0.08 |
| Trabecular thickness (mm) | 0.059±0.009 | 0.063±0.009 | 0.02a |
| Trabecular separation (mm) | 0.54±0.14 | 0.53±0.22 | 0.71 |
| Trabecular volumetric BMD (mgHA/cm3) | 121.4±29.5 | 139.9±38.3 | 0.01a |
| Trabecular area (mm2) | 193.1±43.8 | 181.1±35.6 | 0.16a |
| Total area (mm2) | 245.7±43.7 | 236.5±33.4 | 0.26 |
| Stiffness kN/m | 32,680.6±6,439 | 36,447.9±8,015 | 0.046 a |
| HR-pQCT tibia | |||
| Cortical porosity (%) | 5.6±2.4 | 6.2±2.5 | 0.27 |
| Cortical volumetric BMD (mgHA/cm3) | 806.4±66.0 | 777.4±104.5 | 0.11 |
| Cortical thickness (mm) | 1.25±0.20 | 1.26±0.32 | 0.87 |
| Cortical area (mm2) | 107.1±15.9 | 109.0±28.1 | 0.69 |
| Cortical load fraction (%) | |||
| Proximal | 73.3±9 | 69.3±11 | 0.06 |
| Distal | 33.2±8 | 31.0±10 | 0.27 |
| Trabecular bone volume fraction | 0.117±0.024 | 0.131±0.028 | 0.02a |
| Trabecular number (1/mm) | 1.74±0.38 | 1.80±0.43 | 0.41 |
| Trabecular thickness (mm) | 0.07±0.012 | 0.07±0.012 | 0.13 |
| Trabecular separation (mm) | 0.54±0.18 | 0.52±0.17 | 0.55 |
| Trabecular volumetric BMD (mgHA/cm3) | 139.5±29.0 | 153.3±35.8 | 0.04 |
| Trabecular area (mm2) | 547.3±102.7 | 565.1±107.0 | 0.41 |
| Total area (mm2) | 654.1±100.4 | 673.3±101.3 | 0.36 |
| Stiffness kN/m | 98,740.2±15,923 | 108,922.3±24,909 | 0.002 |
Bold indicates significance at p<0.05.
Significant after adjusting for clinical covariates (years postmenopause, race and weight).
ITS parameters in type 2 diabetes and controls
In unadjusted analyses, total trabecular bone volume (BV/TV) was greater in T2D at the radius and tibia (Table 3). At both the radius and tibia, T2D had a greater plate volume fraction, plate number density, plate–plate junction density, and plate–rod junction density. T2D also had greater axial alignment of trabeculae at both the radius and the tibia, along with greater plate tissue fraction at the radius. Controls, on the other hand, had greater trabecular rod tissue fraction and rod length at the radius.
Table 3.
ITS Results at the Distal Radius and Tibia in Patients With T2D and Controls
| Bone parameter | Control (n=50) | T2D (n=42) | T2D vs. Control % differencea | Clinical covariates β± SEMb | Clinical covariates+vitamin D level β ± SEMc | Clinical covariates+calcium and vitamin D supplements β±SEMd | Clinical covariates+hip aBMD β±SEMe | Clinical covariates+Tb vBMD β±SEMf |
|---|---|---|---|---|---|---|---|---|
| Radius | ||||||||
| BV/TV | 0.254±0.07 | 0.299±0.08 | 17.8 | 0.032±0.016 | 0.031±0.016 | 0.029±0.017 | 0.010±0.015 | 0.005±0.014 |
| pBV/TV | 0.089±0.05 | 0.122±0.07 | 37.4 | 0.023±0.013 | 0.022±0.013 | 0.021±0.013 | 0.008±0.013 | 0.009±0.013 |
| rBV/TV | 0.163±0.03 | 0.172±0.04 | 5.8 | 0.009±0.006 | 0.009±0.006 | 0.008±0.006 | 0.004±0.007 | −0.003±0.005 |
| pBV/BV | 0.331±0.10 | 0.380±0.13 | 14.8 | 0.035±0.024 | 0.034±0.024 | 0.035±0.025 | 0.016±0.025 | 0.021±0.025 |
| rBV/BV | 0.669±0.10 | 0.620±0.12 | −7.3 | −0.036±0.024 | −0.034±0.024 | −0.035±0.025 | −0.016±0.025 | −0.021±0.025 |
| aBV/TV | 0.094±0.04 | 0.115±0.05 | 22.2 | 0.015±0.009 | 0.014±0.010 | 0.013±0.010 | 0.003±0.009 | 0.003±0.009 |
| pTbN, 1/mm | 1.305±0.14 | 1.386±0.16 | 6.2 | 0.090±0.032 | 0.083±0.033 | 0.0.82±0.033 | 0.055±0.033 | 0.014±0.019 |
| rTbN, 1/mm | 1.860±0.12 | 1.905±0.16 | 2.5 | 0.041±0.029 | 0.040±0.030 | 0.035±0.030 | 0.021±0.031 | −0.012±0.024 |
| pTbTh, mm | 0.222±0.03 | 0.234±0.05 | 5.2 | 0.003±0.008 | 0.003±0.009 | 0.003±0.009 | −0.005±0.009 | 0.002±0.009 |
| rTbTh, mm | 0.210±0.01 | 0.209±0.01 | −0.4 | −0.000±0.001 | −0.000±0.001 | −0.000±0.001 | −0.001±0.001 | −0.001±0.001 |
| pTbS, mm2 | 0.158±0.02 | 0.163±0.03 | 3.3 | 0.000±0.004 | 0.001±0.004 | 0.001±0.005 | −0.002±0.005 | 0.002±0.005 |
| rTbL, mm | 0.668±0.02 | 0.655±0.03 | −1.9 | −0.014±0.005 | −0.013±0.005 | −0.013±0.005 | −0.010±0.005 | −0.004±0.003 |
| R-R JuncD, 1/mm3 | 3.00±0.64 | 3.19±0.85 | 6.5 | 0.169±0.154 | 0.169±0.157 | 0.148±0.157 | 0.093±0.163 | −0.044±0.143 |
| P-R JuncD, 1/mm3 | 3.22±0.81 | 3.86±1.05 | 19.9 | 0.640±0.192 | 0.608±0.194 | 0.587±0.196 | 0.405±0.190 | 0.149±0.086 |
| P-P JuncD, 1/mm3 | 1.50±0.43 | 1.83±0.55 | 22.3 | 0.346±0.104 | 0.325±0.105 | 0.320±0.106 | 0.228±0.104 | 0.089±0.052 |
| Tibia | ||||||||
| BV/TV | 0.212±0.06 | 0.304±0.07 | 11.9 | 0.020±0.014 | 0.019±0.014 | 0.016±0.014 | −0.001±0.013 | 0.000±0.011 |
| pBV/TV | 0.12±0.05 | 0.15±0.05 | 21.7 | 0.02-±0.012 | 0.019±0.012 | 0.019±0.012 | 0.006±0.012 | 0.008±0.011 |
| rBV/TV | 0.15±0.04 | 0.15±0.04 | 2.7 | −0.001±0.008 | 0.000±0.008 | −0.002±0.008 | −0.005±0.008 | −0.006±0.007 |
| pBV/BV | 0.450±0.12 | 0.493±0.11 | 9.6 | 0.041±0.026 | 0.038±0.026 | 0.042±0.027 | 0.029±0.027 | 0.033±0.027 |
| rBV/BV | 0.552±0.12 | 0.510±0.11 | −7.6 | −0.040±0.026 | −0.038±0.026 | −0.041±0.026 | −0.029±0.027 | −0.033±0.026 |
| aBV/TV | 0.12±0.03 | 0.13±0.04 | 14.4 | 0.014±0.008 | 0.013±0.008 | 0.012±0.008 | 0.003±0.008 | 0.004±0.007 |
| pTbN, 1/mm | 1.42±0.12 | 1.49±0.12 | 4.9 | 0.074±0.026 | 0.070±0.026 | 0.065±0.026 | 0.048±0.027 | 0.032±0.017 |
| rTbN, 1/mm | 1.76±0.19 | 1.78±0.20 | 1.0 | −0.001±0.040 | 0.004±0.040 | −0.008±0.040 | −0.011±0.004 | −0.025±0.040 |
| pTbTh, mm | 0.23±0.03 | 0.24±0.03 | 3.0 | 0.001±0.006 | 0.001±0.006 | 0.002±0.006 | −0.004±0.006 | 0.000±0.006 |
| rTbTh, mm | 0.21±0.01 | 0.21±0.01 | 0.2 | 0.000±0.001 | 0.000±0.002 | 0.000±0.002 | −0.001±0.001 | −0.001±0.001 |
| pTbS, mm2 | 0.17±0.02 | 0.18±0.02 | 1.9 | 0.001±0.005 | 0.001±0.006 | 0.003±0.006 | −0.001±0.006 | 0.003±0.006 |
| rTbL, mm | 0.65±0.08 | 0.66±0.03 | 1.1 | 0.001±0.013 | 0.001±0.014 | 0.001±0.014 | 0.001±0.014 | 0.004±0.014 |
| R-R JuncD, 1/mm3 | 2.44±0.92 | 2.51±1.01 | 2.6 | −0.057±0.199 | −0.039±0.203 | −0.086±0.203 | −0.087±0.213 | −0.137±0.206 |
| P-R JuncD, 1/mm3 | 3.45±0.75 | 3.86±0.92 | 11.8 | 0.351±0.167 | 0.345±0.169 | 0.285±0.166 | 0.197±0.170 | 0.095±0.124 |
| P-P JuncD, 1/mm3 | 1.87±0.37 | 2.13±0.46 | 13.8 | 0.263±0.088 | 0.252±0.088 | 0.232±0.089 | 0.163±0.089 | 0.108±0.005 |
BV/TV=bone volume fraction; pBV/TV=plate bone volume fraction; rBV/TV=rod bone volume fraction; pBV/BV=plate tissue fraction; rBV/BV=rod tissue fraction; aBV/TV=axial bone volume fraction; pTbN=trabecular plate number density; rTbN=trabecular rod number density; pTbTh=trabecular plate thickness; rTbTh=trabecular rod thickness; pTbS=trabecular plate surface area; rTbL=trabecular rod length; R–R JuncD=rod–rod junction density; P-R JuncD=plate-rod junction density; P–P JuncD=plate–plate junction density.
Bold indicates significance at p<0.05.
Multivariable-adjusted model for T2D relative to Control includes clinical covariates: years postmenopause, race, and weight; bold indicates significance at p<0.05.
Bold indicates significance at p<0.05 when vitamin D level was added to clinical covariates.
Bold indicates significance at p<0.05 when calcium and vitamin D supplements were added to clinical covariates.
Bold indicates significance at p<0.05 when total hip aBMD was added to clinical covariates.
Bold indicates significance at p<0.05 when Tb vBMD was added to clinical covariates.
Adjustment for clinical covariates eliminated some of the differences between T2D and controls in trabecular bone morphology, but other parameters remained significantly different. After adjustment for clinical covariates (including years postmenopause, race, and weight), T2D women continued to have findings consistent with superior plate structural characteristics, including increased plate number density, plate–plate junction density, and plate–rod junction density at both the radius and tibia, as well as greater total trabecular bone volume at the radius. Controls also continued to exhibit greater trabecular rod length at the radius. Adjusting for 25(OH)D level or for calcium and vitamin D supplements did not affect these results (Table 3), nor did adjusting for age instead of years postmenopause (data not shown).
Results were further adjusted for aBMD at the hip, in addition to clinical covariates, to determine if ITS analysis provides information that is incremental to what is available from DXA (Table 3). In these analyses, plate number density and plate–plate junction density at both the radius and tibia, as well as plate–rod junction density and trabecular rod length at the radius tended to remain different. When trabecular vBMD was added to clinical covariates in the multivariate analyses (Table 3), a favoring of plate-like trabecular networks in T2D (plate–plate junction density) persisted at the tibia.
Duration of type 2 diabetes and HR-pQCT parameters
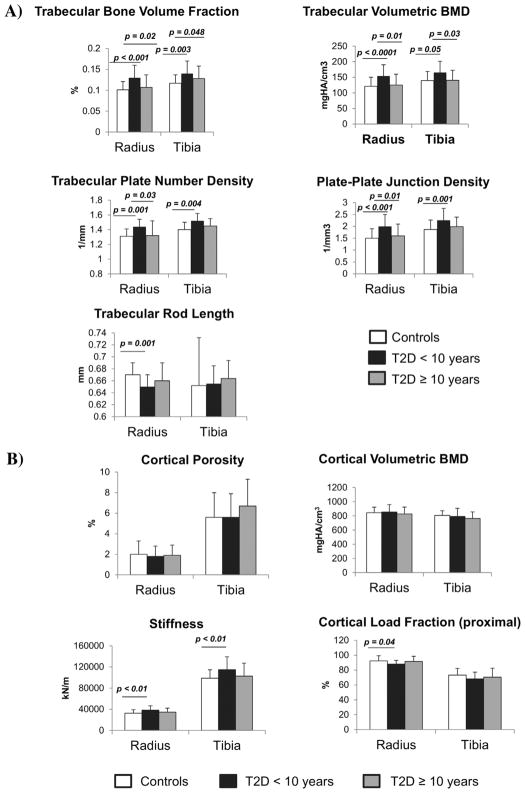
Within T2D subjects, the superior structural characteristics related to the duration of the diagnosis of T2D. Greater trabecular bone volume fraction, trabecular vBMD, and stiffness were present in subjects with T2D duration of<10 years (n=22), but not in those with T2D duration ≥10 years (n=20). Similarly, increased plate-like ITS qualities were present in the earlier stages of T2D, but these were eliminated with longer disease duration (Fig. 1A). Cortical vBMD and cortical porosity did not differ between subjects with T2D duration <10 years as compared with those with T2D duration ≥10 years, although cortical load fraction at the proximal site tended to be lower in early T2D (Fig. 1B). A comparison of only T2D with duration <10 years versus controls revealed differences in the same parameters as when T2D with duration<10 years was compared with T2D with duration ≥10 years, with more parameters becoming significant (radius: increased trabecular number, thickness, rod number density, axial bone volume fraction and cortical area, and decreased trabecular rod length; radius and tibia: increased plate bone volume fraction).
Figure 1.
Trabecular parameters and duration of type 2 diabetes (T2D). Values were compared between controls (n=50), early T2D (T2D duration <10 years; n=22), and late T2D (T2D duration ≥10 years; n=20). In comparison to controls, trabecular bone volume fraction, trabecular volumetric BMD, trabecular plate number density, and plate–plate junction density were increased in early T2D, whereas trabecular rod length was decreased (A). Cortical volumetric BMD, cortical porosity, and cortical load fraction at the distal site (not shown) did not differ between T2D subjects with greater or less than 10 years of disease duration, whereas cortical load fraction (at the proximal site) was decreased and stiffness was increased (B). There was no difference between late T2D and controls in any of the parameters. Values are mean±SD.
Association of ITS analysis with standard HR-pQCT measures
Trabecular and whole bone outcomes from standard HR-pQCT analyses were generally significantly associated with those obtained from ITS analyses at the radius (Table 4). Strong correlations were noted between trabecular vBMD or trabecular thickness and ITS-derived plate volume fraction, plate number, plate connectivity, and axial alignment (r=0.55 to 0.92, p<0.05 for all). Conversely, ITS-derived outcomes reflecting rod-like properties (rod volume fraction, rod number, and rod connectivity) were strongly positively associated with trabecular number (r=0.81 to 0.87, p<0.05), and inversely associated with trabecular separation (r=–0.83 to –0.90, p<0.05). These associations were similar for the tibia (data not shown).
Table 4.
Correlation Coefficients (r) for Standard Whole-Bone and Trabecular HR-pQCT Parameters and ITS Parameters at the Radius
| Standard HR-pQCT parameters
| ||||||||
|---|---|---|---|---|---|---|---|---|
| ITS parameter | Total area | Trab area | Total vBMD | Trab vBMD | Trab number | Trab thickness | Trab separation | Stiffness |
| Whole bone | ||||||||
| BV/TV | −0.11 | −0.14 | 0.74 | 0.73 | 0.69 | 0.54 | −0.50 | 0.06 |
| aBV/TV | −0.11 | −0.10 | 0.60 | 0.58 | 0.36 | 0.65 | −0.20 | 0.03 |
| Plate-like | ||||||||
| pBV/TV | −0.11 | −0.11 | 0.32 | 0.55 | 0.32 | 0.70 | −0.15 | 0.05 |
| pBV/BV | −0.11 | −0.09 | 0.26 | 0.25 | −0.04 | 0.68 | 0.20 | 0.02 |
| pTbN | −0.11 | −0.14 | 0.84 | 0.84 | 0.51 | 0.69 | −0.53 | 0.40 |
| pTbTh | −0.06 | −0.04 | 0.08 | 0.06 | −0.06 | 0.44 | 0.26 | −0.30 |
| pTbS | −0.03 | 0.02 | −0.11 | −0.13 | −0.26 | 0.33 | 0.46 | −0.31 |
| PP JuncD | −0.09 | −0.14 | 0.89 | 0.90 | 0.63 | 0.64 | −0.64 | 0.40 |
| RP JuncD | −0.04 | −0.11 | 0.91 | 0.92 | 0.75 | 0.55 | −0.77 | 0.38 |
| Rod-like | ||||||||
| rBV/TV | 0.03 | −0.04 | 0.66 | 0.67 | 0.87 | 0.03 | −0.90 | 0.19 |
| rTbN | 0.03 | −0.06 | 0.61 | 0.63 | 0.87 | −0.04 | −0.90 | 0.21 |
| rTbTh | 0.03 | 0.07 | 0.20 | 0.19 | −0.15 | 0.51 | 0.15 | 0.02 |
| rTbL | −0.77 | −0.79 | −0.79 | −0.26 | 0.83 | −0.38 | ||
| RR JuncD | 0.05 | −0.03 | 0.49 | 0.51 | 0.81 | −0.16 | −0.83 | 0.14 |
Bolded values indicate significance at p<0.05.
HR-pQCT=high-resolution peripheral quantitative computed tomography; ITS=individual trabecula segmentation; BV/TV=bone volume fraction; pBV/TV=plate bone volume fraction; rBV/TV=rod bone volume fraction; pBV/BV=plate tissue fraction; rBV/BV=rod tissue fraction; aBV/TV=axial bone volume fraction; pTbN=trabecular plate number density; rTbN=trabecular rod number density; pTbTh=trabecular plate thickness; rTbTh=trabecular rod thickness; pTbS=trabecular plate surface area; rTbL=trabecular rod length; R–R JuncD=rod–rod junction density; P–R JuncD=plate-rod junction density; P–P JuncD=plate–plate junction density.
ITS predictors of stiffness
Independent ITS predictors of stiffness at the radius by stepwise multiple regression analysis included BV/TV (standardized effect 0.15, p=0.15) and trabecular plate surface area (pTbS) (standardized effect −0.40, p<0.0001). At the tibia, BV/TV and trabecular plate number density (pTbN) were independent ITS predictors (standardized effect=−0.30, p=0.004 and 0.45, p<0.0001, respectively). These predictors together explained 16%of the variation in estimated stiffness at the radius and 19%at the tibia. Diabetes was not a significant predictor of stiffness apart from the other variables (radius: standardized effect 0.13, p=0.24; tibia: standardized effect=0.04, p=0.70).
Discussion
In this study, ITS-based morphological analyses of HR-pQCT images revealed significant differences in trabecular bone morphology between postmenopausal T2D subjects and controls. T2D subjects had more advantageous trabecular plate-like qualities and less rod-like structural characteristics, with key differences persisting after adjustment for clinical covariates. The differences also persisted after adjustment for hip BMD and trabecular vBMD, suggesting that these alterations in trabecular morphology provide insight beyond that available from DXA and standard HR-pQCT analyses. The observed trabecular differences were present only in T2D subjects with a shorter duration of disease, whereas those with T2D for ≥10 years did not differ from controls in ITS parameters. These trabecular plate qualities, which suggest normal or improved microstructure, thus do not explain the increased fracture risk in T2D.
To our knowledge, this is the first study to use ITS analysis to compare trabecular bonemorphology between T2D and non-T2D women. Using standard analyses of HR-pQCT scans, prior studies in T2D women have demonstrated poor cortical bone quality, particularly increased cortical porosity in most,(12,14,16,17,40) although not all,(41) T2D women. In contrast, trabecular BMD and trabecular microarchitecture were found to be similar or even improved in T2D subjects.(12–14,18) In the current study, standard HR-pQCT trabecular parameters were similar between the two groups, with increases in T2D in trabecular bone volume fraction and trabecular volumetric density at the radius and tibia and trabecular thickness at the radius. Notably, ITS analyses revealed additional differences in trabecularmorphology and alignment not available in standard analyses, namely that T2D women had significantly increased plate number density, plate–plate junction density, and plate–rod junction density at the radius and tibia and decreased rod length at the radius.
Conflicting data exist regarding trabecular bone quality in T2D. In prior studies using standard HR-pQCT measures, trabecular microarchitecture has been shown to be preserved in T2D.(12–14,18,19,40) The scant existing histomorphometry data show no alterations in trabecular bone indices in T2D.(42) However, studies employing other tools suggest that it may be impaired. Using MRI, postmenopausal women with T2D (mean duration of T2D:16.6 years) were found to have 13% larger holes within the trabecular network at the distal radius than nondiabetic women.(20) Moreover, TBS captured a larger portion of the diabetes-associated fracture risk than BMD in a number of reports.(21–23) However, the use of such methods might not be optimal for the assessment of trabecular parameters. MRI assessment of trabecular bone network hole size is a 2D measurement and TBS may not capture aspects of trabecular microstructure assessed by higher resolution imaging modalities. Our finding of advantageous trabecular plate-like qualities by ITS analysis provides evidence for preservation or even enhancement of trabecular parameters.
Extrapolating from other populations, our advantageous ITS analysis findings in T2D would be expected to be associated with a reduced fracture risk. Postmenopausal women with fragility fractures have lower plate number and plate connectivity, along with reduced axial alignment of trabecular bone(27) and a more rod-like trabecular network.(28) Similarly, a loss of plate-like trabecular bone qualities is present in patient populations with conditions predisposing to osteoporosis and fracture, including postmenopausal women with primary hyperparathyroidism,(43) young adults with cystic fibrosis,(44) and patients taking chronic oral glucocorticoids.(45) Therefore, the increased plate-like qualities we found in T2D do not explain the well-established increased fracture risk in these patients.
Notably, the changes in T2D trabecular morphology depended on the stage of diabetes evolution. ITS parameters, as well as standard HRpQCT trabecular parameters and stiffness, were increased in early (<10 years duration) but not late (≥10 years duration) T2D. This might be explained by the fact that protective factors, including large body size and hyperinsulinemia, predominate in early T2D. These features may lead to more plate-like trabeculae at the initial stages of diabetes. Trabecular parameters such as plate tissue fraction were also enhanced in early T2D subjects in comparison with controls. Consistent with this, superior trabecular characteristics have been reported in prediabetic subjects with insulin resistance.(46) With disease progression, however, risk factors including advanced glycation endproduct (AGE) accumulation and the development of chronic complications likely gain ascendency. These later factors could lead to the onset of accelerated aging in the skeleton, in which an overly plate-like trabecular network may convert to a more age-consistent rod-like network with attendant bone loss and skeletal fragility.(47) Consistent with this idea, fracture risk in T2D was found to increase with longer diabetes duration(48) and postmenopausal women with T2D had more rapid bone loss based on DXA than did controls(49); however, these results have not been found in all studies.(50) Notably, cortical load fraction in early T2D at the proximal site tended to be lower than in controls. This might suggest that there is an inverse relationship between trabecular and cortical compartments in T2D over time, with the early overly plate-like trabecular morphology biomechanically compensating for an early deficit in cortical proximal load capacity. Our data also show that standard HR-pQCT measures reflect aspects of trabecular microstructure in this population. In comparing standard trabecular HR-pQCT parameters with ITS parameters, as expected, plate-like ITS measures were highly correlated with trabecular thickness and trabecular density, whereas rod-like measures had strong correlations with trabecular number and separation. Associations between ITS measures and stiffness further showed that plate-like characteristics were a strong contributor to stiffness. When ITS analysis outcomes were considered as predictors, trabecular plate surface area and trabecular plate number were significant predictors of stiffness at the radius and tibia, respectively, in addition to trabecular volume being a predictor at both sites. These data suggest that the amount of plate-like trabecular bone is an important contributor to bone strength. Presumably, this protective effect is abolished in the later stage of T2D.
We considered the possibility that the differences we detected in ITS qualities were attributable to trabecularization of the cortical compartment. With age-related bone loss, intracortical and endosteal remodeling and fragmentation may occur as the cortical component gets smaller and the trabecular component increases in size.(51) Thus, the higher trabecular morphology measurements could have been because of preferential trabecularization of the subendocortical cortex in T2D versus non-T2D. Perhaps this concern could be minimized or further explored by examining the subendocortical region separately. However, the similar cortical thickness and cortical area in our T2D and non-T2D subjects makes this explanation unlikely.
The strengths of this study include the relatively large sample size, and the availability of DXA, HR-pQCT, and ITS analysis data on all subjects, along with the detailed clinical information regarding factors affecting the skeletal health of our cohort. The study also has several limitations. The cross-sectional nature of the design cannot account for the dynamic changes in bone that occur with the progression of T2D. It is also possible that the advantageous T2D trabecular findings were based on metformin use, which has a protective role in bone health.(52) Because almost all the T2D subjects were on metformin, this potentially confounding effect could not be excluded. Also, the microfinite element analyses used a fixed material property and therefore only reflect differences in bone structure. In addition, despite our crossover study, it remains possible that using two generations of HR-pQCT technology injected some variability in our results. Finally, our results pertain to peripheral skeletal sites only, which may not necessarily be representative of trabecular microstructure at the axial skeleton.
In conclusion, ITS analysis demonstrated that postmenopausal women with early T2D had greater plate-like trabecular morphology, whereas nondiabetic women had a more rod-like trabecular network. These early advantages of trabecular plate qualities are eliminated in the later stage of T2D.
Acknowledgments
This work was supported by NIH grant K24-DK074457.
Authors’ roles: Study design: MRR, AMS, JFS and SJS. Study conduct: MRR, AMS, LCB and JFS. Data collection: MRR, SA, and YH. Data analysis: SA, KN, YU DJM, and XEG. Data interpretation: MRR, DJM, and SJS. Drafting manuscript: AMS, LCB, JFS, SJS, and MRR. MRR takes responsibility for the integrity of the data analysis.
Footnotes
Disclosures
The authors have no disclosures.
References
- 1.International Diabetes Federation. [Accessed May 30, 2018];Diabetes Atlas. (8). 2017 Available at http://www.diabetesatlas.org/
- 2.Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture inwomen with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006 Sep;91(9):3404–10. doi: 10.1210/jc.2006-0614. Epub 2006 Jun 29. [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007 Apr;18(4):427–44. doi: 10.1007/s00198-006-0253-4. Epub 2006 Oct 28. [DOI] [PubMed] [Google Scholar]
- 4.Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older U.S. adults. Bone. 2016 Jan;82:9–15. doi: 10.1016/j.bone.2014.12.008. Epub 2015 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Laguna D, Tebe C, Javaid MK, et al. Incident type 2 diabetes and hip fracture risk: a population-based matched cohort study. Osteoporos Int. 2015 Feb;26(2):827–33. doi: 10.1007/s00198-014-2986-9. [DOI] [PubMed] [Google Scholar]
- 6.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007 Sep;166(5):495–505. doi: 10.1093/aje/kwm106. Epub 2007 Jun 19. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. National Diabetes Statistics Report. 2014 Available from: https://www.cdc.gov/diabetes/pdfs/data/2014-report-estimates-of-diabetes-and-its-burden-in-the-united-states.pdf.
- 8.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007 Jun;22(6):781–8. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 9.Ma L, Oei L, Jiang L, Estrada K, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012 May;27(5):319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton LJ, 3rd, Riggs BL, Leibson CL, et al. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab. 2008 Dec;93(12):4804–9. doi: 10.1210/jc.2008-0639. Epub 2008 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petit MA, Paudel ML, Taylor BC, et al. Bone mass and strength in older men with type 2 diabetes: The Osteoporotic Fractures in Men Study. J Bone Miner Res. 2010 Feb 1;25(2):285–91. doi: 10.1359/jbmr.090725. Epub 2009 July 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010 Nov;95(11):5045–55. doi: 10.1210/jc.2010-0226. Epub 2010 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farr JN, Drake MT, Amin S, Melton LJ, 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014 Apr;29(4):787–95. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patsch JM, Burghardt AJ, Yap SP, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013 Feb;28(2):313–24. doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanbhogue VV, Hansen S, Frost M, et al. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. 2016 Feb;174(2):115–24. doi: 10.1530/EJE-15-0860. [DOI] [PubMed] [Google Scholar]
- 16.Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015 Feb;26(2):673–9. doi: 10.1007/s00198-014-2927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samelson EJ, Demissie S, Cupples LA, et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR-pQCT study. J Bone Miner Res. 2018 Jan;33(1):54–62. doi: 10.1002/jbmr.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu A, Yin MT, Stein E, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012 Feb;23(2):635–41. doi: 10.1007/s00198-011-1595-0. Epub 2011 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patsch JM, Rasul S, Huber FA, et al. Similarities in trabecular hypertrophy with site-specific differences in cortical morphology between men and women with type 2 diabetes mellitus. PloS One. 2017;12(4):e0174664. doi: 10.1371/journal.pone.0174664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard JM, Giangregorio LM, Atkinson SA, et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res (Hoboken) 2012 Jan;64(1):83–91. doi: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie WD, Aubry-Rozier B, Lamy O, Hans D Manitoba Bone Density P. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013 Feb;98(2):602–9. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 22.Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014 Jul;25(7):1969–73. doi: 10.1007/s00198-014-2704-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Choi HJ, Ku EJ, et al. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015 Feb;100(2):475–82. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- 24.Walker MD, Liu XS, Zhou B, et al. Premenopausal and postmenopausal differences in bone microstructure and mechanical competence in Chinese-American and white women. J Bone Miner Res. 2013 Jun;28(6):1308–18. doi: 10.1002/jbmr.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XS, Cohen A, Shane E, et al. Individual trabeculae segmentation (ITS)-based morphological analysis of high-resolution peripheral quantitative computed tomography images detects abnormal trabecular plate and rod microarchitecture in premenopausal women with idiopathic osteoporosis. J Bone Miner Res. 2010 Jul;25(7):1496–505. doi: 10.1002/jbmr.50. Epub 2010 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell DM, Tuck P, Ackerman KE, et al. Altered trabecular bone morphology in adolescent and young adult athletes with menstrual dysfunction. Bone. 2015 Dec;81:24–30. doi: 10.1016/j.bone.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein EM, Kepley A, Walker M, et al. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res. 2014;29(5):1101–9. doi: 10.1002/jbmr.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XS, Stein EM, Zhou B, et al. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012 Feb;27(2):263–72. doi: 10.1002/jbmr.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014 Jan;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 30.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005 Dec;90(12):6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 31.Manske SL, Zhu Y, Sandino C, Boyd SK. Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone. 2015 Oct;79:213–21. doi: 10.1016/j.bone.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res. 2011 Jan;26(1):50–62. doi: 10.1002/jbmr.171. Epub 2010 July 2. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal S, Rosete F, Zhang C, et al. In vivo assessment of bone structure and estimated bone strength by first- and second-generation HR-pQCT. Osteoporos Int. 2016 Oct;27(10):2955–66. doi: 10.1007/s00198-016-3621-8. [DOI] [PubMed] [Google Scholar]
- 34.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007 Oct;41(4):505–15. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010 Apr;25(4):882–90. doi: 10.1359/jbmr.091020. [DOI] [PubMed] [Google Scholar]
- 36.Liu XS, Shane E, McMahon DJ, Guo XE. Individual trabecula segmentation (ITS)-based morphological analysis of microscale images of human tibial trabecular bone at limited spatial resolution. J Bone Miner Res. 2011 Sep;26(9):2184–93. doi: 10.1002/jbmr.420. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Zhou B, Liu XS, et al. Trabecular plates and rods determine elastic modulus and yield strength of human trabecular bone. Bone. 2015 Mar;72:71–80. doi: 10.1016/j.bone.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou B, Zhang Z, Wang J, et al. In vivo precision of digital topological skeletonization based individual trabecula segmentation (ITS) analysis of trabecular microstructure at the distal radius and tibia by HRpQCT. Pattern Recognit Lett. 2016 Jun 1;76:83–9. doi: 10.1016/j.patrec.2015.03.012. Epub 2015 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majumdar SR, Leslie WD, Lix LM, et al. Longer duration of diabetes strongly impacts fracture risk assessment: the Manitoba BMD cohort. J Clin Endocrinol Metab. 2016 Nov;101(11):4489–96. doi: 10.1210/jc.2016-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paccou J, Ward KA, Jameson KA, Dennison EM, Cooper C, Edwards MH. Bone microarchitecture in men and women with diabetes: the importance of cortical porosity. Calcif Tissue Int. 2016 May;98(5):465–73. doi: 10.1007/s00223-015-0100-8. Epub 2015 Dec 19. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson AG, Sundh D, Johansson L, et al. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women—a population-based study. J Bone Miner Res. 2017 May;32(5):1062–71. doi: 10.1002/jbmr.3057. Epub 2017 Jan 18. [DOI] [PubMed] [Google Scholar]
- 42.Manavalan JS, Cremers S, Dempster DW, et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012 Sep;97(9):3240–50. doi: 10.1210/jc.2012-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein EM, Silva BC, Boutroy S, et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res. 2013 May;28(5):1029–40. doi: 10.1002/jbmr.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Putman MS, Greenblatt LB, Sicilian L, et al. Young adults with cystic fibrosis have altered trabecular microstructure by ITS-based morphological analysis. Osteoporos Int. 2016 Aug;27(8):2497–505. doi: 10.1007/s00198-016-3557-z. Epub 2016 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutter S, Nishiyama KK, Kepley A, et al. Abnormalities in cortical bone, trabecular plates, and stiffness in postmenopausal women treated with glucocorticoids. J Clin Endocrinol Metab. 2014 Nov;99(11):4231–40. doi: 10.1210/jc.2014-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J Clin Endocrinol Metab. 2016 Aug;101(8):3114–22. doi: 10.1210/jc.2016-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 2016 Feb;4(2):159–73. doi: 10.1016/S2213-8587(15)00283-1. [DOI] [PubMed] [Google Scholar]
- 48.Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014;29(5):1054–60. doi: 10.1002/jbmr.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz AV, Sellmeyer DE, Strotmeyer ES, et al. Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005 Apr;20(4):596–603. doi: 10.1359/JBMR.041219. Epub 2005 March 15. [DOI] [PubMed] [Google Scholar]
- 50.Leslie WD, Morin SN, Majumdar SR, Lix LM. Effects of obesity and diabetes on rate of bone density loss. Osteoporos Int. 2018 Jan;29(1):61–7. doi: 10.1007/s00198-017-4223-9. Epub 2017 Sep 15. [DOI] [PubMed] [Google Scholar]
- 51.Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013 May;54(1):8–20. doi: 10.1016/j.bone.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Kahn SE, Zinman B, Lachin JM, et al. Rosiglitazone-associated fractures in type 2 diabetes: an analysis from A Diabetes Outcome Progression Trial (ADOPT) Diabetes Care. 2008 May;31(5):845–51. doi: 10.2337/dc07-2270. Epub 2008 Jan 29. [DOI] [PubMed] [Google Scholar]